Abstract
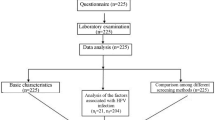
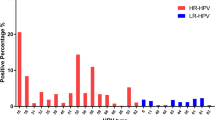
The purpose of this study is to explore the risk factors of HR-HPV (High-Risk Human PapillomaVirus) cervical infection, and to construct an evaluation model through quantification of indicators, so as to provide a basis for the identification of high-risk population, propose a reference for reducing and clearing HR-HPV infection of the cervix and determine a reasonable follow-up period. Computer science and technologies are widely used in the study to calculate and analyze clinical data. The outcome of the study can help to carry forward the prevention of HR-HPV infection, so as to achieve the secondary prevention of cervical cancer. Quantitative analysis indicators model can be used to identify risky people who are vulnerable to HR-HPV and improve the compliance of targeted population to participate in screening. And at the same time, the study can provide reference and basis in the decision of the interval between follow-up visits for HPV testing. Therefor the number of missing HPV screening among risky women is expected to decreased and psychological panic and unnecessary economic burden of patients caused by excessive testing could be avoided.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Bruni, L., et al.: Human Papillomavirus and Related Diseases in the World (Summary Report 17 June 2019), ICO/IARC Information Centre on HPV and Cancer (2019)
Chesson, H.W., Dunne, E.F., Hariri, S., Markowitz, L.E.: The estimated lifetime probability of acquiring human papillomavirus in the United States. Sex. Transm. Dis. 41(11), 660–664 (2014)
Winer, R.L., et al.: Early natural history of incident, type‐specific human papillomavirus infections in newly sexually active young women. Cancer Epidemiol. Biomark. Prev. 20(4), 699–707 (2011)
Tsakogiannis, D., et al.: Sites of disruption within E1 and E2 genes of HPV16 and association with cervical dysplasia. J. Med. Virol. 87(11), 1973–1980 (2015)
van den Heuvel, C.N.A.M., et al.: RNA-based HR-HPV genotyping and identification of HR-HPV transcriptional activity in cervical tissues. Mod. Pathol. 33(4), 748–757 (2020)
Hu, Z., et al.: TALEN-mediated targeting of HPV oncogenes ameliorates HPV-related cervical malignancy. J. Clin. Investig. 125(1), 425–436 (2014)
Egawa, N., Egawa, K., Griffin, H., Doorbar, J.: Human papillomaviruses; epithelial tropisms, and the development of neoplasia. Viruses 7(7), 3863–3890 (2015)
Koh, W.J., et al.: Cervical cancer, version 3.2019, NCCN clinical practice guidelines in oncology. J. Natl. Compr. Canc. Netw. 17(1), 64–84 (2019)
Rumfield, C.S., Roller, N., Pellom, S.T., Schlom, J., Jochems, C.: Therapeutic vaccines for HPV-associated malignancies. Immuno Targets Therapy 9, 167–200 (2020)
de Sanjosé, S., Brotons, M., Pavón, M.A.: The natural history of human papillomavirus infection. Best Pract. Res. Clin. Obstet. Gynaecol. 47, 2–13 (2018)
Heideman, D.A.M., et al.: Clinical validation of the cobas 4800 HPV test for cervical screening purposes. J. Clin. Microbiol. 49(11), 3983–3985 (2011)
Cui, M., et al.: Clinical performance of Roche Cobas 4800 HPV test. J. Clin. Microbiol. 52(6), 2210–2211 (2014)
Madzima, T.R., Vahabi, M., Lofters, A.: Emerging role of HPV self-sampling in cervical cancer screening for hard-to-reach women: focused literature review. Can. Fam. Physician 63(8), 597–601 (2017)
Mchome, B.L., et al.: HPV types, cervical high-grade lesions and risk factors for oncogenic human papillomavirus infection among 3416 Tanzanian women. Sex. Transm. Infect. 97(1), 56–62 (2021)
So, K.A., Lee, I.H., Kim, T.J., Lee, K.H.: Risk factors of persistent HPV infection after treatment for high-grade squamous intraepithelial lesion. Arch. Gynecol. Obstet. 299(1), 223–227 (2018)
Plummer, M., de Martel C., Vignat J., Ferlay J., Bray F., Franceschi S.: Global burden of cancers attributable to infections in 2012: a synthetic analysis. Lancet Global Health 4(9), e609–e616 (2016)
McCredie, M.R.E., et al.: Natural history of cervical neoplasia and risk of invasive cancer in women with cervical intraepithelial neoplasia 3: a retrospective cohort study. Lancet Oncol. 9(5), 425–434 (2008)
Wang, Z.Y., et al.: TruScreen detection of cervical tissues for high-risk human papillomavirus-infected women during the COVID-19 pandemic. Future Oncol. 17(10), 1197–1207 (2021)
Verdoodt, F., Jentschke, M., Hillemanns, P., Racey, C.S., Snijders, P.J., Arbyn, M.: Reaching women who do not participate in the regular cervical cancer screening programme by offering self-sampling kits: a systematic review and meta-analysis of randomised trials. Eur. J. Cancer 51(16), 2375–2385 (2015)
Flanagan, S.M., Wilson, S., Luesley, D., Damery, S.L., Greenfield, S.M.: Adverse outcomes after colposcopy. BMC Womens Health 11, 2 (2011)
Graziottin, A., Serafini, A.: HPV infection in women: psychosexual impact of genital warts and intraepithelial lesions. J. Sex. Med. 6(3), 633–645 (2009)
Acknowledgments
This work was supported by the Education Department Foundation of Jilin Province (Grant No. JJKH20210066KJ).
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this paper
Cite this paper
Gong, L., Xie, H., Wang, L., Zhang, L., Li, S., Miao, B. (2022). Quantitative Analysis Indicators and Risk Factor Assessment of HR-HPV. In: Chu, SC., Lin, J.CW., Li, J., Pan, JS. (eds) Genetic and Evolutionary Computing. ICGEC 2021. Lecture Notes in Electrical Engineering, vol 833. Springer, Singapore. https://doi.org/10.1007/978-981-16-8430-2_14
Download citation
DOI: https://doi.org/10.1007/978-981-16-8430-2_14
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-16-8429-6
Online ISBN: 978-981-16-8430-2
eBook Packages: Intelligent Technologies and RoboticsIntelligent Technologies and Robotics (R0)