Abstract
The water contact angle is a surface analytical technique that provides invaluable information regarding a surface’s chemical and physical characteristics. The surface wetting qualities of the material are achieved by calculating the contact angle of a water drop on a surface. Research regarding these physicochemical interactions is important as minimal changes to the surface, in turn, affect the surface energy notably shown in the observed water contact angle. Changes in the water contact angle also hint at physical and/or chemical alteration of the surface. This chapter introduces the water contact angle method to assess the wettability of surfaces and provides information regarding the hydrophobic or hydrophilic characteristics of different surfaces. The chapter also provides the history, mechanism of operation, advantages, disadvantages of this analysis, and applications of the technique centered on biosensors. The last section of the current chapter describes some of the common errors when using this technique and the possible causes and solutions.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
6.1 Water Contact Angle Analysis for Material Characterization
6.1.1 History of Water Contact Angle Analysis
Water contact angle (WCA) is the main data obtained from wettability studies, identifying the wetting degree in the interaction among a solid and a liquid. The angle is formed at the intersection of the liquid of interest and the particular solid surface, thus projecting the properties and structure of the material’s surface that is in contact with the droplet. The theory and implementation of contact angles have undergone various developments since the 1800s (Fig. 6.1) [1, 2].
Thomas Young, a British polymath and physician, was the first scholar to describe a specific contact angle in a three-phase system analysis in 1805. By examining the adhesion of a liquid to a solid, the correlation between the contact angle and the surface tension for a stable solid was established. This was applied to liquid/vapor, liquid/liquid, and solid/liquid interactions. During the next years, the behavior of liquids on substrate surfaces continued to be researched and has presented new directions to understanding contact angles on the surfaces of various materials [3].
Some months after the publication of Young’s essay about fluid cohesion in 1805, the capillary phenomenon was observed and explored by numerous academics. Nonetheless, at the time there was no reference to contact angle (commonly referred to as angle or angle of contact in early research). Thereafter, Lord Rayleigh in 1890 identified that a liquid droplet can present multiple constant contact angles on a solid surface, however, further analysis on this observation was not provided [4].
Agnes Pockels, one of the collaborators of Lord Rayleigh, gained a deep understanding of the presence of maximum and minimum contact angles, namely hydrophobic and hydrophilic components. In 1914, Pockels published an article with the reported maximum and minimum angles for numerous liquids on solids, discussed their viable applications in the analysis of solid surfaces, and proposed some extensions to the work [1].
In the late 1930s, the effect of liquid film on a solid surface and the impact on the deviation of surface energy was recognized and discussed by Donald H. Bangham and R. I. Razouk, A. N. Frumkin, and B. V. Derjaguin. The work of Frumkin and Derjaguin suggested that a “microscopic” contact angle is made by the molecular forces of the interfaces, and macroscopic contact angles are additionally dependent on some external forces. These scientists also developed the wetting theory which explains the disjoining pressure in a liquid film and the repercussions on the contact angle. In the late nineteenth century, the detection of contact angle hysteresis and related highest and lowest contact angles initiated a pursuit for variables affecting contact angle measurements. Surface roughness and heterogeneity were the foremost aspects for describing contact angle hysteresis in solid interactions [1].
Wenzel [5] in 1936 and later Cassie [6] in 1944 proposed equations to explain the equilibrium contact angle for a liquid on a rough surface, depending on the magnitude of the liquid/solid contact area below the drop. On the one hand, the Wenzel equation is utilized when the drop size can completely penetrate the roughness grooves. However, Cassie’s equation describes when a liquid interacts with a heterogeneous solid surface, while the Cassie–Baxter equation applies to apparent contact angles for rough and porous surfaces with trapped air underneath [1, 6]. However, in 2007, the publication of Lichao Gao and Thomas J. McCarty titled “How Wenzel and Cassie were wrong” analytically demonstrated that contact areas beneath the droplet were independent of the contact angle, but three-phase contact lines are efficient in determining wettability. This opened a discussion regarding the importance of contact lines in contact angle analysis on heterogeneous and irregular surfaces [7, 8].
From the beginning of the twentieth century, experimental advancements of contact angles were investigated for samples of different surface quality. It was, nevertheless, not before the 1960s and 1970s that the impacts of solid surface heterogeneity and roughness on contact angles and contact angle hysteresis gained methodical attention. Rulon E. Johnson Jr. and Robert H. Dettre (1964), and subsequently, A. W. Neumann and R. J. Good (1972), pioneered the modeling of contact angles on heterogeneous and rough surfaces, an advancement that remained rather limited to symmetrical structures. In the 1960s, the theoretical analysis of energetic states of liquids on heterogeneous and rough surfaces begun and continued through the rest of the twentieth century, which provided valuable insights into the knowledge of contact angles. However, certain misleading judgments were further drawn such as advancing contact angles representing or resembling equilibrium contact angles, when they actually differ significantly [1].
In 1969, Johnson and Dettre suggested that if adequate energy was provided for a liquid to subdue the energy blocks while spreading and running amid the metastable states, then both maximum and minimum contact angles may converge to a constant angle. This proposal was briefly investigated until the 1990s and 2000s [1]. It was concluded that the relaxation of liquid on a solid surface to a more stable state at about the weakest system energy is due to external energy through mechanical or acoustic oscillations throughout contact angle measurements [1].
The use of words such as superhydrophobicity, superhydrophilicity, and superwetting dates back to 1991 and 1996 when H. J. Busscher et al. and Tomohiro Onda et al. published the findings on ion-etched Teflon surfaces and wettability of fractal (rough) surfaces, sequentially [1].
In 2009, the centrifugal adhesion balance introduced some applications of centrifugal applications and gravitational forces to cause various regular and lateral force combinations for direct adhesion recording among a fluid drop and a solid surface [1, 9]. Moreover, a microbalance equipped with software was developed to automatically record the interplays of the liquid drop with the surface as a function of the surface position [1].
6.1.2 Mechanism of Operation of Water Contact Angle Analysis
6.1.2.1 Surface Tension and Contact Angle
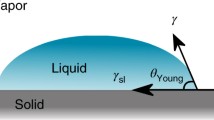
Geometrically, the contact angle is defined by using a tangent line from the contact position along with the liquid–vapor interface in the droplet profile. In another word, WCA is defined as the angle made by the intersection of the liquid–solid interface and the liquid–vapor interface. The term “Three-phase contact line” is commonly used when solid, liquid, and vapor coexist (Fig. 6.2) [10]. For that reason, this technique is often referred to as a water-in-air contact angle.
A large contact angle is recognized when the liquid beads on the surface and a small contact angle is recognized when the liquid spreads over the surface. When a contact angle is smaller than 90°, it indicates that the surface is hydrophilic, and the fluid spreads across a larger area of the surface (Fig. 6.3). However, when contact angles are greater than 90°, it suggests that the surface is hydrophobic, and therefore, the fluid minimizes its contact with the surface and forms a compact liquid droplet. When the droplet turns into a flat puddle, a complete wetting happens, and therefore, the contact angle is ~ 0° which marks a superhydrophilic surface. For superhydrophobic surfaces, however, the water contact angles are regularly larger than 150° representing minimal contact among the surface and the liquid, which explains the lotus effect. The lotus effect is a self-cleaning phenomenon in living beings where rolling water drops on the surface collect particles due to the interplay between the water droplet and the surface nanoscale architecture [11, 12].
Generally, the surface tension of the liquid will take the form of a liquid droplet. Due to the spherical form of small water droplets, the least surface area for a fixed volume is acquired. This intermolecular force to contract the liquid surface into the minimum surface area possible is called surface tension which determines the shape of the liquid droplets. In essence, some external forces including gravity can affect the shape and distort the droplet [3]. Consequently, the contact angle is defined by a mixture of surface tension and external forces including gravity. Thomas Young, for the first time, theoretically described that the contact angle of a liquid drop on an optimal solid surface is defined by the mechanical equilibrium of the drop beneath the action of three interfacial tensions (Eq. 6.1) [3]:
where \({\gamma }_{\text{lv}}\), \({\gamma }_{\text{sv}}\), and \({\gamma }_{\text{sl}}\) denote the liquid–vapor, solid–vapor, and solid–liquid intermolecular tensions, sequentially, and θY is the contact angle. Equation 6.1 is related to Young’s equation, and θY is Young’s contact angle [3].
6.1.2.2 Contact Angle Measurement
The sessile drop method is the renowned method for analyzing the contact angle and directly measuring the tangent angle at a three-phase equilibrium interfacial position. By viewing the drop profile on flat surfaces, the wetting property can be defined by direct recording of contact angle. In a study presented in 1946, W. C. Bigelow et al. claimed that a telescope-goniometer was able to observe the liquid drop profile positioned across the smooth surface and calculate the angle between the three interfacial tensions [10]. Nowadays, an image of the adhered bubble could be transmitted onto a screen, the edges outlined, and the angle calculated via a computer program. The graphical illustration of the sessile drop method is depicted in Fig. 6.4. For measuring the contact angle, the stage for the sample needs to be flattened, so that the droplet does not move during deposition. Subsequently, a droplet of liquid is dispensed onto the surface. A source of light illuminates the droplet from behind, and therefore, an image is projected onto a screen and recorded by a camera for further analysis using program software. The contact angle analysis is conducted by the software, while the images and/or recordings of the phenomenon are stored on the connected computer. Using comparatively high magnifications improve the accuracy of the method and allows the detailed exploration of the intersection between the solid surface and the drop profile [13]. The technique is simple and straightforward in execution and requires a small surface area of substrates and small quantities of liquid to perform the analysis. However, impurities and irregularities on the surface can largely influence the analysis outcomes [10].
The accuracy and reproducibility of the contact angle measurements are mostly affected by the designation of the tangent line and the appropriateness of the operator. Thus, it is vital to define instructions for operators to follow. Additionally, it is recommended that the telescope be shifted slightly down (1–2 degrees) off the horizon so that the proximal edge of the stage is out of focus and a portion of the profile reflected by the sample’s surface is focused. This limits the production of a hazy liquid substrate contact line in the profile. A light source in the background is regularly utilized to support the view, while a distinct light source is selected to inhibit the unfavorable heating of the liquid or specimen. To verify an exact contact angle, it is advised to gradually increase the sessile drop to a diameter of nearly 5 mm employing a micrometer syringe with narrow-gauge stainless steel. In order to avoid distortion of the drop profile, the needle size must be small. Since the drop may be asymmetrical, it is recommended to estimate the contact angles on both corners of the liquid drop and to use the average. In the case of a comparatively large substrate, contact angles should be measured at different points to give an average value that is demonstrative of the complete surface. A microscope detects the interaction between the drop profile and the sheath surface, while the goniometer analyzes the contact angle [14]. Typically, the contact angle of the covering layer is defined by the sessile drop methods in its dry state. Nevertheless, the contact angle of the surface might vary with the neighboring environment, and therefore, the contact angle of a dry membrane might be different from the contact angle of a wet membrane [10].
With the evolution of the theories of wetting procedures, a variety of methods have surged to record contact angles. Some of these techniques are summarized in Table 6.1. In the first group, the direct group, measurements are based on optical imaging techniques, whereas in the second group, the indirect group, the contact angles are determined through force balance [10, 12, 15].
6.1.3 Advantages and Disadvantages of Water Contact Angle Analysis
As previously mentioned, measuring the tangent angle at the three-phase contact point is the commonly applied approach for calculating contact angle. This method is advantageous due to its simplicity of operation. Moreover, it offers information with respect to surface uniformity, porosity, and chemistry without the need for a large sample size. However, considering the quantity of the liquid and substrate, there is a comparatively high risk for error and the intervention of impurities in measurements [10, 16, 17]. Additionally, for materials with a great area, computations at various points are needed, therefore, tedious [17]. Also, the measurement accuracy and reproducibility depend on the reliability of the operator when determining the tangent line which could cause notable error and divergence among various users. Thus, specific guidelines for operators are necessary [10, 17]. Some of the advantages and disadvantages of WCA are detailed in Table 6.2.
6.1.4 Applications of Water Contact Angle Analysis
Surface properties dictate the performance of the materials in their intended applications. For the determination of the hydrophilicity or hydrophobicity of a surface, water contact angle measurement is the customary technique employed to analyze the wettability of a solid material [12]. In some emerging areas of biomedical application, advanced materials with unique wettability properties are needed to evoke desired bioactivities. Therefore, controlling and measuring the surface wettability of the designed materials are of significant importance [20].
An example of a material using specific wettability are platforms patterned with extreme differences in wettability that achieve the selective and sensitive detection of biomolecules by combining different approaches such as surface-enhanced Raman scattering (SERS), fluorescence, colorimetric, electrochemical, and mass spectrometry [21]. This is exemplified in the work undertaken by Sawsan Almohammed et al. where a platform for SERS-based sensing was developed [22]. In order to create wettability gradients and align peptide nanotubes, UV/ozone exposure served as the pattering technique. Silver nanoparticles were incorporated into the peptide nanotubes substrate to test the eligibility of the device to detect an analyte molecule through SERS [22].
Another approach for the detection of biomolecules is the fluorescence detection technique. Fluorescence is a discharge event from a molecule following the beginning electron excitation in a light-absorption procedure [21]. An example is a study carried out by Li Jiang et al. in which different surfaces were modified to develop an appropriate substrate for detecting toxoplasmosis, rubella virus, cytomegalovirus, and herpes infections [23]. Hydrophilic microwells were used to confine analytes, while hydrophobic substrates acted as a barrier for the restrictive spread of microdroplets. Contact angle measurements served to analyze the modified surfaces and understand their nature upon surface treatment (Fig. 6.5). Based on the measurements, it was concluded that agarose-modified slides presented a higher potential for binding to proteins. The developed platform enhances fluorescence sensing and achieves an effective biorecognition procedure. With this research, signal amplification assay with a cyanine dye labeled biotin-streptavidin and nanogold labeling was assessed, revealing a higher sensitivity of the method (Fig. 6.5) [23].

Reproduced (or Adapted) with permission [23], ©2008, Elsevier
Wettable fluorescence detection. a Measurements of contact angles on modified surfaces. b Fluorescence detection enhancement of immunoglobulin M antibodies in subjects with potential infections. The arrows indicate the positive samples.
In order to obtain effective biochips, the microarray quality and spot homogeneity are fundamental elements. Yanxia Chen et al. developed a chip with superhydrophilic microwells on a superhydrophobic substrate [24]. A platform of n-octadecyltrichlorosilane (OTS)-modified nanodendritic SiO2-shell coating with low surface energy and a permeable nanostructure, used as a superhydrophobic surface, had high water contact angles of 157.5 ± 1.8°. However, the superhydrophilic microwells, formed after a UV exposure of the substrate to degrade the OTS, showed a contact angle of around 0° (Fig. 6.6). To probe the sensitivity and accuracy of the platform, a drop of fluorescein isothiocyanate solution was set onto the superhydrophilic microwell followed by a hydrophilic glass and a hydrophobic glass. This resulted in a homogeneous fluorescence spot on the superwettable micropattern, thus overcoming a phenomenon of ring-like morphology which causes inhomogeneous signal and unreliable readout. The study confirms that superwettable micropatterns have excellent potentials as biosensing platforms for biomarker detection while controlling the spot homogeneity [24].

Reproduced (or Adapted) with permission [24], ©2018, Elsevier
Superwettable micropattern developed from superhydrophobic substrates patterning with superhydrophilic microwell arrays. a Illustrative image of homogeneous spot deposition following droplet evaporation. b SEM and TEM images of micropattern coating. c Water contact angles for microwall (i) and microwell substrate (ii). d The methylene blue trihydrate microarray droplets in a superwettable micropattern.
Zi-Xia Zhao et al. also demonstrated the importance of the wettability on the surface modification of a material [25]. It is important to note that once the wettability of a material is altered, its bioactivity is changed as well. The authors modified gold surfaces with hydrophobins, small fungal proteins. After the processing of the surface with hydrophobins, the hydrophilicity of the material improved, altering the WCA from 73.8° to 45.3°. Subsequently, hydrophilic proteins were immobilized on the gold electrode surface modified with hydrophobins preserving its bioactivity. The modified electrode was used to fabricate an amperometric choline biosensor showing a highly effective biocatalytic reaction, therefore exhibiting great potentials as a biosensor [25]. In a similar manner, Adeniyi Olugbenga et al. proved that the wettability modification of electrodes can facilitate the formation of biomolecules on the electrode surface and allow high-ordered immobilization of glucose oxidase on the graphene. Thus, the sensitivity of the material increased which is relevant to the fabrication of electrochemical biosensors [26].
6.1.5 Troubleshooting of Wettability Analysis Technique
The wettability in a material provides indirect information about the physical and chemical properties of a surface. For instance, the roughness and the presence of polar surface functional groups over a material surface can greatly impact the surface wettability. The measurement of contact angle of the droplet with the surface determines the wettability of a variety of specimens (e.g., metals, polymers, and carbon platforms). While the technique appears to be simple to operate, common errors may occur during the test mainly due to following wrong procedures. For instance, the accuracy of the data could be affected if the droplet is not dispensed from the middle of the needle, or the sample holder is inclined. Establishing a correct baseline allows the user to acquire an adequate fitted curve. Moreover, the camera might not be well-positioned, or the surface might be highly irregular that would expectedly result in inconsistent measurements. Furthermore, the values of the water contact angle will not be constant if the droplet is not uniform [17]. The light source is also vital in obtaining a clear image or video, hence, reliable data. If the lamp does not operate well, the user may check the power source or change the bulb. However, it is not recommended for the user to attempt at repairing the lamp [27]. Table 6.3 shows detailed troubleshooting for water contact angle measurement for wettability analysis as well as listing possible causes and solutions.
Abbreviations
- NS:
-
Not specific
- OTS:
-
Octadecyl trichlorosilane
- SERS:
-
Surface-enhanced Raman scattering
- UV:
-
Ultraviolet
- WCA:
-
Water contact angle
References
J. W. Drelich et al., Contact angles: history of over 200 years of open questions. Surface Innov. 8 (1–2), 3–27 (2020)
Y. Yuan, R. Lee, Surface science techniques, in Springer Series in Surface Sciences Techniques, vol. 51, no. 1 (2013). https://doi.org/10.1007/978-3-642-34243-1
T. Young, III. An essay on the cohesion of fluids, in Philosophical Transactions of the Royal Society of London, 95th ed. (1805), pp. 65–87
L. Rayleigh, On the tension of water surfaces, clean and contaminated, investigated by the method of ripples. Philos. Mag. Sci. 5 30 (186), 386–400 (1890). https://doi.org/10.1080/14786449008620040
R.N. Wenzel, Resistance of solid surfaces to wetting by water. Ind. Eng. Chem. 28 (8), 988–994 (1936). https://doi.org/10.1021/ie50320a024
B.D. Cassie, S. Baxter, Wettability of porous surfaces. Trans. Faraday Soc. 40, 546–551 (1944)
L. Gao, T.J. Mccarthy, How Wenzel and Cassie were wrong. Langmuir 23(7), 3762–3765 (2007)
J.W. Drelich, Contact angles: From past mistakes to new developments through liquid-solid adhesion measurements. Adv. Coll. Interface. Sci. 267, 1–14 (2019). https://doi.org/10.1016/j.cis.2019.02.002
R. Tadmor, P. Bahadur, A. Leh, H. N’guessan, R. Jaini, L. Dang, Measurement of lateral adhesion forces at the interface between a liquid drop and a substrate. Phys. Rev. Lett. 103 (26), 266101 (2009)
R.S. Hebbar, A.M. Isloor, A.F. Ismail, Contact angle measurement, in Membrane Characterization (2017), pp. 219–255
A. Lafuma, D. Quéré, Superhydrophobic states. Nat. Mater. 2(7), 457–460 (2003)
I. Ahmad, C.W. Kan, A Review on development and applications of bio-inspired superhydrophobic textiles. Materials 9 (11) (2016). https://doi.org/10.3390/ma9110892
T.T. Chau, A review of techniques for measurement of contact angles and their applicability on mineral surfaces. Miner. Eng. 22(3), 213–219 (2009)
L.R. Fisher, Measurement of small contact angles for sessile drops. J. Colloid Interface Sci. 72(2), 200–205 (1979)
J.W. Song, L.W. Fan, Temperature dependence of the contact angle of water: A review of research progress, theoretical understanding, and implications for boiling heat transfer. Adv. Colloid Interface Sci. 288 (2021). https://doi.org/10.1016/j.cis.2020.102339
T. Zhao, L. Jiang, Contact angle measurement of natural materials. Colloids Surf. B 161, 324–330 (2018). https://doi.org/10.1016/j.colsurfb.2017.10.056
T. Huhtamäki, X. Tian, J.T. Korhonen, R.H.A. Ras, Surface-wetting characterization using contact-angle measurements. Nat. Protoc. 13(7), 1521–1538 (2018). https://doi.org/10.1038/s41596-018-0003-z
R.S. Hebbar, A.M. Isloor, A.F. Ismail, Contact angle measurements. Membrane Charact. 219–255 (2017). https://doi.org/10.1016/B978-0-444-63776-5.00012-7
A. Alghunaim, S. Kirdponpattara, B.M.Z. Newby, Techniques for determining contact angle and wettability of powders. Powder Technol. 287, 201–215 (2016). https://doi.org/10.1016/j.powtec.2015.10.002
C. Aparicio, Y. Maazouz, D. Yang, Measuring wettability of biosurfaces at the microscale. Methods Mol. Biol. 811, 163–177 (2012). https://doi.org/10.1007/978-1-61779-388-2_11
T. Xu, L.P. Xu, X. Zhang, S. Wang, Bioinspired superwettable micropatterns for biosensing. Chem. Soc. Rev. 48(12), 3153–3165 (2019). https://doi.org/10.1039/c8cs00915e
S. Almohammed, S.O. Oladapo, K. Ryan, A.L. Kholkin, J.H. Rice, B.J. Rodriguez, Wettability gradient-induced alignment of peptide nanotubes as templates for biosensing applications. RSC Adv. 6(48), 41809–41815 (2016). https://doi.org/10.1039/c6ra05732b
L. Jiang et al., Development of a fluorescent and colorimetric detection methods-based protein microarray for serodiagnosis of TORCH infections. Biosens. Bioelectron. 24, 376–382 (2008). https://doi.org/10.1016/j.bios.2008.04.019
Y. Chen et al., Superwettable microchips with improved spot homogeneity toward sensitive biosensing. Biosensors and Bioelectronics 102 (July 2017), 418–424 (2018). https://doi.org/10.1016/j.bios.2017.11.036
Z. Zhao et al., Self-assembled film of hydrophobins on gold surfaces and its application to electrochemical biosensing. Colloids Surf. B 71, 102–106 (2009). https://doi.org/10.1016/j.colsurfb.2009.01.011
A. Olugbenga et al., Acetylene-sourced CVD-synthesised catalytically active graphene for electrochemical biosensing. Biosens. Bioelectron. 89, 496–504 (2017)
ChemInstruments, Instruction Manual for Contact Angle Meter Model (2005)
J.T. Korhonen, T. Huhtamäki, O. Ikkala, R.H.A. Ras, Reliable measurement of the receding contact angle. Langmuir 29(12), 3858–3863 (2013). https://doi.org/10.1021/la400009m
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Aguilar Meza, I.B., Ortiz Ortega, E., Hosseinian, H., Rodríguez Vera, A., Rosales López, M.J., Hosseini, S. (2022). Characterization Techniques for Wettability Analysis. In: Material Characterization Techniques and Applications. Progress in Optical Science and Photonics, vol 19. Springer, Singapore. https://doi.org/10.1007/978-981-16-9569-8_6
Download citation
DOI: https://doi.org/10.1007/978-981-16-9569-8_6
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-16-9568-1
Online ISBN: 978-981-16-9569-8
eBook Packages: Physics and AstronomyPhysics and Astronomy (R0)