Abstract
Over the last decade, there has been refinement in both microsurgical techniques as well as endovascular therapy (EVT) for the management of giant intracranial aneurysms (GIAs) and blood blister-like aneurysms (BBAs). Both of them come with their own set of problems in there management. GIAs are treacherous lesions with grave prognosis, and their management is problematic because of the wide atheromatous neck, involved branches, thrombus within, calcified wall, and complex anatomy resulting in a combined surgical morbidity and mortality that remains in the range of 20–30%. Posterior circulation aneurysms have a higher rupture risk (RR) over anterior circulation. While small saccular aneurysms are optimally excluded from circulation by EVT, there is a high failure rate after EVT of GIAs. Failures of EVT are often related to aneurysm morphology, a broad aneurysm neck (high neck: dome ratio), large and giant-size outflow arteries arising from the aneurysm base or walls, and fusiform/dolichoectatic morphology. An aneurysm with a broad neck can result in the herniation of coils into the parent artery lumen. Balloon- and stent-assisted coiling techniques are useful but are associated with the additional risk of parent artery ischemia, perforation, distal thromboembolism, and occlusion of adjacent perforators and branch arteries by the lattice of the stent. The rate of recurrence is also higher in broad neck aneurysms because the hemodynamics at the inflow zone is more complex. The other reasons for failure are incomplete initial obliteration, thrombus within the lumen, poor radiographic visualization of the aneurysm anatomy and its adjacent branches, and tortuosity of the feeding vessel, making catheterization difficult. Flow diverters are exciting, but it is still early days for prime time. Improvements in instrumentation and hardware, application of skull base surgical techniques, revascularization procedures, advances in anesthetic techniques like cerebral protection, adenosine-induced cardiac standstill, rapid ventricular pacing and hypothermic circulatory arrest, and intraoperative indocyanine green (ICG) angiography have made microsurgery a relatively safe and also a cost-effective option over EVT. Treatment of complex aneurysms like GIAs and BBAs is challenging. The modalities of treatment, microsurgery, EVT, or combined should be individualized taking into consideration the patient and pathological factors and available expertise. Although EVT is an attractive option, the high incidence of incomplete treatment, delayed complications, recurrence, and inadequate long-term follow-up data makes microsurgery relevant.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
15.1 Introduction
Giant intracranial aneurysms (GIAs) have, by definition, a minimum diameter of 25 mm [1]. They represent <5% of all intracranial aneurysms, making them rare among all aneurysms [2]. GIAs are treacherous lesions with grave prognosis, and their management is problematic because of the wide atheromatous neck, involved branches, thrombus within, calcified wall, and complex anatomy resulting in a combined surgical morbidity and mortality that remains in the range of 20–30% [3]. Yet, the GIAs need treatment, as these often have a downhill course without treatment, with a mortality rate at 2 and 5 years after diagnosis being 68 and 85%, respectively [4]. Rupture risk (RR) of untreated, unruptured GIAs is 8–10% per year, with a mortality of 65–100% at 1–5 years follow-up [5, 6]. Posterior circulation aneurysms have a higher RR over anterior circulation [7]. Over the last few decades, there has been refinement in both microsurgical techniques and endovascular treatment (EVT). Continuous improvements in EVT offer promise in the management of GIAs. While small saccular aneurysms are optimally excluded from circulation by EVT, there is a high failure rate after EVT of GIAs. Failures of EVT are often related to aneurysm morphology, a broad aneurysm neck (high neck: dome ratio), large and giant-size outflow arteries arising from the aneurysm base or walls, and fusiform/dolichoectatic morphology [8]. An aneurysm with a broad neck can result in the herniation of coils into the parent artery lumen. Balloon- and stent-assisted coiling techniques are useful but are associated with the additional risk of parent artery ischemia, perforation, distal thromboembolism, and occlusion of adjacent perforators and branch arteries by the lattice of the stent [8]. The rate of recurrence is also higher in broad neck aneurysms because the hemodynamics at the inflow zone is more complex. The other reasons for failure are incomplete initial obliteration, thrombus within the lumen, poor radiographic visualization of the aneurysm anatomy and its adjacent branches, and tortuosity of the feeding vessel, making catheterization difficult [8]. Flow diverters are exciting, but it is still early days for prime time. Improvements in instrumentation and hardware, application of skull base surgical techniques, revascularization procedures, advances in anesthetic techniques like cerebral protection, adenosine-induced cardiac standstill, rapid ventricular pacing and hypothermic circulatory arrest, and intraoperative indocyanine green (ICG) angiography have made microsurgery a relatively safer and also a cost-effective option over EVT [9].
The natural history of giant intracranial aneurysms (GIAs) is characterized by progressive growth, thrombosis, and rupture [2], and the natural history of untreated giant cerebral aneurysms is significantly poor. In the International Study for Unruptured Intracranial Aneurysms (ISUIA), Wiebers et al. reported that the 5-year incidence of rupture was 40% for anterior and 50% for posterior circulation giant aneurysms or 8 to 10% per year [6]. Peerless et al. showed that the mortality rate of patients not presenting with hemorrhage was higher than 60% within 2 years, with the prognosis being worse for patients presenting with subarachnoid hemorrhage (SAH) [10]. This poor natural history is attributed to their mass effect on the surrounding brain tissue, higher risk for rupture, location, morphology (saccular versus fusiform), and the presence or absence of laminated thrombus and/or atherosclerotic plaque within the fundus and neck of the aneurysm [11, 12].
Management of Aneurysms not amenable to EVT can be broadly classified into:
-
1.
Hemorrhagic presentation
-
2.
Ischemic presentation
-
3.
Mass effect as presentation
15.2 Operative Techniques
15.2.1 Choice of Operative Approach (Fig. 15.1)
The use of cranial base approaches to enhance exposure and to minimize damage to, and retraction of neural tissue is the golden rule of surgery. In fact, the authors currently utilize dedicated skull base approaches more often in aneurysm surgery than in surgery of skull base tumors. While tumors provide space to be tackled through conventional craniotomy, every millimeter of extra space gained through bone drilling helps significantly in aneurysm surgery. Aggressive drilling of bony structures at the skull base may consume time but ultimately provides a wide and safe corridor. Moreover, drilling is essential to expose the neck and provide proximal vessel control (anterior clinoidectomy for ICA, posterior clinoidectomy, and clivus drilling for BA). For lesions involving the anterior circulation, authors routinely use the frontotemporal (FT) craniotomy.
15.3 Anterior Circulation Aneurysms
15.3.1 Orbitozygomatic-Pterional Approach
The pterional transsylvian approach is the workhorse. It provides access to the entire circle of Willis and branches. Drilling the pterion can further increase basal exposure and bony ridges over the floor of the frontal fossa and OZ osteotomy [13, 14]. Intradural anterior clinoidectomy and carotid exposure in the neck are routinely performed in ophthalmic segment aneurysms. The OZ approach provides a lower trajectory along the skull base, which reduces the need for cerebral retraction. It also enhances access to upper clival lesions. The risks associated with this approach are periorbital bruising, injury to the frontalis nerve, orbital entrapment, diplopia, and blindness. However, these are extremely rare [15, 16].
15.3.2 Interhemispheric Approach
This approach is traditionally used for DACA aneurysms.
15.4 Posterior Circulation Aneurysms
The OZ approach is optimal for lesions of the upper third, and the vertebrobasilar area is best accessed with the far-lateral approach. Lesions involving only the midbasilar zone may require transpetrosal or extended retrosigmoid approaches.
15.4.1 Orbitozygomatic Approach
Additional modification of drilling the anterior and posterior clinoid processes and the clivus itself allows visualization down towards the midbasilar zone [17]. The authors employ extradural anterior clinoidectomy and intradural posterior clinoidectomy for GIAs of the basilar top. It provides excellent exposure of the upper interpeduncular space without excessive frontal lobe traction for high-riding basilar top aneurysms. It also gives an overview of the adjacent vessels and perforators.
15.4.2 Transpetrosal Approaches
The transpetrosal approaches are divided into retrolabyrinthine, translabyrinthine, and transcochlear, depending on the degree of removal of the petrous ridge [18, 19]. This approach is reserved for complex lesions of the midbasilar zone.
15.4.3 Far-Lateral Approach
It provides an excellent exposure from the midbasilar zone down to the intradural vertebral artery, which includes giant aneurysms of the vertebrobasilar, vertebral, and proximal posterior inferior cerebellar arteries [20, 21].
15.4.4 Combined Approaches
If the situation demands, various conventional approaches can be combined to get wider access and control [22]. A combination of supratentorial and infratentorial approaches, for example, a subtemporal craniotomy with a transpetrosal approach, can be extended further inferiorly by the addition of a far-lateral approach to it [23].
For the management of complex aneurysms, including giants, the operating surgeon should have the following in his armamentarium.
15.5 Vascular Control
Proximal and distal vascular control is essential as it gives control during an event of intraoperative rupture of aneurysm. It softens the aneurysm, helps dissection and manipulation from the surrounding neural structures, and facilitates clipping. Proximal control can be easily obtained for GIAs of anterior circulation except if the lesion is more proximal at the level of clinoid and ophthalmic segment. Options available include exposure and control of the cervical carotid artery via a separate neck incision which is the most common, least risky and preferred by the authors. Other options include exposure of the petrous ICA through Glasscock’s triangle, exposure of the clinoidal segment of the ICA after removal of the anterior clinoid process, and endovascular balloon occlusion of the cavernous segment of the ICA [24].
Vascular control of giant aneurysms of the posterior circulation is more difficult owing to the complex anatomy and its proximity to the skull base. There is a high risk of injury to the brainstem perforators if a temporary clip is placed along the middle portion of the basilar artery. Far-lateral exposure can give proximal control over the vertebral arteries, but distal control can be problematic. OZ exposure can give proximal control by access to the basilar trunk, but the contralateral superior cerebellar artery and posterior cerebral artery control are difficult.
Endovascular temporary balloon occlusion can be instrumental particularly in the proximal basilar and vertebral artery regions [25].
The ultimate vascular control is obtained with hypothermic circulatory arrest [26]. Hypothermic circulatory arrest was first applied in neurosurgery in 1938 [27, 28]. In the early 1960s, it was used to treat intracranial aneurysms in several studies; however, it soon lost favor because of its high complication rate due to intraoperative and postoperative coagulopathies. The patients who were earlier candidates for cardiac standstill are now being treated via alternative microsurgical techniques, endovascular therapy, or combined endovascular and microsurgical strategies [26].
Adenosine-induced cardiac asystole has been useful in the treatment of intracranial aneurysms. It gives brief periods (5–10 s) of cardiac arrest, thus facilitating clipping. It has largely obviated the need to use cardiac standstill in the treatment of GIAs [28,29,30].
15.6 Techniques for Clipping
For clipping to happen successfully, the ideal neck should be well defined and favorable to clip as what is commonly seen in a saccular aneurysm. However, the same does not happen if it is a GIA, especially fusiform or dolichoectatic GIAs. These have ill-defined neck and efferent vessels, and perforators may arise from the base or from the body.
The principle of clipping involves reconstruction of the lumen while preserving the branches/perforators and obliteration of the aneurysm. For this, the know-how of technicalities of the aneurysm clip is essential, e.g., the lowest closing force along the clip is located at its tip [31]. Hence instead of one long clip, multiple small clips placed in tandem can be better; they can also be stacked one above the other to prevent migration/slippage [17, 18]. Most frequently, GIAs are associated with atherosclerotic necks, which may prevent the blades of the clip to approximate; discretion is warranted while doing these procedures so as to avoid distal migration and emboli of the plaques in the vessel [32]. Hence preoperative angiographic evaluation can help to decide if a protective bypass is essential while clipping of these aneurysms.
Clipping should ensure that small perforators, especially those arising from the basilar top, ICA bifurcation, proximal MCA aneurysms, are preserved or else significant deficits can happen. Intraoperative ICG/angiography, microvascular Doppler can be used to ensure patency.
15.6.1 Aneurysm with Hemorrhagic Presentation
Illustration 1 (Fig. 15.2)
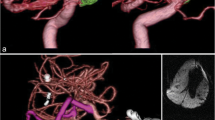
Some GIAs are adherent to the dura of the skull base and do not collapse even with multiple clips unless the wall of the aneurysm is excised and released from the skull base. This is especially true for inferior wall ICA aneurysm and some vertebral aneurysms. Some MCA bifurcation aneurysms are multilobulated and wide neck and incorporate the branches. These need innovative clipping methods after excision of the aneurysmal sac to have a satisfactory outcome. This was the case in a 35-year-old woman who presented to us in a coma and left hemiplegia from WFNS Grade IV SAH. CTA revealed a partially thrombosed giant right MCA aneurysm with significant mass effect, the source of hemorrhage, as well as a large left MCA bifurcation aneurysm. The patient was operated through a right FTOZ craniotomy. Hematoma evacuation, partial excision of the aneurysm wall, and occlusion of the aneurysm with preservation of the parent artery and branches were performed utilizing multiple clips. The patient had a prolonged hospitalization and gradually improved to become independent but was left with residual hemiparesis on the left side. Later at a second stage, the large left MCA bifurcation aneurysm was also successfully clipped without any complication.
(a–c) CTA showing a partially thrombosed giant right MCA bifurcation aneurysm with the branches incorporated in the aneurysm and a large left MCA bifurcation aneurysm. (d) Postoperative DSA after the right side aneurysm surgery shows complete exclusion of the aneurysm and preserved parent right MCA and its branches. (e, f) 1-year follow-up postoperative DSA after the second stage surgery demonstrating occlusion of both MCA aneurysm with preserved normal vessels
Illustration 2 (Fig. 15.3)
A 25-year-old man was admitted to another center in a comatose state with an acute subdural hematoma (SDH) from a giant ICA aneurysm; he had a decompressive craniectomy and gradually improved almost completely. On evaluation, he was found to have a 77 mm partially thrombosed aneurysm involving the whole ICA from the petrous to the supraclinoid segment, the cause of his SDH. He also had a large, wide-necked basilar apex aneurysm. He was referred to us for definitive treatment. After further preoperative workup that included 3D CTA and 3D DSA, it was decided to perform in the first stage, ECA-M2 RAG bypass and trapping of ICA. While preparing to do the distal ECA-M2 anastomosis, it was observed that the length of the graft was not enough because of the large MCF mass caused by the aneurysm. Hence, initially, the ICA was trapped; the aneurysm was opened in the MCF and decompressed. Then interposing a RAG, an ECA-M2 anastomosis was successfully performed. The patient was subsequently referred to the neurointerventionist for taking care of the basilar top aneurysm. The configuration at the basilar top, the wide neck, the right posterior cerebral (PCA), inseparable from the aneurysm made the endovascular proposition unsafe, and the patient was referred back for a microsurgical option. Uneventful microsurgical clipping of the basilar apex aneurysm was thus performed at a second sitting 3 months after the first operation. The second operation was performed after inserting a lumbar drain and combining an OZ craniotomy to the previous FT craniotomy done at the first sitting. An extradural clinoidectomy and intradural posterior clinoidectomy completed the bone work. The aneurysm was completely obliterated with preservation of all perforators and the right PCA by using a combination of fenestrated and large Yasargil titanium clips and employing tandem clipping technique as advocated by Drake.
Illustration 3 (Fig. 15.4)
A 34-year-old male presenting with CT angiogram showing a large distal right ICA aneurysm and WFNS Grade II SAH underwent a right frontotemporal approach and clipping of the aneurysm. Vertical stacking of multiple clips was done in order to achieve obliteration of the aneurysm.
Illustration 4 (Fig. 15.5)
A CT angiogram of a young male presenting with massive WFNS Grade 4 SAH showed a doubtful area of outpouching in the right supracliniod ICA, which was later confirmed to be a blood blister aneurysm on DSA. He underwent a right frontotemporal approach and clipping of the same using a Sundt Encircling clip.
Illustration 5 (Fig. 15.6)
A 57-year-old gentleman presented with profuse right epistaxis, with a bling right eye and left hemiparesis. There was past history of road traffic accidents about a month back. CTA showed presence of a Right petrous ICA pseudoaneurysm. He underwent a right high flow (ECA-M2) bypass with radial artery graft (RAG) followed by clipping of the aneurysm intradurally.
Illustration 6 (Fig. 15.7)
Moyamoya disease presenting with hemorrhage, especially in young adults, can be treated with superficial temporal to middle cerebral artery bypass and clipping of any associated aneurysm at the same time.
15.6.2 Aneurysm with Ischemic Presentation (Fig. 15.8)
As seen in the figure, a left distal MCA thrombosed aneurysm presenting with ischemic changes as seen on diffusion-weighted sequences of the MRI necessitates the need for thrombectomy and clipping of the aneurysm for achieving the optimum outcome of the patient.
15.6.3 Aneurysm with Mass Effect as There Presentation
Illustration 1 (Fig. 15.9): Flow Diversion and Bypass
An elderly gentleman presented with a history of decreased vision in the right eye, MR angiogram showed the presence of pan-dolichocephalic vessels with fusiform dilatations of bilateral ICA, basilar and other vessels. He underwent a high flow (ECA-M2) bypass with RAG followed by ligation of ICA in the neck.
Illustration 2 (Fig. 15.10): Flow Diversion and Bypass
An elderly lady presented with painful right ophthalmoplegia. CTA showed a giant cavernous ICA aneurysm for which she underwent EC-IC bypass with Radial artery graft (RAG) and ligation of ICA in the neck.
Illustration 3 (Fig. 15.11): Combined Approach (Microsurgery and EVT)
A young male presented with a Giant Supraclinoid aneurysm for which he underwent a Pterional approach, extradural anterior clinoidectomy and clipping of aneurysm. Postop angiogram showed residual filling of aneurysm, which was referred for EVT.
Illustration 4: Protective Bypass (Fig. 15.12)
An elderly lady presenting with the progressive visual loss on evaluation was found to have a 4.1 × 3.8 cm Giant ICA Carotid-Ophthalmic segment aneurysm. Clipping of the aneurysm was carried out under protective STMC bypass to avoid ischemia during the prolonged period of temporary clipping of ICA
Illustration 5: Clipping After Failed Coiling (Fig. 15.13)
Residual and recurrent aneurysms are more common after EVT than after microsurgical treatment. Moreover, even after total obliteration of the aneurysm, the patient worsens from the combined mass effect of the thrombosed aneurysm and the coils, as happened in the following case. A 2-year-young male child presented to the author in a drowsy state with a history of rapidly progressive quadriparesis, ataxia, and raised intracranial pressure. The evaluation revealed a 4.5 cm, heavily thrombosed, and calcified basilar top aneurysm with gross hydrocephalus. The child improved significantly following a right ventriculoperitoneal shunt. Our endovascular colleague suggested the coiling of the aneurysm. In view of the complex anatomy and morphology, the patient was treated by endovascular route with coils. The child remained stable for only 48 h before rapidly deteriorating in his sensorium to become comatose with extensor posturing and needed ventilator support. A repeat CT scan showed decompressed ventricles. The combined mass of the coils and the thrombosed giant aneurysm on the brain stem was thought to be the cause of the deterioration. The child was taken up for microsurgery in an attempt to decompress the aneurysm and relieve the mass effect. An FTOZ craniotomy, an extradural anterior clinoidectomy, and an intradural posterior clinoidectomy were performed. Through a transsylvian approach, the aneurysm was approached. The aneurysm was opened, partially decompressed, and with a short period of temporary clipping of the basilar trunk, the aneurysm neck was cleared off of thrombus and coils and clipped. All the branches and perforators were preserved. The patient had a prolonged ICU stay and hospitalization but slowly recovered remarkably. At the current follow-up, the child is going to school and has no disability or deficit other than minimal restriction of ocular motility
(a–d) Plain CT, CTA, MRI, and DSA showing a 4.5 cm giant, calcified, partially thrombosed basilar top aneurysm. (e) Post-EVT DSA demonstrating successful complete occlusion of the aneurysm by coils. (f, g) 5-year follow-up post-microsurgery MRI confirming occluded aneurysm, decompressed brainstem without any ischemia infarct
Illustration 6: Direct Clipping (Fig. 15.14)
Obliteration of the aneurysm by direct microsurgical clipping, the preferred method of microsurgery in cerebral aneurysms, is often not possible in GIAs. However, it is essential to explore the possibility as some GIAs may be best treated by direct clipping, as was possible in the following patient. A 35-year-old man presented to us with history of recent worsening in instability of gait of 5 months’ duration, inability to do fine movements of both hands of 3 months duration, and unprovoked, inappropriate, and uncontrolled spells of laughter of 3 months duration. On examination, he had left-sided deafness, bilateral cerebellar and pyramidal signs, and was unable to walk without support. MRI, DSA, and CTA revealed a partially thrombosed giant (3 cm) basilar top aneurysm with a significant mass effect on the brain stem and associated hydrocephalus. Patient was operated through a right fronto-temporo-orbito-zygomatic (FTOZ) craniotomy with extradural anterior clinoidectomy and intradural drilling of dorsum sellae. A ventricular drain was inserted, and the aneurysm was exposed through a transsylvian route. After defining both the PCAs and dissecting away the perforators, two curved, large titanium clips secured the aneurysm neck. Preoperative ICG dye angiography showed satisfactory, complete occlusion of the aneurysm with a good filling of the basilar and all its branches. Patient made a slow recovery and needed ventilator support and external ventricular drain in the postoperative period. At 3 weeks, the patient was fully conscious, ambulatory with support, and had a right third nerve paresis with mild left hemiparesis. At 3-month follow-up, the patient was independent with complete recovery of third nerve and hemiparesis. Postoperative DSA, CT, and CT angiography demonstrated complete occlusion of aneurysm and no evidence of infarct
Illustration 7 (Fig. 15.15): Microsurgical Clipping Under Deep Hypothermic Circulatory Arrest
Deep hypothermic circulatory arrest technique has evolved but still results in significant mortality and morbidity in a third of the patients. Hence, its use is nowadays limited for giant and complex posterior circulation aneurysms, particularly the basilar apex that has failed or is inappropriate for EVT. It is very rarely employed in author’s practice. The following case is an example of such an approach by us. A 12-year-old boy was referred to us in an altered sensorium after SAH. On admission, his GCS was E1M4V1. After initial resuscitation, endotracheal intubation and artificial ventilation, CT scan brain, CTA of cerebral vessels, and 3D cerebral DSA were performed. The investigations demonstrated bilateral frontal hypodensity, hydrocephalus, and Fisher grade 4 SAH from a giant multilobulated wide-necked basilar top aneurysm. The four-terminal branches of the basilar artery seemed to be arising from the aneurysm. CTA did not reveal the real complex nature of the aneurysm that could be correctly inferred from the 3D DSA. Hence, it was planned to attempt obliteration of the aneurysm under hypothermic circulatory arrest. After insertion of an external ventricular drain, an FTOZ craniotomy, extradural anterior clinoidectomy, and intradural posterior clinoidectomy were performed. After the majority of the dissection at the neck of the aneurysm, circulatory arrest was employed. The aneurysm was successfully obliterated with a combination of fenestrated and angled clips. The right PCA could not be saved. The patient had a prolonged stay in the ICU but finally successfully weaned off the ventilator. He was subsequently discharged to a chronic care hospital with percutaneous endoscopic gastrostomy feeding.
15.7 Literature Review
In microsurgical management of GIAs, Darsaut et al. reported a 69% rate of good clinical outcome. Their study also showed that patients younger than 50 years old had a better clinical outcome as compared with older patients (82% versus 65%), but the difference was not significant [33]. Ota et al., in their microsurgery series, reported a good outcome in 81.8% of patients, and complete occlusion rate in 86.8%. Poor outcomes were secondary to perforating artery infarctions and BA aneurysms, with the rate of perforating artery infarctions in BA aneurysms being 78.6% [34]. According to the ISUIA study, the 5-year rupture risk in patients harboring a very large or giant anterior circulation aneurysm is 15% and 40%, respectively [6]. Based on the ISUIA data, the risk of rupture of these aneurysms projected over a lifetime has been calculated to exceed 87% in a 30yearold patient and 71% in a 50yearold patient [35]. Compared with this grim natural history, the microsurgical intervention is justified. Good outcomes (mRS ≤ 3) were specifically seen in patients with ≤50 years of age. In a similar series by Hauck et al. 92% patients ≤50 years of age had a good outcome (GOS Score 4 or 5), resulting in 88% complete and 4% near complete aneurysm occlusions with an overall surgical morbidity and mortality rate at 8% [36]. In patients older than 70 years, the risk of surgery exceeded the lifetime risk of rupture [35]. A typical presentation of SAH showed a favorable outcome only in 50% of cases. A preoperative mRS score ≤ 1 was shown to have a good outcome in 86% of cases. Sughrue et al., in their series, proposed that indirect aneurysm occlusion (proximal occlusion, distal occlusion or trapping) with or without a bypass has become a more acceptable alternative than hypothermic cardiac arrest [3]. However, it is also associated with its unique complications like bypass graft occlusion and aneurysm thrombosis leading to thrombotic occlusion of perforators or branch arteries in 4 patients (7%) with flawless bypasses in their series. However, unlike non-giant aneurysms that can be trapped safely, giant aneurysm occlusion is often deliberately kept incomplete due to the presence of perforators or branches that would otherwise be trapped. Therefore, thrombosis initiated by bypass and aneurysm occlusion can potentially occlude these same arteries, too [3].
As with most types of technology-heavy fields, the specialty of endovascular neurosurgery has seen a tremendous refinement with the advent of detachable balloons and Guglielmi detachable coils (GDCs) and to the present use of flow diverters and pipeline embolization devices (PED) [37]. A brief literature review of the existing EVT articles is necessary to effectively compare EVT in aneurysms not amenable to EVT to the same being microsurgically clipped. Needless to say, a judicious approach is taken based on the merits of the case, as we have documented earlier in our illustrations. However, GIAs owing to their complexities and multiple anatomical, pathophysiological factors pose a challenge in the management of these lesions by EVT in terms of short-term and long-term results as well as its associated periprocedural morbidities and complications.
EVT management in wide-necked aneurysms, which are typically defined as lesions with necks of 4 mm or more wide or with dome/neck ratios of less than 2, involves stent-assisted coiling (SAC), Balloon-assisted coiling and flow-diverting stents (FDs) [38]. There are technical problems involved in achieving a complete angiographic occlusion after SAC, as a tight coil mass cannot be achieved due to difficulty in maneuvering the coiling microcatheter. In addition, the use of dual antiplatelet therapy often inhibits immediate aneurysmal thrombosis [39]. Overall complication rates of SAC are increased over those of primary coil embolization because of thromboembolic risks from stent placement and hemorrhagic risk from antiplatelet therapy. Shapiro et al., in their review, mentioned the overall incidence of complications to be 19% and mortality rate to be 2.1% [40]. The main drawback of SAC is its heavy dependence on dual antiplatelet therapy, increased risk of hemorrhagic complications, and thromboembolic complications resulting from medication noncompliance or antiplatelet resistance [41,42,43]. Mocco and colleagues reported a procedural mortality rate of 12% with the use of SAC in ruptured aneurysms [44]. Complication rates of SAC are increased over those of primary coil embolization because of thromboembolic risk from stent placement and hemorrhagic risk from antiplatelet therapy [38]. In one review, the overall incidence of complications was reported to be 19% and the mortality rate to be 2.1% [40]. Thromboembolic complications were the primary contributor, responsible for approximately 10% of the overall complication rate and leading to death in 0.6% of cases. Hemorrhagic complications were responsible for 2.2% of overall complications and led to death in 0.9% of cases [38]. Fernandez et al. reported a series with 51 wide-necked aneurysms (necks > 4 mm) treated with coil embolization and achieved complete thrombosis in only 15% of cases [45]. Broad neck aneurysms, vessels arising from aneurysm base or walls, abnormal morphology etc., can result in herniation of coils into the parent artery lumen. BAC and SAC are associated with the additional risk of parent artery ischemia, perforation, distal thromboembolism, and occlusion of adjacent perforators and branch arteries by the lattice of the stent [8]. The rate of recurrence is also higher in broad neck aneurysms because the hemodynamics at the inflow zone is more complex. The other reasons for failure are incomplete initial obliteration, thrombus within the lumen, poor radiographic visualization of the aneurysm anatomy and its adjacent branches, and tortuosity of the feeding vessel, making catheterization difficult [8]. Flow diverters are exciting, but it is still early days for prime time.
The aneurysms most amenable to endovascular treatment are also those that are best treated by surgical techniques, namely, those with well defined, small, narrow necks [45, 46]. Most commonly, giant aneurysms have not been favorable lesions for endovascular therapy because they frequently widen the neck, distort the anatomy of parent and branch arteries at the base, and induce luminal thrombosis. Occlusion is incomplete in a considerable percentage of endovascular treatments leading to recurrent aneurysm, multiple retreatments, occasional re-hemorrhages, and neurological deterioration from progressive aneurysm enlargement. Further, follow-up studies have demonstrated refilling and recurrence of aneurysms thought to have been completely occluded [45,46,47,48,49,50,51,52,53]. Ten-year analysis of saccular aneurysms in the Barrow Ruptured Aneurysm Trial (BRAT) showed no statistical significance difference in poor outcomes (mRS score > 2) or deaths between clipping and coiling on a 10 year follow-up. Of 178 clip-assigned patients with saccular aneurysms, 1 (<1%) was crossed over to coiling, and 64 (36%) of the 178 coil-assigned patients were crossed over to clipping. 2 of 241 (0.8%) clipped saccular aneurysms and 23 of 115 (20%) coiled saccular aneurysms required retreatment (p < 0.001). At the 10-year follow-up, 93% (50/54) of the clipped aneurysms were completely obliterated, compared with only 22% (5/23) of the coiled aneurysms (p < 0.001) [54]. Linfante et al. in their series of 45 GIA’s managed by EVT had 7% mortality, 11.1% experienced ischemic strokes. Good clinical outcome (mRS score ≤ 2) was seen in 86% for anterior circulation cases and 55% for posterior circulation cases in their series (statistically significant, n = 38; p < 0.05) [2]. When GIAs are treated by microsurgical clipping, the mortality rates of both ruptured and unruptured GIAs were reported at 6–22% [5, 55]. Sluzewski et al. reported good clinical outcomes in 79% of very large and giant aneurysms at a median follow-up at 50 months, though 41% of aneurysms were still incompletely occluded even after repeated coiling [56]. A retrospective review that included large and GIAs treated with PED or PED + coils, and the authors found complete or near complete occlusion at the last follow-up in 77% of cases. Of the patients, 12% had symptomatic ischemic complications and 8% had symptomatic hemorrhagic complications, and the overall mortality was 6% [57]. Coiling alone as a treatment option for GIAs has poor long-term outcomes because GIAs are often incompletely occluded and require repeated coiling [53]. Park et al. showed PED in combination with coiling had a higher neurological morbidity and required a longer procedural time versus PED alone [58]. Stent-assisted coiling and PED have shown to be equally effective, with no significant differences in complications and angiographic outcomes [59]. However, good results were seen in Bender et al. in their series of 445 PED procedures, with 85 large (19%) and 4 giant (1%) aneurysms, showed complete occlusion in 72%, 78% and 87% at 6, 12, and 24 months, respectively. Their overall rate of major complications was 3.5% and a 1.1% rate of mortality [60]. In a retrospective analysis by Liang et al. for giant posterior circulation aneurysms, 93.9% resulted in favorable clinical outcomes (mRS score, 0–2) with an overall mortality rate of 6.1% [61]. Darsaut et al. showed that EVT for very large and giant posterior circulation aneurysms was associated with poor clinical outcomes and a low complete obliteration rate [33]. This is also supported by a recent meta-analysis by Cagnazzo et al. that the incidence of treatment-related complications with endovascular treatment of very large and giant posterior circulation aneurysms was greater than that for anterior circulation aneurysms [62]. Siddiqui et al. advised judicious use of flow diversion procedures for large or giant vertebrobasilar aneurysms, owing to the high morbidity and mortality rates of 14.3% and 57.1%, respectively [63]. In a meta-analysis of flow diverter treatment for posterior circulation aneurysms, Wang et al. reported procedure-related mortality rate of 15% and significantly higher rates amongst patients with giant and basilar artery aneurysms [64]. Chalouhi et al. studied 334 large and giant aneurysms (80% anterior circulation) that were coiled at a single institution, 10% were giant aneurysms. Recanalization and retreatment rates were 39% and 33%, respectively [65]. Recanalization is highest in the setting of wide residual aneurysm necks, largely due to coil compaction, growing residual aneurysm neck, and refilling fundus [66,67,68].
Amongst all the above lesions, blood blister aneurysms (BBAs) deserve a special mention as the nuances of management of these treacherous lesions are challenging both microsurgically as well as by EVT. There is limited literature available currently on BBAs in regards to microsurgery versus EVT, without any current established consensus for the management of the same. BBAs are challenging small, bleb-like and ill-defined neck lesions at non-branching sites of the dorsal or anterior wall of the ICA, comprising 0.3–1.7% of all intracranial aneurysms and 6.6% of all ruptured aneurysms [69]. Owing to their fragile and difficult morphology, these lesions are a challenge to manage either surgically or endovascularly. A systematic review and meta-analysis by Zhu et al. of 15 noncomparative studies with a total of 165 target BBAs were studied. Complete occlusion rates were 72%, recurrence occurred in 13% and rebleed in 3% of patients. Procedure-related morbidity and mortality were 26% and 3%, respectively [70]. They concluded that FD was safe and effective, but treatment of BBA should be considered on a case-by-case basis to maximize patient benefits and limit the risk of perioperative complications. Shah et al. in their experience of microsurgery for BBAs observed that surgery provides a superior occlusion rate of up to 90% immediately postoperatively and superior sustained occlusion at follow-up than flow-diverting stents [71]. Kim et al. reported the rate of intraoperative rupture was 16.7%, higher than with FDs, however, 69.5% of patients had a good clinical outcome (mRS score of 0–2) at discharge, and a good long-term outcome in 80.1%, which is comparable to flow-diverting stents. Their study had a complete rate of aneurysm occlusion of 94.4%, and regrowth happened in only 1 case (0.28%) [72]. Thus concluding microsurgery for BBAs seem to have a slight edge over FDs and overlap FDs in term of superior occlusion rates and long-term control with a good postoperative mRS score and without the need for subjecting the patient to heavy antiplatelets which in itself is a reason for morbidity.
15.8 Conclusion
Treatment of complex aneurysms like GIAs and BBAs is challenging. The modalities of treatment, microsurgery, EVT, or combined should be individualized, taking into consideration the patient and pathological factors and available expertise. Although EVT is an attractive option, the high incidence of incomplete treatment, delayed complications, recurrence, and inadequate long-term follow-up data makes microsurgery preferable. The overall outcome of clinical and radiological results is a bit biased towards EVT as the more complex and difficult aneurysms are left for microsurgery. A judicious approach is hence recommended for a complex aneurysms based on their morphology, patient characteristics, departmental technical expertise in both microsurgery and EVT to give the patient the best outcome in terms of quality of life as well as cost affectivity, and the latter is extremely important in developing countries. Microsurgery thus remains an attractive treatment modality, in spite of the recent advances of endovascular techniques.
References
Bull J. Massive aneurysms at the base of the brain. Brain. 1969;92(3):535–70.
Linfante I, Andreone V, Ravelo N, Starosciak AK, Arif B, Shallwani H, et al. Endovascular treatment of giant intracranial aneurysms. Cureus. 2020;12(5):e8290.
Sughrue ME, Saloner D, Rayz VL, Lawton MT. Giant intracranial aneurysms: evolution of management in a contemporary surgical series. Neurosurgery. 2011;69(6):1261–70.
Vishteh AG, David CA, Spetzler RF. Giant aneurysms. In: Sekhar LN, Fessler R, editors. Atlas of neurosurgical techniques, vol. I. Stuttgart: Thieme; 2006. p. 212–21.
Hakma Z, Ramaswamy R, Loftus CM. Mortality rates for giant aneurysms. Acta Neurochir. 2011;153(8):1621–3.
Wiebers DO, Whisnant JP, Huston J, Meissner I, Brown RD Jr, Piepgras DG, et al. Unruptured intracranial aneurysms: natural history, clinical outcome, and risks of surgical and endovascular treatment. Lancet. 2003;362:103–10. https://doi.org/10.1016/s0140-6736(03)13860-3.
Wermer MJ, Van der Schaaf IC, Algra A, Rinkel GJ. Risk of rupture of unruptured intracranial aneurysms in relation to patient and aneurysm characteristics: an updated meta-analysis. Stroke. 2007;38:1404–10.
Quiñones-Hinojosa A, Du R, Lawton MT. Revascularization with saphenous vein bypasses for complex intracranial aneurysms. Skull Base. 2005;15(2):119–32.
Misra BK, Warade AG, Purandare HR. Giant intracranial aneurysms: microsurgery. In: Singh VP, Nair MD, editors. Progress in clinical neuroscience, vol. 29. Stuttgart: Thieme; 2015.
Peerless SJ, Wallace MD, Drake CG. Giant intracranial aneurysms. In: Yeoman’s JR, editor. Neurological surgery: a comprehensive reference guide to the diagnosis and management of neurosurgical problems. 3rd ed. Philadelphia, PA: W.B. Saunders; 1990. p. 1742–63.
Barrow DL, Alleyne C. Natural history of giant intracranial aneurysms and indications for intervention. Clin Neurosurg. 1995;42:214–44.
Dannenbaum MJ, Rahimi SY, Schuette AJ. Natural history of giant intracranial aneurysms. In: Abdulrauf SI, editor. Cerebral revascularization: techniques in extracranial-to-intracranial bypass surgery. Philadelphia, PA: Elsevier; 2011. p. 225–30.
Lawton MT, Spetzler RF. Surgical strategies for giant intracranial aneurysms. Neurosurg Clin N Am. 1998;9(4):725–42.
Sano K, Asano T, Tamura A. Surgical technique. In: Sano K, Tamura A, editors. Acute aneurysm surgery: pathophysiology and management. New York: Springer; 1987. p. 194–246.
Zabramski JM, Kiriş T, Sankhla SK, Cabiol J, Spetzler RF. Orbitozygomatic craniotomy. Technical note. J Neurosurg. 1998;89(2):336–41.
Lawton MT, Spetzler RF. Surgical strategies for giant intracranial aneurysms. Acta Neurochir Suppl (Wien). 1999;72:141–56.
Drake CG. Giant intracranial aneurysms: experience with surgical treatment in 174 patients. Clin Neurosurg. 1979;26:12–95.
Drake CG. The treatment of aneurysms of the posterior circulation. Clin Neurosurg. 1979;26:96–144.
Malis L. Surgical resection of tumors of the skull base. In: Wilkins RH, Rengachary SS, editors. Neurosurgery. New York, NY: McGraw-Hill; 1885. p. 1011–21.
Hammon WM, Kempe LG. The posterior fossa approach to aneurysms of the vertebral and basilar arteries. J Neurosurg. 1972;37(3):339–47.
Sen CN, Sekhar LN. An extreme lateral approach to intradural lesions of the cervical spine and foramen magnum. Neurosurgery. 1990;27(2):197–204.
Lawton MT, Daspit CP, Spetzler RF. Technical aspects and recent trends in the management of large and giant midbasilar artery aneurysms. Neurosurgery. 1997;41(3):513–20.
Baldwin HZ, Miller CG, Van Loveren HR, Keller JT, Daspit CP, Spetzler RF. The far lateral/combined supra- and infratentorial approach. A human cadaveric prosection model for routes of access to the petroclival region and ventral brain stem. J Neurosurg. 1994;81(1):60–8.
Spetzler RF, Riina HA, Lemole GM Jr. Giant aneurysms. Neurosurgery. 2001;49(4):902–8.
Hacein-Bey L, Connolly ES Jr, Mayer SA, Young WL, Pile-Spellman J, Solomon RA. Complex intracranial aneurysms: combined operative and endovascular approaches. Neurosurgery. 1998;43(6):1304–12.
Ponce FA, Spetzler RF, Han PP, Wait SD, Killory BD, Nakaji P, et al. Cardiac standstill for cerebral aneurysms in 103 patients: an update on the experience at the Barrow Neurological Institute. Clinical article. J Neurosurg. 2011;114(3):877–84.
Rothoer RD, Brawanski A. The history and present status of deep hypothermia and circulatory arrest in cerebrovascular surgery. Neurosurg Focus. 2006;20(6):E5.
Groff MW, Adams DC, Kahn RA, Kumbar UM, Yang BY, Bederson JB. Adenosine-induced transient asystole for management of a basilar artery aneurysm. Case report. J Neurosurg. 1999;91(4):687–90.
Heppner PA, Ellegala DB, Robertson N, Nemergut E, Jaganathan J, Mee E. Basilar tip aneurysm – adenosine induced asystole for the treatment of a basilar tip aneurysm following failure of temporary clipping. Acta Neurochir. 2007;149(5):517–20.
Nussbaum ES, Sebring LA, Ostanny I, Nelson WB. Transient cardiac standstill induced by adenosine in the management of intraoperative aneurysmal rupture: technical case report. Neurosurgery. 2000;47(1):240–3.
Atkinson JLD, Piepgras DG. Giant aneurysms: supratentorial. In: Carter LP, Spetzler RF, editors. Neurovascular surgery. New York: McGraw-Hill; 1995. p. 815–28.
Symon L, Vajda J. Surgical experiences with giant intracranial aneurysms. J Neurosurg. 1984;61:100928.
Darsaut TE, Darsaut NM, Chang SD, Silverberg GD, Shuer LM, Tian L, et al. Predictors of clinical and angiographic outcome after surgical or endovascular therapy of very large and giant intracranial aneurysms. Neurosurgery. 2011;68:903–15.
Ota N, Matsukawa H, Noda K, Sato H, Hatano Y, Hashimoto A, et al. Evaluation of microsurgery for managing giant or complex cerebral aneurysms: a retrospective study. World Neurosurg. 2018;115:190–9.
Chang HS. Simulation of the natural history of cerebral aneurysms based on data from the international study of unruptured intracranial aneurysms. J Neurosurg. 2006;104:188–94.
Hauck EF, Wohlfeld B, Welch BG, White JA, Samson D. Clipping of very large or giant unruptured intracranial aneurysms in the anterior circulation: an outcome study. J Neurosurg. 2008;109(6):1012–8.
Park MS, Sanborn MR, McDougall CG, Albuquerque FC. Endovascular approaches to narrow-necked intracranial aneurysms. In: Winn HR, editor. Youman’s & Winn neurological surgery. Philadelphia: Elsevier; 2017. p. 3362–71.
Moon K, Levitt MR, Albuquerque FC, McDougall CG. Endovascular approaches to wide-necked intracranial aneurysms. In: Winn HR, editor. Youman’s & Winn neurological surgery. Philadelphia: Elsevier; 2017. p. 3372–5.
Piotin M, Blanc R. Balloons and stents in the endovascular treatment of cerebral aneurysms: vascular anatomy remodeled. Front Neurol. 2014;5:41.
Shapiro M, Becske T, Sahlein D, Babb J, Nelson PK. Stent-supported aneurysm coiling: a literature survey of treatment and follow-up. AJNR Am J Neuroradiol. 2012;33:159–63.
Goh C, Churilov L, Mitchell P, Dowling R, Yan B. Clopidogrel hyper-response and bleeding risk in neurointerventional procedures. AJNR Am J Neuroradiol. 2013;34:721–6.
Rossen JD, Chalouhi N, Wassef SN, Thomas J, Abel TJ, Jabbour PM, et al. Incidence of cerebral ischemic events after discontinuation of Clopidogrel in patients with intracranial aneurysms treated with stent-assisted techniques. J Neurosurg. 2012;117:929–33.
Fifi JT, Brockington C, Narang J, Leesch W, Ewing SL, Bennet H, et al. Clopidogrel resistance is associated with thromboembolic complications in patients undergoing neurovascular stenting. AJNR Am J Neuroradiol. 2013;34:716–20.
Mocco J, Snyder KV, Albuquerque FC, Bendok BR, Bolos AS, Carpenter JS, et al. Treatment of intracranial aneurysms with the Enterprise stent: a multicenter registry. J Neurosurg. 2009;110:35–9.
Zubillaga AF, Guglielmi G, Viñuela F, Duckwiler GR. Endovascular occlusion of intracranial aneurysms with electrically detachable coils: correlation of aneurysm neck size and treatment results. AJNR Am J Neuroradiol. 1994;15:815–20.
Standard SC, Guterman LR, Chavis TD, Fronckowiak MD, Gibbons KJ, Hopkins LN, et al. Endovascular management of giant intracranial aneurysms. Clin Neurosurg. 1995;42:26793.
Gobin YP, Vinuela F, Gurian JH, Guglielmi G, Duckwiler GR, Massoud TF, et al. Treatment of large and giant fusiform intracranial aneurysms with Guglielmi detachable coils. J Neurosurg. 1996;84:5562.
Gruber A, Killer M, Bavinzski G, Bernd R. Clinical and angiographic results of endosaccular coiling treatment of giant and very large intracranial aneurysms: a 7year, single center experience. Neurosurgery. 1999;45:793803.
Henkes H, Fischer S, Weber W, Miloslavski E, Felber S, Brew S, et al. Endovascular coil occlusion of 1811 intracranial aneurysms: early angiographic and clinical results. Neurosurgery. 2004;54:26880.
Jahromi BS, Mocco J, Bang JA, Gologorsky Y, Siddiqui AH, Horowitz MB, et al. Clinical and angiographic outcome after endovascular management of giant intracranial aneurysms. Neurosurgery. 2008;63:66274.
Klein GE, Szolar DH, Leber KA, Karaic R, Hausegger KA. Basilar tip aneurysm: endovascular treatment with Guglielmi detachable coils—midterm results. Radiology. 1997;205:1916.
Murayama Y, Viñuela F, Ishii A, Nien YL, Yuki I, Duckwiler G, et al. Initial clinical experience with matrix detachable coils for the treatment of intracranial aneurysms. J Neurosurg. 2006;105:1929.
Sluzewski M, Menovsky T, van Rooij WJ, Wijnalda D. Coiling of very large or giant cerebral aneurysms: long-term clinical and serial angiographic results. AJNR Am J Neuroradiol. 2003;24:25762.
Spetzler RF, McDougall CG, Zabramski JM, Albuquerque FC, Hills NK, Nakaji P, et al. Ten-year analysis of saccular aneurysms in the Barrow ruptured aneurysm trial. J Neurosurg. 2019;132(3):771–6.
Wehman JC, Hanel RA, Levy EI, Hopkins LN. Giant cerebral aneurysms: endovascular challenges. Neurosurgery. 2006;59:125–38.
Sluzewski M, van Rooij WJ, Rinkel GJ, Wijnalda D. Endovascular treatment of ruptured intracranial aneurysms with detachable coils: long-term clinical and serial angiographic results. Radiology. 2003;227:720–4.
Adeeb N, Griessenauer CJ, Shallwani H, Shakir H, Foreman PM, Moore JM, et al. Pipeline embolization device in treatment of 50 unruptured large and giant aneurysms. World Neurosurg. 2017;105:232–7.
Park MS, Kilburg C, Taussky P, Albuquerque FC, Kallmes DF, Levy DI, et al. Pipeline embolization device with or without adjunctive coil embolization: analysis of complications from the IntrePED registry. AJNR Am J Neuroradiol. 2016;37:1127–31.
Adeeb N, Griessenauer CJ, Foreman PM, Moore JM, Motei-Langroudi R, Chua MH, et al. Comparison of stent-assisted coil embolization and the pipeline embolization device for endovascular treatment of ophthalmic segment aneurysms: a multicenter cohort study. World Neurosurg. 2017;105:206–12.
Bender MT, Colby GP, Lin L-M, Jiang B, Westbroek EM, Xu R, et al. Predictors of cerebral aneurysm persistence and occlusion after flow diversion: a single-institution series of 445 cases with angiographic follow-up. J Neurosurg. 2018;130:259–67.
Liang F, Zhang Y, Yan P, Ma C, Liang S, Jiang P, et al. Predictors of periprocedural complications and angiographic outcomes of endovascular therapy for large and giant intracranial posterior circulation aneurysms. World Neurosurg. 2019;125:378–84.
Cagnazzo F, Mantilla D, Rouchaud A, Brinjikji W, Lefvre PH, Dargazanli C, et al. Endovascular treatment of very large and giant intracranial aneurysms: comparison between reconstructive and deconstructive techniques—a meta-analysis. AJNR Am J Neuroradiol. 2018;39:852–8.
Siddiqui AH, Abla AA, Kan P, Dumont TM, Jahshan S, Britz GW, et al. Panacea or problem: flow diverters in the treatment of symptomatic large or giant fusiform vertebrobasilar aneurysms. J Neurosurg. 2012;116:1258–66.
Wang CB, Shi WW, Zhang GX, Lu HC, Ma J. Flow diverter treatment of posterior circulation aneurysms. A meta-analysis. Neuroradiology. 2016;58(4):391–400.
Chalouhi N, Tjoumakaris S, Gonzalez LF, Dumont AS, Starke RM, Hasan D, et al. Coiling of large and giant aneurysms: complications and long-term results of 334 cases. AJNR Am J Neuroradiol. 2014;35:546–52.
Katayama Y, Tsubokawa T, Miyazaki S, Furuichi M, Hirayama T, Himi K, et al. Growth of totally thrombosed giant aneurysm within the posterior cranial fossa. Diagnostic and therapeutic considerations. Neuroradiology. 1991;33:168–70.
Horowitz M, Purdy P, Kopitnik T, Dutton K, Samson D. Aneurysm retreatment after Guglielmi detachable coil and nondetachable coil embolization: report of nine cases and review of the literature. Neurosurgery. 1999;44:712–9.
Hasan DM, Nadareyshvili AI, Hoppe AL, Mahaney KB, Kung DK, Raghavan ML. Cerebral aneurysm sac growth as the etiology of recurrence after successful coil embolization. Stroke. 2012;43:866–8.
Peitz GW, Sy CA, Grandhi R. Endovascular treatment of blister aneurysms. Neurosurg Focus. 2017;42(6):E12.
Zhu D, Yan Y, Zhao P, Duan G, Zhao R, Liu J, et al. Safety and efficacy of flow diverter treatment for blood blister like aneurysm: a systematic review and meta-analysis. World Neurosurg. 2018;118:79–86.
Shah SS, Gersey ZC, Nuh M, Ghonim HT, Elhammady MS, Peterson EC. Microsurgical versus endovascular interventions for blood blister aneurysms of the internal carotid artery: systematic review of literature and meta-analysis on safety and efficacy. J Neurosurg. 2017;127:1361–73.
Kim YS, Joo SP, Kim TS. Microsurgical management of ruptured blood blister aneurysms of the internal carotid artery without bypass: a retrospective single-center study of 36 patients over 20 years. World Neurosurg. 2019;128:956–65.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Warade, A.G., Misra, B.K. (2022). Microsurgery of Cerebral Aneurysms Not Amenable to Endovascular Therapy. In: Lv, X. (eds) Endovascular Surgery of Cerebral Aneurysms. Springer, Singapore. https://doi.org/10.1007/978-981-16-7102-9_15
Download citation
DOI: https://doi.org/10.1007/978-981-16-7102-9_15
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-16-7101-2
Online ISBN: 978-981-16-7102-9
eBook Packages: MedicineMedicine (R0)