Abstract
Atherosclerosis is the formation of fibrofatty lesions in the arterial wall, and this inflammatory state of the artery is the main cause of advanced pathological processes, including myocardial infarction and stroke. Dyslipidemic conditions with excess cholesterol accumulate within the arterial vessel wall and initiate atherogenic processes. Following vascular reaction and lipid accumulation, the vascular wall gradually thickens. Together with the occurrence of local inflammation, early atherosclerotic lesions lead to advanced pathophysiological events, plaque rupture, and thrombosis. Ceramide and sphingomyelin have emerged as major risk factors for atherosclerosis and coronary artery disease. Currently, the clinical association between de novo sphingolipid biosynthesis and coronary artery disease has been established. Furthermore, therapeutic strategies to modulate this pathway, especially those involving serine palmitoyltransferase and sphingomyelin synthase, against atherosclerosis, cancer, type 2 diabetes, and non-alcoholic fatty liver disease are actively under development. In this chapter, we focus on the relationship between de novo sphingolipid biosynthesis and coronary artery disease.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
3.1 Introduction
Coronary artery disease (CAD) is the most prevalent type of cardiovascular disease and accounts for millions of deaths worldwide. Stroke and myocardial infarction, which are responsible for a massive number of deaths, are caused by thromboembolism (blood clots clogged in the arteries) via rupture of atherosclerotic plaques in the carotid artery of various organs, including the brain and heart [1,2,3,4,5]. Atherosclerosis, a condition of intimal thickening, is the major cause of vascular diseases and is believed to develop from two different pathological mechanisms. First, lipoproteins, especially low-density lipoprotein (LDL), accumulate in the arterial wall and are oxidized to more toxic lipids. Lipid accumulation in the intima and vascular response to lipids thicken the arterial wall. Second, a local inflammatory reaction (known as the primary cause of CAD) to vessel wall injury caused by infections, immune diseases, and diabetes develops. Atherosclerosis progresses with atherosclerotic plaque formation, which interferes with circulation, leading to the progression of coronary heart diseases, including myocardial infarction, heart failure, stable angina pectoris (chest discomfort), stroke, and claudication [6, 7]. Plaque formation, which is responsible for narrowing of the arterial lumen, is initiated by impaired lipid transport, causing endothelial dysfunction. Since the rupture of these plaques is the most common trigger of stroke and acute thrombosis, lipids are the key players in the pathogenesis of atherosclerosis [8, 9].
Impaired lipid metabolism is associated with various metabolic diseases, including type 2 diabetes, non-alcoholic fatty liver disease, insulin resistance, and cardiomyopathy [10]. Over-nutrition and obesity elevate the plasma levels of fatty acids (FA), which are taken up by non-adipose tissues, and imported FA is oxidized as an energy fuel or stored as an energy reservoir. When the FA uptake exceeds the levels of oxidation, excess FA is utilized for the nonoxidative synthesis of lipid metabolites. These include triacylglycerol, diacylglycerol, and sphingolipid metabolites. Some of these lipids are inert, while others have anomalous effects on cellular function, called lipotoxicity. One possible pathway to consume the FA surplus is the sphingolipid biosynthetic pathway. Thus, ceramide levels are a biomarker for FA surplus, since FA is a substrate for ceramide biosynthesis [11].
Sphingolipids are a heterogeneous class of lipids, primarily known as the building blocks of the plasma membrane. In addition, they are widely described as bioactive signaling molecules, including ceramides, sphingosine 1-phosphate, sphingosines, sphingomyelin (SM), and other complex sphingolipids [12,13,14]. Sphingolipids, critically important for inter- and intracellular signaling, comprise a head group of a sphingoid backbone and an FA side chain [15]. When the FA uptake is excessive and lipid accumulation in tissue is followed, metabolic dysfunction occurs due to dysregulation of sphingolipid metabolism. This results in alteration of plasma sphingolipid levels and inappropriate cellular uptake, causing dysfunction. Clinically, the plasma levels of ceramide or SM are correlated with the progression of CAD and are suggested as independent risk factors for CAD [16, 17]. This review describes how sphingolipids and their role in major cardiovascular conditions have changed over the past few years. The findings thus far have consistently proven that sphingolipids are implicated in the progression of atherosclerosis and CAD. Their possible role as prominent biomarkers may lead to more therapeutic approaches as well as diagnostic tools in the future.
3.2 Sphingolipid Biosynthesis
In mammals, sphingolipids are synthesized de novo in the endoplasmic reticulum (ER) and then transported to the Golgi apparatus for further modification and synthesis of more complex sphingolipids [18, 19]. Alternately, they are catabolized from other sphingolipids via the salvage pathway. Sphingolipids consist of a long-chain base backbone as a common structural element that is generated in the rate-limiting step of the sphingolipid biosynthetic pathway. The biosynthesis of sphingolipids may be different among various species; however, the very first step of the pathway, catalyzed by serine palmitoyltransferase (SPT), is conserved across mammals, plants, bacteria, and fungi [20]. The sphingolipid biosynthetic pathway is necessary for the synthesis of ceramide, SM, and glycosphingolipids, which further act as substrates for the synthesis of complex sphingolipids.
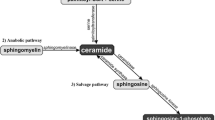
The de novo biosynthetic pathway starts from the condensation reaction of an amino acid, serine, and a fatty acyl-CoA catalyzed by the enzyme complex SPT, followed by 3-ketosphinganine reductase, ceramide synthase, dihydroceramide desaturase, and SM synthase (SMS) (Fig. 3.1) [21]. In this review, we focus on the rate-limiting steps in de novo biosynthesis, SPT and SMS, and major effectors ceramide and SM, which affect vessel wall dysfunction.
The De novo sphingolipid biosynthesis in mammals. The biosynthetic pathway starts with the condensation reaction of palmitoyl CoA and serine, regulated by a rate-limiting enzyme serine palmitoyltransferase (SPT) to form 3-ketosphinganine followed by a series of enzymatic reactions producing ceramide and other complex sphingoid bases. Ceramides are generated in endoplasmic reticulum and transferred to Golgi via vesicular transport or ceramide transfer protein (CERT). Sphingomyelin synthase (SMS) synthesizes sphingomyelin and diacylglycerol (DAG) by transferring phosphocholine from phosphatidylcholine (PC) to ceramide. SPT serine palmitoyltransferase, KDHR 3-keto-dehydrosphingosine reductase, CerS ceramide synthase, SPHK sphingosine kinase, DES dihydroceramide desaturase, CERT ceramide transfer protein, SMS sphingomyelin synthase, PC phosphatidylcholine, DAG diacylglycerol
3.3 Regulation of SPT
SPT is located in the ER membrane and a protein complex consisting of two major subunits, Sptlc1 and Sptlc2, which catalyze the condensation of L-serine and palmitoyl CoA [22]. Sptlc1 and Sptlc2 encode 53- and 63-kDa proteins, respectively, and have homologous sequences with 29% identity [23, 24]. A third major SPT subunit, Sptlc3, has 68% homology with Sptlc2 and 20% homology with Sptlc2. It has been suggested that Sptlc1 is a regulatory subunit, whereas Sptlc2 and Sptlc3 are catalytic subunits with a pyridoxal phosphate-binding domain [25]. The fact that homozygous silencing of Sptlc1 and Sptlc2 in mice is lethal indicates that Sptlc1 and Sptlc2 are essential genes for cell survival [26]. In a previous study, genetic deficiency of Sptlc3 in HepG2 cells resulted in a significant reduction in SPT enzyme activity [27]. The authors proposed that Sptlc3 is an isoform of Sptlc2 and binds to Sptlc1, independently regulating SPT activity to provide sphingolipids for cell requirements. Structural modeling studies using α-oxoamine synthase and bacterial SPT suggested that the active site of SPT is located at the interface of the heteromeric complex, and each subunit contributes to catalytic residues [28, 29]. However, the stoichiometry and topology remain controversial.
Initially, the stoichiometry of the SPT complex was suggested to be a heterodimer composed of Sptlc1 and Sptlc2 in a 1:1 ratio [30]. SPT has been proposed to be an octameric complex of four heterodimers resulting from the binding of Sptlc1 to either Sptlc2 or Sptlc3 [31]. Based on the finding that a third subunit (Tsc3) activates SPT activity markedly and is required for survival at high temperatures in yeast [32], the orthologous SPT small subunits a and b were found to be independent activators of mammalian SPT (ssSPTa and ssSPTb) [33]. However, the mechanism by which this is regulated transcriptionally or post-translationally is not yet known.
SPT enzyme activity is regulated by negative feedback inhibition. Orthologous to the yeast Orm genes, orosomucoid-like (ORMDL) proteins and neurite outgrowth inhibitor (NOGO-B) are found as regulatory transmembrane proteins in the ER. Mammals have ORMDL proteins (ORMDL 1, 2, and 3) that are encoded by separate genes and show different expression patterns in vivo [34, 35]. In this study, Breslow et al. demonstrated that Orm inhibits SPT activity when cellular sphingolipids are sufficient, and silencing of Orm significantly elevates the activity of SPT by sixfold. It differs from the yeast Orm proteins because it does not contain any phosphorylation sites [36]. When cells are treated with myriocin, an SPT inhibitor, to inhibit de novo sphingolipid synthesis, Orm proteins are phosphorylated at multiple sites, and their inhibitory effects on SPT are relieved [37]. Orm deficiency or phosphomimicking mutations drastically enhance SPT activity [34]. Several studies have found that yeast protein kinase 1 phosphorylates Orm proteins when SPT is inhibited by myriocin or when cells have heat stress [38, 39]. The finding that nitrogen starvation in yeast induces phosphorylation of Orm proteins via different mechanisms with no change in SPT activity suggests that Orm has distinct functions in addition to the regulation of de novo sphingolipid biosynthesis [40]. While the mechanism of Orm responsible for SPT activity and de novo sphingolipid synthesis is being elucidated, ORMDL protein-mediated regulation of mammalian SPT has not yet been elucidated. Since ORMDL proteins have no phosphorylation sites, mammalian SPT is regulated differently by these proteins [35]. The mechanism of SPT regulation by ORMDL proteins requires further study.
Additionally, NOGO-B has been identified as a negative SPT regulator in mammals. It is a reticulon (RTN) family protein, which is localized in the tubular ER through two transmembrane domains separated by a loop of the RTN-homology domain with 66 amino acids [41]. Rtn-4, one of the four RTN genes in mammals, produces three major isoforms, NOGO-A, NOGO-B, and NOGO-C, which are expressed in the central nervous system; NOGO-C is expressed in the skeletal muscle at low levels, and NOGO-B is highly expressed in the blood vessels [42]. The biological role of RTNs is to facilitate the formation of tubular ER networks [43]. NOGO proteins function as mediators to inhibit axonal extension of neurons [44] and stimulate migration of endothelial cells by binding to putative receptors [45].
A principal function of NOGO-B is to shape the tubular ER and inhibit SPT activity. Deficiency of NOGO-B elevates SPT activity, and lentiviral re-expression of NOGO-B restores SPT activity to normal levels in murine and human endothelial cells [42]. Co-immunoprecipitation studies confirmed the interaction of SPT with NOGO-B and ORMDL proteins and the close interaction of these proteins. Since SPT is the first enzyme involved in sphingolipid biosynthesis, the absence of endothelial NOGO-B elevates overall sphingolipid metabolites, including sphinganine, ceramide, sphingosine, and sphingosine 1-phosphate. Among the ceramide species, C18-, C20-, and C22-ceramides increased, while C16-, C24-, and C26-ceramides were not altered. This finding suggests the involvement of NOGO-B in SPT as well as ceramide synthases; however, further studies are needed to elucidate the mechanism of sphingolipid metabolism.
Although the importance of SPT has been proposed in cell survival, neuron development, and cardiovascular function, the exact regulatory mechanism has not yet been elucidated. Regulation of SPT by interaction with ORMDMs and NOGO-B should be studied further. In particular, how SPT can sense sphingolipid levels and adjust to the level changes is not yet understood in terms of ORMDL proteins and NOGO-B. Understanding how pathological conditions affect de novo sphingolipid synthesis will contribute to the application of sphingolipid modulation in the development of diagnostic and therapeutic methods.
3.4 Role of Ceramide in Lipoprotein Metabolism and Vascular Regulation
Elevation of circulating and local ceramide levels in atherosclerosis and cardiac dysfunction has been associated with deleterious effects on the vascular wall and cardiomyocytes. Ceramides are present in very low-density lipoprotein (VLDL), LDL, and high-density lipoprotein (HDL) and approximately 3% of total plasma sphingolipids [46]. They are evenly distributed in apoB-containing lipoproteins and HDL. Hussain et al. showed that mice deficient in hepatic and intestinal microsomal triglyceride transfer protein (MTP) (L-I-Mttp−/−) have reduced plasma ceramide levels by 90%, while patients with abetalipoproteinemia who lack apoB-containing lipoproteins have reduced ceramide plasma levels by approximately 80% [47]. These findings suggest that MTP plays an important role in ceramide transport to apoB lipoproteins and apoB lipoprotein assembly. However, it is unclear whether other proteins are involved in the supply of ceramide to lipoproteins.
The total ceramide in plasma is a combined outcome because all cells synthesize ceramides. However, since the apoB lipoproteins in charge of most plasma ceramides are only synthesized in hepatocytes and enterocytes, synthesized ceramides in other cells may mostly be used as components of the cells and not for secretion. In addition, HDL contains a significant amount of plasma ceramides. Phospholipid transfer protein and cholesterol ester transfer protein may transfer ceramide from apoB lipoproteins to HDL during HDL synthesis or reverse cholesterol transport; however, it is not clear how ceramide is transported to HDL.
Pathological conditions with dysregulation of glucose or FA metabolism cause elevation of plasma ceramide levels via the activation of de novo sphingolipid biosynthesis. The accrual of ceramide interferes with endothelial nitric oxide (NO) synthase-derived NO production through the activation of protein phosphatase 2A [48]. Treatment of ex vivo human resistance arterioles with ceramide induces the formation of mitochondrial H2O2, which is associated with an inflammatory response in the endothelium, leading to endothelial dysfunction [49]. This is attributed to ceramide-mediated activation of NADP oxidase and reactive oxygen species (ROS) formation by depleting endothelial NO [50]. Conversely, inhibition of neutral sphingomyelinase (SMase) restores the physiological dysfunction of the arterioles in patients with CAD [49]. Collectively, these findings suggest that ceramide causes endothelial dysfunction.
The contraction of vascular smooth muscle cells (VSMCs) is also regulated by ceramides. SMase and some ceramide species induce sustained vasoconstriction of the cerebral arteries and venules in rats and dogs [51, 52]. Heterozygous dihydroceramide desaturase 1 mice (Des1+/−) have been studied to elucidate the role of ceramide in the vasculature of obese and type 2 diabetes mouse models [48, 53]. The mechanism of action of ceramide in vascular contraction is poorly understood. In part, NOGO-B deficiency in VSMCs causes a selective increase in ceramide species (C18-, C20-, and C22-ceramides) and lower blood pressure [42]. Notably, SPT regulation leads to alteration of various sphingolipid metabolites, including ceramide, and it is difficult to propose that this outcome is derived from altered ceramide levels only. Additionally, ceramide generation from the de novo (SPT) or salvage pathway (SMase) may have distinct effects, such as accumulation in a specific subcellular organelle, and is associated with separate disease generation.
3.5 Role of SM in Atherosclerosis
SM is the most abundant sphingolipid in lipoproteins and cellular membranes and constitutes approximately 87% of total sphingolipids and 20% of total phospholipids in the plasma [46, 54]. ApoB lipoproteins are major sources of plasma SM, as evidenced by L-I-Mttp−/− mice, which have lower plasma SM levels by 73% [47].
Therefore, the involvement of plasma SM levels in atherosclerosis has been studied. Various processes have been implicated in the early development of atherosclerosis, including lipoprotein oxidation, lipoprotein aggregation, endothelial dysfunction, monocyte recruitment, macrophage chemotaxis, foam cell formation, and smooth muscle cell migration and alteration (Fig. 3.2) [55]. There is evidence indicating that SM levels are positively correlated with the development of atherosclerosis. SM accumulates in atheromas from human patients and animal models [56, 57]. Retained atherogenic lipoproteins in the vessel wall are excellent substrates for secretory SMase, making this enzyme a leading candidate for the arterial wall SMase that hydrolyzes LDL-derived SM and causes subendothelial LDL aggregation [57]. Ceramide, a product of SMase, contributes to LDL aggregation in the intima, which is an early step in atherogenesis. The ratio of SM to phosphatidylcholine (PC) is increased five-fold in VLDL from hypercholesterolemic rabbits [58]. ApoE knockout mice, a well-known atherogenic animal model, demonstrated four-fold increased levels compared to WT mice [59]. Moreover, a diet enriched with 1% SM significantly elevated plasma SM levels, LDL aggregation, and atherosclerotic lesions in LDL receptor knockout mice [60]. Based on these findings and clinical information, Jiang et al. proposed that human plasma SM levels and the SM/PC ratio are independent risk factors contributing to CAD [16].
Sphingolipids and atherosclerosis development. LDL accumulation responsible for endothelial dysfunction undergoes oxidative modifications to form oxidized LDL (ox-LDL), inducing an inflammatory reaction causing monocyte infiltration and overexpression of adhesion molecules in endothelial cells. Monocytes circulating in the blood enter into the intima, mature into macrophages, and form foam cells by accumulating ox-LDL aggregates. LDL low-density lipoprotein, ox-LDL oxidized low-density lipoprotein, Cer ceramide, SM sphingomyelin, SMase sphingomyelinase
The following clinical results (Table 3.1) also confirm the correlation between plasma SM levels and CAD. Nelson et al. reported an association between plasma SM levels and three measures of subclinical cardiovascular disease (carotid intimal-medial wall thickness, ankle-arm blood pressure index, and Agatston coronary artery calcium score) among 6,814 middle-aged, asymptomatic adults in the Multi-Ethnic Study of Atherosclerosis [61]. These observations are consistent with the hypothesis that the plasma SM level is a standard cardiovascular disease risk factor that predicts the risk of subclinical disease. In contrast, another study showed that a high plasma SM level was not associated with an increased risk of adjudicated incident CAD in population-based adults free of clinical cardiovascular disease at baseline [63]. Recently, association studies between sphingolipid species and type 2 diabetes depending on pregnancy state, gestational state, and various ethnic groups have been performed [77,78,79]. Indeed, these clinical lipidomic studies demonstrated an association between type 2 diabetes and the level of a certain class of ceramide or SM. These results suggest that the SM level can be a diagnostic risk factor for CAD and type 2 diabetes, depending on the degree of progression and disease status.
There are two possible modulation methods for the sphingolipid pathway to prevent atherosclerosis. The first was to reduce the expression of secretory SMase activity. Previously, SMase-deficient ApoE KO mice showed decreased development of early atherosclerotic lesions and reduced retention of atherogenic lipoproteins compared to ApoE KO mice matched for similar lipoprotein levels [80]. The second was inhibition of de novo SM synthesis by reducing the SM levels in atherogenic lipoproteins synthesized in the liver or intestines and ultimately sub-intimal retention and aggregation. However, heterozygous and total liver-specific ablation of Sptlc2, a major SPT subunit, reduces all major plasma SM and apoB lipoprotein levels but induces jaundice by impairing the development of adherens junctions and causing tumorigenesis [81]. Therefore, a subtle modulation of sphingolipid metabolism in the liver or intestine will be necessary to induce therapeutic effects on lipoprotein metabolism and atherosclerosis.
3.6 SMS and Atherosclerosis
SMS is the last enzyme involved in SM biosynthesis. It catalyzes the formation of SM and DAG by transferring phosphocholine from PC to ceramide. Thus, SMS is a pivotal enzyme for regulating the levels of important lipid signaling mediators and associated pathological conditions.
Among the three members of the SMS gene family found in the cell, SMS1 and SMS2 are selectively distributed in the trans-Golgi apparatus and have catalytic activity [82, 83]. An association study demonstrated that downregulation of SMS2 protects against clinical conditions, including atherosclerosis [84, 85] and hepatosteatosis [86]. Jiang et al. elegantly demonstrated that adenoviral overexpression of SMS1 and SMS2 elevates SM proportions of apoB lipoproteins and decreases SM levels in HDL. ApoB lipoproteins from both SMS1 and SMS2 adenovirus-treated mice were significantly aggregated after treatment with a mammalian SMase, whereas the lipoproteins from control animals did not aggregate [87]. In a subsequent study, SMS2 KO and SMS2 liver-specific transgenic (LTg) mice were constructed and characterized [88]. SMS2 KO mice on a high-fat diet (HFD) had significantly reduced plasma SM levels, while SMS2 LTg mice had increased SM levels, but with no change found in other lipids. ApoB lipoproteins from SMS2 LTg mice displayed a stronger tendency to aggregate after SMase treatment, as shown in the reports of adenoviral overexpression. While SMS2 deficiency increased plasma apoE levels by twofold, SMS2 LTg decreased these levels by 1.8-fold. Moreover, SMS2 KO mice had an activated cholesterol efflux from macrophages, whereas SMS LTg mice had no efflux (efflux prevented) [88]. These results suggest that SMS2 is a key player in the regulation of plasma and liver SM levels in mice.
Disordered apoptosis is important in atherogenesis because the death of lipid-rich foam cells promotes lipid core formation in the vessel wall [89]. Genetic manipulation of SMS activity alters cellular DAG and ceramide, which may contribute to apoptosis. Pharmacological inhibition of SMS reduces cellular DAG levels and activity of protein kinase C (PKC), which is activated by DAG [90]. While ablation of SMS1 or SMS2 significantly reduces DAG levels, overexpression of SMS1 or SMS2 elevates DAG levels in THP-1 macrophages [91]. Cellular DAG is an activator of the conventional novel PKC, a family of serine/threonine kinases that regulate a line of cellular processes, including pro-apoptotic and pro-survival activities. In both CHO cells and THP-1 macrophages, siRNA-mediated knockdown of SMS1 or SMS2 reduced intracellular SM and DAG levels, respectively, and lipopolysaccharide-mediated apoptosis was reduced [91]. In this study, overexpression of SMS1 or SMS2 elevated cellular ceramide levels, as well as SM levels. This mismatch may be attributed to the bidirectional activity of SMS enzymes [83]. In addition, the ratio of ceramide to SM increased in cells overexpressing SMS. This may represent another contributing factor for the increased apoptotic potential of the cells, given that ceramide is a bioactive lipid for pro-apoptotic events. In a recent report, pharmacological inhibition or genetic knockout of SMS2 decreased the generation of M2-type macrophages in vitro and reduced tumor weight and lung metastatic niche formation in a 4T1-triple negative cancer mouse model [92]. These results indicate that modulation of SMS may result in different outcomes depending on the cell type and disease status.
3.7 SMS2 in Macrophages and Endothelial Cells
Macrophages play an important role in the formation of atherosclerotic lesions in the vessel walls. Accumulated LDL in the intima initiates atherogenesis, followed by infiltration of circulating monocytes into the vessel wall, maturation into macrophages, and lipid-laden foam cell formation. Liu et al. transplanted SMS2 knockout bone marrow into LDL receptor knockout (Ldlr−/−) mice, creating a mouse model with macrophage-specific SMS2 deficiency. SMS2 ablation in macrophages reduced the aortic arch, root, and entire aorta compared with transplantation with wild-type macrophages [84]. Further plaque morphology analysis confirmed that SMS2 deficiency in macrophages reduced the necrotic core area and collagen content in atherosclerotic lesions. Downregulation of SMS1 and SMS2 in macrophages resulted in reduced TLR4 on the cell surface [91]. Recently, Prymas et al. demonstrated that silencing of SMS1 and SMS2 led to a depletion of SM in cells, and the TRIF-dependent signaling pathways of TLR4 were inhibited. These results indicate that LPS-induced pro-inflammatory response in macrophages is regulated by SMS via downregulation of SMS and regulates atherogenesis in the vessel wall [93].
Dysfunction of endothelial cells is the pathological basis of atherosclerosis. Oxidative stress and mitochondrial dysfunction are the major causes of endothelial dysfunction [94]. Oxidative stress leads to a depletion of intracellular antioxidants and elevation of ROS levels, causing lipid peroxidation and degeneration of biological macromolecules to develop vascular endothelial dysfunction [95]. The expression of SMS2 in human umbilical vein endothelial cells (EC) was upregulated when cells were treated with H2O2. In addition, SMS2 overexpression promoted apoptosis and macrophage adhesion of H2O2-induced ECs and upregulated the expression of β-catenin. In contrast, the SMS inhibitor Dy105 decreased the levels of endothelial cells and β-catenin. These findings indicate that SMS2 activity is closely associated with endothelial dysfunction via the Wnt/β-catenin signaling pathway, and SMS2 inhibition may inhibit this event.
3.8 Pharmacological Modulation of SPT and SMS2 in Atherogenesis
Since ceramide and SM in the plasma play an important role in the development of atherosclerosis and coronary artery events, pharmacological confirmation of the therapeutic effects and development of the inhibitors of de novo sphingolipid biosynthesis have been reported. For therapeutic purposes, sphingolipid metabolism correcting pathophysiological disease conditions is the most critical. Modulation of SPT and SMS has been actively studied for pharmacological modulation of atherosclerosis and CAD.
3.8.1 SPT
Identification of SPT inhibitors initiated with the characterization of naturally occurring compounds, including myriocin, sphingofungins, and lipoxamycin. These compounds are potent and highly selective for SPT, inhibiting fungal and mammalian SPT in cell-free preparations [96,97,98]. Structurally, these inhibitors resemble the transient intermediate postulated to form during the condensation of serine and palmitoyl CoA. Since these inhibitors act on the first step in the de novo sphingolipid pathway, major sphingolipid metabolites, such as ceramide and SM, are reduced in both cultured cells and in vivo [96,97,98]. Earlier, these compounds have drawn attention as antifungal or immunosuppressive agents.
The association between SPT inhibition and atherosclerosis has been reported with the use of myriocin. Park et al. and Hojjati et al. have reported that oral or intraperitoneal administration of myriocin reduces plasma ceramide and SM levels and atherosclerosis in ApoE KO mice [99, 100]. Although intraperitoneal administration of myriocin did not alter plasma lipid levels, oral administration reduced plasma cholesterol and triglyceride levels. This result suggests that myriocin reduces the absorption of cholesterol in the small intestine. Additionally, myriocin increases insulin sensitivity and reduces non-alcoholic hepatosteatosis [101, 102]. Thus, SPT inhibition by myriocin has direct effects on anti-atherogenic vascular effects and acts as a lipid-lowering agent.
Genetically modified heterozygous Sptlc1 and Sptlc2 mice are healthy and viable despite the embryonic lethality of homozygous ablation. Heterozygous Sptlc1 knockout mice absorbed less cholesterol owing to decreased Niemann-Pick C1-like1 (NPC1L1) and ABCA1 levels and increased ABCG5 levels in the SPT-deficient small intestine. SM levels in the apical membrane of enterocytes also decreased [103]. Together, these results suggest that SPT deficiency reduces cholesterol absorption by downregulating NPC1L1 and ABCA1 proteins in the apical membranes of enterocytes via reduced SM levels in the apical membrane. Therefore, SPT could be an alternative therapeutic target for hypercholesterolemia and atherosclerosis.
In addition to atherosclerosis, cancer is another disease condition that can be modulated by SPT inhibition. Sano et al. synthesized 137 pyrazolopyridine derivatives and validated the relationship between in vitro SPT inhibition and lung cancer cell growth [104]. In a subsequent study, high-throughput screening and medicinal chemistry efforts led to the identification of structurally diverse SPT inhibitors. Their anti-tumor activity was observed in a PL-21 xenograft mouse model [105]. Genin et al. identified novel potent SPT inhibitors for type 2 diabetes and dyslipidemia. These imidazopyridine and pyrazolopiperidine derivatives reduce plasma ceramides, enhance insulin sensitization in diet-induced obese mice, and improve lipid profiles, such as elevation of HDL levels and reduction of triglyceride levels in cholesterol/cholic acid-fed rats. Unfortunately, these compounds cause gastric enteropathy after chronic dosing in rats [106]. Various efforts to regulate SPT pharmacologically for the development of novel therapies are ongoing. The precautionary point is that SPT is the first step in de novo sphingolipid synthesis and alters the cellular levels of various bioactive sphingolipid metabolites. Therefore, fine-tuning of the manipulation of a specific sphingolipid metabolite is important for the future development of therapies for various chronic diseases to improve disease-specific efficacy.
3.8.2 SMS2
SMS-mediated SM synthesis is implicated in atherogenesis, endothelial dysfunction, and macrophage polarization. Recently, a line of compound development has been reported for therapeutic purposes. Adachi et al. identified a 2-quinolone derivative as an SMS2 selective inhibitor with an IC50 of 950 nM and more than 100-fold selectivity for SMS2 over SMS1 using a high-throughput enzymatic assay [85]. Additionally, SMS2-deficient mice are protected from diet-induced obesity, fatty liver, and type 2 diabetes [86, 107, 108]. Mo et al. discovered 4-benzyloxybenzo[d]isoxazole-3-amine derivatives as potent and highly selective SMS2 inhibitors [109]. Among them, the 15w compound had good pharmacokinetic properties in vivo and attenuated chronic inflammation in db/db mice for further development of a therapy against inflammation-associated metabolic dysfunction. Further, 15w showed anti-tumor efficacy against triple-negative breast cancer [92]. Based on these study findings, further medicinal chemistry efforts identified a representative 2-(benzyloxy)-N-arylbenzamide derivative, Ly93, with a high selectivity over SMS1 and a nanomolar range of IC50 [110]. Ly93 dose-dependently reduced apoB secretion from Huh7 cells but also significantly reduced SMS activity and increased cholesterol efflux from macrophages in cell studies. Additionally, it dose-dependently attenuated atherosclerotic lesions in the aortic root and the entire aorta, as well as the macrophage content in lesions in apoE gene knockout mice. In a subsequent study, Huang et al. demonstrated that HFD-induced insulin-resistant C57BL/6 mice treated with Ly93 were more sensitive to insulin than were untreated mice and showed improved insulin tolerance [111]. In particular, phosphorylation of IRS-1, Akt, and GSK-3β increased and sensitized insulin signaling. Yukawa et al. synthesized 1,8-naphthyridin-2-one derivative 37 as a potent and selective SMS2 inhibitor with a nanomolar range of IC50 [112]. This compound reduced hepatic SM levels in mice and exhibited a good pharmacokinetic profile. Collectively, SMS2 is a novel therapeutic target for chronic diseases, including atherosclerosis, inflammation, and insulin resistance; the development of SMS2 inhibitors to find a potent drug candidate in industrial areas is actively ongoing.
3.9 Conclusion
Atherosclerosis is a CAD that is a major cause of mortality worldwide, especially in developed countries. While the current risk factors are predictive parameters for CAD, the broad variability in the development of the disease is difficult to elucidate. Modulation of ceramide and SM in the de novo sphingolipid biosynthetic pathway has been clinically proven to be a new approach for understanding and treating the disease. The development of pharmacological agents to manipulate the levels of ceramide and SM for the treatment of CAD is currently ongoing. Additionally, sphingolipid biosynthesis is tightly associated with the development of cancer, type 2 diabetes, obesity, and non-alcoholic fatty liver disease. SPT and SM are new therapeutic targets for these chronic diseases, and the strategic goal is to manage ceramide and SM in the plasma and target cells. Although fine-tuning is necessary to manage the essential components (sphingolipid metabolites) in the cell, this pathway has a high therapeutic value for the treatment of chronic diseases. Thus, pharmacological modulation of sphingolipid biosynthesis can provide a therapeutic strategy to treat patients with atherosclerosis and CAD.
Abbreviations
- CAD:
-
Coronary artery disease
- SPT:
-
Serine palmitoyltransferase
- SM:
-
Sphingomyelin
- SMS:
-
Sphingomyelin synthase
- MTP:
-
Microsomal triglyceride transfer protein
- SMase:
-
Sphingomyelinase
References
Ding, M., & Rexrode, K. M. (2020). A review of lipidomics of cardiovascular disease highlights the importance of isolating lipoproteins. Metabolites, 10, 163.
Saleem, M., Herrmann, N., Dinoff, A., Marzolini, S., Mielke, M. M., Andreazza, A., et al. (2020). Association between sphingolipids and cardiopulmonary fitness in coronary artery disease patients undertaking cardiac rehabilitation. The Journals of Gerontology: Series A, 75, 671–679.
Mozaffarian, D., Benjamin, E. J., Go, A. S., Arnett, D. K., Blaha, M. J., Cushman, M., et al. (2015). Heart disease and stroke statistics—2015 update: A report from the American Heart Association. Circulation, 131, e29–e32.
Jame, S., & Barnes, G. (2020). Stroke and thromboembolism prevention in atrial fibrillation. Heart, 106, 10–17.
Skagen, K., Skjelland, M., Zamani, M., & Russell, D. (2016). Unstable carotid artery plaque: New insights and controversies in diagnostics and treatment. Croatian Medical Journal, 57, 311–320.
Berry, C. (2017). Stable coronary syndromes: The case for consolidating the nomenclature of stable ischemic heart disease. Circulation, 136, 437–439.
Sakakura, K., Nakano, M., Otsuka, F., Ladich, E., Kolodgie, F. D., & Virmani, R. (2013). Pathophysiology of atherosclerosis plaque progression. Heart, Lung & Circulation, 22, 399–411.
Visscher, M., Moerman, A. M., Burgers, P. C., Van Beusekom, H. M., Luider, T. M., Verhagen, H. J., et al. (2019). Data processing pipeline for lipid profiling of carotid atherosclerotic plaque with mass spectrometry imaging. Journal of the American Society for Mass Spectrometry, 30, 1790–1800.
Libby, P. (2021). The changing landscape of atherosclerosis. Nature, 592, 524–533.
Zhou, Y. T., Grayburn, P., Karim, A., Shimabukuro, M., Higa, M., Baetens, D., et al. (2000). Lipotoxic heart disease in obese rats: Implications for human obesity. Proceedings of the National Academy of Sciences of the United States of America, 97, 1784–1789.
Chaurasia, B., Tippetts, T. S., Monibas, R. M., Liu, J., Li, Y., Wang, L., et al. (2019). Targeting a ceramide double bond improves insulin resistance and hepatic steatosis. Science, 365, 386–392.
Ruangsiriluk, W., Grosskurth, S. E., Ziemek, D., Kuhn, M., des Etages, S. G., & Francone, O. L. (2012). Silencing of enzymes involved in ceramide biosynthesis causes distinct global alterations of lipid homeostasis and gene expression. Journal of Lipid Research, 53, 1459–1471.
Raichur, S., Wang, S. T., Chan, P. W., Li, Y., Ching, J., Chaurasia, B., et al. (2014). CerS2 haploinsufficiency inhibits β-oxidation and confers susceptibility to diet-induced steatohepatitis and insulin resistance. Cell Metabolism, 20, 687–695.
Turpin, S. M., Nicholls, H. T., Willmes, D. M., Mourier, A., Brodesser, S., Wunderlich, C. M., et al. (2014). Obesity-induced CerS6-dependent C16: 0 ceramide production promotes weight gain and glucose intolerance. Cell Metabolism, 20, 678–686.
Seah, J. Y. H., Chew, W. S., Torta, F., Khoo, C. M., Wenk, M. R., Herr, D. R., et al. (2020). Plasma sphingolipids and risk of cardiovascular diseases: A large-scale lipidomic analysis. Metabolomics, 16, 1–12.
Jiang, X. C., Paultre, F., Pearson, T. A., Reed, R. G., Francis, C. K., Lin, M., et al. (2000). Plasma sphingomyelin level as a risk factor for coronary artery disease. Arteriosclerosis, Thrombosis, and Vascular Biology, 20, 2614–2618.
Ichi, I., Nakahara, K., Miyashita, Y., Hidaka, A., Kutsukake, S., Inoue, K., et al. (2006). Association of ceramides in human plasma with risk factors of atherosclerosis. Lipids, 41, 859–863.
Lowther, J., Naismith, J. H., Dunn, T. M., & Campopiano, D. J. (2012). Structural, mechanistic and regulatory studies of serine palmitoyltransferase. Biochemical Society Transactions, 40, 547–554.
Tidhar, R., & Futerman, A. H. (2013). The complexity of sphingolipid biosynthesis in the endoplasmic reticulum. Biochimica et Biophysica Acta (BBA), 1833, 2511–2518.
Iqbal, J., Walsh, M. T., Hammad, S. M., Cuchel, M., Tarugi, P., Hegele, R. A., et al. (2015). Microsomal triglyceride transfer protein transfers and determines plasma concentrations of ceramide and sphingomyelin but not glycosylceramide. Journal of Biological Chemistry, 290, 25863–25875.
Clarke, B. A., Majumder, S., Zhu, H., Lee, Y. T., Kono, M., Li, C., et al. (2019). The Ormdl genes regulate the sphingolipid synthesis pathway to ensure proper myelination and neurologic function in mice. eLife, 8, e51067.
Deng, Y., Rivera-Molina, F. E., Toomre, D. K., & Burd, C. G. (2016). Sphingomyelin is sorted at the trans Golgi network into a distinct class of secretory vesicle. Proceedings of the National Academy of Sciences, 113, 6677–6682.
Weiss, B., & Stoffel, W. (1997). Human and murine serine-palmitoyl-CoA transferase--cloning, expression and characterization of the key enzyme in sphingolipid synthesis. European Journal of Biochemistry, 249, 239–247.
Hanada, K., Hara, T., Nishijima, M., Kuge, O., Dickson, R. C., & Nagiec, M. M. (1997). A mammalian homolog of the yeast LCB1 encodes a component of serine palmitoyltransferase, the enzyme catalyzing the first step in sphingolipid synthesis. The Journal of Biological Chemistry, 272, 32108–32114.
Parthibane, V., Lin, J., Acharya, D., Abimannan, T., Srideshikan, S. M., Klarmann, K., et al. (2021). SSSPTA is essential for serine palmitoyltransferase function during development and hematopoiesis. Journal of Biological Chemistry, 296, 100491.
Hojjati, M. R., Li, Z., & Jiang, X. C. (2005). Serine palmitoyl-CoA transferase (SPT) deficiency and sphingolipid levels in mice. Biochimica et Biophysica Acta, 1737, 44–51.
Hornemann, T., Richard, S., Rutti, M. F., Wei, Y., & von Eckardstein, A. (2006). Cloning and initial characterization of a new subunit for mammalian serine-palmitoyltransferase. The Journal of Biological Chemistry, 281, 37275–37281.
Gable, K., Han, G., Monaghan, E., Bacikova, D., Natarajan, M., Williams, R., et al. (2002). Mutations in the yeast LCB1 and LCB2 genes, including those corresponding to the hereditary sensory neuropathy type I mutations, dominantly inactivate serine palmitoyltransferase. The Journal of Biological Chemistry, 277, 10194–10200.
Yard, B. A., Carter, L. G., Johnson, K. A., Overton, I. M., Dorward, M., Liu, H., et al. (2007). The structure of serine palmitoyltransferase; gateway to sphingolipid biosynthesis. Journal of Molecular Biology, 370, 870–886.
Hanada, K., Hara, T., & Nishijima, M. (2000). Purification of the serine palmitoyltransferase complex responsible for sphingoid base synthesis by using affinity peptide chromatography techniques. The Journal of Biological Chemistry, 275, 8409–8415.
Hornemann, T., Wei, Y., & von Eckardstein, A. (2007). Is the mammalian serine palmitoyltransferase a high-molecular-mass complex? The Biochemical Journal, 405, 157–164.
Gable, K., Slife, H., Bacikova, D., Monaghan, E., & Dunn, T. M. (2000). Tsc3p is an 80-amino acid protein associated with serine palmitoyltransferase and required for optimal enzyme activity. The Journal of Biological Chemistry, 275, 7597–7603.
Han, G., Gupta, S. D., Gable, K., Niranjanakumari, S., Moitra, P., Eichler, F., et al. (2009). Identification of small subunits of mammalian serine palmitoyltransferase that confer distinct acyl-CoA substrate specificities. Proceedings of the National Academy of Sciences of the United States of America, 106, 8186–8191.
Breslow, D. K., Collins, S. R., Bodenmiller, B., Aebersold, R., Simons, K., Shevchenko, A., et al. (2010). Orm family proteins mediate sphingolipid homeostasis. Nature, 463, 1048–1053.
Hjelmqvist, L., Tuson, M., Marfany, G., Herrero, E., Balcells, S., & Gonzalez-Duarte, R. (2002). ORMDL proteins are a conserved new family of endoplasmic reticulum membrane proteins. Genome Biology, 3, 27.
Roelants, F. M., Breslow, D. K., Muir, A., Weissman, J. S., & Thorner, J. (2011). Protein kinase Ypk1 phosphorylates regulatory proteins Orm1 and Orm2 to control sphingolipid homeostasis in Saccharomyces cerevisiae. Proceedings of the National Academy of Sciences, 108, 19222–19227.
Wadsworth, J. M., Clarke, D. J., McMahon, S. A., Lowther, J. P., Beattie, A. E., Langridge-Smith, P. R., et al. (2013). The chemical basis of serine palmitoyltransferase inhibition by myriocin. Journal of the American Chemical Society, 135, 14276–14285.
Roelants, F. M., Breslow, D. K., Muir, A., Weissman, J. S., & Thorner, J. (2011). Protein kinase Ypk1 phosphorylates regulatory proteins Orm1 and Orm2 to control sphingolipid homeostasis in Saccharomyces cerevisiae. Proceedings of the National Academy of Sciences of the United States of America, 108, 19222–19227.
Sun, Y., Miao, Y., Yamane, Y., Zhang, C., Shokat, K. M., Takematsu, H., et al. (2012). Orm protein phosphoregulation mediates transient sphingolipid biosynthesis response to heat stress via the Pkh-Ypk and Cdc55-PP2A pathways. Molecular Biology of the Cell, 23, 2388–2398.
Shimobayashi, M., Oppliger, W., Moes, S., Jeno, P., & Hall, M. N. (2013). TORC1-regulated protein kinase Npr1 phosphorylates Orm to stimulate complex sphingolipid synthesis. Molecular Biology of the Cell, 24, 870–881.
GrandPre, T., Nakamura, F., Vartanian, T., & Strittmatter, S. M. (2000). Identification of the Nogo inhibitor of axon regeneration as a Reticulon protein. Nature, 403, 439–444.
Cantalupo, A., Zhang, Y., Kothiya, M., Galvani, S., Obinata, H., Bucci, M., et al. (2015). Nogo-B regulates endothelial sphingolipid homeostasis to control vascular function and blood pressure. Nature Medicine, 21, 1028–1037.
Voeltz, G. K., Prinz, W. A., Shibata, Y., Rist, J. M., & Rapoport, T. A. (2006). A class of membrane proteins shaping the tubular endoplasmic reticulum. Cell, 124, 573–586.
Fournier, A. E., GrandPre, T., & Strittmatter, S. M. (2001). Identification of a receptor mediating Nogo-66 inhibition of axonal regeneration. Nature, 409, 341–346.
Miao, R. Q., Gao, Y., Harrison, K. D., Prendergast, J., Acevedo, L. M., Yu, J., et al. (2006). Identification of a receptor necessary for Nogo-B stimulated chemotaxis and morphogenesis of endothelial cells. Proceedings of the National Academy of Sciences of the United States of America, 103, 10997–11002.
Hammad, S. M., Pierce, J. S., Soodavar, F., Smith, K. J., Al Gadban, M. M., Rembiesa, B., et al. (2010). Blood sphingolipidomics in healthy humans: Impact of sample collection methodology. Journal of Lipid Research, 51, 3074–3087.
Iqbal, J., Walsh, M. T., Hammad, S. M., Cuchel, M., Tarugi, P., Hegele, R. A., et al. (2015). Microsomal triglyceride transfer protein transfers and determines plasma concentrations of ceramide and sphingomyelin but not glycosylceramide. The Journal of Biological Chemistry, 290, 25863–25875.
Bharath, L. P., Ruan, T., Li, Y., Ravindran, A., Wan, X., Nhan, J. K., et al. (2015). Ceramide-initiated protein phosphatase 2A activation contributes to arterial dysfunction in vivo. Diabetes, 64, 3914–3926.
Freed, J. K., Beyer, A. M., LoGiudice, J. A., Hockenberry, J. C., & Gutterman, D. D. (2014). Ceramide changes the mediator of flow-induced vasodilation from nitric oxide to hydrogen peroxide in the human microcirculation. Circulation Research, 115, 525–532.
Li, H., Junk, P., Huwiler, A., Burkhardt, C., Wallerath, T., Pfeilschifter, J., et al. (2002). Dual effect of ceramide on human endothelial cells: Induction of oxidative stress and transcriptional upregulation of endothelial nitric oxide synthase. Circulation, 106, 2250–2256.
Altura, B. M., Gebrewold, A., Zheng, T., & Altura, B. T. (2002). Sphingomyelinase and ceramide analogs induce vasoconstriction and leukocyte-endothelial interactions in cerebral venules in the intact rat brain: Insight into mechanisms and possible relation to brain injury and stroke. Brain Research Bulletin, 58, 271–278.
Zheng, T., Li, W., Wang, J., Altura, B. T., & Altura, B. M. (2000). Sphingomyelinase and ceramide analogs induce contraction and rises in [Ca(2+)](i) in canine cerebral vascular muscle. American Journal of Physiology. Heart and Circulatory Physiology, 278, 1421–1428.
Zhang, Q. J., Holland, W. L., Wilson, L., Tanner, J. M., Kearns, D., Cahoon, J. M., et al. (2012). Ceramide mediates vascular dysfunction in diet-induced obesity by PP2A-mediated dephosphorylation of the eNOS-Akt complex. Diabetes, 61, 1848–1859.
Nilsson, A., & Duan, R. D. (2006). Absorption and lipoprotein transport of sphingomyelin. Journal of Lipid Research, 47, 154–171.
Tabas, I., Williams, K. J., & Boren, J. (2007). Subendothelial lipoprotein retention as the initiating process in atherosclerosis: Update and therapeutic implications. Circulation, 116, 1832–1844.
Schissel, S. L., Tweedie-Hardman, J., Rapp, J. H., Graham, G., Williams, K. J., & Tabas, I. (1996). Rabbit aorta and human atherosclerotic lesions hydrolyze the sphingomyelin of retained low-density lipoprotein. Proposed role for arterial-wall sphingomyelinase in subendothelial retention and aggregation of atherogenic lipoproteins. The Journal of Clinical Investigation, 98, 1455–1464.
Schissel, S. L., Jiang, X., Tweedie-Hardman, J., Jeong, T., Camejo, E. H., Najib, J., et al. (1998). Secretory sphingomyelinase, a product of the acid sphingomyelinase gene, can hydrolyze atherogenic lipoproteins at neutral pH. Implications for atherosclerotic lesion development. The Journal of Biological Chemistry, 273, 2738–2746.
Rodriguez, J., Catapano, A., Ghiselli, G. C., & Sirtori, C. R. (1976). Turnover and aortic uptake of very low density lipoproteins (VLDL) from hypercholesteremic rabbits as a model for testing antiatherosclerotic compounds. Advances in Experimental Medicine and Biology, 67, 169–189.
Jeong, T., Schissel, S. L., Tabas, I., Pownall, H. J., Tall, A. R., & Jiang, X. (1998). Increased sphingomyelin content of plasma lipoproteins in apolipoprotein E knockout mice reflects combined production and catabolic defects and enhances reactivity with mammalian sphingomyelinase. The Journal of Clinical Investigation, 101, 905–912.
Li, Z., Basterr, M. J., Hailemariam, T. K., Hojjati, M. R., Lu, S., Liu, J., et al. (2005). The effect of dietary sphingolipids on plasma sphingomyelin metabolism and atherosclerosis. Biochimica et Biophysica Acta, 1735, 130–134.
Nelson, J. C., Jiang, X. C., Tabas, I., Tall, A., & Shea, S. (2006). Plasma sphingomyelin and subclinical atherosclerosis: Findings from the multi-ethnic study of atherosclerosis. American Journal of Epidemiology, 163, 903–912.
de Mello, V. D., Lankinen, M., Schwab, U., Kolehmainen, M., Lehto, S., Seppanen-Laakso, T., et al. (2009). Link between plasma ceramides, inflammation and insulin resistance: Association with serum IL-6 concentration in patients with coronary heart disease. Diabetologia, 52, 2612–2615.
Yeboah, J., McNamara, C., Jiang, X. C., Tabas, I., Herrington, D. M., Burke, G. L., et al. (2010). Association of plasma sphingomyelin levels and incident coronary heart disease events in an adult population: Multi-ethnic study of atherosclerosis. Arteriosclerosis, Thrombosis, and Vascular Biology, 30, 628–633.
Fernandez, C., Sandin, M., Sampaio, J. L., Almgren, P., Narkiewicz, K., Hoffmann, M., et al. (2013). Plasma lipid composition and risk of developing cardiovascular disease. PLoS One, 8, e71846.
Pan, W., Yu, J., Shi, R., Yan, L., Yang, T., Li, Y., et al. (2014). Elevation of ceramide and activation of secretory acid sphingomyelinase in patients with acute coronary syndromes. Coronary Artery Disease, 25, 230–235.
Tarasov, K., Ekroos, K., Suoniemi, M., Kauhanen, D., Sylvanne, T., Hurme, R., et al. (2014). Molecular lipids identify cardiovascular risk and are efficiently lowered by simvastatin and PCSK9 deficiency. The Journal of Clinical Endocrinology and Metabolism, 99, 45–52.
Cheng, J. M., Suoniemi, M., Kardys, I., Vihervaara, T., de Boer, S. P., Akkerhuis, K. M., et al. (2015). Plasma concentrations of molecular lipid species in relation to coronary plaque characteristics and cardiovascular outcome: Results of the ATHEROREMO-IVUS study. Atherosclerosis, 243, 560–566.
Yu, J., Pan, W., Shi, R., Yang, T., Li, Y., Yu, G., et al. (2015). Ceramide is upregulated and associated with mortality in patients with chronic heart failure. The Canadian Journal of Cardiology, 31, 357–363.
Laaksonen, R., Ekroos, K., Sysi-Aho, M., Hilvo, M., Vihervaara, T., Kauhanen, D., et al. (2016). Plasma ceramides predict cardiovascular death in patients with stable coronary artery disease and acute coronary syndromes beyond LDL-cholesterol. European Heart Journal, 37, 1967–1976.
Havulinna, A. S., Sysi-Aho, M., Hilvo, M., Kauhanen, D., Hurme, R., Ekroos, K., et al. (2016). Circulating ceramides predict cardiovascular outcomes in the population-based FINRISK 2002 cohort. Arteriosclerosis, Thrombosis, and Vascular Biology, 36, 2424–2430.
Dinoff, A., Saleem, M., Herrmann, N., Mielke, M. M., Oh, P. I., Venkata, S. L. V., et al. (2017). Plasma sphingolipids and depressive symptoms in coronary artery disease. Brain and Behavior: A Cognitive Neuroscience Perspective, 7, e00836.
Wang, D. D., Toledo, E., Hruby, A., Rosner, B. A., Willett, W. C., Sun, Q., et al. (2017). Plasma ceramides, mediterranean diet, and incident cardiovascular disease in the PREDIMED Trial (prevencion con dieta mediterranea). Circulation, 135, 2028–2040.
Lemaitre, R. N., Yu, C., Hoofnagle, A., Hari, N., Jensen, P. N., Fretts, A. M., et al. (2018). Circulating sphingolipids, insulin, HOMA-IR, and HOMA-B: The Strong Heart Family Study. Diabetes, 67, 1663–1672.
Meeusen, J. W., Donato, L. J., Bryant, S. C., Baudhuin, L. M., Berger, P. B., & Jaffe, A. S. (2018). Plasma ceramides. Arteriosclerosis, Thrombosis, and Vascular Biology, 38, 1933–1939.
Peterson, L. R., Xanthakis, V., Duncan, M. S., Gross, S., Friedrich, N., Völzke, H., et al. (2018). Ceramide remodeling and risk of cardiovascular events and mortality. Journal of the American Heart Association, 7, e007931.
Lemaitre, R. N., Jensen, P. N., Hoofnagle, A., McKnight, B., Fretts, A. M., King, I. B., et al. (2019). Plasma ceramides and sphingomyelins in relation to heart failure risk. Circulation, 12, e005708.
Rahman, M. L., Feng, Y. A., Fiehn, O., Albert, P. S., Tsai, M. Y., Zhu, Y., et al. (2021). Plasma lipidomics profile in pregnancy and gestational diabetes risk: A prospective study in a multiracial/ethnic cohort. BMJ Open Diabetes Research & Care, 9, e001551.
Chen, G. C., Chai, J. C., Yu, B., Michelotti, G. A., Grove, M. L., Fretts, A. M., et al. (2020). Serum sphingolipids and incident diabetes in a US population with high diabetes burden: The Hispanic Community Health Study/Study of Latinos (HCHS/SOL). The American Journal of Clinical Nutrition, 112, 57–65.
Rico, J. E., Specker, B., Perry, C. A., & McFadden, J. W. (2020). Plasma ceramides and triglycerides are elevated during pregnancy in association with markers of insulin resistance in hutterite women. Lipids, 55, 375–386.
Devlin, C. M., Leventhal, A. R., Kuriakose, G., Schuchman, E. H., Williams, K. J., & Tabas, I. (2008). Acid sphingomyelinase promotes lipoprotein retention within early atheromata and accelerates lesion progression. Arteriosclerosis, Thrombosis, and Vascular Biology, 28, 1723–1730.
Li, Z., Kabir, I., Jiang, H., Zhou, H., Libien, J., Zeng, J., et al. (2016). Liver serine palmitoyltransferase activity deficiency in early life impairs adherens junctions and promotes tumorigenesis. Hepatology, 64, 2089–2102.
Yamaoka, S., Miyaji, M., Kitano, T., Umehara, H., & Okazaki, T. (2004). Expression cloning of a human cDNA restoring sphingomyelin synthesis and cell growth in sphingomyelin synthase-defective lymphoid cells. The Journal of Biological Chemistry, 279, 18688–18693.
Huitema, K., van den Dikkenberg, J., Brouwers, J. F., & Holthuis, J. C. (2004). Identification of a family of animal sphingomyelin synthases. The EMBO Journal, 23, 33–44.
Liu, J., Huan, C., Chakraborty, M., Zhang, H., Lu, D., Kuo, M. S., et al. (2009). Macrophage sphingomyelin synthase 2 deficiency decreases atherosclerosis in mice. Circulation Research, 105, 295–303.
Adachi, R., Ogawa, K., Matsumoto, S. I., Satou, T., Tanaka, Y., Sakamoto, J., et al. (2017). Discovery and characterization of selective human sphingomyelin synthase 2 inhibitors. European Journal of Medicinal Chemistry, 136, 283–293.
Li, Y., Dong, J., Ding, T., Kuo, M. S., Cao, G., Jiang, X. C., et al. (2013). Sphingomyelin synthase 2 activity and liver steatosis: An effect of ceramide-mediated peroxisome proliferator-activated receptor gamma2 suppression. Arteriosclerosis, Thrombosis, and Vascular Biology, 33, 1513–1520.
Dong, J., Liu, J., Lou, B., Li, Z., Ye, X., Wu, M., et al. (2006). Adenovirus-mediated overexpression of sphingomyelin synthases 1 and 2 increases the atherogenic potential in mice. Journal of Lipid Research, 47, 1307–1314.
Liu, J., Zhang, H., Li, Z., Hailemariam, T. K., Chakraborty, M., Jiang, K., et al. (2009). Sphingomyelin synthase 2 is one of the determinants for plasma and liver sphingomyelin levels in mice. Arteriosclerosis, Thrombosis, and Vascular Biology, 29, 850–856.
Libby, P., Loscalzo, J., Ridker, P. M., Farkouh, M. E., Hsue, P. Y., Fuster, V., et al. (2018). Inflammation, immunity, and infection in atherothrombosis: JACC review topic of the week. Journal of the American College of Cardiology, 72, 2071–2081.
Cerbon, J., & del Carmen, L.-S. R. (2003). Diacylglycerol generated during sphingomyelin synthesis is involved in protein kinase C activation and cell proliferation in Madin-Darby canine kidney cells. The Biochemical Journal, 373, 917–924.
Ding, T., Li, Z., Hailemariam, T., Mukherjee, S., Maxfield, F. R., Wu, M. P., et al. (2008). SMS overexpression and knockdown: Impact on cellular sphingomyelin and diacylglycerol metabolism, and cell apoptosis. Journal of Lipid Research, 49, 376–385.
Deng, Y., Hu, J. C., He, S. H., Lou, B., Ding, T. B., Yang, J. T., et al. (2021). Sphingomyelin synthase 2 facilitates M2-like macrophage polarization and tumor progression in a mouse model of triple-negative breast cancer. Acta Pharmacologica Sinica, 42, 149–159.
Prymas, K., Swiatkowska, A., Traczyk, G., Ziemlinska, E., Dziewulska, A., Ciesielska, A., et al. (2020). Sphingomyelin synthase activity affects TRIF-dependent signaling of Toll-like receptor 4 in cells stimulated with lipopolysaccharide. Biochimica et Biophysica Acta - Molecular and Cell Biology of Lipids, 1865, 158549.
Minuz, P., Fava, C., Vattemi, G., Arcaro, G., Riccadonna, M., Tonin, P., et al. (2012). Endothelial dysfunction and increased oxidative stress in mitochondrial diseases. Clinical Science (London, England), 122, 289–297.
Wu, D., Li, D., Liu, Z., Liu, X., Zhou, S., & Duan, H. (2018). Role and underlying mechanism of SPATA12 in oxidative damage. Oncology Letters, 15, 3676–3684.
Zweerink, M. M., Edison, A. M., Wells, G. B., Pinto, W., & Lester, R. L. (1992). Characterization of a novel, potent, and specific inhibitor of serine palmitoyltransferase. The Journal of Biological Chemistry, 267, 25032–25038.
Mandala, S. M., Frommer, B. R., Thornton, R. A., Kurtz, M. B., Young, N. M., Cabello, M. A., et al. (1994). Inhibition of serine palmitoyl-transferase activity by lipoxamycin. The Journal of Antibiotics, 47, 376–379.
Miyake, Y., Kozutsumi, Y., Nakamura, S., Fujita, T., & Kawasaki, T. (1995). Serine palmitoyltransferase is the primary target of a sphingosine-like immunosuppressant, ISP-1/myriocin. Biochemical and Biophysical Research Communications, 211, 396–403.
Park, T. S., Panek, R. L., Mueller, S. B., Hanselman, J. C., Rosebury, W. S., Robertson, A. W., et al. (2004). Inhibition of sphingomyelin synthesis reduces atherogenesis in apolipoprotein E-knockout mice. Circulation, 110, 3465–3471.
Hojjati, M. R., Li, Z., Zhou, H., Tang, S., Huan, C., Ooi, E., et al. (2005). Effect of myriocin on plasma sphingolipid metabolism and atherosclerosis in apoE-deficient mice. The Journal of Biological Chemistry, 280, 10284–10289.
Holland, W. L., Brozinick, J. T., Wang, L. P., Hawkins, E. D., Sargent, K. M., Liu, Y., et al. (2007). Inhibition of ceramide synthesis ameliorates glucocorticoid-, saturated-fat-, and obesity-induced insulin resistance. Cell Metabolism, 5, 167–179.
Yang, R. X., Pan, Q., Liu, X. L., Zhou, D., Xin, F. Z., Zhao, Z. H., et al. (2019). Therapeutic effect and autophagy regulation of myriocin in nonalcoholic steatohepatitis. Lipids in Health and Disease, 18, 179.
Li, Z., Park, T. S., Li, Y., Pan, X., Iqbal, J., Lu, D., et al. (2009). Serine palmitoyltransferase (SPT) deficient mice absorb less cholesterol. Biochimica et Biophysica Acta, 1791, 297–306.
Sano, O., Kazetani, K. I., Adachi, R., Kurasawa, O., Kawamoto, T., & Iwata, H. (2017). Using a biologically annotated library to analyze the anticancer mechanism of serine palmitoyl transferase (SPT) inhibitors. FEBS Open Bio, 7, 495–503.
Kojima, T., Asano, Y., Kurasawa, O., Hirata, Y., Iwamura, N., Wong, T. T., et al. (2018). Discovery of novel serine palmitoyltransferase inhibitors as cancer therapeutic agents. Bioorganic & Medicinal Chemistry, 26, 2452–2465.
Genin, M. J., Gonzalez Valcarcel, I. C., Holloway, W. G., Lamar, J., Mosior, M., Hawkins, E., et al. (2016). Imidazopyridine and pyrazolopiperidine derivatives as novel inhibitors of serine palmitoyl transferase. Journal of Medicinal Chemistry, 59, 5904–5910.
Li, Z., Zhang, H., Liu, J., Liang, C. P., Li, Y., Teitelman, G., et al. (2011). Reducing plasma membrane sphingomyelin increases insulin sensitivity. Molecular and Cellular Biology, 31, 4205–4218.
Mitsutake, S., Zama, K., Yokota, H., Yoshida, T., Tanaka, M., Mitsui, M., et al. (2011). Dynamic modification of sphingomyelin in lipid microdomains controls development of obesity, fatty liver, and type 2 diabetes. The Journal of Biological Chemistry, 286, 28544–28555.
Mo, M., Yang, J., Jiang, X. C., Cao, Y., Fei, J., Chen, Y., et al. (2018). Discovery of 4-benzyloxybenzo[d]isoxazole-3-amine derivatives as highly selective and orally efficacious human sphingomyelin synthase 2 inhibitors that reduce chronic inflammation in db/db mice. Journal of Medicinal Chemistry, 61, 8241–8254.
Li, Y., Huang, T., Lou, B., Ye, D., Qi, X., Li, X., et al. (2019). Discovery, synthesis and anti-atherosclerotic activities of a novel selective sphingomyelin synthase 2 inhibitor. European Journal of Medicinal Chemistry, 163, 864–882.
Huang, Y., Huang, T., Zhen, X., Li, Y., Mo, M., Ye, D., et al. (2019). A selective sphingomyelin synthase 2 inhibitor ameliorates diet induced insulin resistance via the IRS-1/Akt/GSK-3beta signaling pathway. Pharmazie, 74, 553–558.
Yukawa, T., Nakahata, T., Okamoto, R., Ishichi, Y., Miyamoto, Y., Nishimura, S., et al. (2020). Discovery of 1,8-naphthyridin-2-one derivative as a potent and selective sphingomyelin synthase 2 inhibitor. Bioorganic & Medicinal Chemistry, 28, 115376.
Acknowledgements
This work was supported in part by the Gachon University Research fund of 2019 (GCU-2019-0824) and the National Research Foundation of Korea (NRF) funded by the Korean government (MSIP) to T-. S. P. (2020R1A2C2012833, NRF-2021R1A4A3031661).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive licence to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Park, TS., Devi, S., Sharma, A., Kim, GT., Cho, KH. (2022). De Novo Sphingolipid Biosynthesis in Atherosclerosis. In: Jiang, XC. (eds) Sphingolipid Metabolism and Metabolic Disease. Advances in Experimental Medicine and Biology, vol 1372. Springer, Singapore. https://doi.org/10.1007/978-981-19-0394-6_3
Download citation
DOI: https://doi.org/10.1007/978-981-19-0394-6_3
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-19-0393-9
Online ISBN: 978-981-19-0394-6
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)