Abstract
The degradation of reprocessing materials to nitric acid corrosion in aqueous spent fuel nuclear reprocessing plants is an unwanted and serious issue. The PUREX (Plutonium URanium EXtraction) process employs head-end treatment of chemical or mechanical decladding followed by the dissolution of spent fuel in different nitric acid concentrations (1–14 M HNO3), feed clarification, and chemical conditions of the solution for solvent extraction. These construction materials demand a high integrity and resistance to corrosion for components like fuel dissolvers, evaporators, waste storage tanks, piping, etc. The materials most commonly used for spent reprocessing plants are austenitic grades of type 304L stainless steels (SS), with restricted trace elements of C, B, Si, P, S, Mo, etc., and AISI type 300 series of stabilized grade, Si-based nitric acid grade SS, along with small quantities of refractory metals like titanium and zirconium alloys. Alternatively, aluminium alloys, Ni, Ti and Zr-based bulk metallic glasses alloys, and recently, oxide dispersion strengthened steels have also been investigated. Similarly, the material degradation due to tribocorrosion, i.e., the synergistic interactions between wear and corrosion of reprocessing materials of type 304L SS, Ti-grade 2, Zr-702 and Zircaloy-4 materials in the nitric acid medium are also briefly addressed. In the nuclear reprocessing environment, the tribocorrosion phenomena are encountered in a continuous rotary dissolver, solvent extraction apparatus, moving parts, and also some frictional or moving components are subjected to scratches or wear- or flow-induced vibrations in a nitric acid medium. Despite its practical importance, little basic research has been devoted to understanding the synergistic mechanism involved in a tribocorrosion system of materials used in nuclear reprocessing plants. Finally, the corrosion acceleration process and factors affecting the structural materials specific to the reprocessing environments in the nitric acid environment are presented. It is essential that providing an understanding of the corrosion degradation processes and its mechanism of structural materials will be useful in minimizing the nitric acid corrosion of reprocessing materials.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
In spent nuclear fuel reprocessing plants, nitric acid is the main process medium used for the separation of fission products, unused uranium and plutonium from irradiated nuclear fuels by the well-proven PUREX (Plutonium Uranium, Reduction, EXtraction) process in the form of uranyl nitrate and plutonium nitrate and also for waste storage involving concentrated nitric acid [1,2,3,4,5]. Nitric acid is one of the most widely used acids in the chemical processing industry, and nitric acid is the second most industrial acid after sulfuric acid [6] It is also used for the production of industrial explosives such as nitroglycerin and trinitrotoluene, dyes, plastics, synthetic fibres, etching, pickling and passivation of metal alloys, and most importantly in spent nuclear fuel reprocessing for the recovery of uranium (PUREX process) [7]. It is anticipated that the PUREX process will continue to play an important role in the future advanced nuclear fuel cycles of both the currently used fuels and those anticipated in the near future [3, 5, 8]. However, nitric acid is strongly oxidizing and highly corrosive in nature to many metals and alloys. Thereby, the plant operating conditions: room temperature (liquid/solvent extraction), intermediate (warm, waste storage tanks, etc.), boiling temperature (dissolver, evaporator, etc.), eluted from spent fuel poses a serious risk of degradation or failure of structural materials or even catastrophic plant failure [1,2,3,4,5, 9, 10]. This demands reprocessing plant materials to high corrosion-resistant components like fuel dissolvers, evaporators, waste storage tanks, piping, etc. [5, 9, 12].
Most structural materials and components in the reprocessing plant are exposed to different nitric acid concentrations, including a boiling condition for dissolution of spent fuel, acid concentration for volume reduction, and acid recovery processes [4,5,6,7]. The reprocessing nitric acid medium contains metallic dissolved ions/species eluted from spent fuel, such as uranium, transuranic elements (TRU: Pu, Np, Am, etc.), and fission products (FP: I, Ru, Rh, Pd, Ce, etc.), cations generated as corrosion products of SS (Fe(III), Cr(III), etc.), insoluble solid platinoids species, (Pd, Rh, Ru, etc.). Most of such oxidizing species resulted in a severely corrosive environment that enhances the corrosivity of the solution [3, 7, 11]. Furthermore, the major factors that influence the corrosion process of structural materials in nitric acid include: solute segregation, thermal history, chemical homogeneity and non-metallic inclusion, impurities, nitric acid concentrations and temperature, dissolved species or oxidizing metal ions, welding, cold work, heat transfer, irradiation, etc., which are important factors [3, 5, 9, 12, 13]. Thereby, materials of construction for handling nitric acid depends on the reprocessing applications, type of process equipment and plant operating conditions, acid environment and concentration, and temperature range, its radioactivity, etc., [9, 12, 13]. The construction materials most commonly used for aqueous reprocessing plants are austenitic grades of stainless steels (SS). Austenitic type 304L SS is the primary structural material used in major components of reprocessing plants, i.e., pipework, vessel, tanks, and equipment for handling nitric acid in the range of 60–70% concentrations [3, 5, 13]. Additionally, nitric acid application materials have to be resistant to transpassive corrosion, intergranular corrosion resistance, active corrosion, vapour phase corrosion, and end grain attack (tunnel corrosion), galvanic, selective corrosion of welds, etc., for austenitic stainless steel [12, 13]. Similarly, dissimilar weld corrosion, condensate and trickling corrosion which are common in titanium, and stress corrosion cracking (SCC) can occur in zirconium alloys exposed to hot >70% HNO3 [3, 9, 12]. Therefore, the construction materials used for reprocessing plant equipment need to be carefully chosen considering the nature of the nitric acid environment, thermal history, alloy compositions, concentrations encounter, etc. Austenitic stainless steel with extra low-carbon and restricted levels of C, Si, P, S, and Mo of AISI type 300 series stainless steel (SS), stabilized austenitic grade or higher Cr, Ni-based also called nitric acid grade SS [Uranus 16, Uranus 65, S1N, etc.] aluminum alloys, and to a lesser extent Ti and Zr based alloys are used as reprocessing materials for handling nitric acid [1,2,3, 9, 12], and recently bulk metallic glasses alloys and clad materials ODS steels have been also investigated. On the other hand, niobium alloys are also being explored as the most promising alternative materials instead of Zr and Ti alloys which have a high susceptibility to localized attacks and environmental cracking [12]. International R&D programmes addressing these challenges and many advanced materials used for the reprocessing plant have to date been proposed. Table 1 provides a summary of the common structural materials used at various acid concentration ranges and temperatures for equipment in nitric acid service [7].
2 Corrosion Issues of Austenitic Stainless Steels for Nitric Acid Applications
AISI type 304L SS are extensively used for the fabrication of vessels, tanks, piping and process equipment in nuclear reprocessing plants. However, in a highly oxidizing nitric acid environment (i.e., higher nitric concentrations (i.e., ≤8 M HNO3), temperatures (i.e., ≤80 °C and boiling condition), or in the presence of oxidizing metal ions or species, eluted from spent fuel such as Mn(VII), Ru(IV), Fe(III), Cr(VI), Ce(IV), etc.), SS are susceptible to severe transpassive or intergranular corrosion (IGC), even if the steels are not sensitized [3, 9, 12,13,14,15]. Figure 1 illustrates the typical schematic anodic polarization curve of the corrosion process of austenitic stainless steel in nitric acid media with or without oxidizing ions [16].
Schematic diagram illustrating the corrosion process of type 304L SS in nitric acid median in presence of oxidizing ions [16]
It is obvious that in spent fuel reprocessing plants, which involve various operations in which nitric acid is used at relatively higher concentrations and temperatures up to boiling point and fission products, failures of components occurred [1,2,3,4,5]. In austenitic SS used in spent nuclear fuel reprocessing plants, most of the corrosion failures are associated to uniform (high corrosion rate), (i) transpassive corrosion, (ii) end-grain attack (or tunnel corrosion in forged SS), (iii) IGC due to segregation of impurities at grain boundaries, and (iv) selective corrosion of welds, localized corrosion (pitting and SCC), vapour phase corrosion, galvanic corrosion, etc. [3, 6, 9, 12,13,14,15]. While some of the more apparent conditions leading to these failures in SS and other alloys structural materials have been known and elucidated, the fact that failure still occurs indicates and clearly shows the need for improvement towards understanding the knowledge on the nitric acid corrosion processes. Hence, proper control of trace elements/impurities of alloy chemical composition, steel-making practices (electro slag remelting, which greatly purifies) the alloy, and in processing parameters optimization of the trace elements is essential to achieve improve corrosion resistance for SS in nitric acid application. This has led to the evolution of extra low-carbon and restricted levels of C, Si, P, S, and Mo of nitric acid grade (NAG) SS with: (i) closely controlled chemical composition of alloying elements by adding Si in relatively higher amounts while adjusting the Cr and Ni content, (ii) modified microstructures by the elimination of the defective or weaker sites, to minimize or suppress welds corrosion, and (iii) enhanced passive film stability and resistance against transpassive dissolution or IGC [3, 9, 13,14,15]. AISI type 304L SS of improved varieties with extra low-carbon and restricted levels of C, B, Si, P, S, and Mo (AISI type 304ELC), with Nb (AISI type 347), with Ti (AISI type 321), or higher Si (AISI type 310L SS) and its equivalent with Nb stabilization and higher Cr content, have been investigated and explored for use in several reprocessing plants worldwide [3, 9, 15]. Typical nitric acid grade stainless steel variants being explored for reprocessing application are shown in Table 2.
In studies on the corrosion resistance results of AISI type 304L stainless steel and nitric acid grade (NAG) type 310L SS in boiling 15.65 M HNO3 (69% HNO3) after 240 h show a much lower corrosion rate in type 310L SS (~0.06 ± 0.012 mm/year) than type 304L SS (~0.18 ± 0.02–0.2 ± 0.001 mm/year) that show severe intergranular corrosion (IGC) attack in type 304L SS than in type 310L SS. The improved corrosion resistance of type 310L SS was attributed to higher Cr, and enrichment of the SiO2 layer that further enhances the Cr2O3 passive film stability [13]. On the other hand, transpassive corrosion is an important corrosion degradation process observed in austenitic SS use in a nuclear fuel reprocessing plant; austenitic SS when exposed to higher temperatures or concentrated nitric acid solutions with and without highly oxidizing ions manifests itself as general corrosion with a preferential attack along grain boundaries of SS (i.e., intergranular corrosion, even though the SS is not sensitized). To minimize the transpassive corrosion of stainless steels in nitric acid involves minimizing the grain boundary segregation of traces impurity elements. This can be achieved by modifying the steel-making process (EB-SAR treatment) that involves the following process [3, 15, 17]:
-
adjusting the chemical composition
-
refining by electron beam melting
-
stabilizing minor impurities through the thermo-mechanical treatment so-called SAR process (strained, aged, and recrystallized).
A new Cr–W–Si–Ni base alloy, also called the RW alloy, is being evolved and designed for inhibiting the transpassive corrosion of elements such as Cr, W, and Si, which enhances oxide film stability film in nitric acid media [17].
3 Titanium and Its Alloys for Nitric Acid Service
Titanium is extensively used for handling and producing nitric acid in commercial and industrial applications where stainless steels have exhibited significant uniform or intergranular attack [3, 7, 12]. Titanium and its alloys exhibit higher corrosion resistance compared to conventional austenitic stainless steels for reprocessing plant equipment (e.g., dissolver, reboilers, condensers, heaters, and thermo wells, etc.) in solutions containing 10–70% HNO3 at temperatures from boiling to 315°F (600 °C) [3, 4]. At boiling temperatures and above, titanium corrosion resistance is sensitive to nitric acid purity. However, unlike stainless steel corrosion in nitric acid, the corrosion resistance of Ti is not adversely affected by temperature, concentration, and oxidizing or dissolved metal ions/species (i.e., Fe3+, Cr6+, Si4+, etc.), but rather results in improved corrosion resistance, as the corrosion product or dissolved Ti4+ ions inhibit corrosion in a highly oxidizing nitric acid environment [3,4,5,6,7,8]. Hence, titanium and its alloys have also been used for nitric acid reactors, recycled nitric acid streams such as reboiler loops, heat exchangers, thermowells, and other equipment employed in producing nitric acid [3, 4, 18,19,20]. Therefore, titanium and its alloys are preferred over stainless steel as the dissolver material for a reprocessing application. Titanium is also a candidate material for handling high-level liquid waste of solidification process equipment for the reprocessing of spent nuclear fuel from light water reactors.
The corrosion resistance of Ti and its alloys is highly dependent on nitric acid purity, and Ti is virtually immune to nitric acid corrosion up to 65% at room temperature [3, 4, 18, 20]. Similarly, in a widely used environment of reprocessing plant conditions, i.e. within 35–65% of boiling nitric acid, titanium exhibits low corrosion rates. Although titanium shows excellent resistance to nitric acid over a wide range of nitric acid concentrations and temperatures, it can not be used in red fuming nitric acid because of the danger of pyrophoric reactions. In addition, the corrosion rate varies between ~0.25 and 2.5 mm/year in red fuming acid conditions attributed to pyrophoric reactions [4, 7, 18,19,20]. More than 1.34% water and less than 6% NO2 concentration (NO2/NO ratio) are the general guidelines to avoid pyrophoric reactions [18, 20].
The CP-Ti plate material corrosion is compared in both AR grade and CR grade HNO3 (Fig. 2). In a CR grade HNO3 medium, the corrosion rate was more in the liquid phase than in the vapour and condensate phases. In contrast, the corrosion rate of plate material in AR grade HNO3 was more in the condensate phase, followed by vapour and liquid phases (Fig. 3). The corrosion results of CP-Ti in both AR grade with and without impurities showed more corrosion in the condensate phase followed by vapour and liquid phases. Upon the addition of impurities in AR grade HNO3, the condensate phase corrosion is significantly lowered from 10.56 mpy to 5.43 mpy [21].
Corrosion rate of CR and AR grade nitric acid with and without impurities [21]
Corrosion rates of Ti–Ta–Nb alloy specimens in heat treated as well as welded conditions after 3-phase corrosion test in 11.5 M HNO3 [1]
Furthermore, in hot solutions (100 °C), pure solutions, or vapor condensates of nitric acid, significant general corrosion and trickling acid condensate attack occur in the concentration range of 35 to 65 wt% [3, 9, 20]. Titanium corrosion in the vapor/condensate “trickling” zone is attributed due to frequent replenishment of acid, avoiding adequate Ti4+ ions for the formation of adherent TiO2 film.
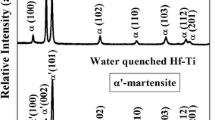
The three-phase corrosion rate results of Ti–5%Ta–1.8%Nb alloy in boiling 11.5 M HNO3 is shown in Fig. 3. The results shows that heat treatment at 1273 K followed by water quenching resulted in better corrosion resistance with low corrosion rates (<1 mpy). This is attributed to beta phases (<5%) in the alpha matrix which is beneficial for dissolving iron and provides homogeneous distribution of alloying elements in order to achieve a low corrosion rate. Similarly, the welded specimen shows lower corrosion rates (<1 mpy) in liquid, vapour, and condensate in boiling 11.5 M HNO3 [1, 3]. Again, the measured corrosion rate for CP-Ti and Ti–5Ta–1.8Nb alloys in boiling liquid, vapour, and condensate phases of 65% nitric acid as shown in Fig. 4 indicates that the Ti–5Ta–1.8Nb alloy shows higher corrosion resistance in all three phases compared to others.
The susceptibility of titanium corrosion to vapour and condensate phase corrosion can be mitigated by alloying with tantalum 5%Ta where corrosion is substantially reduced. Thereby, the addition of refractory metals like Ta and Nb to overcome such corrosion problems are attributed to similar metal ion size, low solubility, and superior oxide film in HNO3 [11, 72]. Hence Ti, Ti–5%Ta, Ti–5%Ta–1.8%Nb, and Ti–4%Nb–4%Zr, etc., were explored as alternate materials of construction for such highly corrosive environments [11, 12, 22].
However, tantalum in Ti–5%Ta–1.8%Nb alloy is expensive, and therefore economically alternatives have also been developed for this purpose, by replacing the costly and scarce tantalum with zirconium without affecting the corrosion properties, such as alloys of Ti–Nb–Zr ternary alloys with high corrosion resistance in all three phases [73, 74]. On the other hand, titanium is also susceptible to corrosion in both weld metal and heat-affected zones in nitric acid [7, 12, 15] which is attributed to preferential corrosion attack at weld regions due to discrete stringers or well-distributed particles becoming deposited on grain boundaries either as iron–titanium intermetallic compounds or as β-phase [12]. To avoid this problem, iron content is maintained below 0.04 wt% in the base metal [5, 12, 15].
The typical alternative materials with higher corrosion resistance for nitric acid application are shown in Table 3. The addition of platinum group metals (PGMs) in Ti (i.e., Pd, Pt, Ru, etc.) for improving corrosion resistance is well known and established. However, the expensive price of the PGMs made this quite unattractive. Lean alloy additions of PGMs such as Pd, Ru, etc., are known to significantly lower the corrosion rate of Ti considerably in both reducing acids and oxidizing media [18,19,20].
This led to the development of ASTM grades with lean levels of noble metals, e.g., ASTM grades 16, 20, 33, and 34 Ti (0.04–0.05 wt% Pd). In such lean PGM Ti alloys, Fig. 5 reveals that the corrosion rates of PGM Ti show a much lower corrosion rate of 0.045 ± 0.002 mm/year in 48 h, 0.055 ± 0.02 mm/year in 96 h, and stabilized to 0.075 ± 0.002 mm/year after 192–240 h [22]. This indicates that lean PGM additions improve corrosion resistance in nitric acid. Moreover, higher corrosion resistance is expected as the addition of noble metals such as Pd and Ru are known to produce a large increase in the passivation ability and improved corrosion resistance [20, 22].
Corrosion rate measured in boiling 15.65 M HNO3 after 240 h exposure of CP Ti and PGM Ti alloys [23]
4 Zirconium and Its Alloys in Nitric Acid Media
Austenitic type 304L stainless steels perform satisfactorily in nitric acid media used in spent nuclear reprocessing plants. Despite its advantages, many corrosion failures in nitric media containing oxidizing ions or species (Cr, VI, Pu VI or higher concentrations of Fe III) are reported attributed to: intergranular corrosion, transpassive corrosion, active corrosion, end grain or tunnelling corrosion, etc.) [3, 9, 12, 24]. Recently, there has been increased interest in zirconium and its alloys for application in nitric acid services. Therefore, an extensive effort to provide a better understanding of the corrosion resistance properties of zirconium and its alloys for nitric acid application have been carried out. Zirconium and its alloys possess high corrosion resistance in severely corrosive nitric acid conditions, and the corrosion rate is typically below 1 mpy up to 70% HNO3 and temperatures up to 260 °C [24,25,26,27].
Zirconium and its alloys exhibit superior resistance to corrosion in high and concentrated nitric acid than stainless steel at elevated temperature; it has been used as structural material successfully for chemical plants for HNO3 or spent nuclear fuel reprocessing plants. Zr and its alloys generally have higher corrosion resistance than stainless steel and titanium [25]. Zirconium and titanium alloys are important constructional materials used as dissolvers and evaporators for spent nuclear fuel plants [3, 26, 27]. Zirconium and its alloys perform much better than stainless steels and possess more advantages in highly aggressive nitric acid environment conditions, such as boiling 65 wt% nitric acid. The corrosion resistance of zirconium in nitric acid is unaffected by temperature, acid concentration, metallic species, or oxidizing ions, and impurities such as seawater, sodium chloride, ferric chloride, iron, etc. [25, 27]. Also seawater, sodium chloride, ferric chloride, and metallic species impurities has no significant effect on the stress corrosion cracking (SCC) susceptibility of zirconium in 70% nitric acid at room temperatures. Unlike titanium, it is unaffected by trickling condensates corrosion above a boiling nitric acid environment [1, 3]. Most of the commercial nuclear reactors (BWR and PHWRs) use zirconium alloys for fuel cladding and pressure tubes for its application in high temperature water-cooled reactors.
Zirconium and its alloys are desirable materials that show excellent corrosion resistance under the most severe corrosive conditions of concentration and temperature, where stainless steel and titanium show corrosion problems for its application in the nitric environment. These materials are highly resistant even to highly aggressive nitric acid corrosion, despite the fact that the operating experience of zirconium materials is less extensive than that of stainless steel and titanium. However, zirconium and its alloys are highly susceptible to local attacks, trace levels of fluoride ions, or fluoride-containing nitric acid and the vapors of chloride-containing nitric acid, and environmental cracking attributed to the low chemical stability and low repassivation rate of oxide film [24,25,26,27]. Furthermore, no enhanced vapor phase corrosion is reported, and they require no dissolved ions to achieve passivity [1, 5]. SCC is induced in zirconium by slow strain rate testing above the azeotrope, and the SSC of zirconium has not been reported in plants operating in nitric acid media [5, 24, 28]. Also, high sustained tensile stresses need to be avoided when zirconium is used to handle at 70% nitric acid at elevated temperatures or >70% nitric acid [28]. Overall, zirconium and its alloys generally show an outstanding corrosion resistance in nitric acid media. In practice, however, for its application in spent nuclear fuel reprocessing facilities certain factors that restrict its application need to be carefully investigated. Some of the important factors include the following [26,27,28,29]:
-
the role of fluoride and chloride content in the nitric acid medium.
-
the heat-transfer surface.
-
nitrogen content and possible insoluble compounds formation despite very low ionic release rates.
-
the role of high sustained tensile stresses on stress corrosion cracking and the SSC acceleration mechanism specific to the reprocessing environments.
The main problems concern [29]:
-
production in the form of the huge size of plates, pipes, and other items.
-
zirconium forming.
-
large-scale zirconium equipment welding.
-
dissimilar welding or joining of zirconium equipment to austenitic stainless steel piping.
In addition, zirconium and its alloys have no complicated microstructures like titanium or stainless steels, and hence, plates, sheets, tubes, and pipes have similar corrosion resistance. Zirconium and its alloys is used at the La Hague facilities for about 80 tons weight and 5500 m of long piping (expected plant life of 30 year) [1, 3]. Rotating dissolvers, acid recovery evaporators and distillation columns, oxalic mother liquor evaporators and heat exchangers, vitrification dust scrubbers, and liquid waste treatment reactors are some of the equipment employed [1, 3, 28]. The corrosion rate of Zircaloy-4 in wrought and weld forms are nearly the same in all three phases (<0.0017 mm/year). Unlike Ti and its alloys, the corrosion rate of Zircaloy-4 and its weldments are similar in all three phases and are not affected by the condensate phase. This is because, unlike Ti, the corrosion resistance of Zircaloy-4 is not influenced by its own ion, i.e., Zircaloy-4 is unaffected by trickling condensates above boiling nitric acid. The corrosion resistance of zirconium is attributed due to the formation of strong and adherent protective ZrO2 oxide formation on the surface, in both liquid phase and condensate/vapour conditions. It is also not sensitive to changes in the concentration of nitric acid, the concentration of its own ion in solution, small amounts of heavy metal ions, and the crystal structure of zirconium [29, 30].
Similarly, studies were carried out on a Zircaloy-4 (Zr-4) mock-up dissolver vessel of several candidate materials of zirconium- and titanium-based alloys in the boiling and vapour phases of simulated dissolver solutions (SDS) containing fission and corrosion product ions in 11.5 M nitric acid. Campaigns up to 5000 h of operation showed that zirconium-702, Zr-4, autoclaved Zr-4, and commercial pure titanium (CP-Ti) exhibited low corrosion rates in the range of 0.08 to 0.23 mm/year (0.003 to 0.009 mils/year). CP-Ti and CPTi weld exhibited marginally higher corrosion rates of 1.0 mm/year (0.04 mils/year), and 1.9 mm/year (0.075 mils/year), respectively; in the vapor phase of the dissolver solution, the lower corrosion rate of 0.08 mm/year (0.003 mils/year) was observed for the autoclaved Zr-4 sample (Fig. 6) [31]. Results on the long term at high temperature, high-concentration Zr-4 test facility of 10 L capacity through 10,000 h of operation in the 11.5 M boiling liquid, vapor, and condensate phases of nitric acid reveal insignificant corrosion rates of 0.025 mm/year (<0,1 mil/year) for Zr-4 (Fig. 7) [32].
Corrosion rate of materials exposed for 2500 h in the boiling liquid and vapor phases of simulated dissolver solutions [31]
Three-phase corrosion rate of Zr-4 samples exposed to boiling liquid, vapor, and condensate phases of nitric acid in hight emperature, high-concentration Zr-4 corrosion testing systems [32]
Therefore, there are no reported issues on the corrosion related failure on its performance; however, the risk of stress corrosion cracking (SCC) of zirconium and its alloys cannot be entirely ignored in extremely oxidizing conditions and also in the presence of fluoride as a contaminant. For this, detail and further investigations are still essential.
5 Corrosion of Oxide Dispersion Strengthened Steels in Nitric Acid Media
Oxide dispersion strengthened (ODS) steels are the most promising class of high-performance materials being developed as an alternative to the standard austenitic steels for the cladding materials of the future sodium fast nuclear reactors. Their major advantages are good thermo physical and mechanical properties at high temperatures; high irradiation resistance; high-temperature mechanical strength and ductility; dimension stability under irradiation; high thermal conductivity; low thermal expansion coefficient; superior void; swelling resistance; and resistance to helium embrittlement and compatibility with major cooling and breeding materials compared to austenitic stainless steels [33,34,35,36]. During the fuel dissolution stage, nitric acids of various concentrations are used, and thereby the cladding material can undergo different forms of corrosion attack. Furthermore, some process specifications impose a limit on the concentration of the corrosion products (mainly iron and chromium) in the dissolution medium. To quantify the potential impact of the use of ODS steels cladding for the fuel reprocessing process, the corrosion behavior of these cladding material steels needs to be studied in order to provide an understanding of the limitations of the life of the material. Studies to develop an understanding of the corrosion resistance of experimental oxide ODS steels as a candidate material for fusion and fast reactors and nuclear reprocessing plants are in progress and being explored [36, 37]. The corrosion rate measured in different nitric acid concentrations of 3 M to 9 M HNO3 shows a comparable or lower corrosion rate of 15% Cr ODS steel than AISI type 304L SS depending on the nitric acid concentration (Fig. 8). However, severe intergranular corrosion attack was revealed in type 304L SS after 240 h exposure, but none in ODS steels. Such an intergranular corrosion attack seen in type 304L stainless steel is undesirable.
Similarly, tempered martensitic ODS steels 9Cr and 11Cr-ODS steel candidate fast reactor fuel pins material developed at JAEA shows that corrosion rate decreased exponentially with effective chromium concentration (Creff) and nitric acid concentration (Fig. 9). However, the addition of vanadium (V) and ruthenium (Ru) lower the corrosion rate, which prevents the active mass dissolution and assists in passivating the surface immediately, and decreases the corrosion rate [38]. In our recent work on Al containing 17Cr ODS steel (Fe–16.78Cr–4.46Al–0.5Ti–0.45Y2O3–0.36Y the corrosion rate in boiling nitric acid tests revealed 0.038 mm/year (1.5 mils per year (mpy)) in 3 M HNO3, 0.075 mm/year (2.9 mpy) in 6 M HNO3, 0.18 mm/year (7.4 mpy) in 9 M HNO3, and 0.24 mm/year (9.8 mpy) in 11.5 M HNO3 after 240 h exposure. The obtained corrosion rate is well below the acceptable limit for nitric acid applications [39].
The corrosion rate measured in different nitric acid concentrations with Ru and V for 30 min at 95 °C on Creff [38]
6 Amorphous Bulk Metallic Glasses Alloys
Bulk metallic glasses (BMGs) are random structures (non-crystalline) lacking rotational and translational symmetries, i.e., lattice parameters of the atomic structure which cannot be defined [40]. The metallic materials with an amorphous structure that are produced directly from the liquid state during cooling are called “glassy metals” or mostly commonly known as “metallic glasses,” and these glassy alloys exhibit glass transition upon heating [40,41,42,43]. Owing to their excellent corrosion resistance, the bulk metallic glasses (BMGs) with an amorphous structure are being explored and considered for service under highly oxidizing nitric acid environments. Among BMG materials, Ni-based metallic glasses or amorphous alloys systems have attracted considerable attention in recent decades because of their commercial importance related to the functional applications for corrosion and thermal oxidation [40, 42]. However, only a few results on the corrosion behavior of the metallic glasses in a nitric acid environment are reported. The corrosion rates of the Ni metalloid-based glassy alloys of Ni65Cr15P16B4 and Ni65Cr10P16B4Ta5 composition were about 1.4 and 0.2 mm/year respectively, in 9 M HNO3 at 124 °C [44]. Similarly, a low corrosion rate of 0.01 mm/year in 9 M HNO3 is observed for the Ni-valve metal based metallic glassy alloys Ni57Nb19Zr19Ta5 and Ni60Nb15Zr5Ti15Ta5 at 124 °C [44, 45] indicates that the corrosion resistance of Ni-valve metal based metallic glasses are better than that of the Ni-metalloid-based metallic glasses. The work on Ti and Zr-free, Ni-valve metal-based glass forming alloys, namely Ni60Nb40, Ni60Nb30Ta10, and Ni50Nb25Zr25 metallic glass were investigated for their performance in concentrated nitric acid.
The potentiodynamic anodic polarization evaluation of Ni60Nb30Ta10 metallic glass and partially crystallized ribbons performed in 11.5 M nitric acid environments (Fig. 10), shows metallic glassy ribbon with higher corrosion resistance compared to crystalline alloys. The formation of crystalline α-Ni phase in the amorphous matrix lowers corrosion resistance [47]. The air-oxidation is a useful processing treatment for improving the corrosion resistance of Ni60Nb40 amorphous alloys. Potentiodynamic polarization results show that oxide film on the 450 °C oxidized sample exhibits a highly protective barrier to the nitric acid when compared to the oxide film at 550 °C [47] (Fig. 11).
Potentiodynamic polarization curves for the as-spun metallic glass and partially crystallized ribbon in 11.5 M HNO3 environments at room temperature [46]
The compositional differences of thermal oxidation at 450 and 550 °C under an air-medium, and their corrosion properties in nitric acid and fluorinated nitric acid: a as-spun Ni60Nb40 ribbon, b the surface enriched with Nb2O5 subjected to thermal oxidation at 450 °C, c thermal oxidation at 550 °C (550AR); and d potentiodynamic polarization curves in 11.5 M HNO3 at room temperature [47]
7 Tribocorrosion of Reprocessing Materials
Tribocorrosion (i.e., wear and corrosion interaction) occurs under a variety of mechanical and corrosion conditions. Similarly, in many nuclear components (shafts, pumps, etc.), a number of such parts operate without the benefit of any lubrication, and any movement with discrete increments or steps will subject the material and component to wear (tribocorrosion). Further, some components are subjected to relative motions because of necessary operational processes (localization and positioning adjustment) or by unwanted effects such as flow-induced vibration [48,49,50,51]. As a result of a wide range of contact kinematics, complex combinations of wear corrosion can occur. Despite its practical importance, little basic research has been devoted to the understanding of the synergistic mechanism involved in tribocorrosion systems of materials used in nuclear reprocessing plants. The understanding of the wear and corrosion synergism in tribocorrosion property correlations will also help to improve the tribocorrosion performance of the materials and coatings. Wear and tribocorrosion studies were carried out on reprocessing materials. The 304L SS exhibited a lower wear rate when compared to Zircaloy-4, Zr-702, and Ti-grade2 (Fig. 3.12). The inferior tribological characteristics of Ti base alloys can be related to low shear strength, low work hardening, and low protecting ability of surface oxides to wear [51]. Further, in comparison with the wear and tribocorrosion behavior of CP-Ti and Ti–5Ta–1.8Nb alloys in nitric acid, the coefficient of friction remains same for both CP-Ti and Ti–5Ta–1.8Nb alloys in dry conditions, and a minor difference is observed for OCP sliding behavior in a nitric acid medium (Fig. 3.13).
Comparative open circuit potential behaviour in 1 M HNO3 with and without sliding of type 304L SS, Zircaloy-4, Zr-702, and Ti-grade-2 [51]
8 Summary
Corrosion continues to occur in virtually all structural or engineering materials, and the degradation of materials due to the corrosion in spent fuel nuclear reprocessing plants of nitric acid is a serious issue. In spent nuclear fuel reprocessing plants, various nitric media are used, i.e., dilute (1–4 M), to concentrated or near to azeotropic concentration (10–14 M), room temperature (liquid/solvent extraction), intermediate (warm, waste storage tanks, etc.) and to boiling temperature (dissolvers, evaporators, etc.) during fuel element dissolutions for the separation of fission products, uranium, and plutonium in the PUREX process. Corrosion processes in nitric acid are affected by a number of factors, which include: welding, cold work, thermal history, chemical homogeneity and non-metallic inclusion NOx gases, dissolved species, radiation, boiling nitric acid solutions, surface and heat transfer, etc. Structural materials used for critical components (e.g., dissolvers, evaporators, waste storage tanks, etc.) in spent nuclear fuel reprocessing plants are required to be resistant to corrosion degradations, and to be designed for the long term, as access for repairs can be difficult, and are not always possible. Corrosion issues and the selection of appropriate structural materials for aqueous spent nuclear fuel reprocessing and the corrosion processes affecting the structural materials, i.e., stainless steel, titanium and zirconium, and their alloys, ODS steels, amorphous BMG alloys, etc. require for the various structural components/equipment (solvent extraction, waste storage tanks, dissolvers, evaporators, vessels, and piping, etc.) are briefly highlighted. Major factors that influence the corrosion resistance of structural materials including: solute segregation, thermal history, chemical homogeneity and non-metallic inclusion, impurities, nitric acid concentrations and temperature, dissolved species or oxidizing metal ions, welding, cold work, heat transfer, irradiation, etc., are also highlighted.
References
Kamachi Mudali U (2017) Materials for hostile corrosive environments.In: Tyagi AK, Banerjee S (eds) Materials under extreme conditions - recent trends and future prospects. Elsevier, pp 91–128
Kamachi Mudali U (2013) Austenitic stainless steels for back end of nuclear fuel cycle. Adv Mat Res 794:530–538
Raj B, Kamachi Mudali U (2006) Materials development and corrosion problems in nuclear fuel reprocessing plants. Prog Nucl Energy 48:283–380
U. Kamachi Mudali, R.K Dayal and J.B Gnanamoorthy, Corrosion studies on materials of construction for spent nuclear fuel reprocessing plant equipment, Journal of Nuclear Materials 203 (1993) pp. 73–82.
Whillock GOH, Worthington SE (2010) Corrosion in nitric acid. In: Richardson TJA, Cottis BRA, Lindsay R, Lyon S, Scantlebury DJD, Stott H, Graham M (eds) Shreir’s corrosion, vol 2. Amsterdam, Elsevier Ltd, pp 1250–1269
Schillmoller CM (1999) Select the best alloys to resist nitric acid. Chem Eng Prog 95:65–69
Ahluwalia HS, Corrosion by nitric acid, ASM handbook. In: SD Cramer, BS Covino Jr (eds) Corrosion: environments and industries, vol 13C, pp 668–673
Herbst RS, Baron P, Nilsson M (2011) Standard and advanced separation: PUREX processes for nuclear fuel reprocessing. In: Advanced separation techniques for nuclear fuel reprocessing and radioactive waste treatment, pp 141–175
Fauvet P, Balbaud F, Robin R, Tran Q-T, Mugnier A, Espinoux D (2008) Corrosion mechanisms of austenitic stainless steels in nitric media used in reprocessing plants. J Nucl Mater 375:552–564
Yamamota T, Tsukui S, Okamoto S, Nagai T, Takeuchi M, Takeda S, Tanaka Y (1998) Gamma-ray irradiation effects on corrosion rates of stainless steel in boiling nitric acid containing ionic additives. J Nucl Sci Technol 35:353–356
Ueno F, Kato C, Motooka T, Ichikawa S and Yamamota M (2008) Corrosion Phenomenon of Stainless Steel in Boiling Nitric Acid Solution Using Large-Scale Mock-Up of Reduced Pressurized Evaporator. J Nucl Sci Technol 45(10):1091–1097
Kato C (2020) Corrosion in nuclear fuel reprocessing plants: corrosion in boiling nitric acid. In: Konings RJM, Stoller RE (eds) Comprehensive nuclear materials, 2nd edn, vol 4. Elsevier, pp 528–563
Ningshen S, Sakairi M (2015) Corrosion degradation of AISI type 304L stainless steel for application in nuclear reprocessing plant. J Solid State Electrochem 19:3533–3542
Ningshen S, Kamachi Mudali U, Ramya S, Raj B (2011) Corrosion behavior of AISI type 304L stainless steel in nitric acid media containing oxidizing species. Corros Sci 53 (1):64–70
Ningshen S, Kamachi Mudali U, Raj B (2009) Assessment of corrosion performance of nitric acid grade stainless steels exposed to nitric acid environments. Corros Sci 51:322–329
Decours J, Decugis J-C, Demay R, Pelras M, Turluer G (1987) Austenitic stainless steels: Assessment of progress in materials performance for reprocessing applications. IAEA-TECDOC-421, pp 117–127
Kiuchi K, Kato T, Motooka H, Hamada S (1999)Technological problems and counter-measures on equipment materials for reprocessing of high burnup fuels. International Atomic Energy Agency, Vienna (Austria); 333 p; ISSN 1011-4289; Jul 2002; pp 210–215; Technical committee meeting on technical and economic limits to fuel burnup extension; San Carlos de Bariloche (Argentina); 15–19 Nov 1999; 16 refs, IAEA-TECDOC-1299
Furuya T, Kawafuku U, Satoh H, Shimogori K, Aoshim A, Takeda S (1991) A Corrosion Testing Method for Titanium in Nitric Acid Environments. ISIJ Int 31(2):189–193
Kamachi Mudali U, Raju VR, Dayal RK (1998) Corrosion of advanced materials in liquid, condensate and vapour phase of nitric acid environments. In: Proceedings of eighth nation congress on corrosion control. Kochi: National Corrosion Council of India, pp 7.3.1–7.3.8
Corrosion resistance of titanium - Titanium Metals Corporation TIMET Report, 1997 Titanium Hearth Technologies, Inc.Pennsylvania, USA
Priya R, Kumar A, Kaliraj K, Vijayalakshmi S, Ningshen S, Philip J, Raju S, Albert SK, Nitric acid test of titanium dissolver material (Ti-Gr2), Report No: IGCAR/MMG/CSTD/2020/388
Ravi Shankar A, Kamachi Mudali U (2013) Refractory metal coatings on titanium to improve corrosion resistance in nitric acid medium. Surf Coat Technol 235:155–164
Ningshen S, Sakairi M, Suzuki K, Okuno O (2015) Corrosion performance and surface analysis of Ti-Ni-Pd-Ru-Cr alloy in nitric acid solution. Corros Sci 91:120–128
Furuya T, Satoh H, Shimogori K, Aoshima A, Takeda S (1984) Corrosion resistance of zirconium and titanium alloy in HNO3 solutions. In: Fuel reprocess, waste manage. Proceedings of the American Nuclear Society International Topical Meeting, vol 1, pp 249–258
Kato C, Ishijima Y, Ueno F, Yamamoto M (2016) The effect of crystal textures on the anodic oxidization of zirconium in a boiling nitric acid solution. J Nucl Sci Technol 53:1371–1379
Auchapt P, Patarin L, Tarnero M (1984) Proc, The International Meeting on Fuel Reprocessing and Waste Management (RECOD’84), vo1 II, p 2, American Nuclear Society, La Grange Park, 1984, ASM, La Grange Park, IL
Nagano H, Kajimura H, Yamanaka K (1995) Corrosion resistance of zirconium and zirconium-titanium alloy in hot nitric acid. Mater Sci Eng, A 198:127–134
Yau T (1986) Zirconium for nitric acid solutions. In: Young C, Durham J (eds) Industrial applications of titanium and zirconium: fourth volume. West Conshohocken, PA zirconium could be attacked, ASTM International, pp 57–68
Chauve H, Decour J, Demay R, Pelras M, Simonne J, Turluer G (1987) Zirconium use for large process components, Materials Reliability in the Back End of the Nuclear Fuel Cycle, IAEA, Vienna. IAEA-TECDOC-421
Tonpe S, Saibaba N, Jayaraj RN, Ravi Shankar A, Kamachi Mudali U, Raj B (2011) Process development for fabrication of Zircaloy–4 dissolver assembly for reprocessing of spent nuclear fuel. Energy Procedia 7:459–467
Jayaraj J, Thyagarajan K, Mallika C, Kamachi Mudali U (2015) Corrosion behaviour of zirconium, titanium and their alloys in simulated dissolver solution of fast breeder reactor spent nuclear fuel using Zircaloy-4 mock-up dissolver vessel. Nucl Technol 191:38–70
Kamachi Mudali U, Ravishankar A, Natarajan R, Saibaba N, Raj B (2013) Applications of zirconium alloys for reprocessing plant components. Nucl Technol 182:349–357
Ukai S (2012) Oxide dispersion strengthened steels. Comp Nucl Mater 4:241–271
Ukai S, Fujiwara M (2002) Perspective of ODS alloys application in nuclear environments. J Nucl Mater 307–311:749–757
Ningshen S, Sakairi M, Sukuki K, Ukai S (2013) Corrosion resistance of 9–15% Cr ODS steels and its comparison with austenitic stainless steel. Adv Mat Res 794:575–582
Ningshen S, Sakairi M, Suzuki K, Ukai S (2014) The corrosion resistance and passive film compositions of 12% Cr and 15% Cr ODS steels in nitric acid media. Corros Sci 78:322–334
Ningshen S, Sakairi M, Suzuki K, Ukai S (2014) “The surface characterization and corrosion resistance of 11% Cr ferritic/martensitic and 9–15% Cr ODS steels for nuclear fuel reprocessing application. J Solid State Electrochem 18(2):411–425
Tanno T, Takeuchi M, Ohtsuka S, Kaito T (2017) Corrosion behavior of ODS steels with several chromium contents in hot nitric acid solutions. J Nucl Mater 494:219–226
Priya R, Ningshen S, Sakairi M, Ukai S (2020) Corrosion behaviour of Al-containing high Cr ferritic oxide dispersion strengthened steel in nitric acid environment. J Nucl Mater 534:Article: 152120
Johnson WL (1986) Thermodynamic and kinetic aspects of the crystal to glass transformation in metallic materials. Prog Mater Sci 30:81–134
U. Kamachi Mudali, S. Scudino, U. Kuhn, J. Eckert and A. Gebert, Polarisation behaviour of the Zr57Ti8Nb25Cu139Ni111Al75 alloy in different microstructural states in acid solutions, Scripta Mater., 50 (2004), pp. 1379–1384
Kamachi Mudali U, Scudino S, Kuhn U, Eckert J, Schultz L, Gebert A (2006) Corrosion behaviour of zirconium based bulk metallic glasses. Trans IIM 59:123–138
Padhy N, Ningshen S, Kamachi Mudali U (2010) Electrochemical and surface investigation of zirconium based metallic glass Zr59Ti3Cu20Al10Ni8 alloy in nitric acid and sodium chloride media. J Alloy Compd 503:50–56
Qin C, Asami K, Kimura H, Zhang W, Inoue A (2008) Surface characteristics of high corrosion resistant Ni–Nb–Zr–Ti–Ta glassy alloys for nuclear fuel reprocessing applications. Electrochemistry Communications 10:1408–1410
Qin C, Asami K, Kimura H, Zhang W, Inoue A (2009) Electrochemical and XPS studies of Ni-based metallic glasses in boiling nitric acid solutions. Electrochim Acta 54:1612–1617
Poddar C, Ningshen S, Jayaraj J (2020) Corrosion assessment of Ni60 Nb30Ta10 metallic glass and its partially crystallized alloy in concentrated nitric acid environment. J Alloy Compd 813:152172
Poddar C, Jayaraj J, Ningshen S, Kamachi Mudali U (2019) Effect of thermal oxidation on the oxide characteristic and corrosion behavior of Ni60Nb40 amorphous ribbon in nitric acid. Appl Surf Sci 479:430–439
Celis JP, Ponthiaux P, Wenger F (2006) Tribocorrosion of materials: Interplay between chemical, electrochemical and mechanical reactivity of surfaces. Wear 261:939–946
Mischler S (2008) Triboelectrochemical techniques and interpretation methods in tribocorrosion: A comparative evaluation. Tribology Int 41:573–583
Priya R, Ningshen S, Vasudevan M, Thyagarajan K, Mallika C, Kamachi Mudali U (2015) Wear and tribocorrosion behaviour of 304L stainless steel welds. Tribol Mater, Surf Interfaces 9:181–189
Priya R, Mallika C, Kamachi Mudali U (2014) Wear and tribocorrosion behaviour of 304L SS, Zr-702, Zircaloy-4 and Ti-grade2, Wear, 310, pp 90–100
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Ningshen, S., Priya, R., Kamachi Mudali, U. (2022). Nitric Acid Corrosion Issues of Spent Fuel Nuclear Fuel Reprocessing Plants Materials. In: Kamachi Mudali, U., Subba Rao, T., Ningshen, S., G. Pillai, R., P. George, R., Sridhar, T.M. (eds) A Treatise on Corrosion Science, Engineering and Technology. Indian Institute of Metals Series. Springer, Singapore. https://doi.org/10.1007/978-981-16-9302-1_3
Download citation
DOI: https://doi.org/10.1007/978-981-16-9302-1_3
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-16-9301-4
Online ISBN: 978-981-16-9302-1
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)