Abstract
Deformity during the early development of golden pompano Trachinotus ovatus has significantly influenced the production capacity of a fish hatchery. However, factors leading to skeletal deformity in this species have never been assessed. In this chapter, the impact of rearing temperature on jaw deformity and BMP gene expression is discussed. Jaw deformity rate of fish larvae increased with the increase of ambient temperature, and the highest malformation rate was recorded at 33 °C. The expressions of the BMP4 and BMP5 genes were positively correlated to the occurrence of jaw malformation. The cultivating water temperature of T. ovatus larvae should be maintained at 26–29 °C. These findings will clarify the role of water temperature in influencing bone deformity in fish larvae and provide a reference point to optimize the environmental condition during the rearing process of golden pompano in hatcheries.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
10.1 Introduction
Temperature is one of the important factors affecting the early development of larval fish through regulating the feeding behavior and metabolism during larval development (Ma 2014; Kestemont and Baras 2001). Besides, studies have shown that unsuitable temperature can cause high mortality and deformity of larval fish (Lein et al. 1997; Ørnsrud et al. 2004; Ludwig and Lochmann 2009). The skeletal malformation is often related to slow growth and high mortality of fish larvae and has continually hindered the production of marine fish in the hatchery (Koumoundouros 2010; Boglione et al. 2013a, b). Jaw abnormality is not only a factor leading to a high mortality rate of fish but also a factor reducing the market value of fish (Cobcroft et al. 2004; Barahona-Fernandes 1982; Ma et al. 2014c). Such malformations have been reported in commercial aquaculture of gilthead sea bream Sparus aurata (Prestinicola et al. 2013; Andrades et al. 1996), striped trumpeter Latris lineata (Cobcroft et al. 2012), and yellowtail kingfish Seriola lalandi (Cobcroft et al. 2004). Lein et al. (1997) suggested that unsuitable rearing temperature can cause jaw malformations in fish. Under inappropriate temperature, significant deformities of skeleton and gill cover have been reported in gilthead seabream Sparus aurata and cranial malformation in European sea bass Dicentrarchus labrax (Georgakopoulou et al. 2007). In pompano Trachinotus ovatus, more than 33% of fish in the same cohort display more than one type of deformities during the larval stage (Zheng et al. 2014; Ma et al. 2014c), but it is not clear whether the temperature can cause jaw malformations in this species. Consequently, in the process of pompano’s larval ontogenesis, to study relationship between temperature and the jaw malformation is very necessary.
Skeletogenesis includes differentiation and proliferation of various cell types, such as osteoblasts, chondrocytes, osteoclasts, and osteocytes which determine the shape, size, and mineral composition of bone structure (Karsenty and Wagner 2002; Nijweide et al. 1986; Phan et al. 2004). The expression of genes mainly underlies the procedure of cell proliferation and differentiation but could also be changed by individual genetic characteristics and biological and nonbiological elements (Boglione et al. 2013a, b). Therefore, it is necessary to examine the structure of gene networks, which will provide an insight into the understanding of the underlying mechanisms of bone deformity. The biological and nonbiological factors could cause bone malformation, while the gene expression is the potential mechanism behind this factor. In some vertebrates, bone formation is controlled by bone morphogenetic proteins (BMPs) at different phases of cell development (e.g., maturing osteoblast, stem cells, hypertrophic chondrocytes, proliferative chondrocytes) (Hogan 1996a, b; Windhausen et al. 2015; Alaee et al. 2014). In the animal kingdom, the function and structure of BMPs are conservative. The function and structure of different BMPs in single species can be seen through their roles in various biological processes (Razdorov and Vukicevic 2012). For example, BMPs 1, 2, and 3 play an essential role in bone fracture repair because these proteins can stimulate the growth of osteoblasts (Grgurevic et al. 2011). BMPs 2, 4, and 6 can regulate skeletogenesis, in particular, chondrocyte differentiation into cartilage, and cell maturation in osteoblast lineages can lead to bone formation (Minina et al. 2001; Rickard et al. 1994; Wan and Cao 2005; Canalis et al. 2003). Although several studies have been conducted to test the expression of BMP genes in various fish species, most of these studies are focused on the changes during embryonic development (Myers et al. 2002; Palomino et al. 2014; Marques et al. 2014, 2015; Tiago et al. 2014). In marine fish, the studies on the expression of BMP genes after incubation and their biological function are limited to the test of nutrient effect such as lipids and vitamins (Villeneuve et al. 2005a, b, 2006). Recently, BMP genes have been used to evaluate the impact of high temperature on the bone abnormality of fish larvae (Ytteborg et al. 2010). The study on BMP expressions in the ontogeny of golden pompano can contribute to the baseline data on the factors relevant to jaw deformity in fish larvae during osteogenesis.
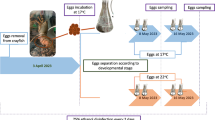
The T. ovatus is an important economic species of the Carangidae family and is a potential species for aquaculture diversification (Guo et al. 2014). Although the digestive function develops early (Ma et al. 2014a, b) and the nutrient requirements of the first feeding T. ovatus larvae have been researched (Ma et al. 2014d), the information on the cause of deformity during the early developmental period is fragmental. Our previous studies have reported the type, position, and frequency of jaw and skeletal deformities in hatchery-reared T. ovatus larvae (Zheng et al. 2014; Ma et al. 2014c). However, factors leading to skeletal deformity in this fish have never been assessed. In this chapter, the impact of rearing temperature on jaw deformity and BMP gene expression is discussed. The results are derived from fish cultured at three constant temperatures of 26, 29, and 33 °C from hatching to 18 days post-hatch (DPH) in a hatchery.
10.2 Growth, Survival, and Jaw Deformity at Different Temperatures
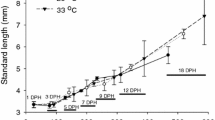
The water temperature is a key to the success of fish hatchery production factors and can significantly impact the quality of fish larvae (Boglione et al. 2013b). The growth of T. ovatus larvae was significantly affected by temperature (P < 0.05, Fig. 10.1). The specific growth rates (SGRs) of fish increase with temperature elevation in the rearing facility. Temperature can affect metabolism, food intake, and growth of fish (Ma 2014; Jobling 1994), and the effect of temperature on larval growth of farmed fish species has been well documented including striped trumpeter Latris lineata (Choa et al. 2010), Australian snapper Pagrus auratus (Fielder et al. 2005), nase Chondrostoma nasus L. (Keckeis et al. 2001), yellowtail kingfish Seriola lalandi (Ma 2014), and haddock Melanogrammus aeglefinus L. (Martell et al. 2005). In T. ovatus, the rapid growth at high temperature probably is related to the high food intake and improved digestive mechanism as evidenced by the early appearance of gastric glands and goblet cells in the gut after 15 DPH at 27–29 °C (Ma et al. 2014b). The growth of fish larvae was expedited when fish were weaned from rotifers to Artemia nauplii. Like Florida pompano Trachinotus carolinus (Riley et al. 2009), the mouth gape of T. ovatus larvae reached 1.05 mm by 12 DPH, which enables the fish to ingest larger food particles such as Artemia nauplii. For this reason, the marked size difference in T. ovatus size between temperature treatments at 18 DPH may also be attributed to the use of enriched rotifers for high-calorie food from 9 DPH onward.
Survival, specific growth rate, and jaw deformity rate of T. ovatus larvae cultured at 26, 29, and 33 °C. Means with the same letter are not significantly different (P > 0.05) (the symbol of “°C” is not shown in the x-axis of the figure) (Ma et al. 2016)
In both artificial and wild environments, fish will go through critical periods in ontogeny and shift from endogenous nutrition to exogenous nutrition (Otterlei et al. 1999; Ma et al. 2012). During the phase of feed transformation, when food provision and light condition are within the range of first feeding requirement for fish larvae, the temperature may be the most decisive factor for fish survival (Kamler 1992; McGurk 1984; Ma 2014; Gardeur et al. 2007). Previous research has indicated that mortality is temperature-dependent in the larvae and juveniles of Pangasianodon hypophthalmus (Baras et al. 2011), Seriola lalandi (Ma 2014), Glyptocephalus cynoglossus (Bidwell and Howell 2001), and Inimicus japonicus (Wen et al. 2013). Ma (2014) argues that there is a temperature-sensitive stage when mortality occurs in fish larvae during early development. In T. ovatus, the lowest survival rate was found when fish were reared at 33 °C (Fig. 10.1), suggesting that the highest level of temperature tolerance has reached for this species.
Jaw abnormality is a crucial point in fish culture because it impacts the quality of fingerlings for further grow-out (Von Westernhagen 1988). In T. ovatus, the rate of jaw deformities rose with the rise of temperature, and the maximum value occurred at 33 °C (Fig. 10.1). The temperature-dependent deformity has also been reported in other species such as Pacific herring Clupea pallasi (Alderdice and Velsen 1971) and Atlantic halibut Hippoglossus hippoglossus (Lein et al. 1997). The fast growth at temperature requires a high level of dissolved oxygen (Rombough 1997) and an adequate amount of nutritional supply. However, unless the feed contains high levels of energy, the fish may not grow very fast (Cahu et al. 2003a, b; Ma 2014). In addition, temperature could interfere with fish development by accelerating or postponing the development of the digestive system, which may be related to the increased rate of skeletal malformation at high temperature. In the present study, the fertilized eggs of T. ovatus hatched at 26 °C, and then yolk sac larvae were acclimated to each of the experimental temperatures (26, 29, and 33 °C) for 5 h on 2 DPH. However, the rapid augment of ambient temperature from 26 to 29 °C or 33 °C may also induce jaw malformation.
10.3 Expression of BMP Genes at Different Temperatures
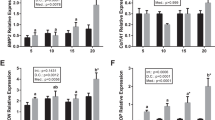
The growth of bone depends on the dynamic balance between the rate of cartilage generation and bone adherence (Breur et al. 1991). BMP2 and BMP4 genes are closely associated with protein synthesis for physiological activities in the crucial period of embryonic development, such as dorsal-ventral axis specification (Graff 1997), apoptosis (Glozak and Rogers 1996; Graham et al. 1994; Zou and Niswander 1996), and epithelio-mesenchymal interactions (Vainio et al. 1993). The BMP2 gene in zebra fish is correlated to the induction and maintenance of ventrolateral cells during the initial stage of development. However, a missense mutation of the BMP2b gene lead to the dorsalized phenotype of the zebra fish swirl mutant, which lacks the cardiogenic mesoderm (Kishimoto et al. 1997). Ytteborg et al. (2010) found that the expression of BMP2 increased when fish are under a high temperature condition. In T. ovatus, the expression of BMP2 was significantly affected by water temperature (P < 0.05, Fig. 10.2). Compared with the fish at 26 °C, the expression of BMP2 in fish showed a trend of increase at 29 °C (Fig. 10.2), which is consistent with the result reported by Ytteborg et al. (2010). However, the reason for low expression of BMP2 in fish at 33 °C remains unclear.
Relative expression levels of bone morphogenetic proteins of T. ovatus larvae at different temperatures on 18 DPH. For BMP2, the reference was the 26 °C BMP2; for BMP4, the reference was the 26 °C BMP4; for BMP5, the reference was the 26 °C BMP5; for BMP10, the reference was the 26 °C BMP10. Means with the same letter are not significantly different (P > 0.05) (Ma et al. 2016)
BMP4 plays a different role in the growth of some vertebrate species (Whitman 1998; Hogan 1996b; Dale and Johns 1999; Mehler et al. 1997; Shi and Massague 2003) and has been used to assess whether the BMP pathway is involved in nutrient deficiency of bone deformities (Villeneuve et al. 2005a, b, 2006) or environmental stress (Ytteborg et al. 2010). According to Villeneuve et al. (2006), the increase of BMP4 and RARγ expressions can diminish the number of osteoblasts for bone generation, and the damage of bone cells is counteracted by the interaction between retinoic acid and BMP4. In T. ovatus, the expression of BMP4 at 29 and 33 °C was significantly higher than those at 26 °C. Jaw deformities of fish at 29 and 33 °C were also significantly higher than those fish at 26 °C. This result is consistent with Ytteborg et al. (2010), such as the results of the study, namely, under the condition of high temperature raising, tend to increase the BMP4 gene expression. When the expression of BMP4 gene was upregulated, the incidence of jaw deformity increased (Villeneuve et al. 2006).
Previous studies have demonstrated that the 60A subgroup (BMP5, 6, 7) is functionally supernumerary and that the collective expression of the 60A subgroup determines the functional change in the early fish development (Kim et al. 2001; Solloway and Robertson 1999). During endochondral ossification, BMP5 can stimulate the mesenchymal cells to coagulate into chondrocytes (Bailon-Plaza et al. 1999; King et al. 1994). Moreover, the mutated BMP5 gene can cause skeletal malformations, indicating the essentiality of BMP5 in skeletal development (Storm et al. 1994; Kingsley et al. 1992; Wolfman et al. 2003). In T. ovatus, BMP5 expression patterns in fish is similar to the expression pattern of BMP4 (Fig. 10.2). Under 29 and 33 °C, the expression level of BMP5 in fish was significantly higher than that under 26 °C. Although the expression level of BMP5 and jaw abnormalities in T. ovatus increased with the increase of rearing temperature, there is no direct evidence to suggest that the expression of BMP5 can regulate the jaw abnormalities.
The BMP10 gene is mainly expressed in the heart of an adult but with a lower chance in the lung and liver (Neuhaus et al. 1999). During the period of heart development, BMP10 is expressed in the ventricular chamber, atrium, and trabeculae in Bulbus cordis (Neuhaus et al. 1999). In zebra fish, a comparatively high BMP10 expression occurs in the liver and heart, but low expression level can be observed in the kidney and brain (Bland 2001). In T. ovatus, feeding temperature had no significant effect on the expression of BMP10, indicating that 18 DPH was insensitive to the expression of BMP10 in ovate cells.
10.4 Conclusion
In summary, temperature significantly regulated the jaw development in larval T. ovatus. Jaw malformation rate in fish larvae increased with the increase of rearing temperature, and the highest malformation rate occurred in fish at 33 °C. To reduce massive malformation, we should control the rearing water temperature at 26–29 °C for T. ovatus larvae. Gene expression analysis indicates that the expression levels of BMP4 and BMP5 were positively correlated to the occurrence of jaw malformations, but the underlying mechanism needs further study.
References
Alaee F, Hong SH, Dukas AG, Pensak MJ, Rowe DW, Lieberman JR (2014) Evaluation of osteogenic cell differentiation in response to bone morphogenetic protein or demineralized bone matric in a critical size defect model using GFP reporter mice. J Orthop Res 32:1120–1128
Alderdice DF, Velsen FPJ (1971) Some effects of salinity and temperature on early development of Pacific herring (Clupea pallasi). J Fish Res Board Can 28:1545–1562
Andrades JA, Becerra J, Fernández-Llebrez P (1996) Skeletal deformities in larval, juvenile and adult stages of cultured gilthead sea bream (Sparus aurata L.). Aquaculture 141:1–11
Bailon-Plaza A, Lee AO, Veson EC, Farnum CE, van der Meulen MC (1999) Bmp-5 deficiency alters chondrocytic activity in the mouse proximal tibial growth plate. Bone 24:211–216
Barahona-Fernandes MH (1982) Body deformation in hatchery reared European sea bass Dicentrarchus labrax (L). Types, prevalence and effect on fish survival. J Fish Biol 21:239–249
Baras E, Raynaud T, Slembrouck J, Caruso D, Cochet C, Legendre M (2011) Interactions between temperature and size on the growth, size heterogeneity, mortality and cannibalism in cultured larvae and juveniles of the Asian catfish, Pangasianodon hypophthalmus (Sauvage). Aquac Res 42:260–276
Bidwell DA, Howell WH (2001) The effect of temperature on first feeding, growth, and survival of larval witch flounder Glyptocephalus cynoglossus. J World Aquacult Soc 32:373–384
Bland RJ (2001) Isolation, characterisation and evolution of zebrafish (Danio rerio) bmp9, bmp10, and gdf11. University of Auckland, New Zealand, p 334
Boglione C, Gavaia P, Koumoundouros G, Gisbert E, Moren M, Fontagne S, Witten PE (2013a) Skeletal anomalies in reared European fish larvae and juveniles. Part 1: Normal and anomalous skeletogenic processes. Rev Aquac 5:S99–S120
Boglione C, Gisbert E, Gavaia P, Witten PE, Moren M, Fontagne S, Koumoundouros G (2013b) Skeletal anomalies in reared European fish larvae and juveniles. Part 2: Main typologies, occurrences and causative factors. Rev Aquac 5:S121–S167
Breur GJ, Vanenkevort BA, Farnum CE, Wilsman NJ (1991) Linear relationship between the volume of hypertrophic chondrocytes and the rate of longitudinal bone-growth plates. J Orthop Res 9:348–359
Cahu C, Zambonino Infante J, Takeuchi T (2003a) Nutritional components affecting skeletal development in fish larvae. Aquaculture 227:245–258
Cahu CL, Infante JLZ, Barbosa V (2003b) Effect of dietary phospholipid level and phospholipid: neutral lipid value on the development of sea bass (Dicentrarchus labrax) larvae fed a compound diet. Br J Nutr 90:21–28
Canalis E, Economides AN, Gazzerro E (2003) Bone morphogenetic proteins, their antagonists, and the skeleton. Endocr Rev 24:218–235
Choa BY, Carter CG, Battaglene SC (2010) Effects of temperature regime on growth and development of post-larval striped trumpeter (Latris lineata). Aquaculture 305:95–101
Cobcroft JM, Pankhurst PM, Poortenaar C, Hickman B, Tait M (2004) Jaw malformation in cultured yellowtail kingfish (Seriola lalandi) larvae. N Z J Mar Freshw Res 38:67–71
Cobcroft J, Shu-chien A, Kuah M, Jaya-Ram A, Battaglene S (2012) The effects of tank colour, live food enrichment and greenwater on the early onset of jaw malformation in striped trumpeter larvae. Aquaculture 356–357:61–72
Dale L, Johns CM (1999) BMP signalling in early Xenopus development. BioEssays 21(9):751–760
Fielder DS, Bardsley WJ, Allan GL, Pankhurst PM (2005) The effects of salinity and temperature on growth and survival of Australian snapper, Pagrus auratus larvae. Aquaculture 250:201–214
Gardeur JN, Mathis N, Kobilinsky A, Brun-Bellut J (2007) Simultaneous effects of nutritional and environmental factors on growth and flesh quality of Perca fluviatilis using a fractional factorial design study. Aquaculture 273:50–63
Georgakopoulou E, Angelopoulou A, Kaspiris P, Divanach P, Koumoundouros G (2007) Temperature effects on cranial deformities in European sea bass, Dicentrarchus labrax (L.). J Appl Ichthyol 23:99–103
Glozak MA, Rogers MB (1996) Specific induction of apoptosis in P19 embryonal carcinoma cells by retinoic acid and BMP2 or BMP4. Dev Biol 179:458–470
Graff JM (1997) Embryonic patterning: to BMP or not to BMP, that is the question. Cell 89:171–174
Graham MA, Francis-West P, Brickell P, Lumsden A (1994) The signalling molecule BMP4 mediates apoptosis in the rhombencephalic neural crest. Nature 372:684–686
Grgurevic L, Macek B, Mercep M, Jelic M, Smoljanovic T, Erjavec I, Dumic-Cule I, Prgomet S, Durdevic D, Vnuk D, Lipar M, Stejskal M, Kufner V, Brkljacic J, Maticic D, Vukicevic S (2011) Bone morphogenetic protein (BMP)1–3 enhances bone repair. Biochem Biophys Res Commun 408:25–31
Guo H, Ma Z, Jiang S, Zhang D, Zhang N, Li Y (2014) Length-weight relationship of oval pompano, Trachinotus ovatus (Linnaeus 1758) (Pisces; Carangidae) cultured in open sea floating sea cages in South China Sea. Indian J Fish 61:93–95
Hogan BLM (1996a) Bone morphogenetic proteins in development. Curr Opin Genet Dev 6:432–438
Hogan BLM (1996b) Bone morphogenetic proteins: multifunctional regulators of vertebrate development. Genes Dev 10:1580–1594
Jobling M (1994) Fish Bioenergetic. Chapman and Hall, London
Kamler E (1992) Early life history of fish: an energetics approach. Chapman and Hall, London
Karsenty G, Wagner EF (2002) Reaching a genetic and molecular understanding of skeletal development. Dev Cell 2:389–406
Keckeis H, Kamler E, Bauer-Nemeschkal E, Schneeweiss K (2001) Survival, development and food energy partitioning of nase larvae and early juveniles at different temperatures. J Fish Biol 59(1):45–61
Kestemont P, Baras E (2001) Environmental factors and feed intake: mechanisms and interactions. In: Houlihan D et al (eds) Food intake in fish. Blackwell Science, Cornwall, pp 131–156
Kim RY, Robertson EJ, Solloway MJ (2001) Bmp6 and Bmp7 are required for cushion formation and septation in the developing mouse heart. Dev Biol 235:449–466
King JA, Marker PC, Seung KJ, Kingsley DM (1994) Bmp5 and the molecular, skeletal, and soft-tissue alterations in short ear mice. Dev Biol 166:112–122
Kingsley DM, Bland AE, Grubber JM, Marker PC, Russell LB, Copeland NG, Jenkins NA (1992) The mouse short ear skeletal morphogenesis locus is associated with defects in a bone morphogenetic member of the tgf beta superfamily. Cell 71:399–410
Kishimoto Y, Lee K, Zon L, Hammerschmidt M, Schulte-Merker S (1997) The molecular nature of zebrafish swirl: BMP2 function is essential during early dorsoventral patterning. Development 124:4457–4466
Koumoundouros G (2010) Morpho-anatomical abnormalities in Mediterranean marine aquaculture. In: Koumoundouros G (ed) Recent advances in aquaculture research. Transworld Research Network, Kerala, India, pp 125–148
Lein I, Holmefjord I, Rye M (1997) Effects of temperature on yolk sac larvae of Atlantic halibut (Hippoglossus hippoglossus L.). Aquaculture 157:123–135
Ludwig GM, Lochmann SE (2009) Effect of temperature on larval sunshine bass growth and survival to the fingerling stage. N Am J Aqualcult 71:260–266
Ma Z (2014) Food ingestion, prey selectivity, feeding incidence, and performance of yellowtail kingfish Seriola lalandi larvae under constant and varying temperatures. Aquac Int 22:1317–1330
Ma Z, Qin JG, Nie Z (2012) Morphological changes of marine fish larvae and their nutrition need. In: Pourali K, Raad VN (eds) Larvae: morphology, biology and life cycle. Nova Science Publishers, Inc., New York, pp 1–20
Ma Z, Guo H, Zhang D, Hu CQ, Jiang S (2014a) Food ingestion, consumption, and selectivity of pompano, Trachinotus ovatus (Linnaeus 1758) under different rotifer densities. Aquac Res 46:2593–2603
Ma Z, Guo H, Zheng P, Wang L, Jiang S, Qin JG, Zhang D (2014b) Ontogenetic development of digestive functionality in golden pompano Trachinotus ovatus (Linnaeus 1758). Fish Physiol Biochem 40(4):1157–1167
Ma Z, Zheng P, Guo H, Zhang N, Jiang S, Zhang D, Qin JG (2014c) Jaw malfromation of hatchery reared golden pompano Trachinotus ovatus (Linnaeus 1758) larvae. Aquac Res 47:1141–1149
Ma Z, Zheng P, Guo H, Zhang N, Wang L, Jiang S, Qin JG, Zhang D (2014d) Effect of weaning time on the performance of Trachinotus ovatus (Linnaeus 1758) larvae. Aquac Nutr 21:670–678
Ma Z, Zhang N, Qin JG, Fu M, Jiang S (2016) Water temperature induces jaw deformity and bone morphogenetic proteins (BMPs) gene expression in golden pompano Trachinotus ovatus larvae. Springerplus 5:1475–1487
Marques CL, Fernandez I, Rosa J, Viegas MN, Cancela ML, Laize V (2014) Spatiotemporal expression and retinoic acid regulation of bone morphogenetic proteins 2, 4 and 16 in Senegalese sole. J Appl Ichthyol 30:713–720
Marques CL, Fernández I, Viegas MN, Cox CJ, Martel P, Rosa J, Cancela ML, Laizé V (2015) Comparative analysis of zebrafish bone morphogenetic proteins 2, 4 and 16: molecular and evolutionary perspectives. Cell Mol Life Sci 73:841–857
Martell DJ, Kieffer JD, Trippel EA (2005) Effect of temperature during early life history on embryonic and larval development and growth in haddock. J Fish Biol 66:1558–1575
McGurk MD (1984) Effects of delayed feeding and temperature on the age of irreversible starvation and on the rates of growth and mortality of Pacific herring larvae. Mar Biol 84:13–26
Mehler MF, Mabie PC, Zhang D, Kessler JA (1997) Bone morphogenetic proteins. Trends Neurosci 20:309–317
Minina E, Wenzel HM, Karp S, Gaffield W, McMahon AP, Vortkamp A (2001) BMP and Ihh/PTHrP signaling interact to coordinate chondrocyte proliferation and differentiation. Development 128:4523–4534
Myers DC, Sepich DS, Solnica-Krezel L (2002) Bmp activity gradient regulates convergent extension during zebrafish gastrulation. Dev Biol 243:81–98
Neuhaus H, Rosen V, Thies RS (1999) Heart specific expression of mouse BMP-10 a novel member of the TGF-b superfamily. Mech Dev 80:181–184
Nijweide PJ, Burger EH, Feyen JH (1986) Cells of bone: proliferation, differentiation, and hormonal regulation. Physiol Rev 66:855–886
Ørnsrud R, Gil L, Waagbø R (2004) Teratogenicity of elevated egg incubation temperature and egg vitamin A status in Atlantic salmon, Salmo salar L. J Fish Dis 27:213–223
Otterlei E, Nyhammer G, Folkvord A, Stefansson SO (1999) Temperature- and size-dependent growth of larval and early juvenile Atlantic cod (Gadus morhua): a comparative study of Norwegian coastal cod and Northeast Arctic cod. Can J Fish Aquat Sci 56:2099–2111
Palomino J, Herrera G, Dettleff P, Martinez V (2014) Growth differentiation factor 9 and bone morphogenetic protein 15 expression in previtellogenic oocytes and during early embryonic development of yellow-tail kingfish Seriola lalandi. Biol Res 47:1–7
Phan TCA, Xu J, Zheng MH (2004) Interaction between osteoblast and osteoclast: impact in bone disease. Histol Histopathol 19:1325–1344
Prestinicola L, Boglione C, Makridis P, Spano A, Rimatori V, Palamara E, Scardi M, Cataudella S (2013) Environmental conditioning of skeletal anomalies typology and frequency in gilthead seabream (Sparus aurata L., 1758) juveniles. PLoS One 8:1–22
Razdorov G, Vukicevic S (2012) The use of mass spectrometry in characterization of bone morphogenetic protein from biological samples. In: Prasain JK (ed) Trandem mass spectrometry-applications and principles. InTech, Rijeka, Croatia, pp 259–284
Rickard DJ, Sullivan TA, Shenker BJ, Leboy PS, Kazhdan I (1994) Induction of rapid osteoblast differentiation in rat bone marrow stromal cell cultures by dexamethasone and BMP-2. Dev Biol 161:218–228
Riley KL, Weirich CR, Cerino D (2009) Development and growth of hatchery-reared larval Florida pompano (Trachinotus carolinus). Fish Bull 107:318–328
Rombough PJ (1997) The effects of temperature on embryonic and larval development. In: Wood CM, McDonald DG (eds) Global warming. Implications for freshwater and marine fish. Cambridge University Press, Cambridge, pp 177–223
Shi Y, Massague J (2003) Mechanisms of TGF-b signaling from cell membrane to the nucleus. Cell 113:695–700
Solloway MJ, Robertson EJ (1999) Early embryonic lethality in Bmp5; Bmp7 double mutant mice suggests functional redundancy within the 60A subgroup. Development 126:1753–1768
Storm EE, Huynh TV, Copeland NG, Jenkins NA, Kingsley DM, Lee SJ (1994) Limb alterations in brachypodism mice due to mutations in a new member of the tgf beta-superfamily. Nature 368:639–643
Tiago DM, Marques CL, Roberto VP, Cancela ML, Laize V (2014) Mir-20a regulates in vitro mineralization and BMP signaling pathway by targeting BMP-2 transcript in fish. Arch Biochem Biophys 543:23–30
Vainio S, Karavanova I, Jowett A, Thesleff I (1993) Identification of BMP-4 as a signal mediating secondary induction between epithelial and mesenchymal tissues during early tooth development. Cell 75:45–58
Villeneuve L, Gisbert E, Delliou HL, Cahu CL, Zambonino-Infante JL (2005a) Dietary levels of all-trans retinol affect retinoid nuclear receptor expression and skeletal development in European sea bass larvae. Br J Nutr 93:791–801
Villeneuve L, Gisbert E, Zambonino-Infante JL, Quazuguel P, Cahu CL (2005b) Effect of nature of dietary lipids on European sea bass morphogenesis: implication of retinoid receptors. Br J Nutr 94:877–884
Villeneuve LAN, Gisbert E, Moriceau J, Cahu CL, Zambonino JL (2006) Intake of high levels of vitamin A and polyunsaturated fatty acids during different developmental periods modifies the expression of morphogenesis genes in European sea bass (Dicentrarchus labrax). Br J Nutr 95:677–687
Von Westernhagen H (1988) Sublethal effects of pollutants on fish eggs and larvae. In: Hoar WS, Randall DJ (eds) Fish physiology. Academic, San Diego
Wan M, Cao X (2005) BMP signaling in skeletal development. Biochem Biophys Res Commun 328:651–657
Wen W, Huang X, Chen Q, Feng L, Wei L (2013) Temperature effects on early development and biochemical dynamics of a marine fish, Inimicus japonicus. J Exp Mar Biol Ecol 442:22–29
Whitman M (1998) Smads and early developmental signaling by the TGF-b super-family. Gene Dev 12:2445–2462
Windhausen T, Squifflet S, Renn J, Muller M (2015) BMP signaling regulates bone morphogenesis in zebrafish through promoting osteoblast function as assessed by their nitric oxide production. Molecules 20:7586–7601
Wolfman NM, McPherron AC, Pappano WN, Davies MV, Song K, Tomkinson KN, Wright JF, Zhao L, Sebald SM, Greenspan DS, Lee SJ (2003) Activation of latent myostatin by the bmp-1/tolloid family of metalloproteinases. Proc Natl Acad Sci U S A 100:15842–15846
Ytteborg E, Baeverfjord G, Torgersen J, Hjelde K, Takle H (2010) Molecular pathology of vertebral deformities in hyperthermic Atlantic salmon (Salmo salar). BMC Physiol 10:1–16
Zheng P, Ma Z, Guo H, Zhang D, Fu M, Zhang N, Jiang S (2014) Osteological ontogeny and malformations in larval and juvenile golden pompano Trachinotus ovatus (Linnaeu 1758). Aquac Res 47(5):1421–1431
Zou H, Niswander L (1996) Requirement for BMP signaling in interdigital apoptosis and scale formation. Science 272:738–741
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 China Agriculture Press
About this chapter
Cite this chapter
Sun, J., Fu, Z., Ma, Z., Yu, G. (2022). High Water Temperature Induces Jaw Deformity and Bone Morphogenetic Protein (BMP) Gene Expression in Golden Pompano Trachinotus ovatus Larvae. In: Ma, Z., Yu, G., Qin, J.G. (eds) Ontogenetic development of pompano Trachinotus ovatus. Springer, Singapore. https://doi.org/10.1007/978-981-19-1712-7_10
Download citation
DOI: https://doi.org/10.1007/978-981-19-1712-7_10
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-19-1711-0
Online ISBN: 978-981-19-1712-7
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)