Abstract
Endocrine fibroblast growth factors (FGFs) require Klotho proteins as an obligate co-receptor to bind to and activate FGF receptor tyrosine kinases. FGF23 is a bone-derived hormone secreted in response to phosphate intake and acts on renal tubules that express αKlotho to increase urinary phosphate excretion per nephron and maintain the phosphate balance. FGF21 is secreted from the liver upon fasting and acts on the suprachiasmatic nucleus in the brain where βKlotho is expressed to induce responses to stress, including activation of the hypothalamus-pituitary-adrenal axis and the sympathetic nervous system. FGF21 is regarded as an antiaging hormone, because mice overexpressing FGF21 live longer than wild-type mice. Both FGF23 and FGF21 start increasing since early stages during the course of chronic kidney disease (CKD) progression. The increase in FGF23 compensates for decrease in the functional nephron number by increasing phosphate excretion per nephron, thereby maintaining phosphate homeostasis. However, FGF23-induced increase in phosphate concentration in the renal tubular fluid damages tubular cells and triggers interstitial fibrosis. In addition, FGF23 causes decrease in the serum level of active vitamin D followed by increase in parathyroid hormone, leading to mineral and bone disorders (CKD-MBD). The increase in FGF21 is necessary to survive CKD, because CKD mice lacking FGF21 exhibit poorer prognosis than wild-type CKD mice. However, FGF21-induced activation of the sympathetic nervous system results in blood pressure dysregulation. Thus, pathophysiology of CKD can be viewed as adverse effects associated with adaptive responses of the FGF-Klotho endocrine system to maintain survival and phosphate homeostasis.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Fibroblast growth factor-23 (FGF23)
- Fibroblast growth factor-21 (FGF21)
- αKlotho
- βKlotho
- Chronic kidney disease (CKD)
- Calciprotein particle (CPP)
1 Discovery of the Klotho Gene
The klotho gene was identified as the gene mutated in a mouse strain that exhibited premature aging (Kuro-o et al. 1997). The founder of this strain was a transgenic mouse carrying exogenous transgene inserted at the chromosome 5. Although expression of the transgene was not detectable in this strain, mice homozygous for the transgene developed complex aging-like symptoms after weaning, including growth arrest, multiple organ atrophy (gonads, thymus, skin, fat, etc.), vascular calcification, cardiac hypertrophy (Faul et al. 2011), sarcopenia, osteopenia (Kawaguchi et al. 1999), emphysematous lung, hearing disturbance (Kamemori et al. 2002), cognition impairment (Nagai et al. 2003), frailty, and premature death around 2 months of age. Because these phenotypes were observed only in homozygotes for the transgene, we hypothesized that the insertional mutation caused by chromosomal integration of the transgene might have disrupted a putative “aging-suppressor gene,” which we named Klotho after a Greek goddess who spins the thread of life.
We found that approximately ten copies of the transgene were integrated in tandem at the 5′-flanking region of an unknown gene at that time, which later turned out to be the klotho gene (Kuro-o et al. 1997). As the mutated allele (kl) was a severe hypomorphic allele, kl/kl mice barely expressed the klotho gene. The klotho gene encodes a single-pass transmembrane protein and is expressed only in a few cell types in wild-type mice, including distal tubular epithelial cells in the kidney, choroid plexus epithelial cells in the brain, and chief cells in parathyroid glands (Ben-Dov et al. 2007). The fact that kl/kl mice exhibited disorders in tissues that did not express the klotho gene endogenously raised the possibility that a humoral factor(s) might mediate the function of the klotho gene. The extracellular domain of Klotho has weak homology to the family 1 glycosidases. However, it was not clear whether Klotho protein would have glycosidase activity, because the two amino acids critical for the enzymatic activity (Asn and Glu) and thus conserved in all the family 1 glycosidases were replaced with other amino acids (Asp and Ala) (Kuro-o et al. 1997).
2 Klotho Protein Function
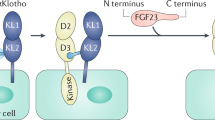
The clue to identification of the Klotho protein function was the report on mice lacking fibroblast growth factor-23 (FGF23) (Shimada et al. 2004b). FGF23 is a member of the fibroblast growth factor (FGF) family, but unlike the other FGF family members that function as paracrine and autocrine factors, it functions as an endocrine factor (hormone) (Goetz et al. 2007). FGF23 had been known to function as a bone-derived “phosphaturic” hormone that promoted urinary phosphate excretion (Shimada et al. 2004a). In response to phosphate intake, FGF23 is secreted from the bone (osteocytes/osteoblasts) and acts on the kidney to suppress phosphate resorption in proximal tubules and increase phosphate excretion per nephron, thereby maintaining the phosphate balance (Fig. 14.1) (Kuro-o 2019). Considering the phosphaturic activity of FGF23, mice lacking FGF23 were predicted to suffer from impaired excretion of phosphate into urine, resulting in phosphate retention. As expected, mice lacking FGF23 developed hyperphosphatemia and vascular calcification (Shimada et al. 2004b). However, they unexpectedly developed aging-like phenotypes, including growth arrest, multiple organ atrophy (gonads, thymus, skin, fat, etc.), sarcopenia, osteopenia, frailty, and premature death around 2 months of age (Shimada et al. 2004b). These phenotypes were reminiscent of those observed in kl/kl mice. In addition, we had known that kl/kl mice developed hyperphosphatemia. These observations prompted us to hypothesize that FGF23 and Klotho might function in the same signaling pathway involved in maintenance of phosphate homeostasis.
At that time, FGF23 was supposed to use FGF receptor tyrosine kinases as its cognate receptor. However, the affinity of any FGF receptor (FGFR) isoforms to FGF23 was too low (KD > 220 nM) to bind to FGF23 at its physiological concentration (~1 pM) (Yu et al. 2005). Hence, identity of the physiological FGF23 receptor had been unknown. The answer was that FGF23 required Klotho to bind to FGFRs. We found that Klotho forms constitutive binary complexes with FGFR1c, FGFR3c, and FGFR4. FGF23 binds to the FGFR-Klotho complexes with high affinity and activates the canonical FGF signaling pathway that culminates phosphorylation of FGFR substrate-2 (FRS2α) and its downstream target extracellular signal-regulated kinases (ERK1/2) (Kurosu et al. 2006). This finding was confirmed later in other laboratories (Urakawa et al. 2006). The fact that Klotho functions as the obligate co-receptor for FGF23 explains why kl/kl mice and mice lacking FGF23 exhibited identical phenotypes.
3 Discovery of the FGF-Klotho Endocrine Axes
After the discovery of the klotho gene, search for the GenBank database identified another gene encoding a single-pass transmembrane protein with ~40% amino acid identity with Klotho and was named βKlotho (Ito et al. 2000). Since then, Klotho was renamed as αKlotho to avoid confusion. Like αKlotho, βKlotho forms complexes with FGFR1c and FGFR4, which is expressed predominantly in adipose tissues and in the liver, respectively (Kurosu et al. 2007). Thus, the βKlotho-FGFR1c complex is expressed in adipose tissues, whereas the βKlotho-FGFR4 complex is expressed in the liver.
In the FGF family, FGF19 and FGF21 also function as endocrine factors besides FGF23. FGF19 is secreted from the intestinal epithelium upon feeding, reaches the liver via the portal circulation, and binds to the βKlotho-FGFR4 complex expressed on hepatocytes to induce metabolic responses to feeding, including suppression of bile acid synthesis and promotion of protein and glycogen synthesis (Inagaki et al. 2005; Kir et al. 2011; Potthoff et al. 2011). On the other hand, FGF21 is secreted from hepatocytes upon fasting and binds to the βKlotho-FGFR1c complex expressed on adipocytes to induce metabolic responses to fasting, including lipolysis (Inagaki et al. 2007; Ogawa et al. 2007; Potthoff et al. 2009). These three FGFs (FGF19, FGF21, and FGF23) are collectively designated as endocrine FGFs and different from the other FGF family members in that they function not as growth factors but as hormones regulating multiple metabolic processes and that they require Klotho family proteins to bind to their cognate FGFRs (Table 14.1) (Kuro-o 2019).
In 2018, crystal structure of the FGFR1c-αKlotho-FGF23 ternary complex and the βKlotho-FGF21 complex was solved (Chen et al. 2018; Lee et al. 2018). As given its namesake, αKlotho protein send out a long “thread” termed the receptor binding arm with intrinsically disordered structure. Once it captures FGFR and takes a fixed structure, a groove is created between αKlotho and FGFR into which FGF23 fits (Fig. 14.2) (Kuro-o 2018a).
4 Phosphate and CKD
Phosphorus is one of the six elements essential for life (H, C, N, O, S, P), but it has never drawn much attention in the medical field until quite recently. The situation has changed since hyperphosphatemia was identified as a major mortality risk for patients with chronic kidney disease (CKD) (Block et al. 1998; Ganesh et al. 2001). CKD is a new disease entity established a few decades ago and defined as any abnormality of the kidney structure and/or function lasting for 3 months or longer (Webster et al. 2017). CKD ensues in patients not only with renal disorders (e.g., chronic glomerulonephritis and polycystic kidney disease) but also with disorders causing renal complications (e.g., diabetes and hypertension). Therefore, CKD is very prevalent in the aging society, affecting more than 10% of the total population (Levey et al. 2005; Hill et al. 2016). Once CKD progresses to renal failure, renal replacement therapy (dialysis or renal transplantation) becomes necessary, which burdens healthcare worldwide. However, many CKD patients die from cardiovascular events before developing renal failure. Regardless of the underlying disorders, CKD progression can be viewed as a process of progressive loss of functional nephrons. CKD is classified from stage 1 (early stage) through stage 5 (end stage) based on the estimated glomerular filtration rate (eGFR) in clinical settings (Table 14.2).
Among several hormones that are increased or decreased during CKD progression, FGF23 is the first to move (Isakova et al. 2011). Serum FGF23 levels start elevating as early as stage 2–3. FGF23 increases phosphate excretion per nephron and compensates for the decrease in the nephron number to maintain the balance between phosphate intake and excretion. Besides functioning as a phosphaturic hormone, FGF23 also functions as a counter-regulatory hormone for active vitamin D (1,25-dihydroxyvitamin D3). FGF23 lowers serum levels of active vitamin D through downregulating renal expression of 1α-hydroxylase necessary for its synthesis and upregulating renal expression of 24-hydroxylase necessary for its degradation (Shimada et al. 2004a). The decrease in active vitamin D induces secretion of parathyroid hormone (PTH), because a robust negative feedback loop exists between active vitamin D and PTH, leading to secondary hyperparathyroidism. Serum phosphate levels start increasing in stage 4–5, when the residual nephron number becomes too low to balance phosphate excretion with phosphate intake. In this way, disturbed mineral metabolism characterized by high FGF23, low active vitamin D, high PTH, and high phosphate ensues in this order during the course of CKD progression, which is designated as CKD-MBD (mineral-bone disorder) (Fig. 14.3). Thus, CKD-MBD can be viewed as a result of an effort to maintain phosphate homeostasis against decrease in the nephron number during CKD progression (Kuro-o 2019).
In rodents, it was reported that renal tubular damage and interstitial fibrosis ensued when phosphate load excreted per nephron exceeded ~1.0 μg/day (Haut et al. 1980). Although the mechanism by which increase in phosphate excretion per nephron damages the kidney remains to be determined, it has been postulated that increased phosphate load per nephron should elevate phosphate concentration in the renal tubular fluid to trigger formation of tiny calcium phosphate precipitations, which might induce tubular damage (Lau 1989). Regardless of the mechanism, renal tubular damage should reduce the nephron number and demand further increase in FGF23 to maintain the phosphate balance unless phosphate intake is reduced. Thus, the increase in FGF23 compensates for the decrease in the nephron number and is indispensable for maintaining the phosphate homeostasis. However, it induces renal tubular damage and fibrosis to trigger a deterioration spiral leading to further nephron loss and acceleration of CKD progression (Kuro-o 2019).
In humans, healthy adults on regular diet excrete ~1.0 g of phosphate into urine. The nephron number in humans is approximately one million per kidney on average (Denic et al. 2017). Therefore, phosphate excretion per nephron is estimated as 0.5 μg/day. The nephron number is decreased with age as a part of the aging process. It has been reported that the nephron number of the elderly people in their 60s or 70s is approximately 50% less than that of the young people in their 20s (Denic et al. 2017). Unless phosphate intake is reduced with age, the amount of urinary phosphate excretion does not change. Therefore, phosphate excretion per nephron can reach 1.0 μg/day in the elderly people and may accelerate kidney aging. In humans, age-associated renal pathology characterized by tubular damage, interstitial inflammation and fibrosis, and glomerulosclerosis is universally observed even in “healthy” individuals and recognized as “aging kidney” (O’Sullivan et al. 2017). Hence, the amount of phosphate taken in from the daily diet potentially contributes to aging kidney in the elderly people.
5 Phosphate Accelerates Aging
As described above, kl/kl mice exhibit premature aging. The fundamental abnormality in kl/kl mice is disturbed phosphate homeostasis (phosphate retention) caused by the inability to induce phosphaturia in response to phosphate intake due to insensitivity to FGF23. Provided that phosphate retention is the cause of premature aging in kl/kl mice, restoration of the phosphate balance by restricting dietary phosphate intake should rescue kl/kl mice from premature aging. Indeed, most of the aging-like phenotypes were alleviated when kl/kl mice were placed on low phosphate diet (Morishita et al. 2001; Stubbs et al. 2007). These observations have led us to the notion that phosphate accelerates aging.
The signs and symptoms of kl/kl mice resemble those of patients with renal failure. Like kl/kl mice, patients with renal failure suffer from vascular calcification, cardiac hypertrophy, sarcopenia, osteopenia, frailty, and high mortality. They also exhibit hyperphosphatemia, hyper-FGF23-emia, and loss of renal αKlotho expression. Furthermore, dietary phosphate restriction and administration of phosphate binders improve their clinical outcomes. Because of the striking similarity to kl/kl mice that have been established as a mouse model of accelerated aging, renal failure patients have been viewed as a clinical model of accelerated aging (Stenvinkel and Larsson 2013). However, a fundamental difference exists between them. The cause of phosphate retention in renal failure patients is abolition of renal function in general. In contrast, the cause of phosphate retention in kl/kl mice is inability to excrete phosphate into urine. Their renal function is otherwise normal. In fact, kl/kl mice have normal serum creatinine levels and never develop renal failure. The complex signs and symptoms displayed by renal failure patients have been collectively designated as uremia and believed to be caused by accumulation of multiple “uremic toxins” that should have been excreted into urine. However, the fact that renal failure patients and kl/kl mice develop very similar pathophysiology suggests that phosphate may be the most important uremic toxin.
6 Calciprotein Particles (CPPs)
As the mechanism by which phosphate accelerates aging, we have proposed “the CPP theory of aging” (Kuro-o 2018b, 2019, 2020). CPPs are mineral-protein complex composed of solid-phase calcium phosphate and serum protein fetuin-A and dispersed as colloids in the blood. Because the blood is supersaturated regarding calcium and phosphate ions, even a slight and transient increase in blood phosphate levels can trigger precipitation of amorphous calcium phosphate. However, the calcium phosphate precipitates never grow and occlude capillaries, because these precipitates are adsorbed by fetuin-A and prevented from growing into large crystals. As a result, a fetuin-A molecule laden with tiny amorphous calcium phosphate precipitates is generated, which is termed primary CPPs. Primary CPPs spontaneously undergo self-aggregation and phase transition of calcium phosphate from the amorphous phase to the crystalline phase to become secondary CPPs (Fig. 14.4) (Kuro-o 2019; Jahnen-Dechent et al. 2020). Primary CPPs function physiologically as a potent inducer of FGF23 expression and secretion (Akiyama et al. 2019), whereas secondary CPPs have the activity that induces cell death in cultured vascular endothelial cells (Di Marco et al. 2008) and renal epithelial cells (Kunishige et al. 2020). CPPs are endocytosed and transported to lysosomes. Accumulation of CPPs in lysosomes increases their luminal pH, which disturbs lysosomal function and autophagic flux, leading to vulnerability to oxidative stress and cell death (Kunishige et al. 2020). CPPs also induce calcification in cultured vascular smooth muscle cells (Reynolds et al. 2004; Ewence et al. 2008; Sage et al. 2011) and innate immune responses in cultured macrophages as if they were a pathogen (Smith et al. 2013). As indicated by a coined word “inflammaging,” chronic inflammation is known to accelerate aging (Franceschi et al. 2000). In addition, recent clinical studies demonstrated that serum CPP levels were associated with clinical parameters for vascular calcification and inflammation (coronary artery calcification scores, aortic pulse wave velocity, hs-CRP, etc.) (Hamano et al. 2010; Smith et al. 2012). Considering the pathogenic activity of secondary CPPs, the correlation observed in these clinical studies may not merely correlation but causation. Phosphate, once precipitated with calcium to become CPPs, may behave like a pathogen that induces chronic noninfectious inflammation and cell damages, eventually accelerating aging.
7 CPPs and Lipoproteins
In mammals, insoluble materials are adsorbed by specific serum proteins and dispersed in the blood as colloids to be transported between organs. Lipids are adsorbed by apoproteins and dispersed in the blood as lipoproteins to be eventually stored in adipose tissues. However, when mistargeted to arteries, atherosclerosis ensues. Likewise, calcium phosphate are adsorbed by fetuin-A and dispersed in the blood as CPPs to be eventually stored in the bone. However, when mistargeted to arteries, vascular calcification ensues. Thus, the two distinct types of arteriosclerosis, atherosclerosis and vascular calcification, can be sublated as a disorder caused by mistargeting of insoluble materials, lipids, and calcium phosphate, respectively. In addition, ectopic accumulation of lipids in the liver and skeletal muscles induces fatty liver and insulin resistance, leading to metabolic syndrome. Likewise, CPPs in extraosseous tissues and extracellular fluid induce cell damage and chronic inflammation, potentially leading to acceleration of aging (Table 14.3).
8 Secreted αKlotho
The extracellular domain of αKlotho is clipped on the plasma membrane by membrane-anchored secretases and released into the extracellular space (Chen et al. 2007; Bloch et al. 2009). The secreted αKlotho exerts multiple functions independently of FGF23 as a humoral factor. It regulates cell surface abundance of several ion channels and transporters. Secreted αKlotho is reported to interact with particular sugar chains of transient receptor potential cation channel subfamily V member 5 and 6 (TRPV5, TRPV6) (Chang et al. 2005; Cha et al. 2008; Alexander et al. 2009) and renal outer medullary potassium channel (ROMK1) (Cha et al. 2009) to prevent them from being internalized, thereby increasing cellular calcium import and potassium export, respectively. It still remains controversial whether secreted αKlotho functions as a lectin (sugar-binding protein) that binds to any specific sugars or an enzyme that hydrolyzes any specific glycosidic bonds in glycans of these glycoproteins. On the other hand, secreted αKlotho reduces the cell surface abundance of TRPC6 through blocking phosphoinositide-3-kinase-dependent exocytosis of TRPC6 (Xie et al. 2012). Sodium-dependent phosphate co-transporters (Npt2a) are downregulated by secreted αKlotho through promoting their endocytosis and degradation (Hu et al. 2010). In addition, secreted αKlotho inhibits activity of several growth factors, including insulin-like growth factor-1 (IGF1) (Kurosu et al. 2005), Wnt (Liu et al. 2007; Zhou et al. 2013), and transforming growth factor-β1 (TGFβ1) (Doi et al. 2006), through distinct mechanisms. Secreted αKlotho inhibits the intracellular IGF1 signaling pathway at the level of receptor tyrosine phosphorylation (Kurosu et al. 2005). The activity of secreted αKlotho that inhibits Wnt signaling depends on its ability to directly bind to Wnt (Liu et al. 2007). Secreted Klotho also binds to the type-II TGFβ receptor (TGFβR2) and inhibits TGFβ1 binding to TGFβR2 (Doi et al. 2006). Of note, transgenic mice that overexpress αKlotho were reported to live longer than wild-type mice (Kurosu et al. 2005). These transgenic mice had higher serum levels of secreted αKlotho than wild-type mice. The ability of secreted αKlotho to inhibit IGF1 signaling might contribute to the extended life span, because adequate suppression of the somatotroph endocrine axis (the growth hormone-IGF1 axis) has been shown to extend life span in various experimental animals (Kenyon 2001).
9 FGF21-βKlotho Endocrine System
FGF21 was originally identified as a hormone secreted from hepatocytes upon fasting and bound to the FGFR1c-βKlotho complex expressed on adipose tissues to induce metabolic responses to fasting (Kharitonenkov et al. 2005; Inagaki et al. 2007; Ogawa et al. 2007). Shortly thereafter, it was reported that FGF21 also had the ability to attenuate responsiveness of hepatocytes to growth hormone (GH) (Inagaki et al. 2008). Thus FGF21 can induce not only metabolic responses resembling those induced by calorie restriction (CR) but also resistance to GH. CR and GH resistance are known to suppress aging and extend life span in various experimental animals (Kenyon 2001; Guarente 2013). In fact, transgenic mice that overexpress FGF21 live much longer than wild-type mice (Zhang et al. 2012). Thus, FGF21 can be regarded as an “antiaging hormone.” However, overexpression of FGF21 was associated with various adverse effects, including disturbed circadian rhythm and overactivation of the sympathetic nervous system and the hypothalamus-pituitary-adrenal axis (Bookout et al. 2013). Recent studies have demonstrated that these adverse effects were dependent on FGF21 acting in the central nervous system.
FGF21 can cross the blood-brain barrier and act on neurons in the suprachiasmatic nucleus (SCN) where βKlotho is expressed (Bookout et al. 2013). As SCN is the center of circadian rhythm, overexpression of FGF21 in mice disturbed circadian behavior with reduced activity in the dark phase and promoted torpor, which is a short-term hibernation-like state with low body temperature to avoid energy expenditure (Inagaki et al. 2007). In addition, activation of SCN by FGF21 stimulated production of corticotropin-releasing hormone (CRH) in the hypothalamus. CRH stimulates the hypothalamus-pituitary-adrenal axis to elevate circulating corticotropin levels and activates the sympathetic nerve system. Thus, FGF21 can be regarded as a stress-responsive hormone (Fig. 14.5) (Bookout et al. 2013).
10 FGF21 and CKD
Besides FGF23, serum FGF21 levels start increasing since early stages in CKD patients (Lin et al. 2011). In a mouse model of CKD (uninephrectomy followed by high phosphate diet feeding), FGF21 is increased significantly as well (Nakano et al. 2019). We hypothesized that the increase in FGF21 might be a response to stress caused by CKD and thus required to survive CKD. To test this hypothesis, we introduced CKD to mice lacking FGF21 (Fgf21−/− mice) and wild-type mice and compared their survival curves. As expected, Fgf21−/− mice showed poorer prognosis than wild-type mice (Fig. 14.6), indicating that FGF21 is necessary to survive CKD (Nakano et al. 2019). We next asked if the increased FGF21 in CKD might induce adverse effects similar to those observed in FGF21 overexpressing transgenic mice. To determine the circadian rhythm of blood pressure in mice, we placed a catheter in the internal carotid artery and monitored their arterial blood pressure continuously over 2 days under the conscious and unrestricted condition using a telemetry system. Although CKD mice showed a normal circadian rhythm in blood pressure (high in nighttime and low in daytime), they showed significantly enhanced blood pressure fluctuation when compared with non-CKD mice (Fig. 14.7a, b) (Nakano et al. 2019). The enhanced blood pressure fluctuation was attributed to augmentation of the blood pressure elevating response during physical activity, which was associated with increase in sympathetic nerve activity and reciprocal decrease in parasympathetic nerve activity. Importantly, the CKD-induced blood pressure dysregulation was not observed in Fgf21−/− mice (Fig. 14.7c, d). In addition, administration of FGF21 alone induced the similar blood pressure dysregulation in non-CKD mice (Fig. 14.7e, f). These findings indicated that FGF21 was necessary and sufficient to induce the blood pressure dysregulation observed in CKD mice (Nakano et al. 2019). This may be also applicable to humans, because CKD patients universally exhibit activation of sympathetic nerve system and augmentation of the blood pressure elevating response during physical activity (Downey et al. 2017).
Survival curves of CKD mice. FGF21 knockout mice (FGF21 KO, N = 20) and wild-type mice (WT, N = 20) were uninephrectomized at 8 weeks of age, placed on high phosphate diet containing 2.0% inorganic phosphate at 12 weeks of age, and then censored at 18 weeks of age. p = 0.017 by log-rank test. (Modified from reference Nakano et al. 2019)
Effects of FGF21 on blood pressure in mice. Blood pressure was measured continuously for 2 days using a telemetry system. Each panel represents overlapped line charts of systolic blood pressure from six mice. The black bars indicate the nighttime. The double arrows indicate the average amplitude of fluctuation of systolic blood pressure. CKD mice were prepared as described in Fig. 14.6. Non-CKD mice were prepared by sham-operation at 8 weeks of age and placed on regular diet containing 0.35% inorganic phosphate throughout the experimental period. (a) Wild-type non-CKD mice. (b) Wild-type CKD mice. (c) Fgf21−/− non-CKD mice. (d) Fgf21−/− CKD mice. (e) Wild-type non-CKD mice injected with an adeno-associated virus vector (AAV) expressing LacZ to serve as a control for (f). (f) wild-type non-CKD mice injected with AAV expressing FGF21. (Modified from reference Nakano et al. 2019)
11 Concluding Remarks
There have been two mainstreams of aging research. One is to investigate the mechanism of longevity evolutionarily conserved among yeasts, nematodes, flies, mice, and primates, leading to the notion that adequate calorie restriction extends life span in various experimental animals (Guarente 2013). The other mainstream is to investigate the mechanism of cell senescence, which revealed the fact that accumulation of senescent cells accelerates aging at the organismal level (Baker et al. 2011). Based on these findings, calorie restriction mimetics (Ingram et al. 2006) and senolytic drugs (drugs that kill senescent cells selectively) (Kirkland et al. 2017) are expected to extend life span in humans. However, even if clinical trials are successful and these medicines indeed extend the average life span, the healthcare system in the aging society can get worse unless the health span extension predominates over the life span extension. By freeing ourselves from these two mainstreams and pursuing the mechanism of aging specific to higher organisms, we may be able to develop a new antiaging medicine. The FGF-Klotho endocrine system is unique to vertebrates and may become a novel target of aging research aiming at extension of health span.
References
Akiyama K, Miura Y, Hayashi H, Sakata A, Matsumura Y, Kojima M, Tsuchiya K, Nitta K, Shiizaki K, Kurosu H, Kuro-o M (2019) Calciprotein particles regulate fibroblast growth factor-23 expression in osteoblasts. Kidney Int 97:702–712
Alexander RT, Woudenberg-Vrenken TE, Buurman J, Dijkman H, van der Eerden BC, van Leeuwen JP, Bindels RJ, Hoenderop JG (2009) Klotho prevents renal calcium loss. J Am Soc Nephrol 20:2371–2379
Baker DJ, Wijshake T, Tchkonia T, LeBrasseur NK, Childs BG, van de Sluis B, Kirkland JL, van Deursen JM (2011) Clearance of p16Ink4a-positive senescent cells delays ageing-associated disorders. Nature 479:232–236
Ben-Dov IZ, Galitzer H, Lavi-Moshayoff V, Goetz R, Kuro-o M, Mohammadi M, Sirkis R, Naveh-Many T, Silver J (2007) The parathyroid is a target organ for FGF23 in rats. J Clin Invest 117:4003–4008
Bloch L, Sineshchekova O, Reichenbach D, Reiss K, Saftig P, Kuro-o M, Kaether C (2009) Klotho is a substrate for alpha-, beta- and gamma-secretase. FEBS Lett 583:3221–3224
Block GA, Hulbert-Shearon TE, Levin NW, Port FK (1998) Association of serum phosphorus and calcium x phosphate product with mortality risk in chronic hemodialysis patients: a national study. Am J Kidney Dis 31:607–617
Bookout AL, de Groot MH, Owen BM, Lee S, Gautron L, Lawrence HL, Ding X, Elmquist JK, Takahashi JS, Mangelsdorf DJ, Kliewer SA (2013) FGF21 regulates metabolism and circadian behavior by acting on the nervous system. Nat Med 19:1147–1152
Cha SK, Ortega B, Kurosu H, Rosenblatt KP, Kuro-o M, Huang CL (2008) Removal of sialic acid involving Klotho causes cell-surface retention of TRPV5 channel via binding to galectin-1. Proc Natl Acad Sci U S A 105:9805–9810
Cha SK, Hu MC, Kurosu H, Kuro-o M, Moe O, Huang CL (2009) Regulation of ROMK1 channel and renal K+ excretion by Klotho. Mol Pharmacol 76:38–46
Chang Q, Hoefs S, van der Kemp AW, Topala CN, Bindels RJ, Hoenderop JG (2005) The beta-glucuronidase klotho hydrolyzes and activates the TRPV5 channel. Science 310:490–493
Chen CD, Podvin S, Gillespie E, Leeman SE, Abraham CR (2007) Insulin stimulates the cleavage and release of the extracellular domain of Klotho by ADAM10 and ADAM17. Proc Natl Acad Sci U S A 104:19796–19801
Chen G, Liu Y, Goetz R, Fu L, Jayaraman S, Hu MC, Moe OW, Liang G, Li X, Mohammadi M (2018) alpha-Klotho is a non-enzymatic molecular scaffold for FGF23 hormone signalling. Nature 553:461–466
Denic A, Lieske JC, Chakkera HA, Poggio ED, Alexander MP, Singh P, Kremers WK, Lerman LO, Rule AD (2017) The substantial loss of nephrons in healthy human kidneys with aging. J Am Soc Nephrol 28:313
Di Marco GS, Hausberg M, Hillebrand U, Rustemeyer P, Wittkowski W, Lang D, Pavenstadt H (2008) Increased inorganic phosphate induces human endothelial cell apoptosis in vitro. Am J Physiol Ren Physiol 294:F1381–F1387
Doi K, Noiri E, Nakao A, Fujita T, Kobayashi S, Tokunaga K (2006) Functional polymorphisms in the vascular endothelial growth factor gene are associated with development of end-stage renal disease in males. J Am Soc Nephrol 17:823–830
Downey RM, Liao P, Millson EC, Quyyumi AA, Sher S, Park J (2017) Endothelial dysfunction correlates with exaggerated exercise pressor response during whole body maximal exercise in chronic kidney disease. Am J Physiol Ren Physiol 312:F917–f924
Ewence AE, Bootman M, Roderick HL, Skepper JN, McCarthy G, Epple M, Neumann M, Shanahan CM, Proudfoot D (2008) Calcium phosphate crystals induce cell death in human vascular smooth muscle cells: a potential mechanism in atherosclerotic plaque destabilization. Circ Res 103:e28–e34
Faul C, Amaral AP, Oskouei B, Hu MC, Sloan A, Isakova T, Gutierrez OM, Aguillon-Prada R, Lincoln J, Hare JM, Mundel P, Morales A, Scialla J, Fischer M, Soliman EZ, Chen J, Go AS, Rosas SE, Nessel L, Townsend RR, Feldman HI, St John Sutton M, Ojo A, Gadegbeku C, Di Marco GS, Reuter S, Kentrup D, Tiemann K, Brand M, Hill JA, Moe OW, Kuro-o M, Kusek JW, Keane MG, Wolf M (2011) FGF23 induces left ventricular hypertrophy. J Clin Invest 121:4393–4408
Franceschi C, Bonafe M, Valensin S, Olivieri F, De Luca M, Ottaviani E, De Benedictis G (2000) Inflamm-aging. An evolutionary perspective on immunosenescence. Ann N Y Acad Sci 908:244–254
Ganesh SK, Stack AG, Levin NW, Hulbert-Shearon T, Port FK (2001) Association of elevated serum PO(4), Ca x PO(4) product, and parathyroid hormone with cardiac mortality risk in chronic hemodialysis patients. J Am Soc Nephrol 12:2131–2138
Goetz R, Beenken A, Ibrahimi OA, Kalinina J, Olsen SK, Eliseenkova AV, Xu C, Neubert TA, Zhang F, Linhardt RJ, Yu X, White KE, Inagaki T, Kliewer SA, Yamamoto M, Kurosu H, Ogawa Y, Kuro-o M, Lanske B, Razzaque MS, Mohammadi M (2007) Molecular insights into the klotho-dependent, endocrine mode of action of fibroblast growth factor 19 subfamily members. Mol Cell Biol 27:3417–3428
Guarente L (2013) Calorie restriction and sirtuins revisited. Genes Dev 27:2072–2085
Hamano T, Matsui I, Mikami S, Tomida K, Fujii N, Imai E, Rakugi H, Isaka Y (2010) Fetuin-mineral complex reflects extraosseous calcification stress in CKD. J Am Soc Nephrol 21:1998–2007
Haut LL, Alfrey AC, Guggenheim S, Buddington B, Schrier N (1980) Renal toxicity of phosphate in rats. Kidney Int 17:722–731
Hill NR, Fatoba ST, Oke JL, Hirst JA, O’Callaghan CA, Lasserson DS, Hobbs FD (2016) Global prevalence of chronic kidney disease - a systematic review and meta-analysis. PLoS One 11:e0158765
Hu MC, Shi M, Zhang J, Pastor J, Nakatani T, Lanske B, Shawkat Razzaque M, Rosenblatt KP, Baum MG, Kuro-o M, Moe OW (2010) Klotho: a novel phosphaturic substance acting as an autocrine enzyme in the renal proximal tubule. FASEB J 24:3438–3450
Inagaki T, Choi M, Moschetta A, Peng L, Cummins CL, McDonald JG, Luo G, Jones SA, Goodwin B, Richardson JA, Gerard RD, Repa JJ, Mangelsdorf DJ, Kliewer SA (2005) Fibroblast growth factor 15 functions as an enterohepatic signal to regulate bile acid homeostasis. Cell Metab 2:217–225
Inagaki T, Dutchak P, Zhao G, Ding X, Gautron L, Parameswara V, Li Y, Goetz R, Mohammadi M, Esser V, Elmquist JK, Gerard RD, Burgess SC, Hammer RE, Mangelsdorf DJ, Kliewer SA (2007) Endocrine regulation of the fasting response by PPARalpha-mediated induction of fibroblast growth factor 21. Cell Metab 5:415–425
Inagaki T, Lin VY, Goetz R, Mohammadi M, Mangelsdorf DJ, Kliewer SA (2008) Inhibition of growth hormone signaling by the fasting-induced hormone FGF21. Cell Metab 8:77–83
Ingram DK, Zhu M, Mamczarz J, Zou S, Lane MA, Roth GS, deCabo R (2006) Calorie restriction mimetics: an emerging research field. Aging Cell 5:97–108
Isakova T, Wahl P, Vargas GS, Gutierrez OM, Scialla J, Xie H, Appleby D, Nessel L, Bellovich K, Chen J, Hamm L, Gadegbeku C, Horwitz E, Townsend RR, Anderson CA, Lash JP, Hsu CY, Leonard MB, Wolf M (2011) Fibroblast growth factor 23 is elevated before parathyroid hormone and phosphate in chronic kidney disease. Kidney Int 79:1370–1378
Ito S, Kinoshita S, Shiraishi N, Nakagawa S, Sekine S, Fujimori T, Nabeshima YI (2000) Molecular cloning and expression analyses of mouse betaklotho, which encodes a novel Klotho family protein. Mech Dev 98:115–119
Jahnen-Dechent W, Büscher A, Köppert S, Heiss A, Kuro-o M, Smith ER (2020) Mud in the blood the role of protein-mineral complexes and extracellular vesicles in biomineralisation and calcification. J Struct Biol 212:107577
Kamemori M, Ohyama Y, Kurabayashi M, Takahashi K, Nagai R, Furuya N (2002) Expression of Klotho protein in the inner ear. Hear Res 171:103–110
Kawaguchi H, Manabe N, Miyaura C, Chikuda H, Nakamura K, Kuro-o M (1999) Independent impairment of osteoblast and osteoclast differentiation in klotho mouse exhibiting low-turnover osteopenia. J Clin Invest 104:229–237
Kenyon C (2001) A conserved regulatory system for aging. Cell 105:165–168
Kharitonenkov A, Shiyanova TL, Koester A, Ford AM, Micanovic R, Galbreath EJ, Sandusky GE, Hammond LJ, Moyers JS, Owens RA, Gromada J, Brozinick JT, Hawkins ED, Wroblewski VJ, Li DS, Mehrbod F, Jaskunas SR, Shanafelt AB (2005) FGF-21 as a novel metabolic regulator. J Clin Invest 115:1627–1635
Kir S, Beddow SA, Samuel VT, Miller P, Previs SF, Suino-Powell K, Xu HE, Shulman GI, Kliewer SA, Mangelsdorf DJ (2011) FGF19 as a postprandial, insulin-independent activator of hepatic protein and glycogen synthesis. Science 331:1621–1624
Kirkland JL, Tchkonia T, Zhu Y, Niedernhofer LJ, Robbins PD (2017) The clinical potential of senolytic drugs. J Am Geriatr Soc 65:2297–2301
Kunishige R, Mizoguchi M, Tsubouchi A, Hanaoka K, Miura Y, Kurosu H, Urano Y, Kuro-o M, Murata M (2020) Calciprotein particle-induced cytotoxicity via lysosomal dysfunction and altered cholesterol distribution in renal epithelial HK-2 cells. Sci Rep 10:20125
Kuro-o M (2018a) Ageing-related receptors resolved. Nature 553:409–410
Kuro-o M (2018b) Klotho and endocrine fibroblast growth factors: marker of chronic kidney disease progression and cardiovascular complications? Nephrol Dial Transplant 34:15–21
Kuro-o M (2019) The Klotho proteins in health and disease. Nat Rev Nephrol 15:27–44
Kuro-o M (2020) Phosphate as a pathogen of arteriosclerosis and aging. J Atheroscler Thromb 28:203–213
Kuro-o M, Matsumura Y, Aizawa H, Kawaguchi H, Suga T, Utsugi T, Ohyama Y, Kurabayashi M, Kaname T, Kume E, Iwasaki H, Iida A, Shiraki-Iida T, Nishikawa S, Nagai R, Nabeshima Y (1997) Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature 390:45–51
Kurosu H, Yamamoto M, Clark JD, Pastor JV, Nandi A, Gurnani P, McGuinness OP, Chikuda H, Yamaguchi M, Kawaguchi H, Shimomura I, Takayama Y, Herz J, Kahn CR, Rosenblatt KP, Kuro-o M (2005) Suppression of aging in mice by the hormone klotho. Science 309:1829–1833
Kurosu H, Ogawa Y, Miyoshi M, Yamamoto M, Nandi A, Rosenblatt KP, Baum MG, Schiavi S, Hu MC, Moe OW, Kuro-o M (2006) Regulation of fibroblast growth factor-23 signaling by klotho. J Biol Chem 281:6120–6123
Kurosu H, Choi M, Ogawa Y, Dickson AS, Goetz R, Eliseenkova AV, Mohammadi M, Rosenblatt KP, Kliewer SA, Kuro-o M (2007) Tissue-specific expression of betaKlotho and fibroblast growth factor (FGF) receptor isoforms determines metabolic activity of FGF19 and FGF21. J Biol Chem 282:26687–26695
Lau K (1989) Phosphate excess and progressive renal failure: the precipitation-calcification hypothesis. Kidney Int 36:918–937
Lee S, Choi J, Mohanty J, Sousa LP, Tome F, Pardon E, Steyaert J, Lemmon MA, Lax I, Schlessinger J (2018) Structures of beta-klotho reveal a ‘zip code’-like mechanism for endocrine FGF signalling. Nature 553:501–505
Levey AS, Eckardt K-U, Tsukamoto Y, Levin A, Coresh J, Rossert J, Zeeuw DDE, Hostetter TH, Lameire N, Eknoyan G (2005) Definition and classification of chronic kidney disease: a position statement from Kidney Disease: Improving Global Outcomes (KDIGO). Kidney Int 67:2089–2100
Lin Z, Zhou Z, Liu Y, Gong Q, Yan X, Xiao J, Wang X, Lin S, Feng W, Li X (2011) Circulating FGF21 levels are progressively increased from the early to end stages of chronic kidney diseases and are associated with renal function in Chinese. PLoS One 6:e18398
Liu H, Fergusson MM, Castilho RM, Liu J, Cao L, Chen J, Malide D, Rovira II, Schimel D, Kuo CJ, Gutkind JS, Hwang PM, Finkel T (2007) Augmented Wnt signaling in a mammalian model of accelerated aging. Science 317:803–806
Morishita K, Shirai A, Kubota M, Katakura Y, Nabeshima Y, Takeshige K, Kamiya T (2001) The progression of aging in klotho mutant mice can be modified by dietary phosphorus and zinc. J Nutr 131:3182–3188
Nagai T, Yamada K, Kim HC, Kim YS, Noda Y, Imura A, Nabeshima Y, Nabeshima T (2003) Cognition impairment in the genetic model of aging klotho gene mutant mice: a role of oxidative stress. FASEB J 17:50–52
Nakano T, Shiizaki K, Miura Y, Matsui M, Kosaki K, Mori S, Yamagata K, Maeda S, Kishi T, Usui N, Yoshida M, Onaka T, Mizukami H, Kaneda R, Karasawa K, Nitta K, Kurosu H, Kuro-o M (2019) Increased fibroblast growth factor-21 in chronic kidney disease is a trade-off between survival benefit and blood pressure dysregulation. Sci Rep 9:19247
O’Sullivan ED, Hughes J, Ferenbach DA (2017) Renal aging: causes and consequences. J Am Soc Nephrol 28:407–420
Ogawa Y, Kurosu H, Yamamoto M, Nandi A, Rosenblatt KP, Goetz R, Eliseenkova AV, Mohammadi M, Kuro-o M (2007) βKlotho is required for metabolic activity of fibroblast growth factor 21. Proc Natl Acad Sci U S A 104:7432–7437
Potthoff MJ, Inagaki T, Satapati S, Ding X, He T, Goetz R, Mohammadi M, Finck BN, Mangelsdorf DJ, Kliewer SA, Burgess SC (2009) FGF21 induces PGC-1alpha and regulates carbohydrate and fatty acid metabolism during the adaptive starvation response. Proc Natl Acad Sci U S A 106:10853–10858
Potthoff MJ, Boney-Montoya J, Choi M, He T, Sunny NE, Satapati S, Suino-Powell K, Xu HE, Gerard RD, Finck BN, Burgess SC, Mangelsdorf DJ, Kliewer SA (2011) FGF15/19 regulates hepatic glucose metabolism by inhibiting the CREB-PGC-1alpha pathway. Cell Metab 13:729–738
Reynolds JL, Joannides AJ, Skepper JN, McNair R, Schurgers LJ, Proudfoot D, Jahnen-Dechent W, Weissberg PL, Shanahan CM (2004) Human vascular smooth muscle cells undergo vesicle-mediated calcification in response to changes in extracellular calcium and phosphate concentrations: a potential mechanism for accelerated vascular calcification in ESRD. J Am Soc Nephrol 15:2857–2867
Sage AP, Lu J, Tintut Y, Demer LL (2011) Hyperphosphatemia-induced nanocrystals upregulate the expression of bone morphogenetic protein-2 and osteopontin genes in mouse smooth muscle cells in vitro. Kidney Int 79:414–422
Shimada T, Hasegawa H, Yamazaki Y, Muto T, Hino R, Takeuchi Y, Fujita T, Nakahara K, Fukumoto S, Yamashita T (2004a) FGF-23 is a potent regulator of vitamin D metabolism and phosphate homeostasis. J Bone Miner Res 19:429–435
Shimada T, Kakitani M, Yamazaki Y, Hasegawa H, Takeuchi Y, Fujita T, Fukumoto S, Tomizuka K, Yamashita T (2004b) Targeted ablation of Fgf23 demonstrates an essential physiological role of FGF23 in phosphate and vitamin D metabolism. J Clin Invest 113:561–568
Smith ER, Ford ML, Tomlinson LA, Rajkumar C, McMahon LP, Holt SG (2012) Phosphorylated fetuin-A-containing calciprotein particles are associated with aortic stiffness and a procalcific milieu in patients with pre-dialysis CKD. Nephrol Dial Transplant 27:1957–1966
Smith ER, Hanssen E, McMahon LP, Holt SG (2013) Fetuin-A-containing calciprotein particles reduce mineral stress in the macrophage. PLoS One 8:e60904
Stenvinkel P, Larsson TE (2013) Chronic kidney disease: a clinical model of premature aging. Am J Kidney Dis 62:339–351
Stubbs JR, Liu S, Tang W, Zhou J, Wang Y, Yao X, Quarles LD (2007) Role of hyperphosphatemia and 1,25-Dihydroxyvitamin D in vascular calcification and mortality in fibroblastic growth factor 23 null mice. J Am Soc Nephrol 18:2116–2124
Urakawa I, Yamazaki Y, Shimada T, Iijima K, Hasegawa H, Okawa K, Fujita T, Fukumoto S, Yamashita T (2006) Klotho converts canonical FGF receptor into a specific receptor for FGF23. Nature 444:770–774
Webster AC, Nagler EV, Morton RL, Masson P (2017) Chronic kidney disease. Lancet 389:1238–1252
Xie J, Cha SK, An SW, Kuro-o M, Birnbaumer L, Huang CL (2012) Cardioprotection by Klotho through downregulation of TRPC6 channels in the mouse heart. Nat Commun 3:1238
Yu X, Ibrahimi OA, Goetz R, Zhang F, Davis SI, Garringer HJ, Linhardt RJ, Ornitz DM, Mohammadi M, White KE (2005) Analysis of the biochemical mechanisms for the endocrine actions of fibroblast growth factor-23. Endocrinology 146:4647–4656
Zhang Y, Xie Y, Berglund ED, Coate KC, He TT, Katafuchi T, Xiao G, Potthoff MJ, Wei W, Wan Y, Yu RT, Evans RM, Kliewer SA, Mangelsdorf DJ (2012) The starvation hormone, fibroblast growth factor-21, extends lifespan in mice. elife 1:e00065
Zhou L, Li Y, Zhou D, Tan RJ, Liu Y (2013) Loss of Klotho contributes to kidney injury by depression of Wnt/beta-catenin signaling. J Am Soc Nephrol 24:771–785
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Kuro-o, M. (2022). Aging and Chronic Kidney Disease Viewed from the FGF-Klotho Endocrine System. In: Mori, N. (eds) Aging Mechanisms II . Springer, Singapore. https://doi.org/10.1007/978-981-16-7977-3_14
Download citation
DOI: https://doi.org/10.1007/978-981-16-7977-3_14
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-16-7976-6
Online ISBN: 978-981-16-7977-3
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)