Abstract
Abdominal tuberculosis is a form of extrapulmonary tuberculosis that can involve the intestine, peritoneum, abdominal lymph nodes, hepatobiliary system, pancreas, or spleen. Microbiological diagnosis of abdominal tuberculosis is difficult because of the paucibacillary nature of disease and antitubercular therapy is prescribed empirically in more than half of the cases. Abdominal tuberculosis closely mimics Crohn’s disease (in case of intestinal tuberculosis) and malignancy (in pelvic-peritoneal tuberculosis, Esophageal tuberculosis, gastroduodenal, hepatobiliary, and pancreatic tuberculosis). Delayed diagnosis of IBD and malignancy because of empirical ATT trial can lead to increased morbidity and mortality; hence, every effort should be made to clinch the diagnosis before considering empirical ATT. For similar reasons, response to antitubercular therapy should be assessed early and it should be one of the criteria for clinically diagnosed cases of tuberculosis. Response to ATT can be assessed by improvement in organ-specific and constitutional symptoms, improvement in lab parameters, immunological response, radiological response, and endoscopic healing. Often, assessment of endoscopic response may be relevant in luminal disease while radiological re-evaluation may be warranted in visceral lesions. Exact timing of response assessment is not known for most locations. However, early mucosal response (at 8 weeks) in ileocolonic tuberculosis could provide an objective early parameter for response assessment.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Gastrointestinal tuberculosis
- tuberculous peritonitis
- peritoneal tuberculosis
- intestinal tuberculosis
- pancreatic tuberculosis
- hepatic tuberculosis
-
1.
Assessment of response should be considered in each patient with abdominal tuberculosis but is mandatory in those who are clinically diagnosed.
-
2.
Objective response criteria (ulcer healing, ascites resolution, disappearance of radiologic lesions) should be preferred over subjective features like weight gain or sense of well-being.
-
3.
Lack of response could be due to misdiagnosis, drug resistance, or sequelae of tuberculosis.
-
4.
Two months is a reasonable time to assess response in intestinal tuberculosis by looking for healing of ulcers (early mucosal response).
-
5.
Role of biomarkers in response assessment is upcoming with a potential role for serum CRP and fecal calprotectin in intestinal TB.
1 Introduction
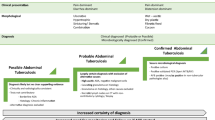
Abdominal tuberculosis (ATB) is a heterogeneous entity encompassing the involvement of various luminal (intestine, esophagus, stomach) and extraluminal (peritoneum, visceral organs, and lymph nodes) structures [1]. Across the various subtypes of abdominal tuberculosis, the major problem in making a diagnosis is the low positivity of microbiological tests [2,3,4]. The yield of various microbiological tests is low and even with a combination of microbiological and histological/cytological tests, the yield remains below 50% in clinical practice. Similar to many other sites of extrapulmonary tuberculosis, clinicians are forced to embark on antitubercular therapy (ATT) on empirical grounds [5]. In such situations, certain precautions need to be ensured: all efforts should be exhausted to make a confirmed diagnosis prior to embarking on the adventure of “empirical therapy” and a close follow-up of such patients be done so as to identify “non-responders” and to look for underlying causes of “non-response.” Response can be assessed by clinical, radiological, microbiological, endoscopic, and immunological or biochemical basis [6]. Clinical response is assessed by improvement in performance status, weight gain, and resolution of systemic and organ-based symptoms. Radiological response can be assessed by a decrease in mesenteric thickening, mural thickening of the bowel wall, resolution of stricture, healing of ulcers, disappearance of lymphadenopathy, and ascites. Microbiological response is difficult to assess in view of paucibacillary nature and low yield by Zeihl–Neelsen (ZN) staining and PCR-based tests but should be considered in non-responding patients to rule out multidrug-resistant tuberculosis. Immune response can be assessed by declining acute phase proteins or modification of cytokines or T-lymphocyte subset [7, 8]. Although it may seem fairly simple, the definitions of “response” and “non-response” are not entirely clear and there are many issues regarding these definitions. There is a need to have clear definitions and guidance for clinicians treating abdominal tuberculosis on basis of the published evidence. The chapter will deal with various armamentariums which have been used to define response, clinical symptoms and monitoring, biomarkers, imaging and endoscopic methods, and address the yin and yang of each of these methods.
1.1 Traditional Definition of Response
In 1969, Logan first suggested the use of “response to therapy” as an important method for the diagnosis of anorectal tuberculosis. This modification of the diagnostic criteria has since been used frequently in clinical practice and also for defining abdominal tuberculosis in research. The definition used by Logan included probable cases with a consistent clinical and radiological profile where the “‘sarcoid’ reaction indistinguishable between Crohn’s disease and tuberculosis” was present and “satisfactory response to chemotherapy” was documented [9]. In the present chapter, we will focus on the definitions of “satisfactory response” for the many subtypes of abdominal tuberculosis.
2 Luminal Tuberculosis
2.1 Intestinal Tuberculosis (ITB)
Intestinal tuberculosis (ITB) is one of the common patterns of involvement in abdominal tuberculosis [10]. The clinical presentation is variable with patients presenting with a mix of constitutional symptoms and localizing features. Abdominal pain and episodes of intestinal obstruction dominate the clinical presentation. The condition closely simulates inflammatory bowel disease, especially Crohn’s disease (CD) [5, 11]. CD has a similar clinical presentation (with abdominal pain, diarrhea), endoscopic findings (ulcers, pseudopolyps), imaging findings (mural thickening), and histological findings (changes of chronicity and granulomatous inflammation). In regions where both these diseases are common, clinicians often find it difficult to conclusively distinguish the two [11]. If even after appropriate evaluation the diagnosis is uncertain, clinicians often embark on “empirical ATT” to sort out the diagnostic confusion [11, 12]. The reasons for preferring “empirical ATT” over empirical therapy for Crohn’s disease are manifold. The therapeutic endpoints for ITB are clear and the treatment usually involves 6 months of therapy. While ATT carries the risk of adverse effects, prescription of steroids/immunosuppressants for presumed CD could be dangerous as it can result in the dissemination of tuberculosis. Also, as we will discuss, the resolution of mucosal ulcers with ATT is a definite method to exclude CD.
2.1.1 Symptomatic Response
The clinical symptomatology of intestinal tuberculosis is dominated by abdominal pain. The underlying causes for abdominal pain may include the presence of strictures or hypertrophic forms of intestinal tuberculosis causing intestinal obstruction. Additional causes could include the formation of adhesions due to concomitant peritoneal involvement. Other symptoms could include diarrhea (especially in cases with extensive ulceration) and constitutional symptoms like fever and weight loss. Some of the studies have evaluated the clinical response to antitubercular therapy in patients with intestinal tuberculosis. In the study by Mouli et al., clinical response was noted in 66% of patients with ITB at 2 months and 99% of them by 6 months. In contrast, 28% of patients with Crohn’s disease (CD) had a symptomatic response at 2 months while 37% of them had a response at 6 months (Tables 21.1) [13]. The study clearly demonstrated that while resolution of symptoms occurred more frequently with ITB, some of the patients with CD also had a symptomatic response with ATT and therefore resolution of symptoms alone may not have adequate discriminative ability. Also, it is not clear if symptoms like fever, abdominal pain, and weight loss differ vis-à-vis the response rates between these patients. Sharma V et al. reported that 83.8% of patients with abdominal tuberculosis responded to 6 months of ATT. Subjective response to treatment was measured by improvement in clinical features like weight gain, increased appetite, defervescence, and improvement of pain abdomen [14]. A study by Anand BS et al. in patients with tubercular ileal stricture reported 91% clinical response at the end of 1 year of ATT. Clinical response was defined as resolution of pain abdomen or vague pain abdomen not requiring analgesics [15].
2.1.2 Mucosal Response
Terminal ileum and right side colon are the most common site of involvement of intestinal tuberculosis [16]. Common endoscopic features are ulcers, nodularity, and luminal narrowing. Mucosal healing is the most well-studied objective response to ATT in patients with ITB. Mouli et al. reported mucosal healing in 100% of ITB patients compared to only 5% of patients with Crohn’s disease at the end of 6 months of ATT [13]. Mucosal healing is a very important tool in differentiation of ITB with CD especially if ATT was started empirically as clinical improvement with ATT can be seen in a significant percentage of patients with CD. Persistence of ulcers on ATT points toward alternate diagnosis like CD. We reported healing of ulcers on colonoscopy as early as 2 months of starting ATT in patients with ITB [17]. In this study, 89% of patients showed complete or partial “early mucosal response.” Causes of non-response were infection by multidrug-resistant (MDR) mycobacteria in one patient and CD in another three patients [17]. This is an important observation, as an early initiation of immunosuppression in case of CD could prevent strictures and provide better long-term outcomes. Also, it is likely to be more cost-effective and can prevent adverse events and costs of continuing ATT [17]. A similar observation was reported by Park et al. in patients with nonspecific ileocecal ulcers. In this study, nine patients of suspected tubercular colitis on median follow-up of 107 days showed mucosal healing. Equal number of patients showed no response to ATT and were later confirmed as IBD or nonspecific colitis [18]. Although the mucosal response is an established tool of response assessment but is limited by invasive nature, patient discomfort, intolerable bowel preparation, and incomplete evaluation in presence of strictures or proximal ileal/jejunal involvement.
2.1.3 Biomarkers
Acute-phase proteins like C-reactive protein (CRP) are frequently elevated in patients with tuberculosis. Studies in patients with pulmonary tuberculosis reported that CRP rapidly declines within the first week after starting ATT [19]. Persistently elevated CRP >20 mg/l is associated with adverse treatment outcomes [20]. We studied the role of serial CRP measurement in patients with suspected abdominal tuberculosis. In this study, 101 of 112 patients with suspected abdominal tuberculosis patients had elevated CRP at baseline. After starting ATT, CRP at 2 and 6 months showed a declining trend in 94 patients, all of them were confirmed as ATB. Out of 7 patients with persistently elevated CRP, 5 were confirmed as alternative diagnosis (3 CD, 1 lymphoma, 1 carcinoma gallbladder with peritoneal carcinomatosis), 1 had ATB with intercurrent infection (Urinary tract infection), and 1 had disseminated tuberculosis with nonhealing ulceration and narrowing at 6 months. This study concluded that a lack of decline in CRP suggests alternate diagnosis or drug-resistant tuberculosis [21]. Studies in patients with pulmonary tuberculosis suggest modification of cytokine and T-lymphocytes subsets after successful ATT [22]. Level of TNF-α decreases and shift of MTB specific TNF-α expressing CD4 T-cells to polyfunctional CD4 (expressing INF-𝜸, TNF-α, and IL-2) with ATT [23]. Tuberculosis causes elevated levels of certain matrix metalloproteinases leads to enzymatic destruction of extracellular matrix and cavity formation. Levels of MMP-1, MMP-3, and MMP-8 elevate in pulmonary tuberculosis and MMP-9 in tubercular meningitis and successful treatment with ATT causes normalization of these MMPs [24]. However, studies regarding change of cytokine profile and MMPs are lacking in patients with abdominal tuberculosis.
Fecal calprotectin could also be used for the assessment of response to therapy. We have reported that fecal calprotectin measured at baseline and at 2 months of ATT provides a better discriminative value than serum CRP to differentiate ITB and CD. Most patients with ITB have an elevated fecal calprotectin but an occasional patient may have a normal level at the baseline. The use of fecal calprotectin and serum CRP could obviate the need for a repeat colonoscopy to assess mucosal response [25].
2.1.4 Imaging
In a small report of 20 patients, 18 patients were followed using CT Enterography and seven by using gastrointestinal ultrasound (GIUS). The definition of complete response was reduction in lesion by 50% or significant decrease in bowel thickness, lymph node size, and bowel enhancement. Similar definitions and a decline in Limberg score by two grades was considered as a definition of response on GIUS. Limberg score, utilized frequently in Crohn’s disease, grades bowel involvement from 0–4 using parameters like bowel wall thickness, and vascularity in bowel wall and perienteric fat and mesentery. With ATT, the thickness of the bowel wall reduced, and mural stratification was better visualized. However, changes in vascularity did not seem to be pronounced. Although limited by the small numbers, the study showed the feasibility of the use of GIUS for response assessment in these patients [26].
Jain R et al. studied sonographic findings in 56 patients with early abdominal tuberculosis and also assessed the response of ATT on sonographic findings. Early tuberculosis was defined as no history of intestinal obstruction and normal barium study. Compared to healthy control (n = 30), presence of thickened (≥15 mm) and echogenic mesentery and mesenteric lymphadenopathy suggest abdominal tuberculosis. Other findings included dilated small bowel loops (n = 38), minimal ascites (n = 17), matted small bowel loops (n = 5), and omental thickening (n = 3). After starting ATT, regression of all of these lesions was noted on serial follow-up USG at 1, 3, 6, and 12 months of ATT [27].
Kedar RP et al. studied US findings in 90 patients with abdominal tuberculosis. Common findings include concentric bowel wall thickening (n = 51), ulcers (n = 8), ascites (n = 36), abdominal lymphadenopathy (n = 23), adhesions (n = 14), peritoneal thickening (n = 13), cold abscess (n = 10), club sandwich sign (n = 5) and peritoneal nodules (n = 3). The presence of fibrinous strands in ascites, loculated ascites, presence of caseation (central echo poor areas in lymph nodes) & calcification of lymph nodes, bowel thickening at ileocecal junction and subhepatic location were highly suggestive for diagnosis of tuberculosis. Follow-up of 38 patients with US at 3 months was available and regression of bowel wall thickening, ascites, lymph node size, and cold abscess was noted in these patients [28].
In a study that used magnetic resonance enterography and diffusion-weighted imaging, the apparent diffusion coefficient (ADC) values were calculated pretreatment and posttreatment. Of 31 diseased segments, 29 segments showed diffusion restriction. On posttreatment imaging, eight patients had complete resolution on conventional MR imaging and the hyperintense signal on T2W as well as the enhancement on posttreatment also resolved. The ADC values showed an increase in those having response to therapy. In other patients who were eventually diagnosed with Crohn’s disease, there was no increase in the ADC values suggesting that ADC values could be an objective non-invasive parameter for evaluation of response to therapy. Unfortunately, no data is yet available at 2 months, and therefore it is unclear if this change in ADC values could occur early or if the response is detectable only at the end of therapy (6 months) [29].
Response to ATT can also be assessed by change in abnormal metabolic activity by using 18 FDG PET CT. Chen et al. reported that PET CT was better than sputum or CT alone at 2 months of ATT for response assessment in 35 south Korean patients with MDR pulmonary TB. FDG PET can identify the presence of cavity, nodule, and consolidation as well as metabolic activity [30]. V. Martinez et al. reported the role of FDG PET as an early non-invasive marker for therapeutic response to ATT. Out of 21 patients, 10 had extrapulmonary tuberculosis {Ovarian TB (n = 3), Bone (n = 1), and lymphadenitis (n = 6)}, 10 had disseminated TB (pulmonary and lymph nodes) and 1 had pulmonary tuberculosis. Median SUV max at baseline was 8.6 and 1 month after ATT was 5.3, with a median fall of 31%. 19/21 patients showed a fall in SUV max as well as clinical improvement 1 month after ATT. One of the two patients with no response on FDG PET was later diagnosed as NHL, while other patients had drug-sensitive tuberculosis [31]. The role of FDG-PET in patients with abdominal tuberculosis is reported in case reports. Park et al. reported a case of disseminated TB (right pleural effusion and right psoas abscess) where FDG-PET CT at baseline showed metabolic activity and on repeat scan at 9 months of ATT showed that regional hyperactivity previously revealed disappeared completely [32].
Anand et al. reported the role of barium series in the assessment of response to ATT in 39 patients with tubercular strictures. Trial was completed by 34 (87%) of the patients, clinical response was reported in 31 (91%) of patients, rest of the 3 required surgery. Barium series after 1 year of ATT (Streptomycin 1 gm/day for 3 months, Rifampicin 450 mg/day for 1 year & Isoniazid 300 mg/day for 1 year) was available in 23 patients, 16 (70%) patients showed complete response to ATT. Of 7 (30%) patients with no response at 1 year, two patients showed response to another 1 year of ATT [15]. Another study by Appasani S et al. in 41 patients with abdominal tuberculosis reported most common site of stricture was at ileocecal region (n = 16, 36%), followed by ileum (n = 9, 21%), jejunum (n = 9, 21%), gastroduodenal (n = 6, 14%) and both jejunum and ileum in 4(9%) of patients. After 6–12 months of ATT barium series showed complete response in 11 (27%) patients, partial response in 11 (27%) patients, no response in 9 (22%) patients and worsening in 10 (24%) of the patients. Clinical improvement was reported in 80% of the patients while the radiological response was noted only in 54% of the patients [33]. Barium studies are not routinely used nowadays as cross-sectional techniques are being preferred.
2.2 Gastroduodenal Tuberculosis
Gastroduodenal tuberculosis is an uncommon form of intestinal tuberculosis. Common clinical features include recurrent vomiting, gastric outlet obstruction (GOO), pain abdomen and constitutional symptoms like fever, anorexia and weight loss. Involvement of other sites reported to be present in close to 40% of patients with the commonest sites being ileocecal, pulmonary and lymph nodal tuberculosis. Common endoscopic findings are presence of duodenal or pre-pyloric strictures, ulcers, growth, and extrinsic compression. Diagnostic yield of endoscopic and lymph node biopsy is low. We did a systematic review on gastroduodenal tuberculosis and found that only one-third of patients had granulomatous inflammation and only 3.6% of patients had AFB positivity, reasons being uncommon disease, paucibacillary nature, and submucosal involvement. Response to standard antitubercular therapy for 6 months and endoscopic dilatation of strictures is good. Response can be assessed clinically, symptoms like vomiting and GOO usually improve by 4–6 weeks and constitutional symptoms subside after the first month of ATT [34]. A study by Puri AS et al. reported 12 patients with gastroduodenal tuberculosis who presented with gastric outlet obstruction and managed with ATT for 6 months and endoscopic balloon dilatation. Patients were followed up clinically, endoscopically and serial upper GI barium series. Resolution of strictures was documented by passing of standard gastroscope and free passage of contrast on barium series [35]. Studies by Dalal A and Amarapurkar DN reported resolution of dyspeptic symptoms & vomiting and there was weight gain after starting ATT. Upper GI endoscopy was repeated which showed ulcer healing and resolution of strictures [36,37,38]. Study by Upadhyaya VD reported free passage of contrast on barium series after starting ATT [39]. These patients also can be followed by a trans-abdominal ultrasound to document normalization of wall thickness and resolution of lymphadenopathy. Endoscopic ultrasound may be useful to document resolution especially if the predominant disease is submucosal [40].
2.3 Esophageal Tuberculosis
Esophageal tuberculosis is an uncommon form of extrapulmonary tuberculosis and usually involves secondary to mediastinal lymphadenopathy. Common presentation includes dysphagia, odynophagia, cough, hematemesis, and constitutional symptoms. Esophagoscopy and endosonography guided histopathology/cytology are the common modes of diagnosis while chest radiograph, barium swallow, and CT scan have a supportive role in diagnosis. On endoscopy, mid esophagus is the most common site of involvement with the presence of ulcer, stricture, submucosal bulge, fistula and pseudotumor are common findings. On EUS, presence of hypoechoic mediastinal lymphadenopathy with hyperechoic strands, matted lymph nodes, esophageal wall thickening and adventitial disruption are common findings [41,42,43]. Response to treatment can be assessed on clinical ground as the resolution of local and systemic symptoms. A study by Devarbhavi et al. reported 10 cases of esophageal tuberculosis, presenting as dysphagia, cough, and hemoptysis. After being treated with ATT for 6 months, all except one improved clinically and there was the healing of esophageal ulcers and sinuses/fistulas on follow-up endoscopy [44]. Similarly, Jain SK et al. reported clinical and endoscopic profile in twelve cases of esophageal tuberculosis. Dysphagia, retrosternal pain, cough, fever, and weight loss were reported common symptoms. Esophagoscopy revealed mid and lower esophagus ulcer, strictures, and pseudotumor. After 9 months of ATT, complete clinical and endoscopic recovery was reported in 9 patients, while 3 patients had concomitant carcinoma esophagus and later underwent surgery & radiotherapy [45]. Study by Tang Y et al. reported 35 cases of esophageal tuberculosis and followed up by endoscopy and EUS after 6 months of ATT. Follow-up EUS showed resolution of esophageal mass, esophageal wall thickness normalized and decrease in size of mediastinal lymph nodes with remnant hyperechoic patches was noted [46].
3 Peritoneal Tuberculosis
3.1 Biomarkers
CA 125
Serum CA-125 level can be elevated in patients with pulmonary and extrapulmonary (pleural, peritoneal, pelvic, miliary) tuberculosis. CA-125 is expressed by cells of coelomic epithelium and activation by inflammation and tumor can lead to an increased level in serum as well as in body fluids including pleural fluid and ascites [47, 48] Very high levels of CA-125 are reported in pelvic-peritoneal tuberculosis and it frequently masquerades as malignancy and reduction of serum CA-125 level with treatment is a valuable criterion for differentiation of tuberculosis from malignancy [49, 50]. A study by Yilmaz A et al. reported significantly higher serum CA-125 levels at baseline in patients with active pulmonary tuberculosis compared to healed pulmonary tuberculosis patients and healthy control. Serial serum CA-125 levels at 2, 4, and 6 months of ATT and at 3 years of follow-up showed decreasing trend signifies role in the assessment of response to therapy [51]. Various case reports showed a reduction of CA-125 level after starting ATT in patients with pelvic-peritoneal tuberculosis. High level of CA-125 in ascites is reported by O’Riordian DK et al. and it is one of the markers of activity of tuberculosis [52]. Gurgan T et al. reported that after treatment level of CA-125 declines both in serum and body fluids and thus helps in differentiation from malignancy [53].
3.2 Ultrasonography of Abdomen
Study by Jain R et al. in 56 patients with early abdominal tuberculosis reported the presence of minimal ascites, mesenteric lymphadenopathy, bowel wall, and mesenteric thickening were the most common ultrasonographic (USG) findings. Ascites and omental thickening were reported in 30% (n = 17) and 5% (n = 3) patients respectively. Follow-up ultrasound at 1, 2, 6, and 12 months of ATT showed regression of both ascites and abdominal thickening [27].
Study by Kedar RP et al. reported peritoneal involvement is common in patients with abdominal tuberculosis. Common findings were ascites in 40% (n = 36), adhesions in 15.3% (n = 14), and peritoneal thickening in 14.4% (n = 13) of the patients. Other less common findings were club sandwich sign in 5.5% (n = 5) and peritoneal nodules in 3.3% (n = 3) of the patients. Follow-up of 38 patients with USG at 3 months was available and regression of ascites, bowel thickening and lymphadenopathy was noted [28].
4 Visceral Tuberculosis
4.1 Pancreatic Tuberculosis
Pancreatic tuberculosis is an uncommon form of abdominal tuberculosis and a great mimicker of pancreatic malignancy. Common presentations reported in literature are pain abdomen, anorexia, weight loss, jaundice, fever, and night sweats. Imaging features include solitary or multiple hypoechoic or mixed iso-hypoechoic solid or cystic lesions in pancreas with peripancreatic lymphadenopathy. Dilated pancreatic and common bile ducts, calcification and invasion of surrounding vascular structures are also reported. Other organ system involvement such as lungs, ileocecal junction, peritoneum, spleen and liver, and HIV positivity is reported in up to 50% of patients. EUS guided FNAC of pancreatic lesions or lymph nodes is a common mode of diagnosis and the presence of granuloma is the most common finding. Duration of ATT in available literature varies between 6 and 12 months and the cure rate is ~90% [54,55,56]. Response of therapy can be assessed by resolution of symptoms such as pain abdomen, fever, jaundice, and weight gain. Liver function tests (LFTs) should be monitored more frequently as these patients may have cholestatic jaundice at presentation. Usually, cholestatic symptoms resolves after 2–4 weeks but complete normalization of LFTs might require 10–16 weeks [56]. Worsening of LFTs suggests ATT hepatitis or paradoxical reaction. Resolution of pancreatic mass, decrease in size of lymphadenopathy, and resolution of lesions at distant sites on follow-up USG and CT can be used for assessment of response to therapy. Kim JB et al. in their study of 42 patients reported that at 6 months of ATT only 30% of patients showed complete radiological response while two-third patients had a partial radiological response. In this study, 30 of the 42 patients received ATT for at least 9 months or more [57]. Follow-up EUS for the pancreaticobiliary system also can be used for assessment of response to treatment. A case series of six patients reported resolution of pancreatic mass 16–20 weeks after starting ATT [58]. One case report reported the utility of FDG-PET in the evaluation of response assessment in a patient with pancreatic tuberculosis [59].
4.2 Hepatobiliary Tuberculosis
Hepatobiliary tuberculosis is a rare form of extrapulmonary tuberculosis and usually associated with miliary, pulmonary, or intestinal tuberculosis. Hepatobiliary tuberculosis is classified as miliary tuberculosis and local/focal tuberculosis which is further divided into nodular TB (including tuberculous hepatic abscess, tuberculomas) and into the tubular form (involving intrahepatic ducts). Most common presentations of hepatic tuberculosis reported are pain abdomen, fever, anorexia, and jaundice. Jaundice could be due to granulomatous hepatitis or due to biliary tract involvement secondary to hepatic tuberculoma, biliary stricture, or extrinsic compression due to lymph nodal enlargement [60,61,62]. Alvarez et al. reported that abnormalities on chest X-ray and hepatic calcification on abdominal X-ray are common findings in these patients [62]. Common radiological findings include the presence of hypodense nodular lesions, abscess, features of extrahepatic biliary obstruction, and lymphadenopathy [63, 64]. Treatment is the same as abdominal tuberculosis and duration of ATT in literature varies between 6 and 12 months [61, 62]. Biliary obstruction may require biliary drainage along with ATT [65]. Biliary drainage can be done either by endoscopic retrograde cholangiopancreatography (ERCP) or percutaneous transhepatic biliary drainage (PTBD). Response to therapy is assessed by clinical response, improvement of liver function test (in granulomatous hepatits), and radiological resolution of hepatic lesions (focal or nodular hepatic TB). Alvarez et al. reported good clinical response to standard ATT in two-third of the patients [62]. Clinical response can be assessed by the disappearance of pain abdomen and fever, increase in appetite and weight. Biochemical response can be assessed by improvement of LFT and radiological response can be assessed by a decrease in size of liver, disappearance of hepatic nodules, abscess and lymphadenopathy. Alvarez and Chen et al. reported that strictures due to hepatobiliary tuberculosis may be multifocal and difficult to treat and might require multiple percutaneous or endoscopic intervention [66, 67]. Adverse drug reaction to ATT is common in these patients due to malnutrition, underlying liver disease including cirrhosis and portal hypertension secondary to hepatobiliary tuberculosis. Close monitoring of LFTs is recommended during treatment in these patients. Despite ATT, these patients have high mortality because of concomitant respiratory failure due to miliary tuberculosis, esophageal variceal bleed due to associated cirrhosis and underlying HIV infection.
5 Conclusion
In a study of abdominal TB from South Korea, it was noted that intestinal, peritoneal, and visceral forms had a good response to therapy while those with nodal tuberculosis were less likely to achieve complete response [68].
To conclude, assessment of response to therapy is important in most forms of EPTB including abdominal tuberculosis. The response assessment should be mandatory in patients where the diagnosis is not microbiologically confirmed. While for visceral forms radiological assessment may be appropriate, endoscopic assessment for ulcer healing should be used in luminal forms (Table 21.2).
Abbreviations
- ATB:
-
Abdominal tuberculosis
- ATT:
-
Antitubercular therapy
- ITB:
-
Intestinal tuberculosis
- TB:
-
Tuberculosis
References
Sharma V, Debi U, Mandavdhare HS, Prasad KK. Abdominal tuberculosis and other mycobacterial infections. In: Encyclopedia of Gastroenterology. 2nd ed; 2020. p. 646–59.
Kumar S, Bopanna S, Kedia S, et al. Evaluation of Xpert MTB/RIF assay performance in the diagnosis of abdominal tuberculosis. Intest Res. 2017;15(2):187–94.
Bellam BL, Mandavdhare HS, Sharma K, et al. Utility of tissue Xpert-Mtb/Rif for the diagnosis of intestinal tuberculosis in patients with ileocolonic ulcers. Ther Adv Infect Dis. 2019;6:2049936119863939.
Sharma V, Soni H, Kumar-M P, et al. Diagnostic accuracy of the Xpert MTB/RIF assay for abdominal tuberculosis: a systematic review and meta-analysis. Expert Rev Anti-Infect Ther. 2021;19(2):253–65.
Jena A, Jha DK, Sharma V. Distinguishing intestinal tuberculosis from Crohn’s disease. Lancet Gastroenterol Hepatol. 2021;6(3):159.
Rockwood N, du Bruyn E, Morris T, Wilkinson RJ. Assessment of treatment response in tuberculosis. Expert Rev Respir Med. 2016;10(6):643–54.
Goletti D, Lindestam Arlehamn CS, Scriba TJ, et al. Can we predict tuberculosis cure? What tools are available? Eur Respir J. 2018;52(5):1801089.
Jørstad MD, Dyrhol-Riise AM, Aßmus J, et al. Evaluation of treatment response in extrapulmonary tuberculosis in a low-resource setting. BMC Infect Dis. 2019;19(1):426.
Logan VS. Anorectal tuberculosis. Proc R Soc Med. 1969;62(12):1227–30.
Sharma MP, Bhatia V. Abdominal tuberculosis. Indian J Med Res. 2004;120(4):305–15.
Kedia S, Das P, Madhusudhan KS, et al. Differentiating Crohn's disease from intestinal tuberculosis. World J Gastroenterol. 2019;25(4):418–32.
Banerjee R, Pal P, Mak JWY, Ng SC. Challenges in the diagnosis and management of inflammatory bowel disease in resource-limited settings in Asia. Lancet Gastroenterol Hepatol. 2020;5(12):1076–88.
Pratap Mouli V, Munot K, Ananthakrishnan A, et al. Endoscopic and clinical responses to anti-tubercular therapy can differentiate intestinal tuberculosis from Crohn's disease. Aliment Pharmacol Ther. 2017;45(1):27–36.
Mandavdhare HS, Singh H, Dutta U, Sharma V. A real-world experience with 6 months of antitubercular therapy in abdominal tuberculosis. JGH Open. 2019;3(3):201–5.
Anand BS, Nanda R, Sachdev GK. Response of tuberculous stricture to antituberculous treatment. Gut. 1988;29(1):62–9.
Debi U, Ravisankar V, Prasad KK, et al. Abdominal tuberculosis of the gastrointestinal tract: revisited. World J Gastroenterol. 2014;20(40):14831–40.
Sharma V, Mandavdhare HS, Dutta U. Mucosal response in discriminating intestinal tuberculosis from Crohn's disease-when to look for it? Aliment Pharmacol Ther. 2018;47(6):859–60.
Park YS, Jun DW, Kim SH, et al. Colonoscopy evaluation after short-term anti-tuberculosis treatment in nonspecific ulcers on the ileocecal area. World J Gastroenterol. 2008;14(32):5051–8.
Lawn SD, Obeng J, Acheampong JW, Griffin GE. Resolution of the acute-phase response in west African patients receiving treatment for pulmonary tuberculosis. Int J Tuberc Lung Dis. 2000;4(4):340–4.
Scott GM, Murphy PG, Gemidjioglu ME. Predicting deterioration of treated tuberculosis by corticosteroid reserve and C-reactive protein. J Infect. 1990;21(1):61–9.
Sharma V, Mandavdhare HS, Lamoria S, et al. Serial C-reactive protein measurements in patients treated for suspected abdominal tuberculosis. Dig Liver Dis. 2018;50(6):559–62.
Clifford V, Zufferey C, Street A, et al. Cytokines for monitoring anti-tuberculous therapy: a systematic review. Tuberculosis (Edinb). 2015;95(3):217–28.
Harari A, Rozot V, Enders B, et al. Dominant TNF-α+ mycobacterium tuberculosis-specific CD4+ T cell responses discriminate between latent infection and active disease. Nat Med. 2011;17(3):372–6.
Ong CW, Elkington PT, Friedland JS. Tuberculosis, pulmonary cavitation, and matrix metalloproteinases. Am J Respir Crit Care Med. 2014;190(1):9–18.
Sharma V, Verma S, Kumar-MP, et al. Serial measurements of faecal calprotectin may discriminate intestinal tuberculosis and Crohn’s disease in patients started on antitubercular therapy. Eur J Gastroenterol Hepatol. 2021;33(3):334–8.
Ma L, Zhu Q, Li Y, et al. The potential role of CT enterography and gastrointestinal ultrasound in the evaluation of anti-tubercular therapy response of intestinal tuberculosis: a retrospective study. BMC Gastroenterol. 2019;19(1):106.
Jain R, Sawhney S, Bhargava DK, Berry M. Diagnosis of abdominal tuberculosis: sonographic findings in patients with early disease. AJR Am J Roentgenol. 1995;165(6):1391–5.
Kedar RP, Shah PP, Shivde RS, Malde HM. Sonographic findings in gastrointestinal and peritoneal tuberculosis. Clin Radiol. 1994;49(1):24–9.
Mathur P, Sharma R, Kandasamy D, et al. Can ADC be used as a surrogate marker of response to therapy in intestinal tuberculosis? Abdom Radiol (NY). 2019;44(9):3006–18.
Chen RY, Dodd LE, Lee M, et al. PET/CT imaging correlates with treatment outcome in patients with multidrug-resistant tuberculosis. Sci Transl Med. 2014;6(265):265ra166.
Martinez V, Castilla-Lievre MA, Guillet-Caruba C, et al. (18)F-FDG PET/CT in tuberculosis: an early non-invasive marker of therapeutic response. Int J Tuberc Lung Dis. 2012;16(9):1180–5.
Park YH, Yu CM, Kim ES, et al. Monitoring therapeutic response in a case of Extrapulmonary tuberculosis by serial F-18 FDG PET/CT. Nucl Med Mol Imaging. 2012;46(1):69–72.
Appasani S, Nagi B, Kochhar R, et al. Radiological evaluation of response to anti-tuberculosis treatment in intestinal tuberculosis. Am J Gastroenterol. 2011;106:S91.
Shah J, Maity P, Kumar-M P, Jena A, Gupta P, Sharma V. Gastroduodenal tuberculosis: a case series and a management focused systematic review. Expert Rev Gastroenterol Hepatol. 2021;15(1):81–90.
Puri AS, Sachdeva S, Mittal VV, et al. Endoscopic diagnosis, management and outcome of gastroduodenal tuberculosis. Indian J Gastroenterol. 2012;31(3):125–9.
Dalal A, Puri AS, Sachdeva S, Sakuja P. Nonsurgical management of gastroduodenal tuberculosis: nine-year experience from a tertiary referral center. Endosc Int Open. 2019;7(10):E1248–52.
Amarapurkar DN, Patel ND, Amarapurkar AD. Primary gastric tuberculosis--report of 5 cases. BMC Gastroenterol. 2003;3:6.
Udgirkar S, Surude R, Zanwar V, et al. Gastroduodenal tuberculosis: a case series and review of literature. Clin Med Insights Gastroenterol. 2018;11:1179552218790566.
Upadhyaya VD, Kumar B, Lal R, Sharma MS, et al. Primary duodenal tuberculosis presenting as gastric-outlet obstruction: its diagnosis. Afr J Paediatr Surg. 2013;10(2):83–6.
Zhu R, Zhou Y, Wang H, et al. Gastric tuberculosis mimicking submucosal tumor: a case series. BMC Gastroenterol. 2020;20(1):23.
Park JH, Kim SU, Sohn JW, et al. Endoscopic findings and clinical features of esophageal tuberculosis. Scand J Gastroenterol. 2010;45(11):1269–72.
Puri R, Khaliq A, Kumar M, Sud R, Vasdev N. Esophageal tuberculosis: role of endoscopic ultrasound in diagnosis. Dis Esophagus. 2012;25(2):102–6.
Rana SS, Bhasin DK, Rao C, Srinivasan R, Singh K. Tuberculosis presenting as dysphagia: clinical, endoscopic, radiological and endosonographic features. Endosc Ultrasound. 2013;2(2):92–5.
Devarbhavi HC, Alvares JF, Radhikadevi M. Esophageal tuberculosis associated with esophagotracheal or esophagomediastinal fistula: report of 10 cases. Gastrointest Endosc. 2003;57(4):588–92.
Jain SK, Jain S, Jain M, Yaduvanshi A. Esophageal tuberculosis: is it so rare? Report of 12 cases and review of the literature. Am J Gastroenterol. 2002;97(2):287–91.
Tang Y, Shi W, Sun X, Xi W. Endoscopic ultrasound in diagnosis of esophageal tuberculosis: 10-year experience at a tertiary care center. Dis Esophagus. 2017;30(8):1–6.
Hunter VJ, Weinberg JB, Haney AF, et al. CA 125 in peritoneal fluid and serum from patients with benign gynecologic conditions and ovarian cancer. Gynecol Oncol. 1990;36(2):161–5.
Buamah P. Benign conditions associated with raised serum CA-125 concentration. J Surg Oncol. 2000;75(4):264–5.
Yoshimura T, Okamura H. Peritoneal tuberculosis with elevated serum CA 125 levels: a case report. Gynecol Oncol. 1987;28(3):342–4.
Candocia SA, Locker GY. Elevated serum CA 125 secondary to tuberculous peritonitis. Cancer. 1993;72(6):2016–8.
Yilmaz A, Ece F, Bayramgürler B, et al. The value of ca 125 in the evaluation of tuberculosis activity. Respir Med. 2001;95(8):666–9.
O'Riordan DK, Deery A, Dorman A, Epstein OE. Increased CA 125 in a patient with tuberculous peritonitis: case report and review of published works. Gut. 1995;36(2):303–5.
Gürgan T, Zeyneloğlu H, Urman B, et al. Pelvic-peritoneal tuberculosis with elevated serum and peritoneal fluid ca-125 levels. A report of two cases. Gynecol Obstet Investig. 1993;35(1):60–1.
Saluja SS, Ray S, Pal S, et al. Hepatobiliary and pancreatic tuberculosis: a two decade experience. BMC Surg. 2007;7:10.
Panic N, Maetzel H, Bulajic M, et al. Pancreatic tuberculosis: a systematic review of symptoms, diagnosis and treatment. United European Gastroenterol J. 2020;8(4):396–402.
Sharma V, Rana SS, Kumar A, Bhasin DK. Pancreatic tuberculosis. J Gastroenterol Hepatol. 2016;31:310–8.
Kim JB, Lee SS, Kim SH, et al. Peripancreatic tuberculous lymphadenopathy masquerading as pancreatic malignancy: a single-center experience. J Gastroenterol Hepatol. 2014;29(2):409–16.
Rana SS, Bhasin DK, Srinivasan R, et al. Distinctive endoscopic ultrasound features of isolated pancreatic tuberculosis and requirements for biliary stenting. Clin Gastroenterol Hepatol. 2012;10(3):323–5.
Santhosh S, Bhattacharya A, Rana SS, et al. Pancreatic tuberculosis: evaluation of therapeutic response using F-18 fluoro-2-deoxy-D-glucose positron emission tomography/computed tomography. Indian J Nucl Med. 2014;29(4):257–9.
Chaudhary P. Hepatobiliary tuberculosis. Ann Gastroenterol. 2014;27(3):207–11.
Chong VH. Hepatobiliary tuberculosis: a review of presentations and outcomes. South Med J. 2008;101(4):356–61.
Alvarez SZ. Hepatobiliary tuberculosis. J Gastroenterol Hepatol. 1998;13(8):833–9.
Yu RS, Zhang SZ, Wu JJ, Li RF. Imaging diagnosis of 12 patients with hepatic tuberculosis. World J Gastroenterol. 2004;10(11):1639–42.
Murata Y, Yamada I, Sumiya Y, et al. Abdominal macronodular tuberculomas: MR findings. J Comput Assist Tomogr. 1996;20(4):643–6.
Behera A, Kochhar R, Dhavan S, et al. Isolated common bile duct tuberculosis mimicking malignant obstruction. Am J Gastroenterol. 1997;92(11):2122–3.
Alvarez SZ, Carpio R. Hepatobiliary tuberculosis. Digest Dis Sci. 1983;28:193–200.
Chong VH, Lim KS. Hepatobiliary tuberculosis. Singap Med J. 2010;51(9):744–51.
Cho JK, Choi YM, Lee SS, et al. Clinical features and outcomes of abdominal tuberculosis in southeastern Korea: 12 years of experience. BMC Infect Dis. 2018;18(1):699.
Acknowledgments
None.
Conflicts of Interest
None.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Birda, C.L., Sharma, V. (2022). Response to Therapy in Abdominal Tuberculosis. In: Sharma, V. (eds) Tuberculosis of the Gastrointestinal system. Springer, Singapore. https://doi.org/10.1007/978-981-16-9053-2_21
Download citation
DOI: https://doi.org/10.1007/978-981-16-9053-2_21
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-16-9052-5
Online ISBN: 978-981-16-9053-2
eBook Packages: MedicineMedicine (R0)