Abstract
Biochar is a thermo-chemically synthesized/fabricated product of biomass. Recently, biochar applications have spanned into various disciplines such as environmental remediation, water purification, catalysis, tissue engineering, additive in organic waste compost, electrode material and modifier, etc. Modification of biochar is done for specific applications. It brings out the activation of the raw biochar through physical and chemical treatments. Physical modification is frequently done to achieve the superior quality of biochar. In recent years, the application of physical modification method has gained attention as a cost-effective and greener method. The current chapter comprehensively describes the recent developments in physical treatment processes of biochar, opportunities, challenges along with future prospects.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Rapid upsurge in urbanization and industrialization led to the adaptation of chemical-based agricultural-crop production since green revolution (Mishra et al. 2020). This has caused increased heavy metals, persistent organic pollutants (POPs) and pesticides into the environment and food chain (Bolan et al. 2022; Nie et al. 2020; Sun et al. 2020). For the safety of human health, food chain and environment, the World Health Organization (WHO) has raised it as a matter of serious public concern (WHO 2017). The depletion of soil fertility and productivity is caused due to use of agrochemical products in farming (Maddalwar et al. 2021; Lal 2015). Soil pollution has also caused decreased soil organic matter (SOM), imbalanced nutrient availability, increase global soil acidity by 30% and soil salinization by 20% (Agegnehu et al. 2017). This concern of soil nutrient depletion is directly associated with the world’s food insecurity. Therefore, many conventional methods are being used for the remediation of these agrochemicals for quick and better productivity. Methods like ion exchange, chemical precipitation and adsorption are used for the removal of chemical residues from the soil but they are neither so effective nor very economical (Kumar et al. 2021a; Oliveira et al. 2017). Furthermore, conventional sustainable agricultural amendments like composts and manure utilization are frequently used to improve the productivity. However, long-term application of these has led to increase in ammonia and methane level in soil which led to the upsurge of serious problems like contamination of groundwater and, emission of greenhouse gases (GHGs) (Awasthi et al. 2019). In this case, there is a need for the development of more sustainable and improved agricultural systems which would be utilized for uplifting the rural economies and also establish the proper global agriculture management (Kumar et al. 2021c; Kammann et al. 2015).
Recently application of biochar as adsorbents, additives and soil amendments has gained attention due to its green and cost-effective nature (Kumar et al. 2021b; Mishra et al. 2021; Ramola et al. 2013). Biochar is a porous carbonaceous material produced by thermochemical conversion of biomass (Bolan et al. 2021; Kumar et al. 2020b). In this process the carbon-rich biomass (raw material) is thermochemically decomposed in no or less oxygen environment. Woody biomass (Fuke et al. 2021), cattle residues, manures (Mishra et al. 2021), agriculture waste (Ramola et al. 2021), algae (Kumar et al. 2020a), sewage sludge (Kumar et al. 2020b) and municipal wastes (Kumar et al. 2020c) are generally used as raw material for synthesis of biochar. The chemical composition of biochar depends on the pyrolysis temperature and the type of raw material used (Bolan et al. 2021; Ramola et al. 2020). The reaction atmosphere (either oxidative or reductive), heating rate and duration are also few factors which play major role in the properties and further application of biochar (Mandal et al. 2021). Thermal treatment of biomass at high temperature produces biochar along with biofuels and syngas as by-products. In thermal treatment (<400 °C), chemical changes occur in the plant biomass for attaining structural stability which can be identified and confirmed by Fourier Transform Infrared (FTIR), thermal or elemental analyser Nuclear Magnetic Resonance (NMR), etc. (Qian et al. 2019; Mandal et al. 2021). To improve the efficiency of pristine biochar, post modification is desirable.
The activated biochar produced post modification (either physical or chemical) is efficient, cost-effective, eco-friendly adsorbent and its production could counterbalance the emission of greenhouse gases because of its ability to store carbon in stable form (Xiang et al. 2020; Bolan et al. 2021). There are several modification/treatments method of biochar such as physical, chemical and biological (Kumar et al. 2020b; Yang et al. 2019). However, application of physical treatment of biochar is considered as most effective and economical. The present chapter provides a comprehensive state of the arts related to physical modification technologies of biochar for its sustainable application. Furthermore, this chapter discusses the opportunities and challenges associated with physical modification methods of biochar along with prospects for future research.
2 Biochar Activation/Treatment
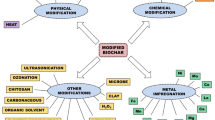
The pristine biochar does not have prominent physico-chemical and functional properties like surface functional group, surface area, porosity, cation exchange capacity and adsorption capacity in comparison to modified or engineered biochar. These properties can be enhanced by the treatment or activation of biochar that is performed in three ways i.e., physical, chemical and biological (Xiao et al. 2020) (Fig. 1). The activation of biochar improves the efficacy of biochar by improving the content of surface functional groups and removing the undesirable material from it (Huang et al. 2020). In comparison to other activation methods, physical activation is considered as more feasible and sustainable due to simpler process and effectiveness (Kumar et al. 2020b, c).
3 Physical Modification of Biochar
Due to high pyrolytic temperature in oxidative environment, the biomass losses more volatiles and gases and clogs the pores of end products (Liu et al. 2015). Therefore, to remove these carbon portions, and also increase the surface properties of biochar, various physical activation methods are applied (Sakhiya et al. 2020). Physical activation includes a sequence of process which is simplified into two steps i.e., activation and carbonization (Sakhiya et al. 2020). In post pyrolysis (partial carbonization), the raw biochar is activated and carbonized with the help of thermal treatment and gasification using inert gas like H2 or He, oxidizing medium like oxygen, steam and carbon dioxide. Apart from these, some other methods of the physical activations are also used such as microwave, plasma treatment, ultrasound and electrochemical (Sajjadi et al. 2019).
3.1 Physical Modification Type I—Gaseous Activation
In this process the biochar is exposed to oxidizing agents like steam, oxygen, ozone and carbon dioxide for a desired volume at 700 °C or above. These agents actually penetrate into the internal structure of biochar and widen the opening of inaccessible pores (Sajjadi et al. 2019).
Steam Activation
The depletion of carbon due to the reactions of water–gas shift is correlated with the pore formation in physical activation. The mechanism of steam activation takes place by three crucial steps i.e., diffusion, chemisorption and gas shift reactions (Cha et al. 2016). In the diffusion process, the water molecules get diffused into the pores and get chemisorbed in the carbon active sites with the formation of oxygen surface complex. This is followed by the decomposition of oxygen surface complex by carbon monoxide (CO) which increase the gasification rate by the production of CO2. The active states of carbon react with hydrogen and get deactivated. This gasification of carbon through water leads to the formation of micropores in biochar (Sajjadi et al. 2019; Akhil et al. 2021).
A study was done by Shim et al. (2015) to evaluate the physicochemical properties, toxicity and steam activation effect on biochar produced from Miscanthus sacchariflorus. The surface area of biochar was found to be less in comparison with the activated biochar, however, no significant difference was observed in the sorption capacities. A comparative model was developed which demonstrates that the steam-activated biochar dominates over the raw biochar in terms of efficiency, acute toxicity and the adsorption efficiency. Similar study was done by Lou et al. (2016) on pine sawdust biochar prepared at 300–550 °C and designed especially for efficient adsorption of phosphate. The results showed removal of phosphate up to 4%, without affecting the pH and other properties of biochar. Another study was carried out by Rajapaksha et al. (2015) for the removal of sulfamethazine (SMT) from the water with the help of steam-activated biochar prepared from invasive plant Sicyos angulatus L. The adsorption capacities of conventional and steam-activated biochar were compared and the results showed enhanced sorption capacity of 37.7 mg/g at pH 3, that was 55% more in comparison to pristine biochar (Table 1).
Carbon dioxide activation
CO2 can be used as an alternative for biomass pyrolysis and activation. Biochar pyrolyzed under CO2 has more O and N. High rate of carbonization refers to the low H/C ratio and high O/C ratio which shows high hydrophilic nature of biochar (Tian et al. 2017). The CO2-activated biochar has proven to be potential adsorbent for pollutants and other applications such as soil amendment. The mechanism of biochar (C) activation with CO2 is done by Boudouard reaction (C + CO2 ↔ 2CO). Here, the dissociation of CO2 takes place on carbon surface to form surface oxide which is desorbed from the surface due to the developed pore and the gaseous product CO is adsorbed on carbon active sites and reverts back to the gasification reaction (Lee et al. 2017). These CO2-activated biochar adsorbents have ability to trap CO2 and utilize it for enhancing physicochemical properties like microporosity, specific surface area, hydrophobicity and aromaticity. Research by Pallarés et al. (2018) showed the activation of barely straw biochar by two methods: steam and CO2. Significant increase in the final biochar yield by 43% was observed that proved that the CO2-activated biochar has much efficacy than the steam activation. Moreover, biochar raw material at different CO2 activation temperature produces various burn off and ash content as depicted in Table 2.
3.2 Physical Modification Type II—Ozone (O3) Activation
O3 has been extensively used in the activation of biochar for oxidizing the carbonaceous materials, as it attacks on both edge carbon atoms and basal plane carbon atoms present in the biochar (Jimenez-Cordero et al. 2015). This is a two-stage process i.e., fast gasification which is followed by the slow surface functionalization. The gasification stage involves the first-order reaction rate, implying that O3 attack on site-specific graphite that leads to the disappearance of O3. From the Fourier-transform infrared spectroscopy (FTIR) analysis, the surface oxides which is formed during reaction are identified, which also includes further detection of aldehydes, ketones, carboxyl, diketones and cyclic carbonyl (carbonates and lactones) present at the edges of carbons graphite. This process increases the acidic oxygen functional groups and oxidation level on the biochar surface. This two-stage activation i.e., gasification and surface functionalization using O3 profoundly impact the adsorption capacity of activated carbon (Sajjadi et al. 2019). The ozone activation of biochar creates the acidic oxygen functional groups which enhance the cation exchange capacity of soil. O3 exposure for 10 min on the high internal surface area of biochar could create sufficient amount of surface oxides i.e. about 20–30% of the total biochar mass.
In a study conducted by El-Nemr et al. (2020) the effectiveness of ozone-modified biochar and unmodified biochar derived from the biomass of Pisum sativum peel waste was investigated for removal of copper (II) from aqueous media. Here the activation/modification of biochar was carried out by ozone followed by labelled ammonium hydroxide as Pea-B and Pea-BO-NH2. The results showed increased adsorption capacities of activated biochar up to 156.25 mg/g. Further characterization of this activated biochar revealed that the amino groups formed in the modified biochar result in the increased adsorption rate. Similarly, in another study by Jimenez-Cordero et al. (2015), biochar was oxidized in presence of O3 and then in inert atmosphere it was subjected to desorption of O2 group at high temperature. High burning off of the biomass due to the high oxidation temperature leads to increase in the number of activation cycle which results in the activation of carbon micropores up to 0.52cm3/g.
3.3 Physical Modification Type III—Thermal Activation
The decomposition of biomass at high temperature in less oxygen environment is known as thermal decomposition. This process involves various phenomena like decomposition, followed by transformation and biomass molecular structural rearrangement (Dodevski et al. 2017). In this process first the dehydration of biomass occurs at low temperature between 100 and 200 °C, after this the temperature increases gradually that leads to decomposition of organic molecules such as cellulose, hemicellulose and lignin. The complete degradation of all biopolymers and aromatic molecules takes place by the rising temperature. Furthermore, these aromatic ring structure stacks up at high temperature and develops a significant quantity of amorphous and crystalline matters on the biochar surface (Kleber et al., 2015). To remove these stacks and improve the efficiency of biochar, the thermal treatment of biochar occurs at very high temperature (600–1500 °C) for 1–2 h. This treatment removes the hydrogen and oxygen atoms with the release of gas which tends to reduce the biochar yield (Sajjadi et al. 2019) (Table 3).
3.4 Physical Modification Type IV—Microwave Activation
The electromagnetic waves of frequency from 300 MHz to 300 GHz having wavelength of 1 m to 1 mm is termed as microwave (Huang et al. 2016). The major benefit of using microwave as activating agent is that it provides uniform and quick internal heating with the help of three mechanisms i.e., ionic conduction, dipole polarization and the interfacial polarization. Here the heat is provided under temperature gradient via dielectric heating which evenly heats the entire volume of material within limited temperature in defined duration, unlike the other conventional method where heat is transferred from surface to the material causing variation of temperature and conduction phenomena (Wang et al. 2018). This procedure of microwave heating generates tiny microplasma spots, found throughout the reaction mixture which results in enhancing the process of chemical reaction and its local temperature, leading to the activation of biochar. This is majorly utilized in the industry for large-scale production. For proper valorization and energy recovery from the biomass, the conventional methods for pyrolysis and activation would not provide appropriate results but the use of process like vacuum pyrolysis, solar pyrolysis and microwave pyrolysis gives rise to the biomass recycling. In microwave activated pyrolysis the solid biochar is produced as the main product which is significantly used for remediation of environmental pollutants (Foong et al. 2020; Wahi et al. 2017) (Table 4).
3.5 Physical Modification Type V—Ultrasound Activation
Sonication has been used widely for the enhancement of mixing and reducing the mass transfer of solid–liquid interactions. The leaching of various minerals like Na, K, S, O, Cl, Fe and Al from carbonaceous material results in the swelling of biochar and improvement in the internal surface area. This action increases the adsorption capacity via modifying the biochar external and internal properties (Kleber et al. 2015). Under the ultrasound irradiation of 1 atm at 65 °C of CO2/H2O-based system, the exfoliation of graphite oxide clusters, leaching of minerals, reductive CO2 fixation and hydrogenation takes place. This ultrasonic treatment induces physical and chemical changes in biochar like water splitting, carboxylation, leaching, hydrogenation and swelling. Ultrasound-treated biochar has higher heating values, reaction rate and high internal surface area (Mulabagal et al. 2015). This method proves to be more effective and efficient as compared to conventional methods because of the much lower energy requirement for activation with increase in carbon content of biochar.
A study was carried out by Nguyen et al. (2021) on biochar generated by the biomass of water bamboo husk (Zizania latifolia) pyrolyzed at 600 °C and then activated using ultrasound activation. The ultrasound irradiation improved the reaction rate up to 80% i.e., the reaction gets completed in 4 h by this method instead of conventional method which took around 24 h. In a study Peter et al. (2020) state that the ultrasound-assisted pre-treatment was efficient in the activation and regeneration of biochar. When the ultrasound of 40 kHz was provided to the pre-treated samples of biochar, the adsorption capacity was increased up to 0.65 mg/g from the conventional technique (0.3 mg/g). The thermodynamic studies state that the ultrasound plays an important role in enhancing the surface area of biochar which results in improved adsorption rate of heavy metals.
3.6 Physical Modification Type VI—Plasma Activation
Like solid, liquid and gas, plasma is the fourth state of matter. It is produced by the electric discharge and does not occur naturally. The voltage and electric current affect the charged as well as neutral particle in the plasma which further affects ionization density and temperature of plasma particles (Karim et al. 2017). This system is classified on the basis of thermodynamic equilibrium state and plasma generation method. Depending upon the application, the thermal plasma reactors have been designed using various equilibrium plasma techniques. These require power levels from 100 W to 10 MW. This is best method for execution of gasification and pyrolysis. On comparing with conventional heating furnaces, the plasma reactor promotes fast reaction rate, high energy density and low formation of tarry compounds. This has a greater advantage of quick start up and shut down which reduce time utilized under pre- and post-process and has benefits like rapid cooling and heating, etc. (Niu et al. 2017). In this process of plasma activation high-energy electron is emitted by the plasma that is responsible for breaking the surface chemical bonds and also expanding the surfaces which directly relates to the adsorption rate of the process. This provides large output in limited time duration with low consumption of resources (Karim et al. 2017).
In a recent study, Zhang et al. (2019a) used plasma treatment technique for the removal of mercury from the biochar. Here, hydrogen sulfide (H2S) plasma modification was utilized for enhancing biochar productivity for the removal of mercury (Hg). The biochar obtained from wheat straw was utilized and modified using sulphur and carboxyl containing functional groups which form mercury sulfide (HgS) and mercury oxide (HgO) and remove Hg in the form of their sulphides and oxides. For better adsorption, low temperature is preferred which controls the physical and chemical adsorption. This has increased the efficiency of biochar from 26.4% to 95.5%. Similarly, another study was conducted by Wang et al. (2018) for the removal of Hg from non-thermal plasma modification using chlorine active sites. Here various biochar was prepared using rice straws, corn, wheat, black bean, millet, etc. via pyrolysis in high pure nitrogen at 600 °C, where non-thermal plasma modification by chlorine increased the HgO removal efficiently from 8.0% to 80%, which proves the efficiency of plasma treatment.
3.7 Physical Modification Type VII—Electrochemical Modification
The electric field modification can be utilized for enhancement of physicochemical properties of biochar. The electric current is applied accordingly for the enhancement of physical properties like porosity and surface area of biochar. Mostly an aluminium-based electrode in the presence of H2SO4 and NaOH solution for limited time period is applied in electrochemical modification of biochar where the acid generates the hypochlorite ions in the presence of NaCl solution and post this electrochemical process the pyrolysis is performed (Jung et al. 2015). These oxidants have the ability of improving the porosity and morphology of biochar. The factors like pH, electrode, and solution play a key role in determining the efficiency of this process. This electrochemical-based system improves porosity and surface area resulting in higher rate of adsorption (Sajjadi et al. 2019).
Moreover, biochar-based energy storage and conversion device is gaining attention these days (Kumar et al. 2020c; Cheng et al. 2017). A study was carried out for electrochemical modification and one-step pyrolysis for preparation of iron-based biochar composite using FeCl3 pre-treated corn straw biomass. This process generates the graphite electrode and developed a sustainable Fe3O4-based magnetic adsorbent. These Fe3O4 nanoparticles were crystalline having uniform dispersion forming rod-like structure of biochar. This modification contributes to the Pb adsorption and also effectively work on the magnetic waste water treatment by biochar adsorbent (Yang et al. 2019).
4 Limitations of Physical Modifications
Physical modification has several advantages over chemical modification as it does not use toxic chemicals which can affect human health as well as cause environmental pollution. Apart from the benefits, physical modifications consume a huge amount of energy which can directly affect the cost involved in the production of biochar. Also, these approaches make biochar production process non-greener. Moreover, this could make biochar activation process non-compatible and non-economical at commercial scale (Sajjadi et al. 2019).
5 Conclusions and Future Prospects
Currently, diverse applications of biochar are gaining global attention. Biochar has been proved as an environment-friendly, cost-effective, thermochemical product of biomass. Biochar is thoroughly applied as adsorbent for the removal of contaminants from wastewater. It also acts as a good carbon storage material in the soil. However, to improve the efficiency of biochar, physical modification is desirable. Therefore, this chapter discussed most prominent physical treatment methods which are required for various purposes and be effectively executed.
To achieve the growing demands of biochar application, further studies and developments are required for the commercial advancement in biochar production as current technologies are only favourable for small-scale production of biochar i.e., up to few tons per day. These low productions are unable to fulfil the demand and also lack in proper utilization of by-products of pyrolysis like tar and non-condensable gas. Similarly, the reaction conditions need to be controlled and optimized to increase the production efficiency of biochar and also make the production process more environment-friendly. The modification of biochar in the coming future should be designed according to its targeted applications like to understand the productivity on various agroecosystems and conducting field experiment for monitoring the long-term effects on soil properties. In the future, the biochar should be modified to have long-lasting effect on immobilization of contaminants and increased reusability.
References
Abioye AM, Ani FN (2015) The characteristics of oil palm shell biochar and activated carbon produced via microwave heating. In: Applied mechanics and materials, vol 695. Trans Tech Publications Ltd., pp 12–15
Agegnehu G, Srivastava AK, Bird MI (2017) The role of biochar and biochar-compost in improving soil quality and crop performance: a review. Appl Soil Ecol 119:156–170
Akhil D, Lakshmi D, Kartik A, Vo DVN, Arun J, Gopinath KP (2021) Production, characterization, activation and environmental applications of engineered biochar: a review. Environ Chem Lett 1–37
Awasthi MK, Sarsaiya S, Wainaina S, Rajendran K, Kumar S, Quan W, Duan Y, Awasthi SK, Chen H, Pandey A, Zhang Z (2019) A critical review of organic manure biorefinery models toward sustainable circular bioeconomy: technological challenges, advancements, innovations, and future perspectives. Renew Sustain Energy Rev 111:115–131
Bolan N, Kumar M, Singh E, Kumar A, Singh L, Kumar S, Keerthanan S, Hoang SA, El-Naggar A, Vithanage M, Sarkar B (2022) Antimony contamination and its risk management in complex environmental settings: a review. Environ Int 158:106908
Bolan N, Hoang SA, Beiyuan J, Gupta S, Hou D, Karakoti A, Joseph S, Jung S, Kim KH, Kirkham MB, Kua HW (2021) Multifunctional applications of biochar beyond carbon storage. Int Mater Rev 1–51
Cha JS, Park SH, Jung SC, Ryu C, Jeon JK, Shin MC, Park YK (2016) Production and utilization of biochar: a review. J Ind Eng Chem 40:1–15
Cheng BH, Zeng RJ, Jiang H (2017) Recent developments of post-modification of biochar for electrochemical energy storage. Bioresource Technol 246:224–233
de Caprariis B, De Filippis P, Hernandez AD, Petrucci E, Petrullo A, Scarsella M, Turchi M (2017) Pyrolysis wastewater treatment by adsorption on biochars produced by poplar biomass. J Environ Manage 197:231–238
Deng H, Li G, Yang H, Tang J, Tang J (2010) Preparation of activated carbons from cotton stalk by microwave assisted KOH and K2CO3 activation. Chem Eng J 163(3):373–381
Dodevski V, Janković B, Stojmenović M, Krstić S, Popović J, Pagnacco MC, Popović M, Pašalić S (2017) Plane tree seed biomass used for preparation of activated carbons (AC) derived from pyrolysis. Modeling the activation process. Colloids Surf, A 522:83–96
El-Nemr MA, Abdelmonem NM, Ismail IM, Ragab S, El Nemr A (2020) Ozone and ammonium hydroxide modification of biochar prepared from Pisum sativum peels improves the adsorption of copper (II) from an aqueous medium. Environ Processes 7(3):973–1007
Foo KY, Hameed BH (2011) Microwave-assisted preparation of oil palm fiber activated carbon for methylene blue adsorption. Chem Eng J 166(2):792–795
Foong SY, Liew RK, Yang Y, Cheng YW, Yek PNY, Mahari WAW, Lee XY, Han CS, Vo DVN, Van Le Q, Aghbashlo M (2020) Valorization of biomass waste to engineered activated biochar by microwave pyrolysis: progress, challenges, and future directions. Chem Eng J 389:124401
Fuke P, Kumar M, Sawarkar AD, Pandey A, Singh L (2021) Role of microbial diversity to influence the growth and environmental remediation capacity of bamboo: a review. Ind Crops Prod 167:113567
Girgis BS, Soliman AM, Fathy NA (2011) Development of micro-mesoporous carbons from several seed hulls under varying conditions of activation. Microporous Mesoporous Mater 142(2–3):518–525
Han L, Qian L, Yan J, Chen M (2017) Effects of the biochar aromaticity and molecular structures of the chlorinated organic compounds on the adsorption characteristics. Environ Sci Pollut Res 24(6):5554–5565
Herath I, Kumarathilaka P, Al-Wabel MI, Abduljabbar A, Ahmad M, Usman AR, Vithanage M (2016) Mechanistic modeling of glyphosate interaction with rice husk derived engineered biochar. Microporous Mesoporous Mater 225:280–288
Huang YF, Chiueh PT, Kuan WH, Lo SL (2016) Microwave pyrolysis of lignocellulosic biomass: heating performance and reaction kinetics. Energy 100:137–144
Huang J, Zimmerman AR, Chen H, Gao B (2020) Ball milled biochar effectively removes sulfamethoxazole and sulfapyridine antibiotics from water and wastewater. Environ Pollut 258:113809
Jimenez-Cordero D, Heras F, Alonso-Morales N, Gilarranz MA, Rodriguez JJ (2015) Ozone as oxidation agent in cyclic activation of biochar. Fuel Process Technol 139:42–48
Jung KW, Hwang MJ, Jeong TU, Ahn KH (2015) A novel approach for preparation of modified-biochar derived from marine macroalgae: dual purpose electro-modification for improvement of surface area and metal impregnation. Biores Technol 191:342–345
Kammann CI, Schmidt HP, Messerschmidt N, Linsel S, Steffens D, Müller C, Koyro HW, Conte P, Joseph S (2015) Erratum: plant growth improvement mediated by nitrate capture in co-composted biochar. Sci Rep 5
Karim AA, Kumar M, Singh SK, Panda CR, Mishra BK (2017) Potassium enriched biochar production by thermal plasma processing of banana peduncle for soil application. J Anal Appl Pyrol 123:165–172
Kleber M, Hockaday W, Nico PS (2015) Characteristics of biochar: macro-molecular properties. In: Biochar for environmental management. Routledge, pp 143–170
Kołtowski M, Charmas B, Skubiszewska-Zięba J, Oleszczuk P (2017a) Effect of biochar activation by different methods on toxicity of soil contaminated by industrial activity. Ecotoxicol Environ Saf 136:119–125
Kołtowski M, Hilber I, Bucheli TD, Charmas B, Skubiszewska-Zięba J, Oleszczuk P (2017b) Activated biochars reduce the exposure of polycyclic aromatic hydrocarbons in industrially contaminated soils. Chem Eng J 310:33–40
Kumar M, Sun Y, Rathour R, Pandey A, Thakur IS, Tsang DC (2020a) Algae as potential feedstock for the production of biofuels and value-added products: opportunities and challenges. Sci Total Environ 716:137116
Kumar M, Xiong X, Sun Y, Yu IK, Tsang DC, Hou D, Gupta J, Bhaskar T, Pandey A (2020b) Critical review on biochar-supported catalysts for pollutant degradation and sustainable biorefinery. Adv Sustain Syst 4(10):1900149
Kumar M, Xiong X, Wan Z, Sun Y, Tsang DC, Gupta J, Gao B, Cao X, Tang J, Ok YS (2020c) Ball milling as a mechanochemical technology for fabrication of novel biochar nanomaterials. Biores Technol 312:123613
Kumar M, Bolan NS, Hoang SA, Sawarkar AD, Jasemizad T, Gao B, Keerthanan S, Padhye LP, Singh L, Kumar S, Vithanage M (2021a) Remediation of soils and sediments polluted with polycyclic aromatic hydrocarbons: To immobilize, mobilize, or degrade? J Hazard Mater 420:126534
Kumar M, You S, Beiyuan J, Luo G, Gupta J, Kumar S, Singh L, Zhang S, Tsang DC (2021c) Lignin valorization by bacterial genus Pseudomonas: state-of-the-art review and prospects. Biores Technol 320:124412
Kumar M, Dutta S, You S, Luo G, Zhang S, Show PL, Sawarkar AD, Singh L, Tsang DC (2021b) A critical review on biochar for enhancing biogas production from anaerobic digestion of food waste and sludge. J Cleaner Prod 127143
Lal R (2015) Sequestering carbon and increasing productivity by conservation agriculture. J Soil Water Conserv 70(3):55A-62A
Lee J, Yang X, Song H, Ok YS, Kwon EE (2017) Effects of carbon dioxide on pyrolysis of peat. Energy 120:929–936
Liu WJ, Jiang H, Yu HQ (2015) Development of biochar-based functional materials: toward a sustainable platform carbon material. Chem Rev 115(22):12251–12285
Lou K, Rajapaksha AU, Ok YS, Chang SX (2016) Pyrolysis temperature and steam activation effects on sorption of phosphate on pine sawdust biochars in aqueous solutions. Chem Speciat Bioavailab 28(1–4):42–50
Maddalwar S, Nayak KK, Kumar M, Singh L (2021) Plant microbial fuel cell: opportunities, challenges, and prospects. Bioresour Technol 341:125772
Mandal S, Pu S, Adhikari S, Ma H, Kim DH, Bai Y, Hou D (2021) Progress and future prospects in biochar composites: application and reflection in the soil environment. Crit Rev Environ Sci Technol 51(3):219–271
Mishra A, Kumar M, Medhi K, Thakur IS (2020) Biomass energy with carbon capture and storage (BECCS). In: Current developments in biotechnology and bioengineering. Elsevier, pp 399–427
Mishra A, Kumar M, Bolan NS, Kapley A, Kumar R, Singh L (2021) Multidimensional approaches of biogas production and up-gradation: opportunities and challenges. Bioresource Technol 125514
Mondal S, Aikat K, Halder G (2016) Ranitidine hydrochloride sorption onto superheated steam activated biochar derived from mung bean husk in fixed bed column. J Environ Chem Eng 4(1):488–497
Mulabagal V, Baah DA, Egiebor NO, Chen WY (2015) Biochar from biomass: a strategy for carbon dioxide sequestration, soil amendment, power generation, and CO2 utilization. Handbook of climate change mitigation and adaptation, pp 1937–1974
Nair V, Vinu R (2016) Peroxide-assisted microwave activation of pyrolysis char for adsorption of dyes from wastewater. Biores Technol 216:511–519
Namazi AB, Allen DG, Jia CQ (2016) Benefits of microwave heating method in production of activated carbon. Canadian J Chem Eng 94(7):1262–1268
Nguyen TT, Chen HH, To TH, Chang YC, Tsai CK, Chen KF, Tsai YP (2021) Development of biochars derived from water bamboo (Zizania latifolia) shoot husks using pyrolysis and ultrasound-assisted pyrolysis for the treatment of reactive black 5 (RB5) in wastewater. Water 13(12):1615
Nie J, Sun Y, Zhou Y, Kumar M, Usman M, Li J, Shao J, Wang L, Tsang DC (2020) Bioremediation of water containing pesticides by microalgae: mechanisms, methods, and prospects for future research. Sci Total Environ 707:136080
Niu Q, Luo J, Xia Y, Sun S, Chen Q (2017) Surface modification of bio-char by dielectric barrier discharge plasma for Hg0 removal. Fuel Process Technol 156:310–316
Oliveira FR, Patel AK, Jaisi DP, Adhikari S, Lu H, Khanal SK (2017) Environmental application of biochar: current status and perspectives. Biores Technol 246:110–122
Pallarés J, González-Cencerrado A, Arauzo I (2018) Production and characterization of activated carbon from barley straw by physical activation with carbon dioxide and steam. Biomass Bioenerg 115:64–73
Peter A, Chabot B, Loranger E (2020) The influence of ultrasonic pre-treatments on metal adsorption properties of softwood-derived biochar. Bioresour Technol Rep 11:100445
Qian TT, Wu P, Qin QY, Huang YN, Wang YJ, Zhou DM (2019) Screening of wheat straw biochars for the remediation of soils polluted with Zn (II) and Cd (II). J Hazard Mater 362:311–317
Rajapaksha AU, Vithanage M, Ahmad M, Seo DC, Cho JS, Lee SE, Lee SS, Ok YS (2015) Enhanced sulfamethazine removal by steam-activated invasive plant-derived biochar. J Hazard Mater 290:43–50
Ramola S, Srivastava RK, Vasudevan P (2013) Effect of biochar application in combination with domestic wastewater on biomass yield of bioenergy plantations. Int J Energy Sect Manag 7(3):355–363
Ramola S, Belwal T, Li CJ et al (2020) Improved lead removal from aqueous solution using novel porous bentonite and calcite-biochar composite. Sci Total Enviro 709:136171. https://doi.org/10.1016/j.scitotenv.2019.136171
Ramola S, Belwal T, Li CJ et al (2021) Preparation and application of novel rice husk biochar-calcite composites for phosphate removal from aqueous medium. J Clean Prod 299:126802 https://doi.org/10.1016/j.jclepro.2021.126802
Sajjadi B, Chen WY, Egiebor NO (2019) A comprehensive review on physical activation of biochar for energy and environmental applications. Rev Chem Eng 35(6):735–776
Sakhiya AK, Anand A, Kaushal P (2020) Production, activation, and applications of biochar in recent times. Biochar 2:253–285
Shim T, Yoo J, Ryu C, Park YK, Jung J (2015) Effect of steam activation of biochar produced from a giant Miscanthus on copper sorption and toxicity. Biores Technol 197:85–90
Sun Y, Kumar M, Wang L, Gupta J, Tsang DC (2020) Biotechnology for soil decontamination: opportunity, challenges, and prospects for pesticide biodegradation. In: Bio-based materials and biotechnologies for eco-efficient construction. Woodhead Publishing, pp 261–283
Tan Z, Lin CS, Ji X, Rainey TJ (2017) Returning biochar to fields: a review. Appl Soil Ecol 116:1–11
Tian S, Tan Z, Kasiulienė A, Ai P (2017) Transformation mechanism of nutrient elements in the process of biochar preparation for returning biochar to soil. Chin J Chem Eng 25(4):477–486
Veksha A, Bhuiyan TI, Hill JM (2016) Activation of aspen wood with carbon dioxide and phosphoric acid for removal of total organic carbon from oil sands produced water: increasing the yield with bio-oil recycling. Materials 9(1):20
Wahi R, Zuhaidi NFQA, Yusof Y, Jamel J, Kanakaraju D, Ngaini Z (2017) Chemically treated microwave-derived biochar: an overview. Biomass Bioenerg 107:411–421
Wang T, Liu J, Zhang Y, Zhang H, Chen WY, Norris P, Pan WP (2018) Use of a non-thermal plasma technique to increase the number of chlorine active sites on biochar for improved mercury removal. Chem Eng J 331:536–544
Xiang W, Zhang X, Chen J, Zou W, He F, Hu X, Tsang DC, Ok YS, Gao B (2020) Biochar technology in wastewater treatment: a critical review. Chemosphere 252:126539
Xiao J, Hu R, Chen G (2020) Micro-nano-engineered nitrogenous bone biochar developed with a ball-milling technique for high-efficiency removal of aquatic Cd (II), Cu (II) and Pb (II). J Hazard Mater 387:121980
Yang F, Zhang S, Sun Y, Du Q, Song J, Tsang DC (2019) A novel electrochemical modification combined with one-step pyrolysis for preparation of sustainable thorn-like iron-based biochar composites. Biores Technol 274:379–385
Yu F, Zhou Y, Gao B, Qiao H, Li Y, Wang E, Pang L, Bao C (2016) Effective removal of ionic liquid using modified biochar and its biological effects. J Taiwan Inst Chem Eng 67:318–324
Zhang T, Walawender WP, Fan LT, Fan M, Daugaard D, Brown RC (2004) Preparation of activated carbon from forest and agricultural residues through CO2 activation. Chem Eng J 105(1–2):53–59
Zhang H, Wang T, Sui Z, Zhang Y, Norris P, Sun B, Pan WP (2019a) Plasma induced addition of active functional groups to biochar for elemental mercury removal. Plasma Chem Plasma Process 39(6):1449–1468
Zhong ZY, Yang Q, Li XM, Luo K, Liu Y, Zeng GM (2012) Preparation of peanut hull-based activated carbon by microwave-induced phosphoric acid activation and its application in Remazol Brilliant Blue R adsorption. Ind Crops Prod 37(1):178–185
Acknowledgements
The authors are thankful to Director, CSIR-National Environmental Engineering Research Institute, Nagpur, India for providing the necessary facilities for this work.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Anerao, P., Salwatkar, G., Kumar, M., Pandey, A., Singh, L. (2022). Physical Treatment for Biochar Modification: Opportunities, Limitations and Advantages. In: Ramola, S., Mohan, D., Masek, O., Méndez, A., Tsubota, T. (eds) Engineered Biochar. Springer, Singapore. https://doi.org/10.1007/978-981-19-2488-0_4
Download citation
DOI: https://doi.org/10.1007/978-981-19-2488-0_4
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-19-2487-3
Online ISBN: 978-981-19-2488-0
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)