Abstract
A thorough preoperative workup is essential not only for surgical planning but also for identification and proactive optimization of comorbidities, maximizing reconstructive success. The patient should be counseled regarding postoperative weight-bearing and ambulation, as failure to comply with prescribed mobility progression increases the risk of reconstructive failure. In patients with diabetes, optimization of perioperative blood glucose levels and management of biomechanical abnormalities are essential for reducing the risk of dehiscence and reoperation. Vascular studies, thrombophilia screening, and serial surgical debridement to achieve a clean wound bed are also important steps in the preoperative workup.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Preoperative procedures
- Care, preoperative
- Preoperative workup
- Surgical planning
- Diabetic complications
- Foot ulcer, diabetic
- Vascular studies
- Comorbidity optimization
-
Thorough preoperative evaluation not only allows for effective surgical planning but also allows the surgeon to identify and proactively manage conditions that may otherwise predispose a patient to reconstructive failure.
-
A multidisciplinary approach is critical for an effective and thorough preoperative workup.
7.1 Introduction
Thorough preoperative workup plays a key role in avoiding perioperative complications and optimizing wound healing and ambulation after reconstructive surgery. Several modalities such as vascular studies, thermograms, and transcutaneous oximetry measurements provide objective data that can guide perioperative management as well as surgical planning. This chapter provides an overview of these available modalities and their utility in planning a successful diabetic foot reconstruction.
7.2 Relevance to Surgical Outcome
Each step of the preoperative evaluation plays an important role in optimizing reconstructive success. Patients and their family members should be counseled early and often regarding the importance of strict adherence to instructions for postoperative weight-bearing and ambulation. A patient who is too aggressive with his or her return to ambulation risks compromised flap perfusion and subsequent flap failure. In patients with diabetes, failure to achieve adequate blood sugar control prior to surgery is associated with an increased risk of dehiscence and reoperation [1]. Proactive identification of arterial and venous pathologies is also of critical importance: endovascular procedures done prior to reconstructive surgery coupled with adequate consideration of vascular pathologies in the surgical plan can help ensure adequate reperfusion of ischemic areas and prevent flap congestion and/or thrombosis [2,3,4]. A thorough assessment for hereditary and/or acquired thrombophilias prior to surgery can help optimize a patient’s perioperative anticoagulation regimen, thereby minimizing the risk of flap thrombosis and subsequent flap failure with a high risk of nonsalvageability [3, 5,6,7,8]. Patients should also undergo a comprehensive biomechanical exam: addressing gait abnormalities is an important step in preventing wound recurrence.
7.3 Preoperative Evaluation and Special Considerations
Our management algorithm (Fig. 7.1) utilizes multidisciplinary collaboration to identify and proactively manage risks for flap failure, optimize comorbidities prior to surgery, and develop a personalized surgical plan. In addition to an early and aggressive focus on comorbidity optimization, the first layer of surgical preparation includes vascular studies to optimize donor and recipient vessel selection, hypercoagulability studies to minimize perioperative clotting risk, and a biomechanical exam to address mechanical factors that may compromise lower extremity wound healing. The next phase of preparation involves serial surgical debridement to prepare a culture-negative wound bed before pursuing further wound management with reconstruction.
Counseling the patient and family should occur frequently throughout the preoperative process, with an emphasis on postoperative ambulation and expectations for the patient’s weight-bearing status as he or she rehabilitates postoperatively. Strict compliance with ambulation instructions is critical for reconstructive success.
7.3.1 Comorbidity Optimization
Comorbid conditions increase surgical complications and should thus be evaluated with thorough history taking and optimized appropriately before surgery [9]. In patients with diabetes, adequate blood sugar control is critical: blood glucose levels above 200 mg/dl and HbA1c above 6.5% increases risk of dehiscence and reoperation by over three and four times, respectively [1]. All patients who smoke should be encouraged to quit or otherwise abstain for a minimum of 4–8 weeks prior to surgery [10]. Postoperatively, patients are in a hypermetabolic state and should undergo preoperative nutritional screening. Malnutrition can be managed with nutritional prehabilitation and exercise therapy [11] and tracked via albumin and prealbumin levels, although utility of these tests as markers of nutrition has recently come into question [12]. In a retrospective review of patients undergoing lower extremity free tissue transfer (FTT) at our institution, albumin levels lower than 2.7 g/dL preoperatively were associated with significantly increased healing times and decreased flap healing rates. Conversely, low prealbumin levels (traditionally defined as lower than 20 mg/dL) were not associated with increased time to flap healing or flap healing rates [13].
7.3.2 Vascular Examination
Flap success is highly dependent on adequate perfusion of the transferred tissue; therefore, a thorough vascular exam is critical to optimizing flap survival. By focusing this exam on the vascular supply defining the 6 foot and ankle angiosomes, a surgeon can (1) predict the viability of a given tissue for harvest, (2) plan for optimal surgical incision placement, and (3) coordinate with a vascular surgeon for preemptive revascularization to ensure adequate reperfusion to areas of ischemic ulceration [2, 3].
7.3.3 Arterial Examination
There are several options for arterial examination, including palpating for pulses, ankle-brachial indices, handheld Doppler examination, computed tomographic (CT) angiography, and catheter arteriography [14]. The contrast dye load required for conventional arteriography is significantly less than that required for lower extremity CT angiography; therefore, arteriography is more renal protective and thus may be preferred in the diabetic patient population, in which many patients have renal insufficiency [14]. In our practice, routine preoperative arteriography identified arterial pathology in 67.8% of patients undergoing FTT for chronic lower extremity wounds [14]. Furthermore, diabetes was associated with the presence of stenosis or occlusion on angiography as well as the need for endovascular intervention [14]. This imaging modality is thus particularly helpful in the preoperative workup of this population.
7.3.4 Venous Examination
Insufficient venous outflow leading to congestion and delayed venous thrombosis is a leading cause of flap loss [15]. Venous studies with lower extremity duplex ultrasound can identify venous anomalies and venous insufficiency that results in high venous pressure and may predispose a patient to congestive complications in the deep or superficial venous system. Preoperative venous studies are therefore useful when selecting recipient veins for FTT and help guide whether the superficial or deep venous system should be used. At our institution, venous duplex ultrasonography detected venous insufficiency (defined as <0.5 seconds of reflux) in 39% of patients undergoing FTT for lower extremity wounds [4]. Deep vein thrombosis requiring anticoagulation was identified in 6.78% of patients [4].
7.3.5 Thrombophilia Assessment
While the literature demonstrates that FTT can be performed with a high success rate in hypercoagulable patients [5], patients with hereditary or acquired factors for thrombophilia are at increased risk of microvascular thrombosis and subsequent flap failure with high rates of nonsalvageability [6,7,8]. Preoperative workup should include a thorough thrombophilia screening to assess propensity for perioperative flap thrombosis, optimize individual anticoagulation protocol, and subsequently obtain hematology consultation, if necessary, prior to surgery [3, 5, 6].
In our practice, all patients are screened for potential thrombophilia via thorough history taking and a preoperative thrombophilia panel [8]. Patients should be asked about any personal or familial history of blood clots, use of blood thinners, previous miscarriage, as well as any diagnoses of clotting disorders, autoimmune disease, and/or purpura fulminans [8]. In addition to complete blood count (CBC), prothrombin time (PT), and partial thromboplastin time (PTT), a comprehensive laboratory workup includes testing for antiphospholipid antibodies; activity levels for antithrombin III, protein C, and protein S; homocysteine and factor VIII levels; genotypes for factor V Leiden G1691A and prothrombin G20210A; testing for MTHFR polymorphisms (A1298C and C677T); and testing for the 4G/5G polymorphism of the plasminogen activator inhibitor-1 (PAI-1) gene [8]. Implementation of this preoperative assessment in our practice revealed that 61% of patients undergoing FTT for lower extremity salvage had at least one thrombophilic trait; 20% of patients were found to have three or more separate diagnoses.
Patients with either known or newly detected thrombophilia should receive a hematology consult to assist with preoperative risk stratification and anticoagulation regimen optimization. Implementation of a risk-stratified anticoagulation algorithm in our practice resulted in lower rates of total (3.0 vs. 19.0%) and partial (10.0 vs. 37.0%) flap loss in the risk-stratified group when compared to non-stratified controls. Successful limb salvage in the setting of postoperative thrombosis was 0% in both groups, reiterating the risk of nonsalvageability in thrombophilic patients who develop thrombosis postoperatively and reinforcing the potential benefits of a risk-stratified anticoagulation protocol [6].
7.3.6 Biomechanical Exam
All patients should undergo biomechanical examination to identify any mechanical issues contributing to wound formation and persistence. If left unaddressed, such issues may lead to wound recurrence. A thorough biomechanical exam is of critical importance in patients with diabetic peripheral neuropathy, as these patients often have altered plantar pressure and stance times [16]. In our practice, we routinely address equinus gait with Achilles tendon lengthening.
7.3.7 Transcutaneous Oximetry
Transcutaneous oximetry (TcPO2) is a noninvasive method that can be used as an adjunctive clinical tool to guide the selection between local wound management and surgical reconstruction. It functions by measuring capillary oxygen content through electrodes placed on the skin. TcPO2 is a valuable tool in determining the likelihood of wound healing in diabetic foot ulcers, with a substantial diagnostic odds ratio (DOR) of 15.8, compared to 1.0 for ankle-brachial index (ABI) [17]. Furthermore, ABI is inaccurate in the presence of arterial calcinosis and toe-brachial index is inappropriate in the presence of an existing ulcer or amputation, making TcPO2 particularly helpful in these cases [17]. A TcPO2 value ≥25 mmHg generally indicates adequate perfusion and significantly improves odds of wound healing [18, 19]. In one study, all wounds with TcPO2 ≥ 40 mmHg achieved healing, while those with measurements < 10 mmHg failed to heal [19]. In addition to compromised healing, low (< 25 mmHg) TcPO2 measures have also been shown to more than double one’s risk of mortality at 1 year [20]. TcPO2 is also significantly correlated with ulcer size and Wagner ulcer grade [21].
The utility of TcPO2 in predicting amputation is less clear, with a DOR of 4.4 compared to 2.9 for ABI [17]. A large prospective cohort by Boyko et al. found that TcPO2 did not significantly correlate with overall amputation rates [22]. Although the risk of amputation based on TcPO2 is inconclusive, it can be used to guide selection of amputation site and should read ≥ 20 mmHg, which confers an 80% chance of wound healing [23, 24].
7.3.8 Thermograms
Thermoanalysis has emerged as an adjunctive method in the early prediction of diabetic foot complications and can thus guide appropriate interventions. Amputations in the diabetic patient are often due to ulcers on the plantar foot, which is prone to ischemic and neuropathic pathophysiology [25]. Infrared thermoanalysis is a fast and noninvasive method used to visualize temperature distribution of the plantar foot without subjecting the patient to radiation [26, 27]. Healthy patients exhibit mirrored symmetry of temperature distribution across both feet, with temperature hottest at the medial longitudinal arch and decreasing distally along the plantar foot [28]. Multiple methods of thermogram analysis exist. Asymmetric temperature analysis is the most common method and assesses for mirrored symmetry of temperature distribution in both feet, with asymmetry suggesting disease [27]. A colder foot on exam may suggest compromised local autonomic control, placing that tissue at risk of ischemic ulceration and warranting further investigation with vascular ultrasound [25, 28]. Contrarily, hot spots may signify areas of inflammation; however, many diabetics will show increased plantar temperatures bilaterally [25]. Temperature distribution analysis is less common and assesses plantar temperature within each individual foot. However, distribution patterns between diabetic patients may be irregular [27]. Nonetheless, evaluation may reveal decreased temperature under the first metatarsal head, fifth metatarsal head, the heel, or the big toe [26]. Thermograms are limited by subjective analysis and susceptibility to the external environment and are most useful for clinical correlation [27].
7.3.9 Considerations for Transplant Patients
Existing evidence demonstrates that free tissue transfer can be successfully performed in patients who have undergone solid organ transplantation and require lifelong immunosuppression [29, 30]. This is particularly important in the diabetic population, as many of these patients may develop chronic kidney disease and eventually require kidney transplantation. Despite demonstrated success of microvascular FTT in this patient population, chronic immunosuppression may put these patients at increased risk of complications such as flap thrombosis, infection, and delayed wound healing [29, 30]. Many immunosuppressive agents can also cause hypertension and thrombocytopenia, which may increase the risk of hematoma formation [30]. Immunosuppressive agents may also exacerbate the atherosclerotic-predisposing effects of diabetes, hypertension, and hyperlipidemia [31], highlighting the importance of aggressive optimization of these comorbidities and preoperative vascular studies in this patient population. Multidisciplinary collaboration between the reconstructive surgeon and the surgical transplant team is essential in ensuring that both the transplanted organ and transferred tissue have adequate monitoring postoperatively [30].
7.3.10 Infection Control
Prior to proceeding with reconstructive surgery, the wound bed must be clear of infection. Serial debridement procedures performed in conjunction with culture-driven antibiotic therapy should continue until negative cultures are obtained. A detailed description of infection control is described in Chaps. 4 and 5 (“Understanding Infection” and “Understanding Wound Bed Preparation,” respectively) of this textbook.
7.4 Conclusion
Comprehensive surgical preparation is key in achieving successful diabetic limb reconstruction. Aggressive management of comorbidities, particularly perioperative blood glucose levels in the patient with diabetes, is essential for reducing the risk of dehiscence and necessity for reoperation. Ancillary clinical testing such as vascular studies, hypercoagulable screening, thermoanalysis, transcutaneous oximetry, and a biomechanical exam can all add vital information necessary for planning and executing the most optimal reconstruction for the presenting patient.
7.5 Case
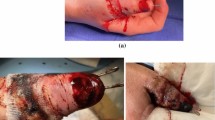
A 43-year-old male with a past medical history significant for type I diabetes mellitus initially presented with a fissure on the left heel (Fig. 7.2). Over the course of the next 3 weeks, the wound developed erythema, swelling, warmth, and sanguineous drainage (Fig. 7.3). The patient also reported severe constant, deep pain of the left lower extremity which he rated as 9+/10 in severity and limited his ability to ambulate. When he presented to the emergency room 3 weeks after initial presentation, the patient was complaining of subjective fever, chills, malaise, and worsening pain and redness of the area. On exam, the patient was afebrile (36.2) but was hypotensive (97/64). Exam of the left heel ulcer revealed fluctuant eschar and purulent drainage (Fig. 7.3b). The area was warm, swollen, and tender to palpation, and erythema was noted, extending distally to the dorsal foot and proximally up the posterior calf. X-ray and CT of the limb were negative for osteomyelitis and subcutaneous emphysema, but the patient was admitted for emergent surgical exploration and debridement due to concern for necrotizing fasciitis.
In the operating room (OR), excision of the ulcer down to fascia was performed, as well as complete excision of the area over the peroneal tendons (Fig. 7.4). The patient returned to the OR on postoperative day one (POD1) for repeat debridement to ensure complete removal of infected tissue. This procedure included partial excision of the lateral wall of the calcaneus bone. The resulting open defect was substantial, and it was determined that definitive closure with FTT may be required (Fig. 7.5). We then proceeded with our management algorithm as outlined above in preparation for free flap reconstruction.
-
Two additional debridement procedures were performed until cultures were negative and a clean wound bed was achieved. Figure 7.6 depicts the clean wound prior to FTT.
-
Vascular surgery was consulted for arteriogram, which was performed 1 week prior to FTT. Findings were significant for an approximately 8–10 cm segment of high-grade subsegmental stenoses of the posterior tibial artery. This finding in conjunction with the location of the patient’s wound necessitated revascularization of the posterior tibial artery with percutaneous transluminal angioplasty.
-
Vascular surgery was also consulted for venous studies. Venous duplex was performed 5 days prior to FTT, and findings were significant for reflux in the external iliac vein and a non-occlusive thrombus in the small saphenous vein at the distal calf.
-
Hypercoagulability studies revealed that the patient had neither the Factor V Leiden G1691A mutation nor the prothrombin G20210A mutation nor the MTHFR polymorphisms (A1298C or C677T). Screening for antiphospholipid antibodies and lupus anticoagulants was also negative. His protein S activity was slightly low (62, normal 65–140), as was his protein C activity (63, normal 70–130). His antithrombin III activity was also slightly low (76, normal 80–125). Homocysteine was within normal limits (6.1, normal 3.2–10.7). The patient’s hypercoagulability studies did not necessitate further workup or specialized intraoperative management.
-
Internal medicine was consulted for optimization of blood glucose levels. On admission, the patient’s hemoglobin A1c was 10.7% and he had glucometer readings as high as 326 mg/dL several days prior to FTT. The patient was a nonsmoker.
At the time of FTT, the defect requiring coverage measured 13 × 9 cm (Fig. 7.6). Closure was performed with an anterolateral thigh (ALT) perforator fasciocutaneous flap (Fig. 7.7) with end-to-side anastomosis to the anterior tibial artery and two venous anastomoses (Fig. 7.8). He was discharged 11 days after FTT. His flap donor and recipient sites are now well healed (Fig. 7.9a, b), and the patient is able to ambulate.
References
Endara M, Masden D, Goldstein J, Gondek S, Steinberg J, Attinger C. The role of chronic and perioperative glucose management in high-risk surgical closures: a case for tighter glycemic control. Plast Reconstr Surg. 2013;132:996–1004. https://doi.org/10.1097/PRS.0b013e31829fe119.
Attinger CE, Evans KK, Bulan E, Blume P, Cooper P. Angiosomes of the foot and ankle and clinical implications for limb salvage: reconstruction, incisions, and revascularization. Plast Reconstr Surg. 2006;117(7 SUPPL):261–93. https://doi.org/10.1097/01.prs.0000222582.84385.54.
Black CK, Kotha VS, Fan KL, Ragothaman K, Attinger CE, Evans KK. Pedicled and free tissue transfers. Clin Podiatr Med Surg. 2019; https://doi.org/10.1016/j.cpm.2019.03.002.
Janhofer DE, Lakhiani C, Kim PJ, et al. The utility of preoperative venous testing for lower extremity flap planning in patients with lower extremity wounds. Plast Reconstr Surg. 2020;145(1):164e–71e. https://doi.org/10.1097/PRS.0000000000006384.
Kotamarti VS, Shiah E, Rezak KM, Patel A, Ricci JA. Does anticoagulation improve flap outcomes in hypercoagulable patients? A systematic review. J Reconstr Microsurg. 2020; https://doi.org/10.1055/s-0039-3400531.
DeFazio M, Economides J, Anghel E, Tefera E, Evans K. Lower extremity free tissue transfer in the setting of thrombophilia: analysis of perioperative anticoagulation protocols and predictors of flap failure. J Reconstr Microsurg. 2019;35(04):270–86. https://doi.org/10.1055/s-0038-1675145.
Wang TY, Serletti JM, Cuker A, et al. Free tissue transfer in the hypercoagulable patient: a review of 58 flaps. Plast Reconstr Surg. 2012;129(2):443–53. https://doi.org/10.1097/PRS.0b013e31823aec4d.
Defazio MV, Hung RWY, Han KD, Bunting HA, Evans KK. Lower extremity flap salvage in thrombophilic patients: managing expectations in the setting of microvascular thrombosis. J Reconstr Microsurg. 2016; https://doi.org/10.1055/s-0035-1571249.
Levy N, Dhatariya K. Pre-operative optimisation of the surgical patient with diagnosed and undiagnosed diabetes: a practical review. Anaesthesia. 2019;74:58–66. https://doi.org/10.1111/anae.14510.
Lumb AB. Pre-operative respiratory optimisation: an expert review. Anaesthesia. 2019;74:43–8. https://doi.org/10.1111/anae.14508.
Gillis C, Wischmeyer PE. Pre-operative nutrition and the elective surgical patient: why, how and what? Anaesthesia. 2019;74:27–35. https://doi.org/10.1111/anae.14506.
Lee JL, Oh ES, Lee RW, Finucane TE. Serum albumin and Prealbumin in calorically restricted, nondiseased individuals: a systematic review. Am J Med. 2015;128(9):1023.e1–1023.e22. https://doi.org/10.1016/j.amjmed.2015.03.032.
Kim KG, Mishu M, Zolper EG, Bhardwaj P, Rogers A, Dekker PK, Fan KL, Evans KK. Nutritional markers for predicting lower extremity free tissue transfer outcomes in the chronic wound population. Microsurgery. 2021 Aug 6. https://doi.org/10.1002/micr.30794. Epub ahead of print. PMID: 34357655.
Janhofer DE, Lakhiani C, Kim PJ, et al. The utility of preoperative arteriography for free flap planning in patients with chronic lower extremity wounds. Plast Reconstr Surg. 2019;143(2):604–13. https://doi.org/10.1097/PRS.0000000000005265.
Fischer JP, Wink JD, Nelson JA, et al. A retrospective review of outcomes and flap selection in free tissue transfers for complex lower extremity reconstruction. J Reconstr Microsurg. 2013; https://doi.org/10.1055/s-0033-1343952.
Fernando M, Crowther R, Lazzarini P, et al. Biomechanical characteristics of peripheral diabetic neuropathy: a systematic review and meta-analysis of findings from the gait cycle, muscle activity and dynamic barefoot plantar pressure. Clin Biomech. 2013;28(8):831–45. https://doi.org/10.1016/j.clinbiomech.2013.08.004.
Wang Z, Hasan R, Firwana B, et al. A systematic review and meta-analysis of tests to predict wound healing in diabetic foot. J Vasc Surg. 2016;63(2):29S–36S.e2. https://doi.org/10.1016/j.jvs.2015.10.004.
Brownrigg JRW, Hinchliffe RJ, Apelqvist J, et al. Performance of prognostic markers in the prediction of wound healing or amputation among patients with foot ulcers in diabetes: a systematic review. Diabetes Metab Res Rev. 2016;32:128–35. https://doi.org/10.1002/dmrr.2704.
Yang C, Weng H, Chen L, et al. Transcutaneous oxygen pressure measurement in diabetic foot ulcers mean values and cut-point for wound healing. J Wound Ostomy Cont Nurs. 2013;40(6):585–9. https://doi.org/10.1097/WON.0b013e3182a9a7bf.
Fagher K, Katzman P, Löndahl M. Transcutaneous oxygen pressure as a predictor for short-term survival in patients with type 2 diabetes and foot ulcers: a comparison with ankle–brachial index and toe blood pressure. Acta Diabetol. 2018;55(8):781–8. https://doi.org/10.1007/s00592-018-1145-8.
Zubair M, Ahmad J. Transcutaneous oxygen pressure (TcPO 2) and ulcer outcome in diabetic patients: is there any correlation? Diabetes Metab Syndr Clin Res Rev. 2019;13(2):953–8. https://doi.org/10.1016/j.dsx.2018.12.008.
Boyko EJ, Seelig AD, Ahroni JH. Limb- and person-level risk factors for lower-limb amputation in the prospective Seattle diabetic foot study. Diabetes Care. 2018;41(4):891–8. https://doi.org/10.2337/dc17-2210.
Ladurner R, Küper M, Königsrainer I, et al. Predictive value of routine transcutaneous tissue oxygen tension (tcpo2) measurement for the risk of non-healing and amputation in diabetic foot ulcer patients with non-palpable pedal pulses. Med Sci Monit. 2010;16(6):273–7.
Wütschert R, Bounameaux H. Determination of amputation level in ischemic limbs: reappraisal of the measurement of TcPO2. Diabetes Care. 1997;20(8):1315–8. https://doi.org/10.2337/diacare.20.8.1315.
Renero-C F-J. The abrupt temperature changes in the plantar skin thermogram of the diabetic patient: looking in to prevent the insidious ulcers. Diabet Foot Ankle. 2018;9(1):1430950. https://doi.org/10.1080/2000625X.2018.1430950.
Astasio-Picado Á, Martínez EE, Gómez-Martín B. Comparison of thermal foot maps between diabetic patients with neuropathic, vascular, neurovascular, and no complications. Curr Diabetes Rev. 2019;15(6):503–9. https://doi.org/10.2174/1573399815666190206160711.
Adam M, Ng EYK, Tan JH, Heng ML, Tong JWK, Acharya UR. Computer aided diagnosis of diabetic foot using infrared thermography: a review. Comput Biol Med. 2017;91:326–36. https://doi.org/10.1016/j.compbiomed.2017.10.030.
Renero-C F-J. The thermoregulation of healthy individuals, overweight–obese, and diabetic from the plantar skin thermogram: a clue to predict the diabetic foot. Diabet Foot Ankle. 2017;8(1):1361298. https://doi.org/10.1080/2000625X.2017.1361298.
Sbitany H, Xu X, Hansen SL, Young DM, Hoffman WY. The effects of immunosuppressive medications on outcomes in microvascular free tissue transfer. Plast Reconstr Surg. 2014; https://doi.org/10.1097/PRS.0000000000000012.
Lee AB, Dupin CL, Colen L, Jones NF, May JW, Chiu ES. Microvascular free tissue transfer in organ transplantation patients: is it safe? Plast Reconstr Surg. 2008; https://doi.org/10.1097/PRS.0b013e31817123b0.
Craig-Schapiro R, Nejim B, Arhuidese I, Malas MB. Aggressive infrainguinal revascularization in renal transplant patients is justifiable. Am J Transplant. 2018; https://doi.org/10.1111/ajt.14636.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Ethics declarations
There are no financial disclosures, commercial associations, or any other conditions posing a conflict of interest to report for any of the above authors.
Rights and permissions
Copyright information
© 2022 The Editor(s) (if applicable) and The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Dekker, P.K., Kim, K.G., Fan, K.L., Evans, K.K. (2022). When and How to Prepare for Surgery. In: Hong, J.P., Suh, H. (eds) Diabetic Foot Reconstruction. Springer, Singapore. https://doi.org/10.1007/978-981-16-9816-3_7
Download citation
DOI: https://doi.org/10.1007/978-981-16-9816-3_7
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-16-9815-6
Online ISBN: 978-981-16-9816-3
eBook Packages: MedicineMedicine (R0)