Abstract
Our lifestyle and dietary structure have changed significantly due to rapid economic growth and improvement of living standards, accelerating occurrence of metabolic disorders such as type II diabetes and other non-communicable diseases. In recent decades, T2DM and its complications have increased dramatically worldwide. As per the recent report, 463 million peoples are living with diabetes, and it has been estimated that the number will rise to 700 million by 2045. T2DM is inferred from multifactorial sources, including genetic and environmental factors. Different therapeutic strategies have adopted, and several medicines developed that work in various ways to promote glycemic management in T2DM, current treatments for T2DM have some drawbacks. Nowadays, the role of microbiota in T2DM pathogenesis has taken into consideration. Some earlier evidences suggest that even the composition of the gut microbiome may lead to T2DM. Since then, tremendous efforts have made to explore the relation between the composition of gut microbiota and T2DM, as well as the role of probiotics in the modulation of gut microbiota. Our current food habits will disturb the gut microbiota composition. Ingestion of probiotics maintain the dysbiosis and produce some secondary metabolites like bacteriocins, short-chain fatty acids (SCFAs), and other organic compounds. These compounds are acting at various levels in controlling metabolic disorders. A recent study has also reported that the dead cells can also be working by maintaining the permeability of intestine barriers. In this chapter, we summarized the relevant results and addressed the close association between intestinal microbiota and T2DM. In this chapter, we summarized the beneficial effects of probiotics on improving glycemic control of T2DM with relevant results and addressed the close association between intestinal microbiota and T2DM in detail.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
7.1 Introduction
Currently significant scientific evidence is available regarding the effects of microbiota on glucose metabolism in T2DM subjects. In this chapter, we briefly discuss disbiotic microbiome of T2DM patients and summarize the most reliable findings for use of probiotic for glycemic control. Probiotics not only control the glucose hemostasis but it also play a significant role in regulating the comorbidities associated with diabetes like obesity, hypertension, inflammation, oxidative stress lipid abnormality, and some brain disorder. All this diseases/ disorders are non-communicable and very much interlinked with the center point of gut environment; once the gut system is maintained properly all other abnormalities can be significantly controlled or improved. Here we also brief the benefits of probiotics in improving glycemic control in T2DM and its associated complication.
7.2 Brief of Gut Microbiota in Type 2 Diabetes (Dysbiosis and T2DM)
Dysbiosis do not directly cause diabetes but it can induce oxidative stress and inflammation, two most common factors in pathophysiology of diabetes.
Earlier, it is believed that the mammal’s gastrointestinal tract is sterile at birth and gut microbial flora colonization in infants start during delivery and further develop during breastfeeding. (Abdul-Ghani and DeFronzo 2010). However, this belief has recently revised that original colonization usually starts during gestation (Walker 2017). Evidence suggests that bacteria of the maternal intestine are generally transferred through the blood circulation of the mothers and later travel into the placenta, and eventually enter the amniotic fluid (Aagaard et al. 2014; Cao et al. 2014). In the human gut, there are mainly five phyla present, and their composition varies based on age and food habits. Newborn intestinal microbial flora generally exhibits low diversity and has a comparatively large concentration of phyla proteobacteria and actinobacteria (Turroni et al. 2008; Rodríguez et al. 2015) and it slowly shifts into a more complex form in adults (Rajilić-Stojanović et al. 2007; Zoetendal et al. 2008). Metagenomics studies have shown that approximately 90% of the bacterial phyla in the adult gut belonged to the phyla of Bacteroidetes and Firmicutes (Blaut and Clavel 2007; Ravel et al. 2014; Rinninella et al. 2019).
These complex microorganism and their metabolites interact differently, in the small and large intestines, with the intestinal epithelial cells (Hsiao et al. 2008). Mucus layers serve as a bacterial insulator at the level of the intestinal barrier. Still, it does not entirely inhibit the diffusion of bacterial fragments across the intestinal barrier and its binding to pattern recognition receptors. This mechanism not only contribute significantly to the defence of the intestinal barrier, but also to an innate and adaptive immune response (Wells et al. 2011).
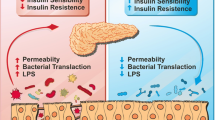
Diet is essential for intestinal microbiota regulation. Due to our modern lifestyle, we are consuming mainly processed food with excess nutrients such as saturated (De La Serre et al. 2010) and polyunsaturated fatty acids (Kankaanpa et al. 2001) or less oligosaccharide (Shoaf et al. 2006) and phytochemicals (Carrera-Quintanar et al. 2018). These food patterns can alter the bacterial metabolic activity. High fat diets affect the gut microbiota resulting in greater intestinal permeability and vulnerability to microbial antigens. Reports have shown that decreased bifidobacterium due to high-fat dietary consumption has been linked with higher LPS concentrations in serum, one of the features of metabolic endotoxemia (Cani et al. 2012). (Metabolic endotoxemia elaborated in Chap. 9). In addition, this typical diet enhances the oxidation of fatty acids in the liver and adipose tissue. Research findings indicate that the reactive oxygen species (ROS) lead oxidative stress because of polyunsaturated fatty acid oxidation and it reduces mucous development. This directly damage the epithelial cell membranes, enhancing the permeability of the intestinal tight junction by stimulating proinflammatory signaling cascades (Muccioli et al. 2010) and indirectly via increasing barrier-disrupting cytokines [TNFα, interleukin (IL) 1B, IL6, and interferon γ (IFNγ)] and decreasing barrier-forming cytokines (IL10, IL17, and IL22) (Rohr et al. 2019). Mild chronic inflammation is one of the characteristic features of metabolic diseases such as obesity and T2DM, which may occur due to the activation of toll-like receptors by LPS, which are present in the cell wall of gram −ve bacteria. Toll-like receptors 4 (TLR4) are present in insulin targeted tissues (Boulangé et al. 2016; Rogero and Calder 2018).
Through activating cytokine-signaling cascades alongside the increased concentration of reactive oxygen species (ROS), these actions may be compromised upon stimulation of TLR4.
Inflammation levels are a key element in the development of insulin resistance, contributing to a deficiency in the action of insulin (Boulangé et al. 2016). Extending the duration of defects in insulin action causes the overproduction of insulin, which leads to a defect in the pancreatic cells, leading to a defect in insulin secretion (Boulangé et al. 2016). It is resulting in T2DM.
The Intestinal tight junction is a multi-protein complex that forms a selective permeable seal between adjacent epithelial cells and demarcates the boundary between apical and basolateral membrane domains.
7.3 Therapeutics for T2DM
Nowadays, T2DM management has become a worldwide epidemic, many therapeutic techniques have been adopted and a wide variety of drugs have been produced to enhance glycemic regulation through improved insulin production and utilization, decrease sugar production and absorption, inhibit glucose re-sorption, and enforce urinary glucose excretion. These are achieved by mainly 5 type of drugs, namely biguanides, sulfonylureas, a-glucosidase inhibitors, and thiazolidinediones (TZDs), which are used to treat hyperglycemia (Chaudhury et al. 2017). Generally, it is commonly accepted that new T2DM therapies have some adverse side effects such as liver disorders, lactic acidosis, and gastrointestinal issues (Manandhar Shrestha et al. 2017). Therefore, alternate methods focused on intestinal microbiota were investigated, indicating promising prospects for the future T2DM intervention (Gérard and Vidal 2019).
7.4 Studies on the Glycemic Control of Gut Microbiota in T2DM
In the last two decades, numerous studies reported the beneficial effects of gut microbiota on metabolic diseases, including T2DM. Meta-analysis of the reports suggest that effect of probiotics on glycemic control is stain specific. Among the widely published findings, the genes of Bifidobacterium, Bacteroides, Faecalibacterium, Akkermansia, and Roseburia have been identified as being negatively associated with T2DM and Ruminococcus, Fusobacterium, and Blautia were positively associated with T2DM. Although Lactobacillus genus is most frequently identified and reported still, most discrepancies in their effect in T2DM were reported (Gurung et al. 2020). Some of recent probiotic clinical trials conducted on T2DM given in Table 7.1.
The exact mechanisms used by probiotics for their advantages were uncertain. Nevertheless, a number of hypothesized processes describe many of their favorable effects. Probiotics control the T2DM in various ways, such as modulate the inflammation (Shen et al. 2018; Maldonado Galdeano et al. 2019), interact with dietary constituents, affect gut permeability (Tian et al. 2016) and lipid metabolism. Mainly the short-chain fatty acids (SCFAs) (acetate, butyrate, propionate) are the major anions in the colon and are largely produced by probiotic bacteria from indigestible polysaccharides (Geirnaert et al. 2017). SCFAs stimulate improvement of intestinal barrier function and upregulation of glucagon-like peptide-1 (GLP-1) (Macfarlane and Macfarlane 2003). GLP-1 is a gut incretin hormone that induces insulin production from the ß cells and inhibits the secretion of glucagon that contribute to glucose homeostasis (Lovshin and Drucker 2009). Probiotic improves the gut physiology and promotes epithelial cell growth by producing vitamins and hormones (Mach and Fuster-Botella 2017; Indira et al. 2019).
Based on the reports, probiotics supplementation is not only reducing the glucose level, but also improving the other metabolic abnormalities linked with diabetes such as hypertension, BMI, lipid profile, oxidative stress markers. Some research has also shown their beneficial effects on mental health. None of the studies has reported any toxic effects on liver and kidney like other synthetic drugs.
7.5 Beneficial Effects of Probiotic for Glycemic Control
Probiotics and their metabolites are involved in various pathways to improve glycemic control (Fig. 7.1) which is explained in the below sections.
7.5.1 Probiotic on Hypertension Associated with Diabetes
There are mainly two forms of hypertension: primary and secondary. Primary hypertension occurs mainly because of genetic variables and unspecific lifestyle, it is characterized as elevated blood pressure, around 95% cases belong to this category, whereas secondary hypertension is attributed to an identifiable cause such as Cushing’s syndrome, obesity, and glucose sensitivity. However, it is still not clear about the etiology of hypertension (Sukor 2011). Indirect involvement of gut microbiota in the regulation of hypertension has been recognized in recent time. As we have seen earlier, in dysbiosis the diversity of microbes increases and Firmicutes/Bacteroidetes ratio changes. These changes accompanied by decrease in acetate and butyrate producing bacteria (Yang et al. 2015). SCFA plays an important role in maintaining blood pressure (BP). Short-chain fatty acid receptors are G-protein coupled receptors GPR41, GPR43, GPR109a, and olfactory receptor OLF79 in mice and OR51E2 in humans. Short-chain fatty acid receptors, such as GPR41 and OLF78, have shown to have inverse roles in blood pressure regulation (Pluznick 2014). Dysbiosis also leads to increased permeability of the intestinal wall. It is an essential factor that influences the bidirectional flow of microbes, cells, metabolites, molecules, and hormones that inevitably interfere with peripheral but also central BP control mechanisms. (Raizada et al. 2017). BP reduction was observed in the late phase of angiotensin II infused wild-type mice, suggestive of the favorable effect of propionate on hypertension (Bartolomaeus et al. 2019).
One study stated that the role of the gut microbiota in steroid enterohepatic circulation and its findings are consistent with the possibility that steroid metabolites contribute to the physiological response to exogenous steroids when reabsorbed in the enterohepatic circulation (Honour 1982). Some other studies reported differences in circulating inflammatory cells in hypertensive individuals compared to controls due to microbial diversity in hypertensive patients. Dysbiosis also contributes to increased T-helper 17 cell activation and is mediated by gut-intrinsic pathways (Kim et al. 2015).
7.5.2 Probiotic on Obesity Related with Diabetes
Obesity is typically associated with metabolic alterations related to glucose homeostasis and cardiovascular risk factors (Eckel et al. 2005). These metabolic alterations are associated with low-grade inflammation that contributes to the onset of these diseases (Olefsky and Glass 2010). Probiotics and prebiotics reduce gut inflammation, which leads to improvement in metabolic dysfunction in obese-insulin resistant model (Thiennimitr et al. 2018). Some studies found that gut microbiota conferred host resistance to high-fat diet-induced obesity through the production of polyunsaturated fatty acid metabolites (Miyamoto et al. 2019). Considering the effect of calorie restriction and weight loss on fetuin-A and SIRT1 levels it can be understood by reducing the appetite and dietary intake and body weight. Studies also found that probiotic supplementation significantly decreased total energy, carbohydrate, fat, and protein intake compared with placebo (Khalili et al. 2019). Other studies found that higher endogenous GLP-1 and GLP-2 production; prebiotic treatment increases the number of enteroendocrine cells producing GLP-1 and GLP-2 (L-cells) in the jejunum and colon (Cani et al. 2012).
7.5.3 Probiotic on Oxidative Stress
In diabetes, free radical formation by non-enzymatic glycation of proteins, glucose oxidation and increased lipid peroxidation causes the damage of enzymes, cellular machinery, and increased insulin resistance. (Asmat et al. 2016). SCFA produced by the probiotic in the digestive system provide nicotinamide adenine dinucleotide phosphate (NADPH) for the synthesis of GSH, induces apoptosis and increases the expression of the pathway of oxidative pathogens. Probiotic supplementation plays a direct role in NO production and reduces the ROS (Asmat et al. 2016; Heshmati et al. 2018). Studies on beneficial effects of probiotic on oxidative stress are given in Table 7.1.
7.5.4 Probiotic on Lipid Management
Glucose and lipid metabolism are related in several ways. Diabetic dyslipidemia, characterized by high triglycerides, LDL particles, and low HDL-C are the most important clinical manifestation of this interaction and this is main cause of cardiovascular diseases (Parhofer 2015; Eid et al. 2019). Cholesterol is the precursor to bile acids. Bile acids are metabolized into secondary bile acids by gut microbiota. Probiotic controls the lipid metabolism by assimilation of cholesterol during growth, binding of cholesterol to cellular surface, disruption of cholesterol micelle, deconjugation of bile salt, and bile salt hydrolase activity (Lye et al. 2010; Jones et al. 2012, 2013; Huang et al. 2014). Gut microbiota regulates bile acid metabolism by reducing the levels of tauro-beta-muricholic acid, a naturally occurring FXR antagonist (Sayin et al. 2013). Mechanisms are explained in more detail in Chap. 8.
7.5.5 Probiotic on Comorbid Brain Disorders Associated with Diabetes
The most common causes of T2DM and brain disorders are poor sleep, lack of exercise, and diet habits (Watkins and Thomas 1998; Yoda et al. 2015). There are numerous studies in the past reporting the effects of diabetes on the brain. Certainly, high glucose levels can damage blood vessels in the brain and thereby increase the risk of stroke. However, its effects are more widely felt than that. High glucose and insulin resistance affect many neuronal processes and contribute to inflammation in the brain. T2DM also appears to increase the risk of Alzheimer’s disease and other dementias (Li et al. 2015). The other ways stress stimulates the hypothalamus–pituitary–adrenal axis (HPA-axis) and the sympathetic nervous system (SNS) are: increased levels of cortisol in the adrenal cortex, and adrenalin and noradrenalin in the adrenal medulla (Smith and Vale 2006; Stephens and Wand 2012). Chronic hypercortisolemia and excessive SNS activity promote insulin resistance, visceral obesity and contribute to T2DM (Pickup and Crook 1998; Wang et al. 2013).
In addition, constant stress also causes immune dysfunction directly or via the HPA or SNS axis, enhancing the production of inflammatory cytokines. High levels of inflammatory cytokines interfere with the regular functioning of pancreatic β-cells, induce insulin resistance and other consequences. The other studies reported that pro-inflammatory cytokines have been found to interact with many of the pathophysiological domains that characterize depression, including neurotransmitter metabolism, neuroendocrine function, synaptic plasticity, and behavior (Fig. 7.2).
Consumption of diet rich in high fats and refined carbohydrates mainly sugar has the ability to disturb the healthy microbiota composition, leading to dysbiosis. Studies showed that dysbiosis increases lipopolysaccharide (LPS) levels, which triggers the production of proinflammatory cytokines in the gut (Zeevi et al. 2015; Agus et al. 2016). Dysbiosis imposes regulatory roles on inflammation and oxidative stress and is a pathogenetic contributor associated with various diseases characterized by a pro-oxidative and pro-inflammatory disorder mainly AD, depression, and T2DM (Luca et al. 2019). The gut–brain axis involves a number of sophisticated channels of communication among many interconnected systems, including the CNS, the autonomic nervous system (ANS), the HPA axis, as well as the GI corticotropin-releasing factor system, and the intestinal immune response system featuring the intestinal mucosal barrier (Carabotti et al. 2015).
Dysbiosis is also confirmed by the high levels of comorbidity among depression and T2DM subjects. This may account for the genetic similarities related to these disorders and contribute to an increase in the risk of dementia. It is strictly associated with metabolism, cognition, and mood (Rowland and Bellush 1989; Hilakivi-Clarke et al. 1990; Thakur et al. 2013; Thakur et al. 2016). Gut microorganisms are capable of producing and delivering neurotransmitters such as serotonin and gamma-aminobutyric acid, which act in the gut–brain axis and modulate food intake and energy balance in the system (Cryan and Dinan 2012; Borre et al. 2014).
Cross talk between the brain and the gut involve many interacting pathways, including the autonomic, neuroendocrine, immune systems as well as bacterial metabolites and neuromodulatory molecules. Bacterial metabolites (SCFAs) like acetate and propionate are mainly produced by the bacteroidetes, while Firmicutes generate most of the butyrate. Butyrate also prevent inflammatory reactions by inhibition of NF-kappaB (NF-κB) (Segain et al. 2000). Propionate is usually utilized by the liver and has also been reported to inhibit NF-κB, as well as boost insulin sensitivity, while acetate is normally released into circulation so that it can enter peripheral tissues, including the brain (Guarner and Malagelada 2003; Al-Lahham et al. 2010; Iwanaga and Kishimoto 2015). Both propionate and acetate have been found to improve insulin sensitivity (Canfora et al. 2015; González Hernández et al. 2020). Acetate and butyrate are structurally related to ketone, acetoacetate, and d-β-hydroxybutyrate, all of which have positive effects in neurological conditions (Stilling et al. 2016; Courchesne-Loyer et al. 2017). Meta-analysis suggests that modulating the composition of the gut microbiota using prebiotics and probiotics may produce beneficial effects on brain disorders associated with diabetes (Schachter et al. 2018).
7.5.6 Other
The beneficial effect of probiotics also extends to chronic liver and kidney disease (Lo et al. 2014; Jia et al. 2018). Our lab observation also finds that the probiotic supplementation has improved the kidney and liver function markers of the diabetic subjects with metabolic syndrome (unpublished observation). Detailed effect of probiotic on liver diseases is mentioned in Chap. 10.
7.6 Conclusion
The available evidence from experimental studies and clinical trials supports that the modulation of the intestinal microbiota by probiotics uptake may be effective towards prevention and management of T2D and other related complications.
References
Aagaard K et al (2014) The placenta harbors a unique microbiome. Sci Transl Med 6(237):237ra65. https://doi.org/10.1126/scitranslmed.3008599
Abdul-Ghani MA, DeFronzo RA (2010) Pathogenesis of insulin resistance in skeletal muscle. J Biomed Biotechnol 2010:1–19. https://doi.org/10.1155/2010/476279
Agus A et al (2016) Western diet induces a shift in microbiota composition enhancing susceptibility to adherent-Invasive E. coli infection and intestinal inflammation. Sci Rep 6. https://doi.org/10.1038/srep19032
Al-Lahham SH et al (2010) Biological effects of propionic acid in humans; metabolism, potential applications and underlying mechanisms. Biochim Biophys Acta:1175–1183. https://doi.org/10.1016/j.bbalip.2010.07.007
Asemi Z et al (2013) Effect of multispecies probiotic supplements on metabolic profiles, hs-CRP, and oxidative stress in patients with type 2 diabetes. Ann Nutr Metab 63(1–2):1–9. https://doi.org/10.1159/000349922
Asemi Z et al (2014) Effects of synbiotic food consumption on metabolic status of diabetic patients: a double-blind randomized cross-over controlled clinical trial. Clin Nutr 33(2):198–203. https://doi.org/10.1016/j.clnu.2013.05.015
Asemi Z et al (2016) Effects of beta-carotene fortified synbiotic food on metabolic control of patients with type 2 diabetes mellitus: a double-blind randomized cross-over controlled clinical trial. Clin Nutr 35(4):819–825. https://doi.org/10.1016/j.clnu.2015.07.009
Asmat U, Abad K, Ismail K (2016) Diabetes mellitus and oxidative stress—a concise review. Saudi Pharm J:547–553. https://doi.org/10.1016/j.jsps.2015.03.013
Bartolomaeus H et al (2019) Short-chain fatty acid propionate protects from hypertensive cardiovascular damage. Circulation 139(11):1407–1421. https://doi.org/10.1161/CIRCULATIONAHA.118.036652
Blaut M, Clavel T (2007) Metabolic diversity of the intestinal microbiota: implications for health and disease. J Nutr 137(3):751S–755S. https://doi.org/10.1093/jn/137.3.751s
Borre YE et al (2014) The impact of microbiota on brain and behavior: mechanisms & therapeutic potential. Adv Exp Med Biol 817:373–403. https://doi.org/10.1007/978-1-4939-0897-4_17
Boulangé CL et al (2016) Impact of the gut microbiota on inflammation, obesity, and metabolic disease. Genome Med. https://doi.org/10.1186/s13073-016-0303-2
Canfora EE, Jocken JW, Blaak EE (2015) Short-chain fatty acids in control of body weight and insulin sensitivity. Nat Rev Endocrinol:577–591. https://doi.org/10.1038/nrendo.2015.128
Cani PD et al (2012) Involvement of gut microbiota in the development of low-grade inflammation and type 2 diabetes associated with obesity. Gut Microbes:279. https://doi.org/10.4161/gmic.19625
Cao B et al (2014) Placental microbiome and its role in preterm birth. NeoReviews 15(12):e537–e545. https://doi.org/10.1542/neo.15-12-e537
Carabotti M et al (2015) The gut-brain axis: Interactions between enteric microbiota, central and enteric nervous systems. Ann Gastroenterol 28(2):203–209. www.annalsgastro.gr (Accessed: 15 Feb 2021)
Carrera-Quintanar L et al (2018) Phytochemicals that influence gut microbiota as prophylactics and for the treatment of obesity and inflammatory diseases. Mediat Inflamm 2018. https://doi.org/10.1155/2018/9734845
Chaudhury A et al (2017) Clinical review of antidiabetic drugs: implications for type 2 diabetes mellitus management. Front Endocrinol 8:6. https://doi.org/10.3389/fendo.2017.00006
Courchesne-Loyer A et al (2017) Inverse relationship between brain glucose and ketone metabolism in adults during short-term moderate dietary ketosis: a dual tracer quantitative positron emission tomography study. J Cereb Blood Flow Metab 37(7):2485–2493. https://doi.org/10.1177/0271678X16669366
Cryan JF, Dinan TG (2012) Mind-altering microorganisms: the impact of the gut microbiota on brain and behaviour. Nat Rev Neurosci:701–712. https://doi.org/10.1038/nrn3346
De La Serre CB et al (2010) Propensity to high-fat diet-induced obesity in rats is associated with changes in the gut microbiota and gut inflammation. Am J Physiol Gastrointest Liver Physiol 299(2). https://doi.org/10.1152/ajpgi.00098.2010
Eckel RH, Grundy SM, Zimmet PZ (2005) The metabolic syndrome. Lancet:1415–1428. https://doi.org/10.1016/S0140-6736(05)66378-7
Eid S et al (2019) New insights into the mechanisms of diabetic complications: role of lipids and lipid metabolism. Diabetologia:1539–1549. https://doi.org/10.1007/s00125-019-4959-1
Ejtahed HS et al (2011) Effect of probiotic yogurt containing lactobacillus acidophilus and Bifidobacterium lactis on lipid profile in individuals with type 2 diabetes mellitus. J Dairy Sci 94(7):3288–3294. https://doi.org/10.3168/jds.2010-4128
Firouzi S et al (2017) Effect of multi-strain probiotics (multi-strain microbial cell preparation) on glycemic control and other diabetes-related outcomes in people with type 2 diabetes: a randomized controlled trial. Eur J Nutr 56(4):1535–1550. https://doi.org/10.1007/s00394-016-1199-8
Geirnaert A et al (2017) Butyrate-producing bacteria supplemented in vitro to Crohn’s disease patient microbiota increased butyrate production and enhanced intestinal epithelial barrier integrity. Sci Rep 7(1):1–14. https://doi.org/10.1038/s41598-017-11734-8
Gérard C, Vidal H (2019) Impact of gut microbiota on host glycemic control. Front Endocrinol 29. https://doi.org/10.3389/fendo.2019.00029
González Hernández MA et al (2020) The relationship between circulating acetate and human insulin resistance before and after weight loss in the DiOgenes study. Nutrients 12(2). https://doi.org/10.3390/nu12020339
Guarner F, Malagelada JR (2003) Gut flora in health and disease. Lancet:512–519. https://doi.org/10.1016/S0140-6736(03)12489-0
Gurung M et al (2020) Role of gut microbiota in type 2 diabetes pathophysiology. EBioMedicine 51:102590. https://doi.org/10.1016/j.ebiom.2019.11.051
Heshmati J et al (2018) A systematic review and meta-analysis of the probiotics and synbiotics effects on oxidative stress. J Funct Foods:66–84. https://doi.org/10.1016/j.jff.2018.04.049
Hilakivi-Clarke LA et al (1990) Behavior of streptozotocin-diabetic mice in tests of exploration, locomotion, anxiety, depression and aggression. Physiol Behav 48(3):429–433. https://doi.org/10.1016/0031-9384(90)90339-6
Honour J (1982) The possible involvement of intestinal bacteria in steroidal hypertension. Endocrinology 110(1):285–287. https://doi.org/10.1210/endo-110-1-285
Hsiao WWL et al (2008) The microbes of the intestine: an introduction to their metabolic and signaling capabilities. Endocrinol Metab Clin North Am:857–871. https://doi.org/10.1016/j.ecl.2008.08.006
Huang Y et al (2014) Lactobacillus acidophilus ATCC 4356 prevents atherosclerosis via inhibition of intestinal cholesterol absorption in apolipoprotein E-knockout mice. Appl Environ Microbiol 80(24):7496–7504. https://doi.org/10.1128/AEM.02926-14
Indira M et al (2019) Bioactive molecules of probiotic bacteria and their mechanism of action: a review. 3 Biotech 9(8):306. https://doi.org/10.1007/s13205-019-1841-2
Iwanaga T, Kishimoto A (2015) Cellular distributions of monocarboxylate transporters: a review. Biomed Res Foundation:279–301. https://doi.org/10.2220/biomedres.36.279
Jia L et al (2018) Efficacy of probiotics supplementation on chronic kidney disease: a systematic review and meta-analysis. Kidney Blood Press Res 43(5):1623–1635. https://doi.org/10.1159/000494677
Jones ML et al (2012) Cholesterol-lowering efficacy of a microencapsulated bile salt hydrolase-active lactobacillus reuteri NCIMB 30242 yoghurt formulation in hypercholesterolaemic adults. Br J Nutr 107(10):1505–1513. https://doi.org/10.1017/S0007114511004703
Jones ML et al (2013) Cholesterol lowering with bile salt hydrolase-active probiotic bacteria, mechanism of action, clinical evidence, and future direction for heart health applications. Expert Opin Biol Ther:631–642. https://doi.org/10.1517/14712598.2013.758706
Kankaanpa PE et al (2001) The influence of polyunsaturated fatty acids on probiotic growth and adhesion. FEMS Microbiol Lett 194(2):149–153. https://doi.org/10.1111/j.1574-6968.2001.tb09460.x
Khalili L et al (2019) The effects of lactobacillus casei on glycemic response, serum sirtuin1 and fetuin-a levels in patients with type 2 diabetes mellitus: a randomized controlled trial. Iran Biomed J 23(1):68–77. https://doi.org/10.29252/IBJ.23.1.68
Kim S et al (2015) Hypertensive patients exhibit gut microbial dysbiosis and an increase in th17 cells. J Hypertens 33:e77–e78. https://doi.org/10.1097/01.hjh.0000467562.03337.a5
Kobyliak N et al (2018) Effect of alive probiotic on insulin resistance in type 2 diabetes patients: randomized clinical trial. Diabetes Metab Syndr 12(5):617–624. https://doi.org/10.1016/j.dsx.2018.04.015
Li X, Song D, Leng SX (2015) Link between type 2 diabetes and Alzheimer’s disease: from epidemiology to mechanism and treatment. Clin Interven Aging:549–560. https://doi.org/10.2147/CIA.S74042
Lo RS, Austin AS, Freeman JG (2014) Is there a role for probiotics in liver disease? Sci World J 2014:1–7. https://doi.org/10.1155/2014/874768
Lovshin JA, Drucker DJ (2009) Incretin-based therapies for type 2 diabetes mellitus. Nat Rev Endocrinol:262–269. https://doi.org/10.1038/nrendo.2009.48
Luca M et al (2019) Gut microbiota in Alzheimer’s disease, depression, and type 2 diabetes mellitus: the role of oxidative stress. Oxid Med Cell Longev. https://doi.org/10.1155/2019/4730539
Lye HS, Rahmat-Ali GR, Liong MT (2010) Mechanisms of cholesterol removal by lactobacilli under conditions that mimic the human gastrointestinal tract. Int Dairy J 20(3):169–175. https://doi.org/10.1016/j.idairyj.2009.10.003
Macfarlane S, Macfarlane GT (2003) Regulation of short-chain fatty acid production. Proc Nutr Soc 62(1):67–72. https://doi.org/10.1079/pns2002207
Mach, N. and Fuster-Botella, D. (2017) Endurance exercise and gut microbiota: a review, J Sport Health Sci, 179–197. doi: https://doi.org/10.1016/j.jshs.2016.05.001
Mafi A et al (2018) Metabolic and genetic response to probiotics supplementation in patients with diabetic nephropathy: a randomized, double-blind, placebo-controlled trial. Food and Function 9(9):4763–4770. https://doi.org/10.1039/c8fo00888d
Maldonado Galdeano C et al (2019) Beneficial effects of probiotic consumption on the immune system. Ann Nutr Metab 74(2):115–124. https://doi.org/10.1159/000496426
Manandhar Shrestha JT et al (2017) Adverse effects of oral hypoglycemic agents and adherence to them among patients with type 2 diabetes mellitus in Nepal. J Lumbini Med Coll 5(1):34. https://doi.org/10.22502/jlmc.v5i1.126
Mazruei Arani N et al (2019) The effects of probiotic honey consumption on metabolic status in patients with diabetic nephropathy: a randomized, double-blind, controlled trial. Probiotics Antimicrob Proteins 11(4):1195–1201. https://doi.org/10.1007/s12602-018-9468-x
Miyamoto J et al (2019) Gut microbiota confers host resistance to obesity by metabolizing dietary polyunsaturated fatty acids. Nat Commun 10(1):1–15. https://doi.org/10.1038/s41467-019-11978-0
Mohseni S et al (2018) The beneficial effects of probiotic administration on wound healing and metabolic status in patients with diabetic foot ulcer: a randomized, double-blind, placebo-controlled trial. Diabetes Metab Res Rev 34(3). https://doi.org/10.1002/dmrr.2970
Moroti C et al (2012) Effect of the consumption of a new symbiotic shake on glycemia and cholesterol levels in elderly people with type 2 diabetes mellitus. Lipids Health Dis 11:29. https://doi.org/10.1186/1476-511X-11-29
Muccioli GG et al (2010) The endocannabinoid system links gut microbiota to adipogenesis. Mol Syst Biol 6. https://doi.org/10.1038/msb.2010.46
Olefsky JM, Glass CK (2010) Macrophages, inflammation, and insulin resistance. Annu Rev Physiol 72(1):219–246. https://doi.org/10.1146/annurev-physiol-021909-135846
Ostadrahimi A et al (2015) Effect of probiotic fermented milk (Kefir) on glycemic control and lipid profile in type 2 diabetic patients: a randomized double-blind placebo-controlled clinical trial. Iran J Public Health 44(2):228–237. http://ijph.tums.ac.ir (Accessed 2 July 2020)
Parhofer KG (2015) Interaction between glucose and lipid metabolism: more than diabetic dyslipidemia. Diabetes Metab J:353–362. https://doi.org/10.4093/dmj.2015.39.5.353
Pickup JC, Crook MA (1998) Is type II diabetes mellitus a disease of the innate immune system? Diabetologia:1241–1248. https://doi.org/10.1007/s001250051058
Pluznick J (2014) A novel SCFA receptor the microbiota and blood pressure regulation. Gut Microbes 5(2):202–207. https://doi.org/10.4161/gmic.27492
Raizada MK et al (2017) Report of the National Heart, Lung, and Blood Institute working group on the role of microbiota in blood pressure regulation: current status and future directions. Hypertension:479–485. https://doi.org/10.1161/HYPERTENSIONAHA.117.09699
Rajilić-Stojanović M, Smidt H, De Vos WM (2007) Diversity of the human gastrointestinal tract microbiota revisited. Environ Microbiol:2125–2136. https://doi.org/10.1111/j.1462-2920.2007.01369.x
Ravel J et al (2014) Human microbiome science: vision for the future, Bethesda, MD, July 24 to 26, 2013. Microbiome 2(1):16. https://doi.org/10.1186/2049-2618-2-16
Razmpoosh E et al (2019) The effect of probiotic supplementation on glycemic control and lipid profile in patients with type 2 diabetes: a randomized placebo controlled trial. Diabetes Metab Syndr 13(1):175–182. https://doi.org/10.1016/j.dsx.2018.08.008
Rinninella E et al (2019) What is the healthy gut microbiota composition? A changing ecosystem across age, environment, diet, and diseases. Microorganisms 7(1). https://doi.org/10.3390/microorganisms7010014
Rodríguez JM et al (2015) The composition of the gut microbiota throughout life, with an emphasis on early life. Microb Ecol Health Dis 26:1–17. https://doi.org/10.3402/mehd.v26.26050
Rogero MM, Calder PC (2018) Obesity, inflammation, toll-like receptor 4 and fatty acids. Nutrients. https://doi.org/10.3390/nu10040432
Rohr MW et al (2019) Negative effects of a high-fat diet on intestinal permeability: a review. Adv Nutr. https://doi.org/10.1093/advances/nmz061
Rowland NE, Bellush LL (1989) Diabetes mellitus: stress, neurochemistry and behavior. Neurosci Biobehav Rev 13(4):199–206. https://doi.org/10.1016/S0149-7634(89)80054-5
Sayin SI et al (2013) Gut microbiota regulates bile acid metabolism by reducing the levels of tauro-beta-muricholic acid, a naturally occurring FXR antagonist. Cell Metab 17(2):225–235. https://doi.org/10.1016/j.cmet.2013.01.003
Schachter J et al (2018) Effects of obesity on depression: a role for inflammation and the gut microbiota. Brain Behav Immun:1–8. https://doi.org/10.1016/j.bbi.2017.08.026
Segain JP et al (2000) Butyrate inhibits inflammatory responses through NFκB inhibition: implications for Crohn’s disease. Gut 47(3):397–403. https://doi.org/10.1136/gut.47.3.397
Shen Z et al (2018) Insights into Roseburia intestinalis which alleviates experimental colitis pathology by inducing anti-inflammatory responses. J Gastroenterol Hepatol (Australia) 33(10):1751–1760. https://doi.org/10.1111/jgh.14144
Shoaf K et al (2006) Prebiotic galactooligosaccharides reduce adherence of enteropathogenic Escherichia coli to tissue culture cells. Infect Immun 74(12):6920–6928. https://doi.org/10.1128/IAI.01030-06
Smith SM, Vale WW (2006) The role of the hypothalamic-pituitary-adrenal axis in neuroendocrine responses to stress. Dialogues Clin Neurosci:383–395. https://doi.org/10.31887/dcns.2006.8.4/ssmith
Stephens MAC, Wand G (2012) Stress and the HPA axis: role of glucocorticoids in alcohol dependence. Alcohol Res 34:468–483. /pmc/articles/PMC3860380/ (Accessed 15 Feb 2021)
Stilling RM et al (2016) The neuropharmacology of butyrate: the bread and butter of the microbiota-gut-brain axis? Neurochem Int:110–132. https://doi.org/10.1016/j.neuint.2016.06.011
Sukor N (2011) Secondary hypertension: a condition not to be missed. Postgrad Med J 87(1032):706–713. https://doi.org/10.1136/pgmj.2011.118661
Thakur AK, Chatterjee SS, Kumar V (2013) Beneficial effects of Brassica juncea on cognitive functions in rats. Pharm Biol 51(10):1304–1310. https://doi.org/10.3109/13880209.2013.789917
Thakur AK et al (2016) Beneficial effects of an Andrographis paniculata extract and andrographolide on cognitive functions in streptozotocin-induced diabetic rats. Pharm Biol 54(9):1528–1538. https://doi.org/10.3109/13880209.2015.1107107
Thiennimitr P et al (2018) Lactobacillus paracasei HII01, xylooligosaccharides, and synbiotics reduce gut disturbance in obese rats. Nutrition 54:40–47. https://doi.org/10.1016/j.nut.2018.03.005
Tian P et al (2016) Antidiabetic (type 2) effects of lactobacillus G15 and Q14 in rats through regulation of intestinal permeability and microbiota. Food Funct 7(9):3789–3797. https://doi.org/10.1039/c6fo00831c
Tonucci LB et al (2017) Clinical application of probiotics in type 2 diabetes mellitus: a randomized, double-blind, placebo-controlled study. Clin Nutr 36(1):85–92. https://doi.org/10.1016/j.clnu.2015.11.011
Turroni F et al (2008) Human gut microbiota and bifidobacteria: from composition to functionality. Antonie van Leeuwenhoek 94(1):35–50. https://doi.org/10.1007/s10482-008-9232-4
Walker WA (2017) The importance of appropriate initial bacterial colonization of the intestine in newborn, child, and adult health. Pediatr Res:387–395. https://doi.org/10.1038/pr.2017.111
Wang X et al (2013) Inflammatory markers and risk of type 2 diabetes: a systematic review and meta-analysis. Diabetes Care:166–175. https://doi.org/10.2337/dc12-0702
Watkins PJ, Thomas PK (1998) Diabetes mellitus and the nervous system. J Neurol Neurosurg Psychiatry:620–632. https://doi.org/10.1136/jnnp.65.5.620
Wells JM et al (2011) Epithelial crosstalk at the microbiota-mucosal interface. Proc Natl Acad Sci U S A 108(SUPPL. 1):4607–4614. https://doi.org/10.1073/pnas.1000092107
Yang T et al (2015) Gut dysbiosis is linked to hypertension. Hypertension 65(6):1331–1340. https://doi.org/10.1161/HYPERTENSIONAHA.115.05315
Yoda K et al (2015) Association between poor glycemic control, impaired sleep quality, and increased arterial thickening in type 2 diabetic patients. PLoS One 10(4). https://doi.org/10.1371/journal.pone.0122521
Zeevi D et al (2015) Personalized nutrition by prediction of glycemic responses. Cell 163(5):1079–1094. https://doi.org/10.1016/j.cell.2015.11.001
Zoetendal EG, Rajilić-Stojanović M, De Vos WM (2008) High-throughput diversity and functionality analysis of the gastrointestinal tract microbiota. Gut:1605–1615. https://doi.org/10.1136/gut.2007.133603
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Subramani, S.K., Raghuwanshi, S., Sharma, R. (2022). Preventive and Therapeutic Role of Probiotics in Type-2 Diabetes and Its Associated Complications. In: Chopra, K., Bishnoi, M., Kondepudi, K.K. (eds) Probiotic Research in Therapeutics. Springer, Singapore. https://doi.org/10.1007/978-981-16-8444-9_7
Download citation
DOI: https://doi.org/10.1007/978-981-16-8444-9_7
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-16-8443-2
Online ISBN: 978-981-16-8444-9
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)