Abstract
Emerging contaminants (ECs) consist of pharmaceuticals, personal care products, pesticides, organic solvents and dyes, and other nitrogen and phosphorus contaminants that cause adverse effects on environment and human health. Many of these compounds are presently not tested for municipal water systems. This chapter focuses on the application of pristine and modified biochar as adsorbents for emerging contaminants from water and soil. The first part of the chapter describes ECs problem in general, presenting different types of emerging contaminants and challenges related to their removal from soil and water. Further, the biochar applications for removal of ECs are presented, based on the detailed review of literature providing characterization of the adsorbents and the different approaches that can be followed to enhance biochar sorption capacity for ECs. A description of the main challenges in designing the biochar properties for its multifunctional application as adsorbent is also discussed.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Emerging contaminants (ECs) have recently been recognized as new and significant environmental pollutants representing a wide group of chemicals, such as pharmaceuticals, personal care products (PPCPs), endocrine-disrupting compounds (EDCs), and industrial effluents (Lei et al. 2015). Due to their rapidly increasing use in industry, transport, agriculture, and urbanization, these chemicals are entering the environment at elevated levels (Gavrilescu et al. 2015). The presence of ECs in natural water systems and soils is a major concern worldwide as these substances, even in very low concentrations, may have negative effects on human health, causing environmental risks to animals and plants. EC originates from various anthropogenic sources and are distributed throughout the environmental matrices (Sehonova et al. 2018). The most significant source of hazardous substances are wastewaters and effluents produced during anthropogenic activities. ECs, when released into the environment, undergo biodegradation processes, causing contaminant transformation induced by the presence of specific constituents in matrix-like organic matter and microorganisms. One of the major problems is that ECs found in industrial and municipal wastewater treatment plants cannot be easily remediated through conventional treatment technologies (Fan et al. 2018). Among all treatment methods that have been developed, adsorption is the one most pertinent and promising one for removing organic and inorganic micropollutants (Sophia and Lima 2018).
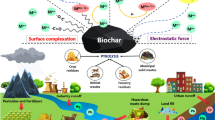
Many different materials have been investigated as ECs adsorbents, however, the most attention has been paid to carbon-based materials like activated carbons, carbon nanotubes, peat, or graphene. Recently, efforts have been made to propose an alternative carbon source for generating ECs sorbent at lower costs. Carbon production from several wastes such as agricultural crop and forestry residues, biodegradable fraction of municipal wastes or animal manure seems to be a win–win solution solving two major environmental problems—of waste management and wastewater treatment. Biochar is a highly stable material, having strong adsorption capabilities and natural origin. In terms of microscopic structure biochar possess porous characteristics, large surface area, and aromaticity, that can be designed by adjusting raw material type and temperature of pyrolysis. In particular, while higher proportions of aliphatic carbons and functional groups are typical of biochar pyrolyzed at low temperatures, biochar obtained at higher temperatures contains mainly polyaromatic carbons and has a higher microporosity, which enhances hydrophobic organic compounds adsorption (Chen et al. 2008). Many emerging organic micropollutants are more polar than “conventional” contaminants (e.g., polycyclic aromatic hydrocarbons) and may have numerous acidic and/or basic functional groups (Kim et al. 2016) that are able to interact with bonding sites on biochar surface.
However, the mechanisms of ECs adsorption on biochar can be related to various processes depending on multiple factors including, (1) structural properties of the adsorbate, (2) physiochemical properties of the sorbent surface such as specific surface area, pore size distribution, surface functionality, or ash content, and (3) the conditions at which the sorption is carried out such as pH and the nature of the matrix (Azhar et al. 2016; Peiris et al. 2017). In general, pristine biochar has lower adsorption capacity than activated carbon (Kearns et al. 2019). Therefore, in the recent years, a number of studies in the scientific literature have dealt with production of the engineered or modified biochar having desirable properties. Majority of these studies are focused on biochar modifications, enabling effective immobilization of pesticides. The extensive and inefficient use over the last decades resulted in serious soil and water contamination (Liu et al. 2018). Modulation of biochar properties, in order to obtain higher retention of particular chemicals, is very often based on optimization of process engineering to improve its adsorption capacity and hence to increase the economic benefits of its implementation. The simplest modifications concern the pyrolysis route variables. One of them is temperature as either high or low has both positive and negative effects on sorption of chemicals, one by larger surface area and the other by a large number of functional groups (Gaffar et al. 2021). Nevertheless, in the recent years, biochar studies are mainly focused on designing a sorbent with precisely defined properties, selective to the particular chemical class of compounds. In order to obtain biochar with superior, carefully modulated, and application-oriented properties, different modification methods of biochar are tested. They refer to the pre-treating biomass using chemical reagent (so called—activators) before or after the pyrolysis process (Tan et al. 2016) or activation with the use of physical and chemical methods—to achieve the desired purpose (Liu et al. 2018). The multifunctionality of biochar can also be enhanced by its coating with nanoparticles (e.g., chitosan, graphene, graphene oxide, carbon nanotubes, ZnS nanocrystals, layered double hydroxides, nanoscale zero-valent iron, graphitic C3N4) (Tan et al. 2016). Such approach combines the advantages of biochar matrix and functional nanoparticles, improving and modifying surface functional groups, surface area, porosity, and thermal stability of biochar, which contribute to better performance of contaminants removal (Leng et al. 2021).
As removal of emerging contaminants from aqueous and soil medium is a relatively new application of biochar, in this chapter, we will summarize the state of the art about its use in environmental decontamination.
2 Pharmaceuticals
The definition of a PPCP broadly includes any product that has healthcare or medical purpose for humans or animals. These products are usually subdivided into categories on the basis of their nature and use. Pharmaceuticals is a large group of chemical substances, however, based on their use and occurrence in environment, these antibiotics and anti-inflammatory drugs are most frequently detected as water and soil contaminants.
2.1 Antibiotics
Biochar has been extensively investigated as an effective sorbent of several groups of antibiotics (tetracycline, sulfonamides, fluoroquinolones, macrolides) and many different mechanisms of antibiotics adsorption have been described depending on biochar and contaminant properties. In general, biochar is considered a good sorbent for hydrophobic organic contaminants because of its relatively high hydrophobicity and aromaticity (Lian et al. 2014). Unlike activated carbon (AC), biochar is generally not fully carbonized. As a result, both carbonized and non-carbonized domains regulate its sorptive behavior (Chen et al. 2008). Sorption of antibiotics on biochar can occur through a variety of mechanisms, including π–π electron–donor–acceptor interaction, nucleophilic addition, electrostatic attraction, pore filling, partitioning into noncarbonized fraction, and formation of charge-assisted hydrogen bonding (CAHB) with surface oxygen groups (Ahmed et al. 2017).
Biochar capacity for antibiotics can be modified by the specific interactions between micropollutant and biochar surface groups. Presence of surface functional groups on biochar (e.g., phenyl, amino, alcohol, and ketone) and moieties, acting either as H donors (amino, amide carbonyl, and hydroxyl groups) or H acceptors (carbonyl and dimethylamino groups), deliberately modifies the surface of biochar, enhancing the sorption interaction (Jing et al. 2014). Ahmed et al. (2017) described that hydroxyl groups on aromatic rings are stronger adsorbents for sulfonamides, in comparison to the low-temperature biochar, containing more carboxyl groups acting as π acceptors. The sorption magnitude depends mainly on the physicochemical properties of the micropollutants (Li et al. 2019), type of solid matrices, surface area, porosity, pore diameter, and environmental conditions (Pavlović et al. 2014). Preliminary research presented very divergent results of biochar capacity for antibiotics adsorption, however, in most cases, pristine biochar had significantly lower adsorption rates compared to even simply modified biochar, e.g., by steam activation.
Steam activation and acid modifications are commonly used to introduce oxygen-containing functional groups (e.g., carboxylic, carbonyl, ether, and phenolic hydroxyl groups) onto biochar surfaces, increasing its hydrophilic nature. Opposite effects—an increase in biochar hydrophobicity and aromaticity can be obtained during heat treatments at high temperature >600 °C. In terms of designing a biochar with high sorption capacity for pharmaceuticals, preparation of materials with aromatic structure and low content of volatile chemical is desired. This function can be obtained either by high-temperature pyrolysis or heat activation of biochar obtained during low-temperature processes. Biochar deashing by material acid-washing can also be an efficient method of increasing biochar sorption capacity (Srinivasan and Sarmah 2015, Luo et al. 2018) for pharmaceuticals. Shimabuku et al. (2016) comparing low and high ash biochar derived from wood wastes observed that high ash biochar contains a surface rich in oxygen due to metal oxides, which weaken the sorption capacity for sulphmetoxazole. In contrast, certain studies have reported an opposite behavior, where the sorption capacity of low-temperature biochar was higher, though this mechanism and the effect of pyrolysis temperature on antibiotics sorption efficiency is still uncertain and needs further investigation. Competition between components for sorption sites is one of the major concerns when biochar is applied to natural mediums.
Different matrix components present in natural waters and soil, e.g., humic acid or metal cations can compete with the antibiotics for biochar sorption sites, resulting in lower sorption efficiency. Antibiotic adsorption to soils depends on the chemical species and soil properties including pH, clay minerals, and organic matter content, but also on the concentration and type of divalent cations present in the soil solution (Kim et al. 2011, Wang et al. 2018). Organic matter can strongly interact with the polar fraction of BC through H-bonding and with hydrophobic fraction through π−π EDA interactions (Lian et al. 2015). In general, humic acids are able to mitigate antibiotics sorption on biochar, mainly due to sorption competition (Xie et al. 2014). Sun et al. (2016) described that biochar sorption capacity for sulfamethoxazole can be greatly enhanced by interaction with low molecular weight organic acids (LMWOAs) present in rhizosphere. Metal ions including K+, Cu2+, Cd2+, or Pb2+ coexisting in soil solution tends to have the opposite effect, enhancing antibiotic sorption on biochar, due to formation of very stable complexes (Jia et al. 2013; Han et al. 2013). Similarly in the presence of clay minerals, e.g., kaolinite in soil and water sediments, antibiotics sorption capacity of biochar may increase what was described by Bair et al. (2016). When antibiotic is entering soil it is primarily degraded by abiotic rather than biotic processes, that is why most of the groups like suflonamides are not readily biodegradable, but leachable (Rajapaksha et al. 2014b). It means that they can be easily transferred from soil to groundwater or taken up by edible plants, due to its low molecular weight (Rajapaksha et al. 2014a). Biochar application to soil can directly or indirectly modify these conditions, mitigating the problem of antibiotics transfer in the food chain by decreasing antibiotics bioavailability and leachability (Vithanage et al. 2014). Large surface area and aromatic surface properties developed during high-temperature pyrolysis as well as low ash content, tends to enable biochar to effectively adsorb most of the antibiotic groups present in water and soils.
Indirectly biochar can modify soil properties like pH, cation exchange capacity, or organic matter content, enhancing or reducing the sorption capacity of soil. In general, ionizable molecules sorption process is pH depended. Solution pH affects both the ionization of the pharmaceuticals and the surface charge of the biochar which, in turn, influences the different mechanisms of pharmaceutical adsorption onto biochar (Ndoun et al. 2021, Chen et al. 2018). Kahle and Stamm (2007) described that antibiotic sorption on organic sorbents decreased with the increase of pH. The highest sorption efficiency is usually observed in very narrow pH, e.g., 5.5–7.0 or 4.0–4.25 depending on the testing speciation of the ionizable antibiotic (Teixidó et al. 2011; Ahmed et al. 2017). Similar findings were observed by Ndoun et al. (2021) describing pH-depended adsorption capacity for sulfapyridine on two different biochars derived from cotton gin waste and guayule bagasse. The removal of sulfapyridine was approximately 70% at pH 10–11 but was significantly reduced (40%) at pH 7. Biochar surface becomes negatively charged at lower pH, and the adsorption capacity is improved due to cation-π bonding and electrostatic attractive force (Jia et al. 2013, Qin at al. 2020). At pH > 6.0 negative charge-assisted hydrogen bond (CAHB) dominates the adsorption as the antibiotics and biochar both have negative surface charge (Masrura et al. 2020). As most pharmaceuticals charge is pH depended, adjusting pH and dose of biochar used to achieve the highest sorption capacity is very challenging in a natural system that has contaminants present in mixture.
2.2 Anti-inflammatory Pharmaceuticals
Non-steroidal anti-inflammatory drugs (NSAIDs) are an extensive range of medication used to treat and alleviate pain as well as inflammation (Mlunguza et al. 2019). NSAIDs are regarded as emerging pollutants in water bodies and they are among the most detectable organic pollutants in the aquatic systems. Long-term exposure of NSAIDs has created an urgent need of finding a proper solution to minimize the risk of NSAIDs transfer in the food chain. As this group of pharmaceuticals is represented in the environment by wide groups of chemicals (paracetamol, ibuprofen, ketoprofen, diclofenac, aspirin) the removal of these substances from water and soil by application of sorbents is challenging. The adsorption efficiency of NSAIDs depends on their biodegradability and other physicochemical properties such as the likelihood of immobilization by organic or mineral constituents, water solubility and their tendency to volatilize (Quintana et al. 2005). However, various sorbents showed different efficiency of the process depending mainly on their sorption capacity and surface properties. The knowledge about biochar utility for NSAIDs removal from wastewaters is still insufficient. Hence, very limited data suggest that biochar adsorption capacity for inflammatory drugs and their metabolites can be more compared with often studied activated carbons (Yi et al. 2016; Luo et al. 2020).
Modified biochar application for NSAID removal from wastewaters is reported in relatively high number of publications, nonetheless, studies of pristine biochar are rare. Composite formation, coating, chemical or steam activation are commonly studied methods that are employed for increasing biochar sorption capacity for anti-inflammatory pharmaceuticals. These modifications increase carbon content (Mondal et al. 2016), number of oxygen functional groups on biochar surface or abilities of the material to adsorb negatively or positively charged ions (de Souza dos Santos et al. 2020). In terms of inflammatory drugs mechanism of adsorption on biochar is similar to the one of antibiotics. Nevertheless, substance molecular size and partitioning, hydrophobicity, and porosity of the sorbent are crucial when considering biochar as NSAIDs sorbents. Jung et al. (2015) studied competitive sorption of diclofenac (DCF), naproxen (NPX), and ibuprofen (IBP) on loblolly pine chip biochar and observed difference in adsorption capacity for these three pharmaceuticals, depending mostly on molecule size. Smaller molecules are able to occupy smaller biochar pores making process of adsorption more efficient. In addition, chemical properties of some inflammatory drugs, e.g., salicylic acid and the formation of carboxylate anions increase the likelihood of H-bonding with phenolic hydroxyls on biochar surface. Mechanisms of NSAIDs adsorption on pine wood biochar was studied by Essandoh et al. (2015) showing higher efficiency of the process at low pH. Increase of pH causes increased electrostatic repulsion of adsorbate carboxylate anions, decreasing the adsorption capacity. Biochar adsorption strength is also dependent on surface negative charge density. Nonetheless, this property for both biochar and NSAIDs molecule is a complex function of pH, because surface carboxylic acids, phenols, and aliphatic hydroxyls ionize at different pH ranges and their concentrations also differ, that makes it very difficult to predict occurrence of optimum adsorption. Tran et al. (2020) observed that morphological characteristics (especially pore size distribution) of the biochar should be also considered when designing a proper sorbent for pharmaceuticals. In the study, two different kinds of biochar (spherical and non-spherical) exhibited similar physicochemical properties, but their adsorption affinities and behaviors toward paracetamol molecules in solution were remarkably dissimilar due to differences in porosity.
Chakraborty et al. (2018) studied pristine and steam-activated Aegle marmelos (wood apple) fruit shell biochar that showed promising efficiency for ibuprofen removal, pointing out that biochar surface modification and development of highly porous biochar during high-temperature pyrolysis or steam activation increase the sorption efficiency up to 95%. Similar findings were described by Show et al. (2020) where tamarind seeds biochar showed high efficiency for ibuprofen removal from an aqueous solution. Recently, a lot of attention has been paid to caffeine (1,3,7-trimethylxanthine) that is used in cold and flu medicines and as a stimulant in beverages. Álvarez-Torrellas et al. (2016) reported that natural organic matter has high capacity for caffeine (CF) removal, while Keerthanan et al. (2020) confirmed that biochar was successful in CF removal from wastewaters. Knowledge about biochar efficiency for NSAIDs adsorption from soil is still very limited and there is an urgent need to develop scientific projects dedicated to soil—biochar—NSAIDs interactions.
2.3 Antidepressants
Antidepressant pharmaceuticals are typical anthropogenic pollutants. The knowledge about antidepressant medicine sorption on biochar is limited, however, some data can be found on biochar sorption capacity for nitrazepam, diazepam, and fluoxetine from aqueous solution. Fernandes et al. (2019) evaluated the use of twelve biochars derived from different forest and agri-food wastes for removal of fluoxetine. Raw material type had a great influence on sorption capacity and significant difference between pine biochar (36% of efficiency) versus eucalyptus residue (100% of efficiency) was found. This dissimilarity in behavior of biochar can be related to different physical (particle fractionation and pore size distribution, specific surface area) and chemical, e.g., abundance of surface functional groups and biochar alkalinity, properties. Typical to other pharmaceuticals, removal of antidepressant by biochar is pH-dependent and the maximum efficiency can be obtained at pH between 4 and 6. Differences observed between sorption efficiency at different pH values can be related to electrostatic interactions between chemical compounds and biochar. According to various studies, biochar at pH ranging between 5.0 and 9.5 becomes positively charged, contributing to electrostatic repulsion. The other factor that should be considered as antidepressant sorption mechanism on biochar is the role of its surface functional groups, which become deprotonated when pH increases and therefore negatively charged, thus favoring adsorption of ECs (de Souza dos Santos et al. 2020). Nazal et al. (2021) studied the efficiency of macroalgae biochar for nitrazepam removal from wastewaters presenting high efficiency of the process (up to 98%). Similar results were obtained by Escudero-Curiel et al. (2021) describing high efficiency of commercially available biochar on antidepressant sorption and enhanced sorption capacity in the presence of Fe3+ cations in the solution.
3 Personal Care Products Components
Recently a lot of attention has been paid to personal care and household products containing endocrine-disrupting chemicals (EDCs). EDCs can mimic the biological activity of natural hormones, occupy hormone receptors, or interfere in the transport and metabolic processes of natural hormones (Danzo 1998; Diamanti-Kandarakis et al. 2009). The main source of EDCs in the environment is municipal sewage sludge and manure. As a result of this material usage in soil fertilization and irrigation of agricultural lands from surface water reservoirs, EDCs enter soil and can be easily leached to groundwaters (Sun et al. 2011). EDCs are relatively hydrophobic organic compounds that’s why conventional biological wastewater treatment processes have shown satisfying results for contaminant removal (Ahmed et al. 2018a). Adsorption of EDCs on carbonaceous materials has been studied using mainly activated carbon, while biochar studies are limited.
3.1 Synthetic and Natural Hormones
A lot of studies have been done recently to study on potential sorption of estrogens and estradiols (natural and synthetic) on biochar. Sorption mechanisms are similar to other described ECs. Biochar can act as either an electron acceptor or donor depending on the extent of functionalization and graphitization of its surface (Peiris et al. 2017). Generally, EDA interactions take place in between the phenolic moiety of estrogen and the electron-accepting moieties attached to biochar (Liu et al. 2019b). H-bonding occurs between the phenolic and hydroxyl groups of the estrogens and oxygen surface functional groups on biochar (Ahmed et al. 2018b). Another mechanism that has been reported is the electrostatic force of attraction that occurs between the phenolate ion of the estrogen and the positively charged surface of biochar (Liu et al. 2019a). Estrogen adsorption capacity is pH dependent. Over a wide range of pH (pH 3–12), the hydroxyl groups attached to the arene rings on the biochar surface can function as electron donors, whereas the electron-deficient carbon atom of the carbonyl groups can function as electron acceptors (Peiris 2020). Sun et al. (2011) reported higher adsorption capacities for synthetic estrogen ethinyl estradiol due to an increase in oxygen surface functional groups on the hydrochar produced from poultry litter and swine solids. Alizadeh et al. (2018) demonstrated that biochar could act as an efficient adsorbent in removing two manure-borne estrogens from sandy soil. As Guo et al. (2019) reported, biochar addition significantly improved the adsorption rates and capacities for 17-ethinyl estradiol and perfluorooctane sulfonate removal in sediments. Zhou et al. (2020) tested graphene-like magnetic sawdust biochar showing that it is a promising adsorbent of 17-estradiol (E2) from wastewaters. Sorptive uptake of estrogens from natural water systems is influenced by the different matrix components such as natural organic matter (NOM). Organic matter carries negative charge and a lot of carboxyl and phenolic moieties exhibiting competitive effect and significantly reducing removal of estrogens (Dong et al. 2018). Regkouzas and Diamadopoulos (2019), reported that sewage sludge biochar can be effectively used to remove organic micropollutants from water or treated wastewater in realistic initial concentrations, with removal rates ranging between 35 and 99%. Bisphenol A (BPA) is one of the EDCs that widely occurs in the environment mainly due to plastic disposal and abiotic degradation. BPA has affinities to human estrogen being called “environmental estrogen” (Takeshita et al. 2001). Little is known about sorptive behavior of pristine biochar in BPA removal mechanism. Recently, magnetic biochar or biochar obtained during hydrothermal carbonization was used to assess the efficiency for BPA removal from water. Wang and Zhang (2020) tested biochar prepared from discarded pomelo peels modified with FeSO4 and FeCl3 solution. Results suggested that magnetic biochar is easy to remove from solution after adsorption of large amounts of BPAs. Heo et al. (2019) enhanced adsorption of BPA and sulphmethoxazole by magnetic CuZnFe2O4–biochar composite. Wang et al. (2019) examined the adsorption behavior of BPA to peanut shell biochar and effects of cationic, anionic, and nonionic surfactants. Results demonstrated that many of the chemical substances present in wastewaters may affect the adsorption behavior, inhibiting the process of BPA adsorption on biochar sorbents. Sengottian et al. (2020) prepared biochar from Casuarina equisetifolia L. and Wrightia tinctoria showed that biochars obtained during hydrothermal carbonization have better sorption capacity for PBA compared to activated carbon. This phenomena was also, previously described by Sun et al. (2011) showing that hydrothermally produced biochars are able to adsorb a wider spectrum of both polar and nonpolar organic contaminants than thermally produced biochar.
3.2 Pesticides
Large group of more than an hundred pesticides widely used in agriculture and households can be listed as potential endocrine disruptors (Mnif et al. 2011). Use of modified biochar for pesticides sorption is widely recognized, showing very promising application of these materials in soil and water remediation. Some of the proposed biochar modification methods are based on the pretreatment of biomass and its loading with different materials (Liu et al. 2018). A good example of such practice is the work of Han et al. (2021), where different ways were identified to efficiently convert biomass wastes into biochar with potential for organic contaminants retention. In this study, FeCl3 and AlCl3 were added to two typical biomass wastes (rice straw and poultry litter) during pyrochar and hydrochar production. Results showed that added metal activators significantly changed the properties of biochar. Al (III) induced the carbonization pyrochar at 250 °C and Fe (III) stimulated the formation of aromatic structures. Applied metal activators increased the stability and adsorption efficiency of both pyrochar and hydrochar for polar and nonpolar contaminants. Yavari et al. (2020) aimed to synthesize chitosan-modified biochar with the objective of producing an efficient biosorbent for removal of imazapic and imazapyr herbicides in soil. Such improvement resulted in increase of the cation exchange capacity of the material, which evoked 76 and 84% sorption of imazapic and imazapyr, respectively, in case of soil amended with chitosan-modified biochar.
An interesting example of physical modification of biochar is work of Kearns et al. (2019), who tested biochar generated from updraft gasifiers under conditions of simultaneous co-pyrolysis thermal air activation (CPTA). Adsorption of anionic (2,4-D) and neutral (simazine) herbicides from surface water containing dissolved organic matter were investigated. 2,4-D adsorption by ≥850 °C CPTA biochar was 10 times more in comparison with biochar generated from a conventional pyrolysis. It was due to the increase in mesopores and removal of pyrolysis tars in CPTA biochar.
Many studies revealed that biochar produced through microwave-assisted pyrolysis may be effective for the remediation of soil contaminated with organic pollutants, including pesticides (Yavari et al. 2015; Lam et al. 2019; Li et al. 2016). Microwave-based technology is an alternative heating method and, thanks to its fast, volumetric, selective, and efficient heating, it has already been successfully used in biomass pyrolysis for biochar and biofuel production (Li et al. 2016). Biochars generated via this method are characterized with high yields (>60 wt%) and BET surface areas (450–800 m2/g). Clay and Malo (2012) produced maize stover and switchgrass biochar, under the temperature range of 350–670 °C using microwave. Based on their findings, maize stover biochar shows a higher affinity for weak cationic pesticides such as atrazine while the biochar made from switchgrass strongly retained anionic 2,4-D herbicide. A group of Lam et al. (2019) combined a microwave-assisted pyrolysis of palm kernel shells with steam activation and obtained a sorbent with a microporous structure and high surface area (419 m2/g). The designed activated carbon was then tested as an adsorbent of 2,4-D from surface water in agricultural land, indicating high potential to remove the herbicide.
Klasson et al. (2013) utilized steam activation for preparation of almond shell biochar at 800 °C. The biochar increased its specific surface area to 344 m2 g-1 which resulted in 100% removal of dibromochloropropane from a municipal water well. Removal of thiacloprid and thiamethoxam from water (ultrapure and from natural reservoir in Brazil), using activated and magnetized biochars produced from exhausted husk, and dry tannin from barks of black wattle was done by Matos et al. (2017). The magnetized biochar was obtained by mixing dry input material (dry tannin biomass) with the mixture of FeCl3 and FeCl2, followed by the pyrolysis at 400 °C. The amount of thiacloprid and thiamethoxam adsorbed per gram of biochar was maximum for activated adsorbent (1.02 and 0.97 mg/g, respectively), while for the magnetized biochar it was found to be 0.73 mg/g (thiacloprid) and 0.40 mg/g (thiamethoxam). Baharum et al. (2020) evaluated the adsorption of organophosphorus pesticide—diazinon from aqueous solutions onto chemically activated coconut shell biochar. The carbonized coconut shell biochar (BC1), activated using electrical muffle furnace (BC2), was further chemically modified with phosphoric acid (BC3) and sodium hydroxide (BC4) and tested in batch experiment with diazinon. The modified BC3 and BC4 were reported with maximum diazinon removal at pH 7 (84.55% and 87.93%, respectively).
Deashing of biochar is another popular way of its chemical modification. Wheat and rice biochars prepared at 400 and 600 °C and their deashed counterparts were tested as potential sorbents of sulfonylurea herbicide-pyrazosulfuron-ethyl (Manna et al. 2020). This chemical modification of both types of biochar, based on reaction with a mixture of 10% HF 1 M HCl (v/v), resulted in enhanced and effective herbicide adsorption by a factor of 2–3. Zhang et al. (2018) elucidated the sorption affinity of biochar for neonicotinoid pesticides (midacloprid, clothianidin and thiacloprid) on sorption mechanisms of 24 biochar samples, which were further deashed with acids. This treatment increased the relative percentage contents of organic carbon, bulk oxygen content, aromaticity and O-containing functional groups, surface area, and pore volume of biochar. As a result, they efficiently adsorbed the studied neonicotinoids and multiple mechanisms were involved in sorption, indicating that the ash can bind neonicotinoids by specific interactions playing a negative role in the sorption process. Cederlund et al. (2016) studied the ability of thermally treated and magnetized wood-based biochar produced by slow pyrolysis to adsorb bentazone, chlorpyrifos, diuron, glyphosate, and MCPA to assess its potential use as a filter material to prevent point source pollution in agriculture. They modified the biochar by its heating (450 °C) which resulted in an increase of the specific surface area and the wettability of the biochar, at the same time increasing the adsorption of bentazone and MCPA. Additional treatment with iron salts, which partially coated the biochar with an iron oxide (particularly, with magnetite), decreased the specific surface area of the tested sorbent, but increased the adsorption of glyphosate. Mixing the modified biochar fractions allowed to optimize the sorbent properties and improved its sorption abilities toward the studied agrochemicals.
Al Bahri et al. (2012) designed a sorbent fabricated in conventional wet, chemical impregnation method of grape seeds with phosphoric acid and applied it for the adsorption of diuron from water. In terms of physical properties of biochar the best results (surface area of 1139 m2 g-1 and mesopore volume of 0.24 cm3 g-1) were obtained for grape seeds to phosphoric acid ratio of 1:3 and a carbonization temperature of 500 °C. Its utility to adsorb diuron was high and comparable to the one of powdered activated carbon. Modification of biochar via slow pyrolysis of ammonium di-hydrogen phosphate (ADP) pretreated biomass from corn straw was the subject of the studies of Zhao et al. (2013). They compared ADP-pretreated biochar with the same one produced conventionally, and observed the enhancement of specific surface area, porosity, and the micropore volume of the modified biochar, which significantly increased atrazine sorption.
3.3 Triclosan
Triclosan (TCS) is a broad-spectrum antibacterial agent present as an active ingredient in some personal care products such as soaps, toothpastes, and sterilizers. It is an endocrine-disrupting compound and its increasing presence in water resources as well as in biosolid-amended soils used in farming, its potential for bioaccumulation in fatty tissues and toxicity in aquatic organisms are of concern to human and environmental health, mainly due to its probable role in breast and hepatic tumourigenesis (Olaniyan et al. 2016). Biochar application for triclosan removal from aqueous phase was tested showing better efficiency for triclosan removal by biochar obtained during high-temperature pyrolysis and preconditioning of biochar using inorganic acids. Using such modified biochar, maximum adsorption of 872 μg triclosan/g biochar was achieved using biochar pyrolyzed at 800 °C, at pH between 5 and 9 (Tong et al. 2016). Kimbell et al. (2018) tested biosolid–biochar columns that demonstrated high efficiency of triclosan removal from water and wastewaters solutions, however, contaminant removal was reduced in the presence of other organic pollutants and inorganic nutrients (ammonium and phosphates) in the solution. This finding can limit the carbonaceous materials application for wastewater treatment. Use of municipal biosolids in agriculture present a concern with potential uptake and bioaccumulation of pharmaceutical compounds from biosolids into agronomic plants. Bair et al. (2020) studied the effects of biochar application to minimize triclosan uptake by lettuce and carrots, showing even 67% reduction of triclosan in carrot roots after soil amending with 100 t/ha walnut shell biochar. However, the results of Phandanouvong-Lozano et al. (2018) determined that biochar application to soil can reduce the bioavailability of triclosan to soil microbes, inhibiting biodegradation process of the contaminant, which is an undesired mechanism during soil remediation.
4 Organic Solvents and Dyes
Trichloroethylene (TCE), representing chlorinated hydrocarbons, has been widely used as a solvent in industry, dry cleaning, and food processing, but also as an ingredient in printing dyes, household care products like degreasers, spot removers, mold release agents, paints and as a general anesthetic or analgesic (Bakke et al. 2007). TCE is one of the most hazardous volatile organic (VOCs) compounds which has been proven to be carcinogenic and mutagenic (Shukla et al. 2014). For the remediation of contaminated soil or groundwater with TCE, biotic and abiotic methods have been employed, however, abiotic methods like sorption seem to be more efficient. Biochar has been recently tested as TCE sorbent from wastewaters showing an increase of sorption capacity with increased temperature of pyrolysis (Dong et al. 2017). As nanoscale zero-valent iron (NZVI) has been widely used for the degradation of trichloroethylene (TCE) in contaminated water, NZVI impregnated biochar is described as a win–win solution enhancing TCE adsorption from wastewaters mitigating the problem of NVZI aggregation due to its high surface energy and magnetic interaction in solution (Lawrinenko et al. 2017; Li et al. 2017). Siggins et al. (2020) studied pristine biochars (spruce wastes, herbal pomace and oak bark) pyrolyzed at >600 °C. The results demonstrated that waste-derived biochars were capable of adsorbing >99.5% TCE, however, the efficiency of the process increases when very fine biochar material is utilized in this process.
Similarly, to other ECs, high-temperature biochar is more effective in removing TCE (Ahmad et al. 2014; Zhang et al. 2015; Puppa et al. 2020). The fate of TCE in the environment depends on the medium (air, soil, or water) into which it is released. TCE is not easily biodegradable and hydrolyzed as a hydrophobic substance. However, different groups of microorganisms have been recently found to participate in TCE biodegradation in soil (Shukla et al. 2014) and bioremediation strategies seem to be more effective in contaminant removal than standard applications of adsorbents. That is why trichloroethylene sorption studies on biochar in soil are rare. Sorption of hydrophobic organic compounds in soil is depended on organic matter (OM) content and probably (there is no study on the topic) biochar application, especially to soils poor in OM will enhance TCE sorption process. Synthetic dyes are extensively used in many fields of up-to-date technology, e.g., in various branches of the textile industry, leather tanning, food technology, or cosmetics production. Due to large wastewater yield, high concentrations of aromatic pollutants, dark color, weakly biodegradable components, alkaline properties, and a variety of complex substances, the dyeing industry wastewater caused serious water pollution (Forgacs et al. 2004). Due to its high surface area and proper microporous structure, biochar proved to be an effective adsorbent for both cationic and anionic dyes. Electrolytes in solution effectively neutralized the negative charge of biochar and induced the dimerization of cationic dyes, thus enhancing the adsorption of both ionic forms of dyes by biochar (Qiu et al. 2009). Fan et al. (2016) studied methylene blue adsorption by municipal sewage sludge and tea waste biochar. The results demonstrated that biochar adsorption capacity for dyes increased with the pyrolysis temperature and many different mechanisms were involved in adsorption process, such as electrostatic interaction, ion exchange, surface complexation, physical diffusion, and others, suggesting the use of biochar as very promising for dyes removal from wastewaters. Similar findings were described by Sumalinog et al. (2018) testing the efficiency of methylene blue adsorption on biochar obtained from the slow pyrolysis of municipal solid waste (MSW), suggesting that dye adsorption occurs mainly due to chemical reactions on biochar surface in a wide range of pH values. Finally, Zhang et al. (2020b) presented green biochar/iron oxide composite as an effective adsorbent for dye substances removal from industrial effluents, showing that modified biochar has very good dye removal capacity.
5 Nitrogen and Phosphorus Contaminants
Various agricultural chemicals, such as fertilizers are being used to increase the output of crops. The risk posed by the contamination of water with excessively posed phosphorus and nitrogen from agricultural runoff is of great concern (Salimova et al. 2020). Also, wastewaters containing high concentrations of inorganic salts, organic compounds including nitrogenous molecules (urea), and other metabolic waste components became a source of N, P, K in the environment. Agricultural runoffs and natural surface water contamination with wastewaters lead to the process of eutrophication, causing depletion of drinking water supplies. Numerous studies have shown the applications of biochar for nutrient (ammonia and phosphate) recovery from aqueous solutions and urine. Nutrient extraction from human urine is a sustainable option for waste disposal and an economically feasible way to produce soil conditioner/fertilizer (Masrura et al. 2020). Raw materials and pyrolysis conditions can influence different biochar properties, however, the affinity of pristine biochar for the removal of oxyanions is relatively low due to the electrostatic repulsion by negatively charged BC surface. Biochars activated by metals have a significantly higher capacity to adsorb nitrate and phosphate than their unmodified counterparts. For oxyanions such as NO3− and PO43−, mainly positively charged metal oxides, increasing the number of active sites in biochar and its surface charge, are responsible for their sorption (Wang et al. 2020a, b). Its mechanism occurs mainly via ion exchange and interactions with oxygen-containing functional groups on biochar surfaces and is related to the catalytic action of the composite material (Wang et al. 2020a, b; Zhang et al. 2020a).
5.1 Nitrates
For NO3−, the sorption mechanisms are governed by multiple interactions. Electrostatic attraction and ionic bonds with exchangeable cations from the biochar is postulated as a primary route of nitrates retention. Biochar is a potentially effective sorbent for NO3− in water treatment and soil applications. Fidel et al. (2018) evaluated nitrates sorption rates to acid-washed biochar produced from red oak and corn stover at different pyrolysis temperatures and pH range. Additionally, they quantified NO3− sorption, as well as Cl− displacement for corn stover biochar to examine the sorption mechanisms. Nitrates sorption was the maximum (1.4–1.5 mg N g−1) at 600 °C and pH of 3.5–4. NO3− displaced Cl− from previously CaCl2-saturated corn stover biochar, which supports the predominance of ion exchange mechanisms. Viglašová et al. (2018) fabricated a biochar/montmorillonite composite, using bamboo as raw material and tested its utility in the adsorption studies for removal of nitrates from aqueous solutions. Montmorillonite was distributed across the biochar surface which resulted in enhancement of the maximum adsorption capacity of the composite to 9 mg of NO3− per gram of the sorbent.
A variety of different activating agent solutions (HCl, NaCl, KCl, MgCl2, ZnCl2, AlCl3, FeCl3, MgCl2 with HCl) were added to the pristine corncob biochar, to study its utility to remove NO3− in wastewaters (Long et al. 2019). Among the studied variants, FeCl3 modified biochar with the highest positive surface charge was further tested to remove nitrates from aqueous solutions. Through the formation of iron nitrate hydrate (Fe(NO3)3·9H2O) on the biochar surface, higher nitrate adsorption, than that of pristine biochar, was achieved. In the study of Hafshejani et al. (2016), chemically modified biochar (developed from sugarcane bagasse) was used for the nitrate removal from aqueous solution. The results showed that the maximum percentage of NO3− adsorption was achieved at equilibrium pH of 4.64, after 60 min of contact time and with an adsorbent dose of 2 g L−1. Additionally, competing anions (carbonate and chloride ions) have shown maximum and minimum influence on the NO3− adsorption of the studied BC.
5.2 Phosphates
Effective and economical phosphate removal from wastewater can be very often achieved by magnetic modification of biochar. Precipitation by metal oxides in BC is the primary mechanism for PO43- removal. Ajmal et al. produced magnetically modified biochar by co-precipitation of Fe(II) and Fe(III) ions in their presence, from wood and rice husks. Its utility as PO43− sorbent in wastewaters was tested to be twice as efficient (25–28 mg g−1) than that of the unmodified biochar. According to the results, the driving PO43− sorption mechanism on magnetic biochar is simultaneously the electrostatic attraction, surface precipitation, and complexation, while for the original biochar, the sorption mainly depends on electrostatic attraction. Sometimes, however, the input material itself can exhibit properties enabling it to efficiently sorb the destined pollutant. One such example is sewage sludge that after pyrolysis acts as an excellent PO43− sorbent (the maximum sorption capacity of 303.5 mg g−1) in the wide pH range of the studied eutrophic waters (Yin et al. 2019). This ideal adsorption ability was mainly due to the abundance of metal oxides and functional groups in sewage sludge that are capable of effectively removing phosphates. Magnetic modification of biochar was also used to study the phosphates adsorption mechanisms in soil, under field conditions. Wu et al. (2019) pyrolyzed peanut shells and modified them with MgO. Such activation of biochar resulted in 20% increase in phosphates adsorption than unmodified biochar. Electrostatic attraction, precipitation, and exchangeable anions contributed to the adsorption of phosphate and MgO-modified biochar, which possessed the greatest adsorption capacity and its application in soil increased the available P content and resulted in higher rice yields in the field experiments. Iron-modified corn straw biochar was used as an adsorbent to remove phosphorus from agricultural runoff (Liu et al. 2015). The surface of the activated biochar was covered by small iron granules, which were identified as Fe3O4. The tested biochar was packed in a column and the agricultural runoffs with a total phosphorus concentration of 1.86–2.47 mg L−1 were applied with a hydraulic retention time of 2 h. The efficiency to remove the phosphorous from effluent was 99% and its concentration was less than 0.02 mg L−1. At the studied biochar-to-soil rate (5%), the stem, root, and bean of broad bean plants demonstrated increased growth rates of 91%, 64%, and 165%, respectively.
Chemical modification of biochar with the use of polyethyleneimine (PEI) was conducted to test the efficiency of amine-functionalized biochar to remove phosphates (Li et al. 2020). Biomass pyrolyzed in the study was bamboo. PEI was successfully grafted onto biochar which enhanced P adsorption. The most optimal conditions for P adsorption were pH 3, low ionic strength, and low concentration of coexisting ions, such as HCO3−, SO42−, NO3−, and Cl−. The electrostatic interaction between P and surface functional groups of PEI-modified biochar served as the primary mechanism controlling the adsorption process.
Ramola et al. (2021) prepared a biochar–mineral composite by using rice husk and calcite as raw materials. Prepared biochar–calcite composite was an optimized adsorbent for phosphate with maximum removal of 87.3% and adsorption capacity of 1.76 mg/g. The optimized conditions of pyrolysis were—700 °C, 2.3 h, and 4.2:1 (w/w) of rice husk and calcite ratio. Introduction of calcite increased the efficiency of biochar for phosphate removal that was mainly controlled by adsorption onto the surface of biochar–mineral composite and electrostatic interactions. However, the presence of other ions in the solution, reduced the amount of phosphate removal by biochar–mineral composite.
The cosorption of both oxyanions: NO3− and PO43− was evaluated by Yin et al. (2018) in the studies assessing the effectiveness of eutrophic waters remediation by magnetically modified BC with different Al contents (i.e., 5, 10, 15, and 20 wt %). Biochar with 15% Al content exhibited optimal NO3− adsorption capacity (the maximum sorption capacity of 89.58 mg g-1), whereas for PO43− it was the highest for 20% Al amendment (57.49 mg g-1). pH = 6 and pH < 6 were advantageous to NO3− and PO43− adsorptions, respectively, which occurred mainly through chemisorption. Li et al. (2016) also tested modified biochar for NO3− and PO43− removal from water. They produced biochar from wheat straw in low-temperature pyrolysis (450 °C), activated with hydrochloric acid (HCl), and coated with different amounts of iron (FeCl3 · 6H2O). Activation with HCl and coating with iron significantly increased biochar adsorption capacity at the optimal ratio of iron to biochar for iron-coated biochar (0.70). The active substance of the optimal-modified biochar (OMB) was amorphous FeOOH. The maximum adsorption capacities were 2.47 at pH 3 and 16.58 mg g−1 at pH 6, for nitrates and phosphates, respectively.
5.3 Ammonia
Ammonium (NH4+) is one of the common forms of reactive nitrogen (N) in water and wastewater. As biochar usually carries negative surface charge, its adsorption capacity for NH4+ cations is high. NH4+ adsorption depends more on oxygen functional groups and sorbent porosity and low-temperature biochar seems to be more efficient in ammonium removal. Non-modified, low-temperature biochar (<400 ºC) can adsorb 60–79% of ammonia and up to 60% of phosphorus from aqueous solution (Chen et al. 2011; Xie et al. 2015). Oxygen-containing functional groups such as C=O, –COOH, and –COC– contribute substantially to NH4+ adsorption because of hydrogen bonds and electrostatic interaction between NH4+ and biochar (Cai et al. 2016). Nitrogen recovery efficiency by biochar increased with solution pH from 7 to 12. At high pH, the zeta potential of biochar decreased leading to enhanced adsorption of positively charged ions (Yang et al. 2020). Tang et al. (2019) tested digested sludge biochar and demonstrated that the final amount of adsorbed ammonium increased significantly (p < 0.001) as the initial pH increased from 2 to 6, and decreased slightly (p = 0.6) when pH increased to 8. These results also show that at acidic conditions, ammonium removal capacity is low, mainly due to the competition between H+ and NH4+ in the solution for adsorption to biochar surface functional groups. Significant reduction of ammonia adsorption capacity was observed when pH reached 10, which is due to the effects of pH on the form of ammonium, i.e., it converts to NH3 (Vu et al. 2017). Designing a proper material for ammonia removal—type of biomass, biochar production temperature, and residence time on NH4+ adsorption are significant. Salimova et al. (2020) demonstrated that orange tree biochar has a strong ability to remove ammonia from water, while tea tree biochar did not develop proper properties during the same pyrolysis temperature and efficiency of this material for NH4+ was very low. Similar findings were described by Xue et al. (2019) testing seven types of food waste-based biochar (meat and bone, starchy staples, leafy stemmed vegetables, nut husks, fruit pericarp, bean dreg, and tea leaves) achieving even 92.6% of ammonia nitrogen removal, depending on raw material. Raw materials like fruit pericarp and nut husk indicated better effective adsorption, but starchy staples, meat, and bone were least effective. Similarly with the increase of pyrolysis temperature the adsorption capacity of biochar decreased. The efficiency of biochar for ammonium adsorption also depends on initial ammonium concentration. Biochar deashing and acid modifications with inorganic acids seems to be a sufficient method of enhancing biochar sorption capacity for ammonium (Vu et al. 2017).
6 Microplastic
Although microplastic (MP) is not yet on the list of emerging contaminants, its wide occurrence in aquatic and terrestrial ecosystem brings concerns about possible impacts of this microcontaminant on living organisms, including human beings. Biochar application for wastewater and water purification from microplastic seems to be a good strategy to find cost-effective and efficient MP sorbent. The advantage of MP removal is that the main mechanisms are based on physical sorption on the porous structure of adsorbent and it is not necessarily important to obtain materials with high surface area or large number of oxygen functional groups which makes the biochar-based—microplastic sorbent production less expensive and easier to obtain in standard pyrolysis reactors. Siipola et al. (2020) described low-cost spruce and pine bark-derived biochar as good adsorbent for microplastic removal in water purification process. As MPs is adsorbed mainly on biochar micropores, this microporosity can be obtained during steam activation or ball-milling. The more micropores are generated on biochar surface, the more efficient MPs removal is. Similarly, observation was described by Wang et al. (2020a, b) using biochar samples produced at three different temperatures from corn straw and a hardwood biochar to compare retention of spherical microplastics. Results of the study showed that removal efficiency was higher than 95% when microporous biochar were used for trapping MPs.
Limitations
Although biochar has proven to be one of the most efficient adsorbents in removing ECs, certain disadvantages are making it a less attractive option for many applications. Numerous sorption mechanisms, mostly tested under very controlled conditions in pure solutions or batch experiments will not be sufficient to extrapolate on real soils and natural waters conditions. That is why obtained results should be handled with care and further investigation are necessary to predict the efficiency of biochar for the removal of EC in natural systems.
There is a lot of uncertainty about optimum parametric conditions of the process, e.g., pH of medium, adsorbent dose, pharmaceutical concentrations, contact time, or temperature. There are also doubts about the use of proper biomass for adsorbent production as the amount of the biochar necessary to clean wastewaters are estimated in million tons. Finally, what to do with spent biochar waste? How to separate biochar from soil or solution? These are the questions with no answer at this moment. Pretreatments or post-treatments of biochar will be necessary in most of the cases to enhance the adsorption capacity. However, these procedures may significantly increase the cost of biochar use for wastewater treatment. High costs due to the activation process, lack of biodegradability of adsorbed ECs by microbes, and high regeneration cost are some of the limitations of biochar used in this process.
7 Conclusions and Future Prospects
As the problem of emerging contaminants released to the environmental media occurs globally with an unknown effect to human health, there is an urgent need to develop new, cost-effective, and ecological-friendly methods of water and soil treatment to mitigate potential risk related to presence of ECs in the food chain. In this context, the presented review highlights the wide application potential of biomass waste to become a precursor of biochar, i.e., highly efficient material for the removal of emerging contaminants from wastewaters. The number of scientific papers describing efficiency of different biochars for ECs removal from wastewaters and aqueous solutions is gaining a growing interest for this material as an emerging contaminant adsorbent. Designing a proper sorbent should ensure immobilization of high concentrations of contaminants present in media. Therefore, adsorption capacity and affinity to micropollutants molecules should be enhanced by biochar surface modifications.
References
Ahmad M, Moon DH, Vithanage M, Koutsospyros A, Lee SS, Yang JE, Lee SE, Jeon C, Ok YS (2014) Production and use of biochar from buffalo-weed (Ambrosia trifida L.) for trichloroethylene removal from water: Production and use of biochar from Ambrosia trifida for TCE removal from water. J Chem Technol Biotechnol 89(1):150–157. https://doi.org/10.1002/jctb.4157
Ahmed M, John L, Ngo H, Johir M, Sornalingam K (2018a) Sorptive removal of phenolic endocrine disruptors by functionalized biochar: competitive interaction mechanism, removal efficacy and application in wastewater. Chem Eng J 335:801–811. https://doi.org/10.1016/j.cej.2017.11.041
Ahmed M, Zhou J, Ngo H, Johir M, Sornalingam K (2018b) Sorptive removal of phenolic endocrine disruptors by functionalized biochar: competitive interaction mechanism, removal efficacy and application in wastewater. Chem Eng J 335:801–811. https://doi.org/10.1016/j.cej.2017.11.041
Ahmed M, Zhou J, Ngo H, Guo W, Johir M, Belhaj D (2017) Competitive sorption affinity of sulfonamides and chloramphenicol antibiotics toward functionalized biochar for water and wastewater treatment. Biores Technol 238:306–312. https://doi.org/10.1016/j.biortech.2017.04.042
Al Bahri M, Calvo L, Gilarranz MA, Rodriguez JJ (2012) Activated carbon from grape seeds upon chemical activation with phosphoric acid: application to the adsorption of diuron from water. Chem Eng J 203:348–356. https://doi.org/10.1016/j.cej.2012.07.053
Alizadeh S, Prasher S, ElSayed E, Qi Z, Patel RM (2018) Effect of biochar on fate and transport of manure-borne estrogens in sandy soil. J Environ Sci 73:162–176. https://doi.org/10.1016/j.jes.2018.01.025
Álvarez-Torrellas S, Rodríguez A, Ovejero G, Gómez JM, García J (2016) Removal of caffeine from pharmaceutical wastewater by adsorption: influence of NOM, textural and chemical properties of the adsorbent. Environ Technol 37(13):1618–1630. https://doi.org/10.1080/09593330.2015.1122666
Azhar MR, Abid HR, Sun H, Periasamy V, Tadé MO, Wang S (2016) Excellent performance of copper based metal organic framework in adsorptive removal of toxic sulfonamide antibiotics from wastewater. J Colloid Interface Sci 478:344–352. https://doi.org/10.1016/j.jcis.2016.06.032
Baharum NA, Nasir HM, Ishak MY, Isa NM, Hassan MA, Aris AZ (2020) Highly efficient removal of diazinon pesticide from aqueous solutions by using coconut shell-modified biochar. Arab J Chem 13(7):6106–6121. https://doi.org/10.1016/j.arabjc.2020.05.011
Bair DA, Anderson CG, Chung Y, Scow KM, Franco RB, Parikh SJ (2020) Impact of biochar on plant growth and uptake of ciprofloxacin, triclocarban and triclosan from biosolids. J Environ Sci Health B 55(11):990–1001. https://doi.org/10.1080/03601234.2020.1807264
Bair DA, Mukome FND, Popova IE, Ogunyoku TA, Jefferson A, Wang D, Hafner SC, Young TM, Parikh SJ (2016) Sorption of pharmaceuticals, heavy metals, and herbicides to biochar in the presence of biosolids. J Environ Qual 45(6):1998–2006. https://doi.org/10.2134/jeq2016.03.0106
Bakke B, Stewart PA, Waters MA (2007) Uses of and exposure to trichloroethylene in U.S. industry: a systematic literature review. J Occup Environ Hyg 4(5):375–390. https://doi.org/10.1080/15459620701301763
Cai Y, Qi H, Liu Y, He X (2016) Sorption/desorption behavior and mechanism of NH 4 + by biochar as a nitrogen fertilizer sustained-release material. J Agric Food Chem 64(24):4958–4964. https://doi.org/10.1021/acs.jafc.6b00109
Cederlund H, Börjesson E, Lundberg D, Stenström J (2016) Adsorption of pesticides with different chemical properties to a wood biochar treated with heat and iron. Water Air Soil Pollut 227(6):203. https://doi.org/10.1007/s11270-016-2894-z
Chakraborty P, Banerjee S, Kumar S, Sadhukhan S, Halder G (2018) Elucidation of ibuprofen uptake capability of raw and steam activated biochar of Aegle marmelos shell: isotherm, kinetics, thermodynamics and cost estimation. Process Saf Environ Prot 118:10–23. https://doi.org/10.1016/j.psep.2018.06.015
Chen B, Chen Z, Lv S (2011) A novel magnetic biochar efficiently sorbs organic pollutants and phosphate. Biores Technol 102(2):716–723. https://doi.org/10.1016/j.biortech.2010.08.067
Chen B, Zhou D, Zhu L (2008) Transitional adsorption and partition of nonpolar and polar aromatic contaminants by biochars of pine needles with different pyrolytic temperatures. Environ Sci Technol 42(14):5137–5143. https://doi.org/10.1021/es8002684
Chen T, Luo L, Deng S, Shi G, Zhang S, Zhang Y, Deng O, Wang L, Zhang J, Wei L (2018) Sorption of tetracycline on H3PO4 modified biochar derived from rice straw and swine manure. Biores Technol 267:431–437. https://doi.org/10.1016/j.biortech.2018.07.074
Clay SA, Malo DD (2012) The influence of biochar production on herbicide sorption characteristics. In: Hasannen M (ed) Herbicides—properties, synthesis and control of weeds. In Tech, New York, pp 59–74. https://doi.org/10.5772/31492
Danzo BJ (1998) The effects of environmental hormones on reproduction. Cell Mol Life Sci (CMLS) 54(11):1249–1264. https://doi.org/10.1007/s000180050251
de Souza dos Santos GE, Ide AH, Duarte JLS, McKay G, Silva AOS, Meili L (2020) Adsorption of anti-inflammatory drug diclofenac by MgAl/layered double hydroxide supported on Syagrus coronata biochar. Powder Technol 364:229–240. https://doi.org/10.1016/j.powtec.2020.01.083
Diamanti-Kandarakis E, Bourguignon J-P, Giudice LC, Hauser R, Prins GS, Soto AM, Zoeller RT, Gore AC (2009) Endocrine-disrupting chemicals: an endocrine society scientific statement. Endocr Rev 30(4):293–342. https://doi.org/10.1210/er.2009-0002
Divband Hafshejani L, Hooshmand A, Naseri AA, Mohammadi AS, Abbasi F, Bhatnagar A (2016) Removal of nitrate from aqueous solution by modified sugarcane bagasse biochar. Ecol Eng 95:101–111. https://doi.org/10.1016/j.ecoleng.2016.06.035
Dong H, Zhang C, Hou K, Cheng Y, Deng J, Jiang Z, Tang L, Zeng G (2017) Removal of trichloroethylene by biochar supported nanoscale zero-valent iron in aqueous solution. Sep Purif Technol 188:188–196. https://doi.org/10.1016/j.seppur.2017.07.033
Dong X, He L, Hu H, Liu N, Gao S, Piao Y (2018) Removal of 17β-estradiol by using highly adsorptive magnetic biochar nanoparticles from aqueous solution. Chem Eng J 352:371–379. https://doi.org/10.1016/j.cej.2018.07.025
Escudero-Curiel S, Penelas U, Sanromán MÁ, Pazos M (2021) An approach towards Zero-Waste wastewater technology: fluoxetine adsorption on biochar and removal by the sulfate radical. Chemosphere 268:129318. https://doi.org/10.1016/j.chemosphere.2020.129318
Essandoh M, Kunwar B, Pittman CU, Mohan D, Mlsna T (2015) Sorptive removal of salicylic acid and ibuprofen from aqueous solutions using pine wood fast pyrolysis biochar. Chem Eng J 265:219–227. https://doi.org/10.1016/j.cej.2014.12.006
Fan S, Tang J, Wang Y, Li H, Zhang H, Tang J, Wang Z, Li X (2016) Biochar prepared from co-pyrolysis of municipal sewage sludge and tea waste for the adsorption of methylene blue from aqueous solutions: kinetics, isotherm, thermodynamic and mechanism. J Mol Liq 220:432–441. https://doi.org/10.1016/j.molliq.2016.04.107
Fan S, Wang Y, Li Y, Wang Z, Xie Z, Tang J (2018) Removal of tetracycline from aqueous solution by biochar derived from rice straw. Environ Sci Pollut Res 25(29):29529–29540. https://doi.org/10.1007/s11356-018-2976-0
Fernandes MJ, Moreira MM, Paíga P, Dias D, Bernardo M, Carvalho M, Lapa N, Fonseca I, Morais S, Figueiredo S, Delerue-Matos C (2019) Evaluation of the adsorption potential of biochars prepared from forest and agri-food wastes for the removal of fluoxetine. Biores Technol 292:121973. https://doi.org/10.1016/j.biortech.2019.121973
Fidel RB, Laird DA, Spokas KA (2018) Sorption of ammonium and nitrate to biochars is electrostatic and pH-dependent. Sci Rep 8(1):17627. https://doi.org/10.1038/s41598-018-35534-w
Forgacs E, Cserháti T, Oros G (2004) Removal of synthetic dyes from wastewaters: a review. Environ Int 30(7):953–971. https://doi.org/10.1016/j.envint.2004.02.001
Gaffar S, Dattamudi S, Baboukani AR, Chanda S, Novak JM, Watts DW, Wang C, Jayachandran K (2021) Physiochemical characterization of biochars from six feedstocks and their effects on the sorption of atrazine in an organic soil. Agronomy 11(4):716. https://doi.org/10.3390/agronomy11040716
Gavrilescu M, Demnerová K, Aamand J, Agathos S, Fava F (2015) Emerging pollutants in the environment: present and future challenges in biomonitoring, ecological risks and bioremediation. New Biotechnol 32(1):147–156. https://doi.org/10.1016/j.nbt.2014.01.001
Guo W, Lu S, Shi J, Zhao X (2019) Effect of corn straw biochar application to sediments on the adsorption of 17α-ethinyl estradiol and perfluorooctane sulfonate at sediment-water interface. Ecotoxicol Environ Saf 174:363–369. https://doi.org/10.1016/j.ecoenv.2019.01.128
Han X, Liang C, Li T, Wang K, Huang H, Yang X (2013) Simultaneous removal of cadmium and sulfamethoxazole from aqueous solution by rice straw biochar. J Zhejiang Univ Sci B 14(7):640–649. https://doi.org/10.1631/jzus.B1200353
Heo J, Yoon Y, Lee G, Kim Y, Han J, Park CM (2019) Enhanced adsorption of bisphenol A and sulfamethoxazole by a novel magnetic CuZnFe2O4–biochar composite. Biores Technol 281:179–187. https://doi.org/10.1016/j.biortech.2019.02.091
Jia M, Wang F, Bian Y, Jin X, Song Y, Kengara FO, Xu R, Jiang X (2013) Effects of pH and metal ions on oxytetracycline sorption to maize-straw-derived biochar. Biores Technol 136:87–93. https://doi.org/10.1016/j.biortech.2013.02.098
Jing X-R, Wang Y-Y, Liu W-J, Wang Y-K, Jiang H (2014) Enhanced adsorption performance of tetracycline in aqueous solutions by methanol-modified biochar. Chem Eng J 248:168–174. https://doi.org/10.1016/j.cej.2014.03.006
Jung C, Boateng LK, Flora JRV, Oh J, Braswell MC, Son A, Yoon Y (2015) Competitive adsorption of selected non-steroidal anti-inflammatory drugs on activated biochars: experimental and molecular modeling study. Chem Eng J 264:1–9. https://doi.org/10.1016/j.cej.2014.11.076
Kahle M, Stamm C (2007) Time and pH-dependent sorption of the veterinary antimicrobial sulfathiazole to clay minerals and ferrihydrite. Chemosphere 68(7):1224–1231. https://doi.org/10.1016/j.chemosphere.2007.01.061
Kearns JP, Shimabuku KK, Knappe DRU, Summers RS (2019) High temperature co-pyrolysis thermal air activation enhances biochar adsorption of herbicides from surface water. Environ Eng Sci 36(6):710–723. https://doi.org/10.1089/ees.2018.0476
Keerthanan S, Rajapaksha SM, Trakal L, Vithanage M (2020) Caffeine removal by Gliricidia sepium biochar: influence of pyrolysis temperature and physicochemical properties. Environ Res 189:109865. https://doi.org/10.1016/j.envres.2020.109865
Kim E, Jung C, Han J, Her N, Park CM, Jang M, Son A, Yoon Y (2016) Sorptive removal of selected emerging contaminants using biochar in aqueous solution. J Ind Eng Chem 36:364–371. https://doi.org/10.1016/j.jiec.2016.03.004
Kim K-R, Owens G, Kwon S-I, So K-H, Lee D-B, Ok YS (2011) Occurrence and environmental fate of veterinary antibiotics in the terrestrial environment. Water Air Soil Pollut 214(1–4):163–174. https://doi.org/10.1007/s11270-010-0412-2
Kimbell LK, Tong Y, Mayer BK, McNamara PJ (2018) Biosolids-derived biochar for triclosan removal from wastewater. Environ Eng Sci 35(6):513–524. https://doi.org/10.1089/ees.2017.0291
Klasson KT, Ledbetter CA, Uchimiya M, Lima IM (2013) Activated biochar removes 100 % dibromochloropropane from field well water. Environ Chem Lett 11(3):271–275. https://doi.org/10.1007/s10311-012-0398-7
Lam SS, Su MH, Nam WL, Thoo DS, Ng CM, Liew RK, Yuh Yek PN, Ma NL, Nguyen Vo DV (2019) Microwave pyrolysis with steam activation in producing activated carbon for removal of herbicides in agricultural surface water. Ind Eng Chem Res 58:695–703. https://doi.org/10.1021/acs.iecr.8b03319
Lawrinenko M, Wang Z, Horton R, Mendivelso-Perez D, Smith EA, Webster TE, Laird DA, van Leeuwen JH (2017) Macroporous carbon supported zerovalent iron for remediation of trichloroethylene. ACS Sustainable Chem Eng 5(2):1586–1593. https://doi.org/10.1021/acssuschemeng.6b02375
Lei M, Zhang L, Lei J, Zong L, Li J, Wu Z, Wang Z (2015) Overview of emerging contaminants and associated human health effects. Biomed Res Int 2015:1–12. https://doi.org/10.1155/2015/404796
Leng L, Xiong Q, Yang L, Li H, Zhou Y, Zhang W, Jiang S, Li H, Huang H (2021) An overview on engineering the surface area and porosity of biochar. Sci Total Environ 763:144204. https://doi.org/10.1016/j.scitotenv.2020.144204
Li C, Zhu X, He H, Fang Y, Dong H, Lü J, Li J, Li Y (2019) Adsorption of two antibiotics on biochar prepared in air-containing atmosphere: influence of biochar porosity and molecular size of antibiotics. J Mol Liq 274:353–361. https://doi.org/10.1016/j.molliq.2018.10.142
Li H, Chen YQ, Chen S, Wang XL, Guo S, Qiu YF, Liu YD, Duan XL, Yu YJ (2017) Wheat straw biochar-supported nanoscale zerovalent iron for removal of trichloroethylene from groundwater. PLoS ONE 12(3):e0172337. https://doi.org/10.1371/journal.pone.0172337
Li J, Lv G, Bai W, Liu Q, Zhang Y, Song J (2016) Modification and use of biochar from wheat straw (Triticum aestivum L.) for nitrate and phosphate removal from water. Desalin Water Treat 57(10):4681–4693. https://doi.org/10.1080/19443994.2014.994104
Li T, Tong Z, Gao B, Li YC, Smyth A, Bayabil HK (2020) Polyethyleneimine-modified biochar for enhanced phosphate adsorption. Environ Sci Pollut Res 27(7):7420–7429. https://doi.org/10.1007/s11356-019-07053-2
Lian F, Sun B, Chen X, Zhu L, Liu Z, Xing B (2015) Effect of humic acid (HA) on sulfonamide sorption by biochars. Environ Pollut 204:306–312. https://doi.org/10.1016/j.envpol.2015.05.030
Lian F, Sun B, Song Z, Zhu L, Qi X, Xing B (2014) Physicochemical properties of herb-residue biochar and its sorption to ionizable antibiotic sulfamethoxazole. Chem Eng J 248:128–134. https://doi.org/10.1016/j.cej.2014.03.021
Liu F, Zuo J, Chi T, Wang P, Yang B (2015) Removing phosphorus from aqueous solutions by using iron-modified corn straw biochar. Front Environ Sci Eng 9(6):1066–1075. https://doi.org/10.1007/s11783-015-0769-y
Liu S, Li M, Liu Y, Liu N, Tan X, Jiang L, Wen J, Hu X, Yin Z (2019a) Removal of 17β-estradiol from aqueous solution by graphene oxide supported activated magnetic biochar: adsorption behavior and mechanism. J Taiwan Inst Chem Eng 102:330–339. https://doi.org/10.1016/j.jtice.2019.05.002
Liu S, Liu Y, Jiang L, Zeng G, Li Y, Zeng Z, Wang X, Ning Q (2019b) Removal of 17β-Estradiol from water by adsorption onto montmorillonite-carbon hybrids derived from pyrolysis carbonization of carboxymethyl cellulose. J Environ Manage 236:25–33. https://doi.org/10.1016/j.jenvman.2019.01.064
Liu Y, Lonappan L, Brar SK, Yang S (2018) Impact of biochar amendment in agricultural soils on the sorption, desorption, and degradation of pesticides: a review. Sci Total Environ 645:60–70. https://doi.org/10.1016/j.scitotenv.2018.07.099
Long L, Xue Y, Hu X, Zhu Y (2019) Study on the influence of surface potential on the nitrate adsorption capacity of metal modified biochar. Environ Sci Pollut Res 26(3):3065–3074. https://doi.org/10.1007/s11356-018-3815-z
Luo J, Li X, Ge C, Müller K, Yu H, Huang P, Li J, Tsang DCW, Bolan NS, Rinklebe J, Wang H (2018) Sorption of norfloxacin, sulfamerazine and oxytetracycline by KOH-modified biochar under single and ternary systems. Biores Technol 263:385–392. https://doi.org/10.1016/j.biortech.2018.05.022
Luo R, Li X, Xu H, Sun Y, Wu J (2020) Effects of Temperature, solution pH, and ball-milling modification on the adsorption of non-steroidal anti-inflammatory drugs onto biochar. Bull Environ Contam Toxicol 105(3):422–427. https://doi.org/10.1007/s00128-020-02948-0
Manna S, Singh N, Purakayastha TJ, Berns AE (2020) Effect of deashing on physico-chemical properties of wheat and rice straw biochars and potential sorption of pyrazosulfuron-ethyl. Arab J Chem 13:1247–1258. https://doi.org/10.1016/j.arabjc.2017.10.005
Matos TTS, Schultz J, Khan MY, Zanoelo EF, Mangrich AS, Araújo BR, Navickiene S, Romão LPC (2017) Using magnetized (Fe3O4/biochar nanocomposites) and activated biochar as adsorbents to remove two neuro-active pesticides from waters. J Braz Chem Soc 28:1975–1987. https://doi.org/10.21577/0103-5053.20170042
Masrura SU, Dissanayake P, Sun Y, Ok YS, Tsang DCW, Khan E (2020) Sustainable use of biochar for resource recovery and pharmaceutical removal from human urine: a critical review. Crit Rev Environ Sci Technol 1–33. https://doi.org/10.1080/10643389.2020.1818497
Mlunguza NY, Ncube S, Nokwethemba Mahlambi P, Chimuka L, Madikizela LM (2019) Adsorbents and removal strategies of non-steroidal anti-inflammatory drugs from contaminated water bodies. J Environ Chem Eng 7(3):103142. https://doi.org/10.1016/j.jece.2019.103142
Mnif W, Hassine AIH, Bouaziz A, Bartegi A, Thomas O, Roig B (2011) Effect of endocrine disruptor pesticides: a review. IJERPH 8(6):2265–2303. https://doi.org/10.3390/ijerph8062265
Mondal S, Aikat K, Halder G (2016) Biosorptive uptake of ibuprofen by chemically modified Parthenium hysterophorus derived biochar: equilibrium, kinetics, thermodynamics and modeling. Ecol Eng 92:158–172. https://doi.org/10.1016/j.ecoleng.2016.03.022
Mutavdžić Pavlović D, Ćurković L, Blažek D, Župan J (2014) The sorption of sulfamethazine on soil samples: isotherms and error analysis. Sci Total Environ 497–498:543–552. https://doi.org/10.1016/j.scitotenv.2014.08.018
Nazal MK, Rao D, Abuzaid N (2021) First investigations on removal of nitrazepam from water using biochar derived from macroalgae low-cost adsorbent: kinetics, isotherms and thermodynamics studies. Water Pract Technol wpt2021040. https://doi.org/10.2166/wpt.2021.040
Ndoun MC, Elliott HA, Preisendanz HE, Williams CF, Knopf A, Watson JE (2021) Adsorption of pharmaceuticals from aqueous solutions using biochar derived from cotton gin waste and guayule bagasse. Biochar 3(1):89–104. https://doi.org/10.1007/s42773-020-00070-2
Olaniyan LWB, Mkwetshana N, Okoh AI (2016) Triclosan in water, implications for human and environmental health. Springerplus 5(1):1639. https://doi.org/10.1186/s40064-016-3287-x
Peiris C (2020) Biochar based sorptive remediation of steroidal estrogen contaminated aqueous systems: a critical review. Environ Res 11
Peiris C, Gunatilake SR, Mlsna TE, Mohan D, Vithanage M (2017) Biochar based removal of antibiotic sulfonamides and tetracyclines in aquatic environments: a critical review. Biores Technol 246:150–159. https://doi.org/10.1016/j.biortech.2017.07.150
Phandanouvong-Lozano V, Sun W, Sanders JM, Hay AG (2018) Biochar does not attenuate triclosan’s impact on soil bacterial communities. Chemosphere 213:215–225. https://doi.org/10.1016/j.chemosphere.2018.08.132
Puppa LD, Ducousso M, Batisse N, Dubois M, Verney V, Xavier V, Delor-Jestin F (2020) Poplar wood and tea biochars for trichloroethylene remediation in pure water and contaminated groundwater. Environ Challenges 1:100003. https://doi.org/10.1016/j.envc.2020.100003
Qin F, Peng Y, Song G, Fang Q, Wang R, Zhang C, Zeng G, Huang D, Lai C, Zhou Y, Tan X, Cheng M, Liu S (2020) Degradation of sulfamethazine by biochar-supported bimetallic oxide/persulfate system in natural water: Performance and reaction mechanism. J Hazard Mater 398:122816. https://doi.org/10.1016/j.jhazmat.2020.122816
Qiu Y, Zheng Z, Zhou Z, Sheng GD (2009) Effectiveness and mechanisms of dye adsorption on a straw-based biochar. Biores Technol 100(21):5348–5351. https://doi.org/10.1016/j.biortech.2009.05.054
Quintana J, Weiss S, Reemtsma T (2005) Pathways and metabolites of microbial degradation of selected acidic pharmaceutical and their occurrence in municipal wastewater treated by a membrane bioreactor. Water Res 39(12):2654–2664. https://doi.org/10.1016/j.watres.2005.04.068
Rajapaksha AU, Vithanage M, Lim JE, Ahmed MBM, Zhang M, Lee SS, Ok YS (2014a) Invasive plant-derived biochar inhibits sulfamethazine uptake by lettuce in soil. Chemosphere 111:500–504. https://doi.org/10.1016/j.chemosphere.2014.04.040
Rajapaksha AU, Vithanage M, Zhang M, Ahmad M, Mohan D, Chang SX, Ok YS (2014b) Pyrolysis condition affected sulfamethazine sorption by tea waste biochars. Bioresour Technol 166:303–308. https://doi.org/10.1016/j.biortech.2014b.05.029
Ramola S, Belwal T, Li C, Liu Y, Wang Y, Yang S, Zhou C (2021) Preparation and application of novel rice husk biochar–calcite composites for phosphate removal from aqueous medium. J Cleaner Prod 299:126802. https://doi.org/10.1016/j.jclepro.2021.126802
Regkouzas P, Diamadopoulos E (2019) Adsorption of selected organic micro-pollutants on sewage sludge biochar. Chemosphere 224:840–851. https://doi.org/10.1016/j.chemosphere.2019.02.165
Salimova A, Zuo J, Liu F, Wang Y, Wang S, Verichev K (2020) Ammonia and phosphorus removal from agricultural runoff using cash crop waste-derived biochars. Front Environ Sci Eng 14(3):48. https://doi.org/10.1007/s11783-020-1225-1
Sehonova P, Svobodova Z, Dolezelova P, Vosmerova P, Faggio C (2018) Effects of waterborne antidepressants on non-target animals living in the aquatic environment: a review. Sci Total Environ 631–632:789–794. https://doi.org/10.1016/j.scitotenv.2018.03.076
Sengottian M, Venkatachalam CD, Ravichandran SR, Sekar S, Thirumoorthi A, Selvakumar KA, Sankaralingam L (2020) Bisphenol A adsorption using hydrothermal carbonization derived biochar resulting from Casuarina equisetifolia L. and Wrightia tinctoria. Erode, India, p 130003
Shimabuku KK, Kearns JP, Martinez JE, Mahoney RB, Moreno-Vasquez L, Summers RS (2016) Biochar sorbents for sulfamethoxazole removal from surface water, stormwater, and wastewater effluent. Water Res 96:236–245. https://doi.org/10.1016/j.watres.2016.03.049
Show S, Karmakar B, Halder G (2020) Sorptive uptake of anti-inflammatory drug ibuprofen by waste biomass–derived biochar: experimental and statistical analysis. Biomass Conv Bioref. https://doi.org/10.1007/s13399-020-00922-8
Shukla AK, Upadhyay SN, Dubey SK (2014) Current trends in trichloroethylene biodegradation: a review. Crit Rev Biotechnol 34(2):101–114. https://doi.org/10.3109/07388551.2012.727080
Siggins A, Abram F, Healy MG (2020) Pyrolysed waste materials show potential for remediation of trichloroethylene-contaminated water. J Hazard Mater 390:121909. https://doi.org/10.1016/j.jhazmat.2019.121909
Siipola V, Pflugmacher S, Romar H, Wendling L, Koukkari P (2020) Low-cost biochar adsorbents for water purification including microplastics removal. Appl Sci 10(3):788. https://doi.org/10.3390/app10030788
Sophia A, Lima E (2018) Removal of emerging contaminants from the environment by adsorption. Ecotoxicol Environ Saf 150:1–17. https://doi.org/10.1016/j.ecoenv.2017.12.026
Srinivasan P, Sarmah AK (2015) Characterisation of agricultural waste-derived biochars and their sorption potential for sulfamethoxazole in pasture soil: a spectroscopic investigation. Sci Total Environ 502:471–480. https://doi.org/10.1016/j.scitotenv.2014.09.048
Sumalinog D, Capareda S, de Luna M (2018) Evaluation of the effectiveness and mechanisms of acetaminophen and methylene blue dye adsorption on activated biochar derived from municipal solid wastes. J Environ Manage 210:255–262. https://doi.org/10.1016/j.jenvman.2018.01.010
Sun B, Lian F, Bao Q, Liu Z, Song Z, Zhu L (2016) Impact of low molecular weight organic acids (LMWOAs) on biochar micropores and sorption properties for sulfamethoxazole. Environ Pollut 214:142–148. https://doi.org/10.1016/j.envpol.2016.04.017
Sun K, Ro K, Guo M, Novak J, Mashayekhi H, Xing B (2011) Sorption of bisphenol A, 17α-ethinyl estradiol and phenanthrene on thermally and hydrothermally produced biochars. Biores Technol 102(10):5757–5763. https://doi.org/10.1016/j.biortech.2011.03.038
Takeshita A, Koibuchi N, Oka J, Taguchi M, Shishiba Y, Ozawa Y (2001) Bisphenol-A, an environmental estrogen, activates the human orphan nuclear receptor, steroid and xenobiotic receptor-mediated transcription. Eur J Endocrinol 513–517. https://doi.org/10.1530/eje.0.1450513
Tan X, Liu Y, Gu Y, Xu Y, Zeng G, Hu X, Liu S, Wang X, Liu S, Li J (2016) Biochar-based nano-composites for the decontamination of wastewater: a review. Biores Technol 212:318–333. https://doi.org/10.1016/j.biortech.2016.04.093
Tang Y, Alam MS, Konhauser KO, Alessi DS, Xu S, Tian W, Liu Y (2019) Influence of pyrolysis temperature on production of digested sludge biochar and its application for ammonium removal from municipal wastewater. J Clean Prod 209:927–936. https://doi.org/10.1016/j.jclepro.2018.10.268
Teixidó M, Pignatello JJ, Beltrán JL, Granados M, Peccia J (2011) Speciation of the ionizable antibiotic sulfamethazine on black carbon (Biochar). Environ Sci Technol 45(23):10020–10027. https://doi.org/10.1021/es202487h
Tong Y, Mayer BK, McNamara PJ (2016) Triclosan adsorption using wastewater biosolids-derived biochar. Environ Sci: Water Res Technol 2(4):761–768. https://doi.org/10.1039/C6EW00127K
Tran HN, Tomul F, Thi Hoang Ha N, Nguyen DT, Lima EC, Le GT, Chang C-T, Masindi V, Woo SH (2020) Innovative spherical biochar for pharmaceutical removal from water: Insight into adsorption mechanism. J Hazard Mater 394:122255. https://doi.org/10.1016/j.jhazmat.2020.122255
Viglašová E, Galamboš M, Danková Z, Krivosudský L, Lengauer CL, Hood-Nowotny R, Soja G, Rompel A, Matík M, Briančin J (2018) Production, characterization and adsorption studies of bamboo-based biochar/montmorillonite composite for nitrate removal. Waste Manage 79:385–394. https://doi.org/10.1016/j.wasman.2018.08.005
Vithanage M, Rajapaksha AU, Tang X, Thiele-Bruhn S, Kim KH, Lee S-E, Ok YS (2014) Sorption and transport of sulfamethazine in agricultural soils amended with invasive-plant-derived biochar. J Environ Manage 141:95–103. https://doi.org/10.1016/j.jenvman.2014.02.030
Vu TM, Trinh VT, Doan DP, Van HT, Nguyen TV, Vigneswaran S, Ngo HH (2017) Removing ammonium from water using modified corncob-biochar. Sci Total Environ 579:612–619. https://doi.org/10.1016/j.scitotenv.2016.11.050
Wang F, Zeng Q, Su W, Zhang M, Hou L, Wang Z-L (2019) Adsorption of bisphenol A on peanut shell biochars: the effects of surfactants. J Chem 11
Wang H, Fang C, Wang Q, Chu Y, Song Y, Chen Y, Xue X (2018) Sorption of tetracycline on biochar derived from rice straw and swine manure. RSC Adv 8(29):16260–16268. https://doi.org/10.1039/C8RA01454J
Wang J, Zhang M (2020) adsorption characteristics and mechanism of bisphenol A by magnetic biochar. Int J Environ Res Public Health 17
Wang X, Guo Z, Hu Z, Zhang J (2020a) Recent advances in biochar application for water and wastewater treatment: a review. PeerJ 8:e9164. https://doi.org/10.7717/peerj.9164
Wang Z, Sedighi M, Lea-Langton A (2020b) Filtration of microplastic spheres by biochar: removal efficiency and immobilisation mechanisms. Water Res 184:116165. https://doi.org/10.1016/j.watres.2020.116165
Wu L, Wei C, Zhang S, Wang Y, Kuzyakov Y, Ding X (2019) MgO-modified biochar increases phosphate retention and rice yields in saline-alkaline soil. J Clean Prod 235:901–909. https://doi.org/10.1016/j.jclepro.2019.07.043
Xie M, Chen W, Xu Z, Zheng S, Zhu D (2014) Adsorption of sulfonamides to demineralized pine wood biochars prepared under different thermochemical conditions. Environ Pollut 186:187–194. https://doi.org/10.1016/j.envpol.2013.11.022
Xie T, Reddy KR, Wang C, Yargicoglu E, Spokas K (2015) Characteristics and applications of biochar for environmental remediation: a review. Crit Rev Environ Sci Technol 45(9):939–969. https://doi.org/10.1080/10643389.2014.924180
Xue S, Zhang X, Ngo HH, Guo W, Wen H, Li C, Zhang Y, Ma C (2019) Food waste based biochars for ammonia nitrogen removal from aqueous solutions. Biores Technol 292:121927. https://doi.org/10.1016/j.biortech.2019.121927
Yang W, Feng T, Flury M, Li B, Shang J (2020) Effect of sulfamethazine on surface characteristics of biochar colloids and its implications for transport in porous media. Environ Pollut 256:113482. https://doi.org/10.1016/j.envpol.2019.113482
Yavari S, Malakahmad A, Sapari NB (2015) Biochar efficiency in pesticides sorption as a function of production variables—a review. Environ Sci Pollut Res 22(18):13824–13841. https://doi.org/10.1007/s11356-015-5114-2
Yavari S, Abualqumboz M, Sapari N, Hata-Suhaimi HA, Nik-Fuaad NZ, Yavari S (2020) Sorption of imazapic and imazapyr herbicides on chitosan-modified biochars. Int J Environ Sci Technol 17:3341–3350. https://doi.org/10.1007/s13762-020-02629-9
Yi S, Gao B, Sun Y, Wu J, Shi X, Wu B, Hu X (2016) Removal of levofloxacin from aqueous solution using rice-husk and wood-chip biochars. Chemosphere 150:694–701. https://doi.org/10.1016/j.chemosphere.2015.12.112
Yin Q, Liu M, Ren H (2019) Biochar produced from the co-pyrolysis of sewage sludge and walnut shell for ammonium and phosphate adsorption from water. J Environ Manage 249:109410. https://doi.org/10.1016/j.jenvman.2019.109410
Yin Q, Ren H, Wang R, Zhao Z (2018) Evaluation of nitrate and phosphate adsorption on Al-modified biochar: influence of Al content. Sci Total Environ 631–632:895–903. https://doi.org/10.1016/j.scitotenv.2018.03.091
Zhang M, Ahmad M, Al-Wabel MI, Vithanage M, Rajapaksha AU, Kim HS, Lee SS, Ok YS (2015) Adsorptive removal of trichloroethylene in water by crop residue biochars pyrolyzed at contrasting temperatures: continuous fixed-bed experiments. J Chem 2015:1–6. https://doi.org/10.1155/2015/647072
Zhao X, Ouyang W, Hao F, Lin C, Wang F, Han S, Geng X (2013) Properties comparison of biochars from corn straw with different pretreatment and sorption behaviour of atrazine. Bioresour Technol 147:338–344. https://doi.org/10.1016/j.biortech.2013.08.042
Zhang M, Song G, Gelardi DL, Huang L, Khan E, Mašek O, Parikh SJ, Ok YS (2020a) Evaluating biochar and its modifications for the removal of ammonium, nitrate, and phosphate in water. Water Res 186. https://doi.org/10.1016/j.watres.2020.116303
Zhang P, O’Connor D, Wang Y, Jiang L, Xia T, Wang L, Tsang DCW, Ok YS, Hou D (2020b) A green biochar/iron oxide composite for methylene blue removal. J Hazard Mater 384:121286. https://doi.org/10.1016/j.jhazmat.2019.121286
Zhou Y, Liu S, Liu Y, Tan X, Liu N, Wen J (2020) Efficient removal 17-estradiol by graphene-like magnetic sawdust biochar: preparation condition and adsorption mechanism. Int J Environ Res Public Health 15
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Medyńska-Juraszek, A., Ćwieląg-Piasecka, I. (2022). Engineered Biochar as Adsorbent for Removal of Emerging Contaminants from Aqueous and Soil Medium. In: Ramola, S., Mohan, D., Masek, O., Méndez, A., Tsubota, T. (eds) Engineered Biochar. Springer, Singapore. https://doi.org/10.1007/978-981-19-2488-0_10
Download citation
DOI: https://doi.org/10.1007/978-981-19-2488-0_10
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-19-2487-3
Online ISBN: 978-981-19-2488-0
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)