Abstract
Plants and microbes are the reservoirs of many structural and biological distinctive properties, which can be used to combat against environmental pollutants. They are the primary producers of ecosystems and transfer the primary productivity in the form of carbon energy to higher trophic levels in the food chain. They play a pivotal role in protecting the environment by reducing greenhouse gas emission, excess nutrients, heavy metal degradation, and other pollutants. They are found in every kind of habitat such as terrestrial, aquatic, and desert. A direct correlation has been identified among different plant and microbial communities, at different pollution levels, and different heavy metals in their habitats. Besides this, species composition of plants and microbes depends on different types of habitats and abiotic environmental factors. They consume excess amount of nutrients from the land and wastewater and take up CO2 from the environment by the process of photosynthesis maintain the biological oxygen demand (BOD) and chemical oxygen demand (COD) of the habitat and restore the original conditions of natural habitat by reducing environmental and soil pollution. Further, their biomass can be used for bioenergy production, food production, and novel biochemical production for human and animal welfare. Further, their residual part can be utilized as fodder to cattle. The main aim of the article is to overview the advances in current and futuristic techniques for phytoremediation.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
The removal of heavy metal contamination has become a challenge for the environment, as various physical and chemical techniques used to remediate the toxicants are environmentally disruptive and have the chances of secondary contamination. Therefore, in the present scenario, bioremediation techniques offer an economic and environment-friendly approach as a suitable alternative to remediate the contaminants. The use of plants and their products, as well as microbes, is playing a pivotal role in phytoremediation (Guleri et al. 2020). The word phytoremediation is derived from the Greek word “phyton” meaning “plant” and “remedium” means “to correct.” It helps in the removal of elemental pollutants to lower their availability in the soil. The environment has been continuously loading with the heavy metal pollutants as a result of the progression of urbanization and industrialization, which is significantly contributing to the deterioration of the environment health across the globe, hence becoming a major concern (Siddiqua et al. 2021; Ashraf et al. 2019). Other than plants, microalgae are the largest group of primary producers comprising 40–60% of aquatic diversity. They play predominant role in pollution reduction in aquatic ecosystems. Microalgae are single-celled and primary food source of second trophic organisms such as copepods (Dhanker et al. 2013). They are the best source to make the adsorbent model for the reduction in pollutants from their environment having several advantages over chemical adsorbent models (Guleri et al. 2020). Besides having prominent role in phytoremediation, they secrete many bioactive compounds, which act as sanitizer deterrents and the influence reproduction and growth rate of invertebrates (Dhanker et al. 2015) and aquatic stages of mosquito larvae (Dhanker et al. 2020). The anthropogenic sources, i.e., petroleum industries (Pitche 2016), agricultural practices (Hamzah et al. 2016), chemicals (Iqbal et al. 2016), mining (Chen et al. 2016), sewage (Farahat and Linderholm 2015), and electroplating (Muradoglu et al. 2015), are the chief originating medium for metalloids and heavy metals. However, the role of heavy metals in plant growth is indisputable, but it is restricted to a specific quantity. On this basis, they can be categorized as essential and nonessential heavy metals. Ni, Fe, Cu, Mn, and Zn are referred as essential heavy metals as they are essentially required up to a certain extent for various physiological and biochemical reactions (Cempel and Nikel 2016), while Pb, Cd, As, and Hg are referred as nonessential heavy metals with unknown functions in plants (Fasani et al. 2018). The excess amount of heavy metals results in alteration of physiological and biochemical processes and deteriorates the crop and human health (Haris et al. 2020; Rehman et al. 2017). This is a very alarming situation for the various components of environment, especially the heavy metal contamination in soil that may ultimately lead to the biomagnification. Most physical and chemical applications are expensive. Therefore, phytoremediation is the best suitable alternative for the removal of heavy metal contaminants by lowering their availability in the soil, hence preventing them to enter the food chain. This approach is plant-based, economic, easily available, and eco-friendly and reduces the risk of disposal. Sometimes, it is helpful in restoring the lost metals Ni, Fe, Zn, and Se to soil through phytomining and biofortification (Hussain et al. 2021). In this regard, plant selection for a specific metal is a key process for the final outcome. The main aim is to overview the advances in current and futuristic techniques for phytoremediation.
2 Need of Bioremediation of Heavy Metal-Infected Areas
2.1 Bioremediation
The process of bioremediation employs the use of microorganisms, which are present in nature or used deliberately to recover and repair the heavy metal-contaminated environment in the ecosystem. Bioremediation is an inventive and optimistic approach applied for retrieval and reduction in heavy metals in polluted sites. It is cost-effective and environment-friendly, with minimal health hazards and methodological way of revitalizing environment, polluted with toxic metals by making use of the characteristic biological mechanism of plants and microorganisms (Dhankar et al. 2020). In contrast to the conventional physical and chemical strategies, which are often costly, dangerous to life, and it is not much effective, when metals are at low concentration, and produce a substantial amount of sludge, which is toxic (Ekperusi and Aigbodion 2015). Blaylock et al. (1997) reported bioremediation as effective and economical, and he was able to save 50–65% of the cost of one acre Pb polluted soil by using bioremediation treatment in contrast to conventional methods such as landfill and excavation (Jain and Arnepalli 2016). Therefore, there is a need to pave a novel, systematic, cost-effective, and eco-friendly perspective for the retrieving inorganic metals (chromium, mercury, cadmium, and lead) added into the biosphere to shield the environment. The basic principle of bioremediation includes reducing the solubility of toxic heavy metals by altering pH, the redox reactions, and adsorption of hazardous waste from infected environment (Hussain and Dhanker 2021; Gadd 2010). Principally, bioremediation is a state of art used to neutralize and counteract heavy metal-contaminated soil and water, either ex situ or in situ. In this concern, microbes are considered as an important contrivance for retrieving metals as they can concentrate, remove, and make less soluble heavy metals from environment.
2.2 Need for Bioremediation in Heavy Metal-Affected Areas
During the last centenary natural processes, anthropogenic activities, unmanaged use of agrochemical, etc., have defiled areas of highly developed countries with high contamination of heavy metals. Heavy metal studies have defined its elements, which are present in nature possessing a high density and atomic weight, five times more than water. Toxic heavy metals are regularly present in natural waters but in tracer amounts, but many of them are toxic even when low. Nevertheless, the low levels of metals like arsenic, lead, cadmium, nickel, mercury, chromium, cobalt, zinc, and selenium are highly toxic and affect growth and development. Heavy metals if not properly assimilated in the body become injurious and start depositing in the body tissues. They make a pathway to enter the human body through water, air, food, or absorption through the skin surface when they are in contact with humans in pharmaceutical, manufacturing, agricultural, industrial, or residential areas (Dhankar et al. 2021). The chemical methods are cost-effective, which includes the use of heavy chemicals for washing such as leaching of heavy metals/mobilization into environment segments using chelating agents. Lately, the researchers shed light on heavy metal bioremediation as an emerging tool to conventional techniques. This could be done by new hopeful technology known as phytoremediation also called botanical remediation. On the basis of the mode of action, phytoremediation is further divided into subclasses such as phytostabilization, phytofiltration, phytovolatilization, and phytoextraction. Phytoremediation is a method for either converting or recovering toxic heavy metals into a less toxic form and/or get rid of these elements from the contaminated zone or downgrading organic substances and eventually mineralizing organic compounds into CO2, H2O, and N2 gas, availing dead or living biomass. The method of bioremediation is applicable for both water and soil media through in situ and ex situ procedure. A major concern is adopting a relevant biosorbent in terms of economy and potential. Redox reaction is carried out by microbes and hence alters the bioremediation due to metal mobilization/immobilization (Ahamd et al. 2019).
2.3 Bioremediation Mechanism
The microorganism is ubiquitously present exhibiting resistance toward heavy metals and adapts themselves in a contaminated environment. On the cell wall, certain polymeric substances (present extracellularly) attach to heavy metals by phenomena like micro-precipitation or proton exchange of metals (Fomina and Gadd 2014). Due to the presence of amino, phosphoryl carboxyl, and sulfo groups, biomass surfaces exhibit a negative charge as potential ion exchange sites and metal sinks. Redox process, ion exchange, adsorption, precipitation, complexation, and electrostatic attraction are various channels involved in the bioremediation mechanism. Microbes may inculcate mobilization/immobilization of heavy metals through redox reactions and thus influence bioremediation. As, Fe, Hg, and Cr like heavy metals undergo oxide reduction processes (Glass 2000). Bioremediation process is hastened by changing an element from its stationary and insoluble form in sediments into its soluble and mobile phase. Many detrimental effects have been observed because of mobilization when metal ions are redistributed and released from their insoluble state from sediments into the soluble state (Fomina and Gadd 2014). Microbial metal immobilization serves as pool for metals by adopting different mechanisms (both in situ and ex situ) like bioconversion, bioaccumulation, biosorption, and/or intra/intercellular precipitation (as oxalates of Cd, Ni Zn, Cu, Co) employed in different ways (Comte et al. 2008; Mosa et al. 2016). Furthermore, due to impairment, an element can be easily drained from its aqueous phase in groundwater or wastewater (Klaus-Joerger et al. 2001; Ahluwalia and Goyal 2007). Cell wall and plasma membrane-like cellular inclusions act as fences and explore the entry of metal ions toward cells (Poirier et al. 2013). Bioaccumulation and biosorption are more attractive options to substitute traditional methods for bioremediation of heavy metals. Bioaccumulation sheds light to heavy metal uptake by living biomass (metabolism-dependent/active uptake) and is characterized by the absorption of contaminants by living cells/biomass (Haris et al. 2021). Biosorption is more superior in contrast to active bioaccumulation, as it is metabolized independently; however, it is largely dependent on the biomass/biosorbent type and contaminants present (Paliwal et al. 2012). Bioremediation of heavy metals in their metallic nanoparticles form, with the help of bacteria and the use of genetically engineered microbes as a part of bioremediation mechanism, has also been manipulated (Klaus-Joerger et al. 2001; Paliwal et al. 2012; Poirier et al. 2013; Mosa et al. 2016). Bioremediation serves many advantages, but also certain limitations, which should be kept into consideration while applying these methods (Table 11.1).
With the recent researches in the biotechnological field, genetically modified plants can be favorable in the phytoremediation approaches for making environment upstanding (Fasani et al. 2018). Futuristic studies should be highlighted on the combined use of more than one phytoremediation application for the successful interaction with heavy metal and ameliorated polluted area under field conditions.
3 Mechanism of Detoxification of Heavy Metals by Microbes
The society is dealing with many health and environmental challenges such as discharged household and industries, agricultural seepage, and other biotoxic substances containing practices. Hence, the emergence for the propelling need of cost-effective, eco-friendly, novel, and efficient solution to shelter the ecosystem for the amelioration of inorganic metals (Hg, Cr, Pb, and Cd) is present in the environment. In this context, current advancements in microbes-based heavy metal remediation have emerged as alternative promising conventional techniques. Heavy metals are nonbiodegradable and toxic to the microbes. The diverse forms of microbes have matured to generate potent mechanisms for detoxification to mitigate the detrimental effects of aforesaid metals. The microbes with bioremediation potential, especially in reference to environmental safety, have been underestimated for a long time. Furthermore, the application of bacteria, biofilm, genetically altered microbes, algae, fungi, and microbial cells of immobilized nature has the capability to remove heavy metals through biosorption. For the sake of a sustainable environment, the heavy metal removal is preferred by the use of microbes resulting in the synergetic effects with many folds increasing (Yang et al. 2015). Heavy metals are not only the most important and long-lasting pollutant in the environment but economically very significant in industry. The current scenario of the ecosystem is combating pollution load due to heavy metals, which have been a toxic situation to the living organisms (Wu et al. 2010; Su 2014; Siddiquee et al. 2015; Okolo et al. 2016).
3.1 Microbial Remediation of Heavy Metals
Microorganisms follow different detoxification mechanisms to combat and survive amid of metal contamination. Microbes use various mechanisms to cope up metal toxicity such as the production of exopolysaccharide (EPS), biotransformation, extrusion, and use of enzymes (Dixit et al. 2015; Wu et al. 2010). The microbes respond to metals retained by the environment through many evolved ingenious metal detoxification and resistance mechanisms (Kumar et al. 2021). These mechanisms include electrostatic interaction, precipitation, ion exchange, surface complexation, on redox process alone or together (Yang et al. 2015).
Microorganisms decontaminate pollution by volatilization, valence conversion, and extracellular chemical precipitation (Tripathi and Hussain 2022). The microbes have negatively charged extracellular surface owing to the presence of anions that help microbes to increase the binding of cations (Gavrilescu 2004). The anionic sites on the microbial surface have the groups such as alcohol, phosphoryl, sulfonate, hydroxyl, sulfhydryl, ester, carboxyl, amine, thiol groups, and thioether, which facilitate the adsorption of metal (Gavrilescu 2004) (Fig. 11.1).
3.2 Toxicity of Heavy Metals to Microbes
Contamination by heavy metals is the potent source to cause toxic effects on microorganisms, and it is correlated with the preoccupied dose and accumulation of heavy metal (Rasmussen et al. 2000). Contamination of heavy metal involves several mechanisms, that is, destructing ion regulation, reacting as redox catalysts in the production of reactive oxygen species (ROS), breaking fatal enzymatic functions, and directly affecting the formation of DNA and protein (Hildebrandt et al. 2007). The biochemical and physiological properties of microbes are altered by the exposure of heavy metals. Metals such as cadmium (Cd) and chromium (Cr) have the capacity of calculating denaturation and oxidative injury of microbes and reducing the detoxification capacity of microbes.
Reactivity of metals such as Cr (III) can alter the activity and formation of enzymes due to the presence of thiol and carboxyl groups. The DNA with the negative charge on the phosphate group could interact with the complexes of cationic Cr (III) present intracellularly, and it might affect transcription and replication leading to the mutations.
Heavy metals cause severe injuries to DNA, lipids, proteins, and other cytoplasmic inclusions due to Fenton and Haber–Weis reactions, which catalyze the production of ROS. These ROS act as soluble electron carriers. Some metals are mainly responsible for DNA damage because they could enable the stabilizing superoxide radicals (Booth et al. 2015). Enzymatic interactions could be restricted through the configuration changes induced by competitive and noncompetitive inhibitors as heavy metals. The ionic imbalance is the result of the heavy metal adhesion on the cell surface and sometimes inflow through the transmembrane carrier or ion channels (Chen et al. 2014).
Lead (Pb) and cadmium (Cd) pose damaging effects on the cell membrane of microbes and cause DNA activity. This detrimental effect is responded due to the metal displacement from ligand interactions or native binding sites (Olaniran et al. 2013). The altered function of nucleic acid causes disrupted cell membrane, much functional disturbance, arrested enzyme activity, and oxidative phosphorylation (Fashola et al. 2016), which ultimately affect the growth, metabolism, and morphology of microbes (Fig. 11.2)
.
3.3 Heavy Metal Detoxification by Microbes
Microbes use different mechanisms to survive under metal exposure, which are extrusion, biotransformation, production of exopolysaccharide (EPS), use of enzymes, and synthesis of metallothioneins. The process involves procedures, electrostatic reactions, precipitation and exchange of ion, surface complexation, and redox interactions. Microbes withstand heavy metals by methylation, metal oxidation, organic–metal complex, enzymatic decrease, metal decrease along with efflux pumps, metal–ligand degradation, extracellular and intracellular sequestration of metals, removal by the permeable barrier, and presence of metal chelators such as metallothioneins and biosurfactants (Saghafi et al. 2019).
3.4 Biosorption Mechanism
The heavy metals uptake by microbes could be categorized into metabolically independent biosorption, that is prevalent on the cell surface, while metabolism-dependent bioaccumulation involves redox reaction, species transformation, and sequestration processes. A variety of metal ion compounds form complex for intracellular sequestration in the cytoplasm. The surface ligands interact with metals and increase their concentration within microbial cells, which are further transported to intracellular compartments. The metals are stored intracellularly by bacterial cells that have been efficiently utilized, especially in the effluent treatment methods. The rigidity of fungal cell wall is due to the presence of lipids, mineral, chitin, polyphosphates, protein, and polysaccharide with nitrogen constituents. The cell wall could be helpful in decontamination of metal ions by precipitation from the extracellular surface and intracellular components and uptake influence the valence conversion of some fungi aggravating metal accumulation to their spores and mycelium. The external surface of fungal cell wall can behave like a ligand and eliminate inorganic metals (Ayangbenro and Babalola 2017).
Microalgae are used as a bioindicator for the health of the aquatic ecosystem. The presence and absence of a particular type of species indicate the quality parameters or pollution level of any aquatic ecosystem. Many microalgae species, especially diatoms, have very unique structural cell structure, which makes them a perfect resource of pollutant degradation and accumulator of heavy metal (Tripathi and Hussain 2021; Hildebrand et al. 2012). On one hand, they absorb excess amounts of nutrients from wastewater and utilize it in increasing their population. On the other hand, microalgae use carbon dioxide for increasing their productivity like this they sequester excess amounts of greenhouse gas and contribute to the reduction in global warming (Janani and Kumar 2018). For example, Thalassiosira sp. is main diatom sp. having a dominant role in pollution reduction in water bodies. Heavy metals, cadmium, and copper are observed in almost every kind of aquatic habitats. Accumulation of these metals is observed in many microalgal species such as Thalassiosira weissflogii.
3.5 Extracellular Sequestration
The metal ions are extracellularly sequestered in cellular components, the periplasm, or complexation of metal ions as insoluble compounds. Copper-resistant Pseudomonas syringae strains result in copper-inducible proteins CopA, CopB (periplasmic proteins), and CopC (outer membrane protein), which attach with copper ions and microbial colonies. Recent studies have reported extraction of copper from contaminated soil by sulfur-oxidizing bacteria, followed by electrokinetic treatment using a DC current to further acidify the soil and collect the mobilized metals at the cathode. Iron-reducing bacteria such as Geobacter spp. and sulfur-reducing bacteria like Desulfuromonas spp. are able to reduce metals to less or nontoxic metals. Biological methods used for extraction of metals other than metal reduction include the organic acids production by heterotrophic organisms and the formation of sulfuric acid by oxidation of sulfur (e.g., by Thiobacillus spp.). G. metallireducens, an anaerobe, is able to reduce manganese (Mn), from lethal Mn. G. sulfurreducens and G. metallireducens are able to reduce chromium (Cr) from the lethal Cr (VI) to less toxic Cr (III). Sulfate-reducing bacteria produce hydrogen sulfide causing precipitation of metal cations (Frers 2009).
3.6 Extracellular Barrier of Preventing Metal Entry Into Microbial Cell
The plasma membrane, cell wall, or capsule fences metal ions from entering into the cell. Adsorption of metal ions is facilitated by ionizable groups of the bacterial cell wall. Pseudomonas aeruginosa biofilm cells show higher resistance toward copper, lead, and zinc ions than planktonic cells, whereas peripheral cells of the biofilm were killed. Accumulation of metal ions by extracellular polymers of biofilm takes place and then protects bacterial cells inside the biofilm.
3.7 Methylation of Metals
Metal toxicity is increased by methylation as a result increase in lipophilicity followed by increased permeability across cell membranes. Methylation plays a pivotal role in metal decontamination. Methylated compounds are usually explosive; for instance, Hg (II) can be biomethylated by bacteria such as Bacillus spp., Escherichia spp., Clostridium spp., and Pseudomonas spp. to gaseous methyl mercury (Ayangbenro and Babalola 2017).
3.8 Reduction in Heavy Metal Ions by Microbial Cell
Microbial cells convert the oxidation state of metal ions, hence reducing their toxicity. Bacteria for energy generation use metals and metalloids as electrons donors or acceptors. Metal ions are reduced by enzymatic activities, which result in the formation of the less toxic form of mercury and chromium inorganic metals.
4 Mechanism of Detoxification of Heavy Metals By Plants
The population has been increasing day by day, and the United Nations in 2018 estimated that the global births would be 74 million per year until 2027 through the Food and Agricultural Organization. This would be raising urbanization, industrialization, deforestation, agriculture, and energy supply to fulfill the global demands. The result is an increase in pollution and making the soil and water of poor quality. Human military activities, industrial mining, waste disposal, and farming practices are responsible for large-scale contamination with heavy metals and organic compounds in soil and water (Ayangbenro and Babalola 2017). The group of chemical elements of relatively high atomic number, atomic weight, and high densities are referred as heavy metals. The commonly found metalloids or heavy metals include lead (Pb), mercury (Hg), cadmium (Cd), zinc (Zn), arsenic (As), chromium (Cr), copper (Cu), and nickel (Ni). These are originated from natural and anthropogenic sources such as oil and gas industries (Neff et al. 2011), use of phosphate fertilizers in cropping (Hamzah et al. 2016; Rafique and Tariq 2016), sewage sludge (Farahat and Linderholm 2015), metal mining and smelting (Chen et al. 2016), and electroplating and burning fossil (Muradoglu et al. 2015). According to their roles in the biological system, heavy metals are grouped into essential and nonessential heavy metals. Zn, Cu, Ni, Fe, and Mn are referred to as essential heavy metals that require in physiological and biochemical activities of plants but become toxic when in large amount. Hg, Pb, As, and Cd are categorized as nonessential heavy metals that are highly toxic and decline agricultural productivity (Fasani et al. 2018).
The heavy metals are biodegradable-resistant and cannot be degraded by any physio- and biological processes, and they get accumulated into the human body through the food chain, persist in the environment, and increase in concentration eventually by biomagnification (Suman et al. 2018; Sarwar et al. 2010). Hence, heavy metals create a negative impact on the ecosystem and human health. Therefore, it is crucial to detoxify and remediate water and soil. Since from the decade, conventional methods of soil decontamination, which are pumping and treat, stabilization, soil washing, air stripping precipitation, thermal and adsorption, have been applied (Sheoron et al. 2011; Wuanna and Okieimen 2011; Rehman et al. 2017; Dal Carso et al. 2019). But these conventional techniques are expensive and furthermore do not really solve the problem. So, it is an urgent requirement for substitute practices, which are elegant, cost-effective, and capable to clean up heavily contaminated sites efficiently (Meagher 2000). A new alternative eco-friendly and cost-effective technology known as phytoremediation could be achieved. Phytoremediation is plant-based approach in which high-efficient metal-accumulated plants are used to make contaminated soil nontoxic. This method is also known as bioremediation, botanical bioremediation, or green remediation. In this technique, wild or genetically modified plants species are used to collect a large number of heavy metals from the soil so that it could lower the bioavailability and remove elemental pollutants from the soil.
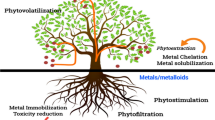
4.1 Methods of Phytoremediation
Several methods of phytoremediation are as follows:
4.1.1 Phytoextraction
This excludes organic compounds or metals from the soil due to their accumulation in the biomass. The roots absorb substances from the soil or water and concentrate it above the ground in the plant biomass (Ali et al. 2013). Plants that are hyperaccumulators can uptake higher amount of contaminants (Rascio and Navari-Izzo 2011).
4.1.2 Phytodegradation
It is also called phytotransformation in which plants are utilized to store and degrade or mineralize inside the plant cells by specific enzymes that include nitroreductases, dehalogenases, and laccases (Rylott and Bruce 2008).
4.1.3 Phytostabilization
The bioavailability of pollutants is reduced by binding or immobilizing them in the soil matrix. Plants make the substances limited to leach from the soil (Lone et al. 2008).
4.1.4 Phytovolatilization
In this technique, plants use pollutants, and transform and release them into the atmosphere from growth matrix (Ruiz and Daniell 2009).
4.1.5 Phytofiltration
The root system or other submerged parts of the plants absorb, precipitate, and concentrate the contaminants specifically heavy metals or sometimes radioactive elements (Javed et al. 2019).
4.1.6 Rhizodegradation
It is also known as phytostimulation in which growing roots of plants stimulate the proliferation of microbes in degrading rhizosphere. They utilize metabolites and exudates of the plants as a source of energy and carbon (Ali et al. 2013; Jacob et al. 2018).
4.1.7 Phytomining
Some metals can be restored from plant parts that have been applied by humans in potash. It may be profitable too. Besides accumulating toxic and unwanted minerals in their parts, plants also absorb contaminated organic compounds such as polychlorinated biphenyl (PCB).
4.2 Advantages and Limitations of Phytoremediation
It is in situ and passive technique, which has minimized environmental impact and contributed to landscape improvisation. This is useful to maintain the fertility of the soil (Othman Yahia and Daniel 2018). It is responsible to reduce dispersal of pollutants and dust by wind, leaching, surface run-off, and transportation of soil contaminants. Overall, it is low-cost and eco-friendly technique. Besides offering several advantages, phytoremediation has some limitations, which should be considered when seeking to apply it. Though it is of low cost but a time taking technology. Its treatment is slower than that of the traditional methods. The concentration of pollutants and other toxins could determine or exceed the tolerance limit of the plants. Usually, plants are selective in metal accumulation. If the concentration and toxicity of metals are more in the soil then it can be lethal for the plants too (Marques et al. 2009).
4.2.1 Genetic Engineering in Phytoremediation
To overcome the limitations of phytoremediation, recombinant DNA technology has been proved an effective technique to improve the tolerance potential of plants toward heavy metals. To make the plant genetically modified, the foreign gene of interest is selected from a particular plant species or animal or bacteria, inserted, and transferred into the genome of the target. This process produces the inherited desired gene after DNA recombination and confers specific traits to that particular plant or animal or bacteria (Maurya et al. 2020; Chaurasia et al. 2021). This is comparatively unparalleled to the traditional method of breeding and advantageous to produce plants with desired traits such as phytoremediation (Marques et al. 2009). It is the most common strategy to produce GMPs for increasing the heavy metal efficiency in the plants to enhance phytoremediation.
5 New Innovative Approaches for Removal of Heavy Metals
Phytoremediation approach is not a new technique in terms of contaminant removal, but no doubt it has been overshadowed for a long time. Plants have been reported to remediate the wastewater even 300 years ago. This technique involves many different applications using plants, plant products, and microbes. The approaches include multiple processes such as degradation, filtration, extraction, stabilization, and volatilization.
5.1 Phytodegradation
Plant’s metabolic processes convert the toxic organic pollutants into less toxic substances. Microbes associate the process of degradation (Marques et al. 2009). For example, Populus species and Myriophyllum spicatum have an enzymatic system to degrade the contaminants (Rylott and Bruce 2008).
5.2 Phytofiltration
This technique is also referred to as rhizofiltration. Plants absorb the contaminants through an aqueous medium, filtered by roots. Plants with high accumulation capacity or high root biomass and high tolerance potential are favorable for phytofiltration, for instance, Phragmites australis, Helianthus annuus, Fontinalis antipyretica, Brassica juncea, species of Populus, Lemna, Salix, and Callitriche (Prasad 2004; Pratas et al. 2012).
5.3 Phytoextraction
This technology is also referred to as phytosequestration or phyto-absorption or phytoaccumulation. The variety of studied plants are capable of storing the contaminants in their aerial parts. The major absorbing metals are Cd, Ni, Cu, Zn, Pb, and sometimes for Se and As, e.g., Pteris vittata, Elsholtzia splendens, Thlaspi caerulescens, and Alyssum bertolonii (Van der Ent et al. 2013).
5.4 Phytostabilization
It prevents the direct exposure of soil contaminants to wind, water, humans, and animals as they are covered by the plant community. Tolerant plant species are utilized to immobilize the translocation of metal and metalloids and restrict them in via dose zone (Nanthi et al. 2011). This technique is also effective in preventing secondary waste. But it needs the monitoring to check the stabilizing conditions.
5.5 Phytovolatilization
The process to absorb the contaminants, convert them into nontoxic simpler forms, and release them into the atmosphere by plants is referred to as phytovolatilization. Some rhizobia and their associates help in the degradation of organic contaminants (Jacob et al. 2018), e.g., Astragalus bisulcatus, Brassica napus, Stanleya pinnata, Liriodendron tulipifera, Nicotiana tabacum, and Arabidopsis thaliana (Ali et al. 2013).
5.6 Current Techniques
These abovementioned techniques have been improvised and categorized on the basis of contamination source. The techniques with some variations are stated mentioned:
5.6.1 Hydraulic Barrier
The deep root system of large trees absorbs water in large quantities from the soil, resulting in major soaking of contaminants, which eventually evaporate through transpiration (Dushenkov 2003; Pratas et al. 2012), for example, Populus species.
5.6.2 Vegetation Cover
It includes the trees, herbs, and shrubs to cover the landfills or tailings. This plant surface decreases the pollutant spread as the roots favor the aeration to microbial population, which increase the degradation of pollutants (Nanthi et al. 2011; Van der Ent et al. 2013; Sabir et al. 2015). The main limiting factor of this technique is difficulty in plant root development in tailings.
5.6.3 Constructed Wetlands
This is the oldest process for wastewater treatment and is not considered as pure phytoremediation process due to the inclusion of multiple techniques, i.e., adsorption, filtration, precipitation, and ion exchange (Ali et al. 2013; Jacob et al. 2018). This is mainly present in the areas where water level remains at or near the surface for some seasons in the year. It consists of microbes, algae, vascular phytoplanktons, and organic soils.
5.6.4 Phytodesalination
This is a recent approach to extract salt from saline soils through halophytes. This can be considered the extended part of phytoextraction owing to its peculiarities.
6 Conclusion and Future Prospective
Increasing metal pollution has become a menace for food, agricultural production, and health due to their persistence hence their exponential growth in the environment. To minimize and mitigate heavy metal contamination, multiple techniques have been explored. Genetic engineering approach has emerged as a futuristic potential aid to alter the target plant species with competent traits such as fast growth, good adaption, high biomass production and accumulation, and high heavy metal tolerance to various geological and climatic conditions. Furthermore, microbes and chelating agents can be used either to ameliorate soil health or to increase heavy metal bioaccessibility, which hastens heavy metal accumulation in plants. Phytoremediation is suitable for heavy metal remediation of water, sediments, and soil. The effectiveness of phytoremediation processes could be determined by many available monitoring tools. Despite a few limitations and disadvantages of the phytoremediation process, it is found an effective method for cleaning and recovery of the environment. In the current scenario, bioremediation of heavy metals is exhibiting a positive promising approach for detoxification and metal biosorption, especially from genetically modified microbes and biofilm-mediated techniques such as microbial fuel cell-based techniques and microbial gene transfer. The cell wall of biosorbents composed of polysaccharides and peptidoglycan favors the binding of metal uptake. It has multiple advantages in terms of cost-effectiveness, broad spectrum of pH for high metal affinity, temperature, faster kinetics, and safe technology. Furthermore, extended research intervention is required to be employed on biofilms for gene transfer in bioremediation. Contamination could be significantly decreased with the advances in the arena of environmental biotechnology using genomics in many fields of biology. Phytoremediation is mainly applied technology to decontaminate the site by using plants and related microbes. Purposefully, selected plant species could be efficiently used at metal-polluted sites and different strategies could be devised for future innovations such as chelate-assisted and microbe-assisted methods and genetic engineering, and methods. It would greatly enhance the future of phytoremediation.
References
Ahamd G, Nishat Y, Haris M, Danish M, Hussain T (2019) Efficiency of soil, plant and microbes for the healthy plant immunity and sustainable agricultural system. In: Varma A, Tripathi S, Prasad R (eds) Plant-microbe interface. Springer, Cham. https://doi.org/10.1007/978-3-030-19831-2_15
Ahluwalia SS, Goyal D (2007) Microbial and plant derived biomass for removal of heavy metals from wastewater. Bioresour Technol 98(12):2243–2257
Ali H, Khan E, Sajad MA (2013) Phytoremediation of heavy metals concepts and applications. Chemosphere 91:869–881
Ashraf S, Ali Q, Zahir ZA, Ashraf S, Asghar HN (2019) Phytoremediation: environmentally sustainable way for reclamation of heavy metals polluted soils. Ecotox Environ Safe 174:714–727
Ayangbenro AS, Babalola OO (2017) A new strategy for heavy metal polluted environments: a review of microbial biosorbents. Int J Environ Res Public Health 14:94
Blaylock MJ, Salt DE, Dushenkov S, Zakharova O, Gussman C, Kapulnik, Ensley BD, Raskin I (1997) Enhanced accumulation of Pb in Indian mustard by soil-applied chelating agents. Environ Sci Technol 31:860–865
Booth SC, Weljie AM, Turner RJ (2015) Metabolomics reveals differences of metal toxicity in cultures of Pseudomonas pseudoalcaligenes KF707 grown on different carbon sources. Front Microbiol 6:827
Cempel M, Nikel G (2016) Nickel: a review of its sources and environmental toxicology. Pol J Environ Stud 15:375–382
Chen S, Yin H, Ye J (2014) Influence of co-existed benzo[a]pyrene and copper on the cellular characteristics of Stenotrophomonas maltophilia during biodegradation and transformation. Bioresour Technol 158:181–187
Chen B, Stem AF, Castell N, Gonzalez Caslanedo Y, De La Campa AS, La D, Rosa J (2016) Modeling and evaluation of urban pollution events of atmospheric heavy metals from a large Cu-smelter. Sci Total Environ 539:17–25
Dhankar R, Goyal S, Kumar K, Hussain T (2021) Bacterial community response to pesticides polluted soil. In: Mandal S et al (eds) Recent advancement in microbial biotechnology-agricultural and industrial approach. Elsevier, San Diego, pp 339–355. https://doi.org/10.1016/B978-0-12-822098-6.00010-0
Dhankar R, Tyagi P, Kamble SS, Gupta D, Hussain T (2020) Advances in fungi: rejuvenation of polluted sites. In: Sharma VK, Shah MP, Parmar S, Kumar A (eds) Fungi bio-prospects in sustainable agriculture, environment and nano-technology, vol 2. Elsevier, San Diego, pp 251–275. https://doi.org/10.1016/B978-0-12-821925-6.00012-5. isbn:978-0-12-821925-6
Chaurasia U, Kumar A, Maurya DK, Yadav SK, Hussain T, Maurya VK (2021) Role of nano-biotechnology in agriculture and allied sciences. In: Mallick MA, Solanki MK, Kumari B, Verma SK (eds) Nanotechnology in sustainable agriculture. CRC Press, Boca Raton, pp 69–96
Comte S, Guibaud G, Baudu M (2008) Biosorption properties of extracellular polymeric substances (EPS) towards Cd, Cu and Pb for different pH values. J Hazard Mater 151(1):185–193
Dal Carso G, Fasani E, Manara A, Visioli G, Furini A (2019) Heavy metal pollution: state of the art and initiation in phytoremediation. Int J Mol Sci 20:3412
Dhanker R, Kumar R, Tseng LC, Hwang JS (2013) Ciliate (Euplotes sp.) predation by Pseudodiaptomus annandalei (Copepoda: Calanoida) and effects of mono- and pluri-algal diets. Zool Stud 52:34–44
Dhanker R, Molinero JC, Kumar R, Tseng LC, Ianora A, Hwang JS (2015) Responses of the estuarine copepod Pseudodiaptomus annandalei to diatom polyunsaturated aldehydes: Reproduction, survival and postembryonic development. Harmful Algae 43:74–81
Dhanker R, Tiwari A, Dahms HU, Kumar R, Hwang JS (2020) Influence of three diatom aldehydes against the dengue vector Aedes aegypti (Diptera: Culicidae). Am J Plant Sci 10(10):1749–1762
Dixit R, Wasiullah D, Malaviya (2015) Bioremediation of heavy metals from soil and aquatic environment: an overview of principles and criteria of fundamental processes. Sustain For 7(2):2189–2212
Dushenkov S (2003) Trends in phytoremediation of radionuclides. Plant Soil 249:167–175. https://doi.org/10.1023/A:1022527207359
Ekperusi O, Aigbodion F (2015) Bioremediation of petroleum hydrocarbons from crude oil-contaminated soil with the earthworm: Hyperiodrilus africanus. Biotech 5:957–965
Farahat E, Linderholm HW (2015) The effects of long-term wastewater irrigation on accumulation and transfer of heavy metals in cupressus sempervirens leaves and adjacent soil. Sci Total Environ 51:1–7
Fasani E, Manara A, Martini F, Furini A, Dal Corso G (2018) The potential of genetic engineering of plants for the remediation of soils contaminated with heavy metals. Plant Cell Environ 41:1201–1232
Fashola MO, Ngole-Jeme VM, Babalola OO (2016) Heavy metal pollution from gold mines: environmental effects and bacterial strategies for resistance. Int J Environ Res 13(11):1047
Fomina M, Gadd GM (2014) Biosorption: current perspectives on concept, definition and application. Bioresour Technol 160:3–14
Frers CE (2009) El uso de plantasacuáticasen el tratamiento de aguasresiduales. El Planeta Azul, Carmen de Areco
Gadd GM (2010) Metals, minerals and microbes: geomicrobiology and bioremediation. Microbiology 156(3):609–643
Gavrilescu (2004) Removal of heavy metals from the environment by biosorption. Eng Life Sci 4(3):219–232
Glass DJ (2000) Economic potential of phytoremediation. In: Raskin I, Ensley BD (eds) Phytoremediation of toxic metals. Using plants to clean up the environment. Wiley, New York, pp 15–31
Guleri S, Singh K, Kaushik R, Dhankar R, Tiwari A (2020) Phycogenic synthesis of nanoparticles supported on adsorbent models for the water remediation. J Microbiol Biotechnol Food Sci 10(1):98–106
Hamzah A, Hapsari RI, Wisnubroto EJ (2016) Phytoremediation of cadmium contaminated agricultural land using indigenous plants. Int J Environ Agric 2:8–14
Haris M, Shakeel A, Ansari MA, Hussain T, Khan AA, Dhankar R (2020) Sustainable crop production and improvement through bio-prospecting of fungi. In: Sharma VK, Shah MP, Parmar S, Kumar A (eds) Fungi bio-prospects in sustainable agriculture, environment and nano-technology, vol 1. Elsevier, San Diego. https://doi.org/10.1016/B978-0-12-821394-0.00016-0. isbn:978-0-12-821394-0
Haris M, Shakeel A, Hussain T, Ahmad G, Khan AA (2021) New trends in removing heavy metals from industrial wastewater through microbes. In: Shah MP (ed) Removal of emerging contaminants through microbial processes. Springer, Singapore. https://doi.org/10.1007/978-981-15-5901-3_9
Hildebrand M, Davis AK, Smith SR, Traller JC, Abbriano R (2012) The place of diatoms in the biofuels industry. Biofuels 3:221–240
Hildebrandt U, Regvar M, Bothe H (2007) Arbuscular mycorrhiza and heavy metal tolerance. Phytochemistry 68(1):139–146
Hussain T, Dhanker R (2021) Science of microorganisms for the restoration of polluted sites for safe and healthy environment. In: Shah M, Rodriguez-Couto S (eds) Microbial ecology of wastewater treatment plants. Elsevier, Amsterdam, pp 127–144. https://doi.org/10.1016/C2019-0-04695-X
Hussain K, Haris M, Qamar H, Hussain T, Ahmad G, Ansari MS, Khan AA (2021) Bioremediation of waste gases and polluted soils. In: Panpatte DG, Jhala YK (eds) Microbial rejuvenation of polluted environment. Microorganisms for sustainability, vol 26. Springer, Singapore. https://doi.org/10.1007/978-981-15-7455-9_5
Iqbal M, Iqbal N, Bhatti IA, Ahmad N, Zahid M (2016) Response surface methodology application in optimization of cadmium adsorption by shoe water: a good option of waste mitigation by waste. Ecol Eng 88:265–275
Jacob J, Chinnannan K, Saratale R, Prabakar KS, Desika KK, Pugazhendhi A (2018) Biological approaches to tackle heavy metal pollution: a survey of literature. J Environ Manag 217:56–70. https://doi.org/10.1016/j.jenvman.2018.03.077
Jain S, Arnepalli D (2016) Biominerlisation as a remediation technique: a critical review. In Proceedings of the Indian Geotechnical Conference (IGC2016); Chennai, India, pp 15–17
Janani S, Kumar S (2018) Performance analysis of different textile effluent treatment processes involving marine diatom Odontella aurita. Environ Technol Innov 11:153–164
Javed M, Tanwir K, Akram MS, Shahid M, Niazi NK, Lindberg S (2019) Chapter 20 – phytoremediation of cadmium-polluted water/sediment by aquatic macrophytes: role of plant-induced pH changes. In: Hasanuzzaman M, Prasad MNV, Fujita M (eds) Cadmium toxicity and tolerance in plants. Academic, London, pp 495–529
Klaus-Joerger T, Joerger R, Olsson E, Granqvist C (2001) Bacteria as workers in the living factory: metal accumulating bacteria and their potential for materials science. Trends Biotechnol 19(1):15–20
Kumar A, Hussain T, Susmita C, Maurya DK, Danish M, Farooqui SA (2021) Microbial remediation and detoxification of heavy metals by plants and microbes. In: Shah M et al (eds) The future of effluent treatment plants-biological treatment systems. Elsevier, Amsterdam, pp 589–614. https://doi.org/10.1016/B978-0-12-822956-9.00030-1
Lone MI, Zhen L, He L, Stoffella PJ, Yang X (2008) Phytoremed Heavy Metals Pollut Soils Water 9(3):210–220
Marques AP, Rangel AO, Castro PM (2009) Remediation heavy metals contaminated soils. Phytoremediation as a potentially promising clean up technology. Crit Rev Environ Sci Technol 39:622–654
Maurya DK, Kumar A, Chaurasiya U, Hussain T, Singh SK (2020) Modern era of microbial biotechnology: opportunities and future prospects. In: Solanki MK, Kashyap PL, Ansari RA, Kumari B (eds) Microbiomes and plant health. Elsevier, Massachusett, pp 317–343. https://doi.org/10.1016/B978-0-12-819715-8.00011-2
Meagher RB (2000) Phytoremediation of toxic elemental and organic pollutants. Curr Opin Plant Biol 3:153–162
Mosa KA, Saadoun I, Kumar K, Helmy M, Dhankher OP (2016) Potential biotechnological strategies for the cleanup of heavy metals and metalloids. Front Plant Sci 7:14
Muradoglu F, Gundoglo M, Ercisli S, Encu T, Balta F, Jaafar HZ (2015) Cadmium toxicity affects chlorophyll a and b content, antioxidant enzyme activities and mineral nutrient accumulation in strawberry. Research 48:11
Sabir M, Ejaz A, Waraich K, Rehman H, Öztürk M, Ahmad HR, Muhamad S (2015) Phytoremediation: mechanisms and adaptations soil remediation and plants (prospects and challenges). Elsevier, London, pp 85–105
Neff J, Lee K, Deblois EM (2011) Produced water overview of composition, fates and effects. In: Lees K, Neff J (eds) Produced water, environmental risks and advances in mitigation technologies. Springer, New York, pp 3–54
Nanthi S, Jin B, Park H, Robinson B, Naidu R, Young K (2011) Phytostabilization: a green approach to contaminant containment. Adv Agron 112:147–159
Okolo VN, Olowolafe EA, Akawu I, Okoduwa S (2016) Effects of industrial effluents 581 on soil resource in challawa industrial area. J Global Ecol Environ 5(1):10
Olaniran AO, Balgobind A, Pillay B (2013) Bioavailability of heavy metals in soil: Impact on microbial biodegradation of organic compounds and possible improvement strategies. Int J Mol Sci 14(5):10197–10228
Othman Yahia A, Daniel L (2018) Organic soil amendments influence soil health, yield and phytochemicals of globe artichoke head. Biol Agric Hortic 34:1–10
Poirier I, Hammann P, Kuhn L, Bertrand M (2013) Strategies developed by the marine bacterium Pseudomonas fluorescens BA3SM1 to resist metals: a proteome analysis. Aquat Toxicol 128–129:215–232
Paliwal V, Puranik S, Purohit HJ (2012) Integrated perspective for effective bioremediation. Appl Biochem Biotechnol 166(4):903–924
Prasad MNV (2004) Phytoremediation of metals and radionuclides in the environment: the case for natural hyperaccumulators, metal transporters, soil-amending chelators and transgenic plants. In: Prasad MNV (ed) Heavy metal stress in plants: from biomolecules to ecosystems, 2nd edn. Springer, Berlin, pp 345–391
Pratas J, Favas PJC, Paulo C, Rodrigues N, Prasad MNV (2012) Uranium accumulation by aquatic plants from uranium-contaminated water in Central Portugal. Int J Phytoremediation 14:221–234
Pitche RJ (2016) Oil and gas production waste water: soil contamination and pollution prevention. Appl Environ. Soil Sci 2016:2707989
Rafique N, Tariq SR (2016) Distribution and source apportionment studies of heavy metals in soil cotton/wheat field. Environ Monit Assess 188:309
Rehman MZU, Rizwan M, Ali S, Ok YS, Ishaque W, Saifullah (2017) Remediation of heavy metal contaminated soil by using Solanum nigrum: a review. Ecotox Environ Safe 143:236–248
Rascio N, Navari-Izzo F (2011) Heavy metals accumulating plants: how and why do they do so? And what makes them so interesting? Plant Sci 180(2):169–181
Rylott EL, Bruce NC (2008) Plants disarm soil: engineering plants for the phytoremediation of explosives. Trends Biotechnol 27(7):73–81
Ruiz ON, Daniell H (2009) Genetic engineering to enhance mercury phytoremediation current opinion. Biotechnology 20:213–219
Rasmussen LD, Sørensen SJ, Turner RR, Barkay T (2000) Application of a mer-lux biosensor for estimating bioavailable mercury in soil. Soil Biol Biochem 32(5):639–646
Siddiquee S, Rovina K, Azad SA (2015) Heavy metal contaminants removal from wastewater using the potential filamentous fungi biomass: a review. J Microbial Biochem Technol 7(6):384–393
Siddiqua KS, Farooqui SA, Hussain T, Mohamed HI (2021) Microbial enzymes and their role in phytoremediation. In: Mohamed HI, El-Beltagi HEDS, Abd-Elsalam KA (eds) Plant growth-promoting microbes for sustainable biotic and abiotic stress management. Springer, Cham. https://doi.org/10.1007/978-3-030-66587-6_22
Saghafi D, Delangiz N, Lajayer BA, Ghorbanpour M (2019) An overview on improvement of crop productivity in saline soils by halotolerant and halophilic PGPRs. Biotech 9(7):261
Suman J, Uhlik O, Viktorona J, Macek T (2018) Phytoextraction of heavy metals: a promising tool for clean up polluted environment. Front Plant Sci 9:1476
Sarwar N, Malhi SS, Zia MH, Naeem A, Bibi S, Farid G (2010) Role of mineral nutrition in minimizing Cadmium accumulation by plants. J Sci Food Agric 90:925–937
Sheoron V, Sheoron A, Ponia P (2011) Role of hyperaccumulators in Phytoextraction of metals from contaminated mining sites: a review function for Cadmium binding peptides. Plant Physiol 92:1086–1093
Su C (2014) A review on heavy metal contamination in the soil worldwide: situation, impact and remediation techniques. Environ Skept Crit 3:24–38
Tripathi S, Hussain T (2021) Treatment of industrial wastewater through new approaches using algae biomass. In: Shah M (ed) The future of effluent treatment plants-biological treatment systems. Elsevier, Amsterdam, pp 89–112. https://doi.org/10.1016/B978-0-12-822956-9.00006-4
Tripathi S, Hussain T (2022) Biofilltration treatment of wastewater through microbial ecology. In: Shah M (ed) An innovative role of Biofilltration in Wastewater Treatment Plants (WWTPs). Elsevier, pp 19–44. https://doi.org/10.1016/B978-0-12-823946-9.00005-X
Van der Ent A, Baker A, Reeves RD, Pollard AJ, Schat H (2013) Hyperaccumulators of metal and metalloid trace elements: facts and fiction. Plant Soil 362:319–334
Wu G, Kang H, Zhang X, Shao H, Chu L, Ruan C (2010) A critical review on the bio-removal of hazardous heavy metals from contaminated soils: issues, progress, eco-environmental concerns and opportunities. J Hazard Mater 174(1-3):1–8
Wuanna RA, Okieimen FE (2011) Heavy metals in contaminated soils: a review of sources, chemistry, risks and best available strategies for remediation. ISRN Ecol 2011:402647
Yang T, Chen M, Wang J (2015) Genetic and chemical modification of cells for selective separation and analysis of heavy metals of biological or environmental significance. Trends Anal Chem 66:90–102
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Saxena, K., Hussain, T., Dhanker, R., Jain, P., Goyal, S. (2022). Phytoremediation: A Sustainable Solution to Combat Pollution. In: Arora, S., Kumar, A., Ogita, S., Yau, Y.Y. (eds) Biotechnological Innovations for Environmental Bioremediation. Springer, Singapore. https://doi.org/10.1007/978-981-16-9001-3_11
Download citation
DOI: https://doi.org/10.1007/978-981-16-9001-3_11
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-16-9000-6
Online ISBN: 978-981-16-9001-3
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)