Abstract
A good diet may deliver micronutrients such as vitamins A, B6, B12, C, and D and minerals such as iron, copper, zinc, and selenium that have been implicated to have key roles for supporting immunity with reducing host infections. Most studies have shown that once the subject was infected, the immune system will be enhanced, which will require high levels of metabolic rate, energy requirements, different biosynthesis substances and regulatory molecules, which are obtained from dietary sources. Consequently, a healthy diet will result in a healthy gut by achieving well-balanced gut microbiota which enhances the immune system. The human gut microbiota consists of two major two groups: Firmicutes and Bacteroidetes. Some of these can beneficial, some can be detrimental to the host. Their composition can be modified by small changes in diet when beneficially supports the body’s repair, growth, and immunity. Dietary sources can be converted into beneficial metabolic end-products such as short chain fatty acids, i.e., acetate, propionate, and butyrate, fermented by the beneficial gut microbiota such as Lactobacillus and Bifidobacterium. This is achieved by an indirect nutrient strategy using pro/prebiotic. The gut microbiota cooperates with their hosts for metabolic and nervous systems development, in addition to the function of the immune system regulation via dynamic bidirectional communication known as the gut–brain axis. Indeed, studies have shown a correlation with anxiety, pain, cognition, and mood regulation in animal models studies, related to gut microbiota due to dietary carbohydrates. In addition, specific studies have demonstrated the link of gut microbiota on neurodevelopmental disorders, autism spectrum disorder, and Parkinson diseases. Furthermore, factors such as direct and indirect micronutrients, affecting and the gut–brain microbiome are anticipated with the use of probiotics and prebiotics as functional foods.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Gut–brain axis
- Microbiome
- Parkinson disease
- Neuroinflammation
- Neurodevelopmental disorder
- Oxidative stress
1 Introduction
The human body is populated with microorganisms, inside and outside, i.e., mouth, gut, skin, and any body cavity. However, significant number of bacteria with different organisms colonizing has been recognized within the gut (the colon), referred to as colonic or gut microbiota (GM). The gene set known as the gut microbiome (about three million genes) is more than the human genome by 150 times (Rowland et al. 2018; Zhang et al. 2010). An abnormal impaired GM composition and activity, i.e., gut dysbiosis, has been implicated in the development of intestinal permeability, inflammation in intestinal and peripheral tissues, e.g., adipose tissue, muscles, liver, and brain, altered glucose and energy levels and homeostasis, i.e., metabolic diseases (Blumberg and Powrie 2012; Gentile and Weir 2018; Valdes et al. 2018; Zmora et al. 2019; Willson and Situ 2017; Singh et al. 2017). Additionally, disruption of the intestinal integrity (known as leaky gut) has been associated with dysbiosis that has been linked with different diseases, e.g., obesity, cardiovascular diseases, autoimmune diseases, type2 diabetes (T2D), and inflammatory bowel diseases (IBD) (Rowland et al. 2018; Blumberg and Powrie 2012; Khalil et al. 2013; Pickard et al. 2017; Kohane et al. 2012; Kang et al. 2013).
Certain species of colonic bacteria are involved in neural development and functioning, brain development as modulation of brain physiology, mood sleep, and eating behaviours. The colonic microbiota could alter human behaviour through the central nervous system (CNS) by which called gut–brain axes (El-Ansary et al. 2013) however, such linkage is still unclear. It has been suggested by different animal models that such communication regulates immunity and autoimmune diseases because of the role of microbiota in the host defence; comparing the exposure and non-exposure to pathogenic infected bacterial and pro/pre/antibiotics. They work as a vulnerable area in the GIT that could be characterized by the influence of pathogenic bacteria in all the characteristics of physiology and CNS neuroinflammation induction. It has been confirmed within a study that transferred human gut microbiota samples obtained from autism spectrum disorder (ASD) subjects to animal models (mice) shown indication of the autistic behaviour hallmarks (Sharon et al. 2019). However, it is unclear whether the microbiome contributes to the symptoms. Additionally, the free-casein and glutamine diets showed an influence on the microbiota composition and activities on specific allergens. Other brain physiology, mood, and behaviours are connected to depression and anxiety; the main psychic troubles spread widely as about 1 in every 100 people experience mania and depression. The rates of depression and anxiety are increasing worldwide in both Eastern and Western cultures such as Egyptian and Saudia and the UK due to economic problems (Rajeh et al. 1993; El-Metwally et al. 2019). Possibly such cases could be managed by certain dietary consumptions (pro/prebiotics) in correlation to the colonic microbiota compositions and activities by their metabolic end-products; short chain fatty acid (SCFA) which in turn will exhibit a healthy immune system and good human brain activity (Pickard et al. 2017; Rowland et al. 2018).
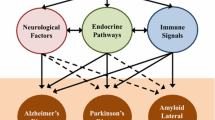
Parkinson disease (PD) is the second most common neurodegenerative disorder after Alzheimer’s disease (AD), which affects approximately seven million people worldwide mainly the elderly. Such patients are well known for their malnutrition caused by the decrease of food intake, malabsorption, accelerated nutrient loss and increase of nutritional requirement. Therefore, nutritional therapy is necessary for them to improve their nutritional status. The depressive disorder interferes with such people to do things in their lives normally. These cause pain for not only the patient, but also those who care about them.
The gut–brain dialogue associated with different neurodegenerative diseases should be examined for gaining a better understanding to provide a clear understanding of the pathogenesis and possible therapeutic strategies of such disorders concerning the colonic microbiota (El-Ansary et al. 2013). Thus, the current chapter aims to cover the concept of colonic microbiota in correlation to the gut–brain axis offering various pathways with different dietary patterns linking the gut and the brain, gut–brain cross talk microbiome, in simple term: how the intestinal microbiota influences our brain and how our brain, in turn, affects them.
2 Human Gut Microbiome
The human gut microbiome is a huge selection of intestinal bacteria that reach about 1500 species (Salminen et al. 2005; Young 2012). GM consists of four main phyla, i.e., Firmicutes, Bacteroidetes, Actinobacteria, and Eubacterium which were estimated recently by more than 1014 bacteria/g of colon contents (Sender et al. 2016). The major two groups are Firmicutes, the predominantly gram-positive bacteria, i.e., Bacilli, Clostridia, and Erysipelotrichia, and Bacteroidetes, the gram-negative bacteria. Gut microbiota highest concentration occurs in the colon, 1014/mL and comprises up to 60% of the faecal weight. However, the lowest occurs in the stomach, <103/mL and that depends on different factors such as pH and transit time (Kolida and Gibson 2007; Sender et al. 2016). Such colon matters have a huge diversity of bacterial species between individuals depending on many factors such as age and environmental factors, mainly diets (Wu et al. 2011). Such diversity of the GM has many functions as they are promoting the gastrointestinal tract (GIT) function, infection protection, metabolism regulation in addition to the immune response modulations. Colonic microbiome dysbiosis has been implicated in several human diseases including obesity and cardiovascular disease in addition to autism (Rowland et al. 2018). Additionally, the total species depend on illness, antibiotics, immune system, digestive and other secretions, stress, and diets especially probiotic and prebiotic sources (Kolida and Gibson 2007; Sekirov et al. 2010; Pickard et al. 2017).
Different dietary carbon sources such as dietary carbohydrates or protein indicated different bacterial species and levels in either healthy or ulcerative colitis patients (Rowland et al. 2018; Scott et al. 2013; Wu et al. 2011; Willson and Situ 2017; Khalil et al. 2013). Any diet of such different dietary sources normally converts to the metabolic end-products through the fermentation process and forms the SCFA, i.e., acetate, propionate, and butyrate, which detected mostly within the faecal samples with healthy or unhealthy subjects (Pickard et al. 2017). However, their ratio is different depending on the availability of dietary carbon sources and the health status of the studied models. The normal ratio obtained from faecal samples collected from healthy volunteers eating a healthy and balanced diet is in between 3:1:1 and 10:2:1 in molar ratio for acetate: propionate: butyrate, respectively (Rowland et al. 2018; Macfarlane et al. 1992; Steliou et al. 2012). Therefore, any changes that appear with the evaluation with such SCFA will be depending on the balanced or unbalanced, either gut microbiota or substrate available. Indeed, our previous data with ulcerative colitis patients presented an alternate intestinal microbiota that was reflected by alternate SCFA levels in comparison to healthy subjects. Their rational levels were depended on the dietary carbon sources available (Khalil et al. 2013). Again, different dietary sources either by using pro/prebiotics have been studied in correlation to the gut microbes and their metabolic end-products with either healthy or unhealthy peoples (Rowland et al. 2018).
3 Diet, Micronutrient, and Immune Function
Most studies have shown that once subject was infected, the immune system will be enhanced that will require high levels of metabolic rate, energy requirements, different biosynthesis substances and regulatory molecules, which obtained from dietary sources. Some micronutrients such as vitamin A, B6, B12, C, and D; minerals such as iron, copper, zinc, and selenium have been implicated to have key roles for supporting immunity with reducing the risk of infections (Rowland et al. 2018). Also, amino and fatty acids play a vital role. Therefore, it is very important for any host to consume an adequate amount of nutrients with different plant-based and animal-based foods so the immune system can work and deal with pathogens effectively (Calder 2020; Rowland et al. 2018). Consequently, a healthy diet will result in healthy gut by achieving well-balanced GM which can benefit the immune system in educating and regulating the host immunity (Thomas and Versalovic 2010).
Human gut microbiota composition modification can be achieved by small changes in diet (nutrients in foods support the body’s repair, growth, and wellness) with special reference to the dietary sources available to the gut microbiota. One way is using the pro/prebiotic strategy. Probiotics known as living bacteria are competing with the pathogens for available nutrients. They can interact well with the host’s gut epithelium and gut-associated immune tissues, affecting the immune function (Thomas and Versalovic 2010; Hemarajata and Versalovic 2013; Ahern and Maloy 2020). Different researchers studied the effects of many probiotic organisms alone or in a combination on the host health status, or the effects of different dietary sources on the probiotic species activities and compositions mainly within the immune functions and inflammatory conditions (Lomax and Calder 2009; Boge et al. 2009; Khalil et al. 2013). Conversely, modelling the gut microbiome is very well documented in many studies using either probiotic or prebiotic supplementations. For examples, some Lactobacilli and Bifidobacteria have been used as probiotics for GM modifications within the immune system responses (Thomas and Versalovic 2010; Hemarajata and Versalovic 2013; Ahern and Maloy 2020). Additionally, probiotic species show active effects if they have been used with prebiotic supplementations, known as synbiotic (mix of both pre/probiotics). Prebiotic, which is a non-digestible oligosaccharide, acts as fuels for some types of bacteria by enhancing their growth, especially the probiotics species such as Bifidobacteria and Lactobacilli (Rowland et al. 2018). Also, different prebiotic sources such as fibre, fermented and plant foods maintain well-balanced gut microbiota in addition to supporting the immune responses (Hill et al. 2014; Rowland et al. 2018). Conversely, any deficiency in either probiotics and/or prebiotics will lead to an unbalanced colonic microbiome, i.e., dysbiosis (Pickard et al. 2017).
Indeed, certain gut microbiota species are involved in neural development and functioning between animal models, affect a variety of complex behaviours such as social, emotional in addition to brain development and function. The colonic microbiota cooperates with their hosts for metabolic and nervous systems development, in addition to the function of the immune system regulation via dynamic bidirectional communication along the ‘gut–brain axis’. Also, it has been associated with a variety of psychiatric disorders as well as modulation of brain physiology, mood, and behaviours which in turn might help to discover new therapeutic strategies combating mood disorders.
4 Gut–Brain Axis
The rates of depression, anxiety, and combined anxiety depression are increasing worldwide, in both eastern and western cultures (El-Metwally et al. 2019). A Saudi research demonstrated that rates of anxiety and depression are about 35% and 15%, respectively (Albugami et al. 2018). Also, western countries such as UK showed high rates of depressive symptoms among young students using the screening test, a study applied to young university students from different universities in the UK. The outcome illustrated that about 60% of depression symptoms with factors behind such as economics problems recognized among lower economic class students. Also, an Egyptian study noticed a seasonal related pattern of mania and depression. The peak of depression was common in December, while mania was more common in the summer as the weather was humid and hot fewer rates of depression were recorded. Such condition levels depend on many factors worldwide such as dietary consumptions in addition to the host colonic microbiota composition and activities known as the gut–brain axis.
Gut–brain microbiota was correlated well to certain species (composition and activity) (van De Sande and van Buul 2014) that shows a correlation in neural development and functions, modulation of brain physiology, mood, and behaviours. Recently, the term gut–brain axis has been used as it refers to the biochemical signalling transversely between the GIT and the nervous system (Montiel-Castro et al. 2013). GM shows an effective role for obtaining healthy brain functions through both enteric and central nervous systems development. These were examined after bacterial non/colonization in the gut between using germ-free models (El-Ansary et al. 2013). The non-colonized model shows alteration in the neurotransmitters (chemical produced from the brain and passes through the nerve cell signals), in both enteric and central systems. This led to changes in the functions of sensory and motor within the gut which could be reversed (van De Sande and van Buul 2014).
The gut–brain axis has its function in two ways: the first one is the autonomic projections that regulate digestive reflexes, signals travelling from the gut to the brain that influence satiety (El-Metwally et al. 2019). The second one is the stress and anxiety impacts that affect the gut function and sensitivity through the vagus nerve (the most complex longest organ emanates from the brain and consists of 12 pairs of cranial, transmits the signals to or from the brain to organs and tissues (El-Metwally et al. 2019; El-Ansary et al. 2013)). Therefore, it controls the transmitting process from the gut to the brain, e.g., transmit the gut feelings especially the sensory to the brain. The afferent signals are the ones that sent nerve receptors into the brain, while efferent signals pass nerve receptors from the brain to the body (El-Ansary et al. 2013).
The signals that go through the vagus nerve and travelling from the gut to the brain have been linked previously to modulating mood and distinctive types of fear and anxiety, in form of electrical circuit linking our heart, lungs, and gut, to the brain-base (van De Sande and van Buul 2014). The following section explains the communication between the gut and the brain, and how they can affect each other on the risk of different diseases.
4.1 How Gut Microbiota Can Influence the Brain?
The gut microbiota has shown strong influences on human health and diseases involving brain health, mood, and behaviour. The gut has its nervous system (enteric nervous system, ENS). There are many ways for the GM communications within neural, immune, and endocrine which is affecting brain function and behaviours. For instance, GM shown a correlation with anxiety, pain, cognition, and mood regulation in animal models studies (El-Metwally et al. 2019; van De Sande and van Buul 2014). Different micronutrients are essential substrates for brain function, for example, dietary carbohydrates (whole grains, fruits, vegetables, and legumes) that was linked to mood via mood-boosting brain chemical, serotonin. The activity is declined by any craving such as carbohydrate consumptions. Indeed, prebiotics is normally used to prevent mood disorders safely and effectively by improving the brain’s nutritional milieu that may augment the effectiveness of antidepressant medication (van De Sande and van Buul 2014). It might be due to the secretion of neuroactive components in the lumen of the intestinal which then can pass through blood–brain Barrier causing certain cognitive and behavioural problems. Also, GM correlated well with the maturation and activation of microglial cells which has been involved in different diseases by their metabolic end-products, i.e., SCFA (Carabotti et al. 2015). Different SCFA such as butyric acid are mainly released into the bloodstreams and influence the neurodevelopment when crossing the blood–brain barriers. Butyric acid has a very important role in the gut by reducing the pH for eliminating the pathogenic bacterial besides being the main fuel for the epithelium, so they are able to restore the macrophages with possible therapeutic impacts (El-Ansary et al. 2013). The acids are the main dietary for some probiotics such as Bacteroides fragilis, Lactobacillus, and Bifidobacterium. Thus, having foods rich in probiotic such as fermented milk or fermented dairy products may help to cure and reduce the symptoms of neurological disorders. Indeed, a study showed autistic children have low levels of B. fragilis. However, supplemented B. fragilis to human and mice show low levels of anxiety (Canitano and Scandurra 2008; Peñagarikano et al. 2011; Round and Mazmanian 2010).
In contrast, having the pathogenic bacteria, i.e., Clostridium difficile, may cause gut diseases such as diarrhoea and malabsorption or well known as the leaky gut. This could be again due to having low levels of butyric acid. It also results in low levels of immunity as the tissues of the immune system could be affected by mistaken identity. Such bacterial species have been associated with diarrhoea as they are mainly propionic acid producer especially within high dietary carbohydrates fermentation and that has been shown within autism spectrum disorder (ASD) children (Wakefield 2002; van De Sande and van Buul 2014). Autism disorder is a set of complex neurodevelopmental disorders defined by impaired social interaction, delayed, and disordered language, repetitive or stereotypic behaviour, and a restricted range of interests. Children with autism have many issues to be concerned such as nutrition, intestinal permeability, inflammatory processes that should correlate well with underlying pathology, e.g., gut–brain axis, immunity, the interaction between animal models and genome-microbiome (de Theije et al. 2011). Gut dysbiosis has been implicated in the development of intestinal permeability, associated with both inflammations in intestinal and peripheral tissues, e.g., adipose tissue, muscles, liver, and brain, and altered glucose and energy homeostasis, i.e., metabolic diseases. Mucosal immune cells make up to 70% of the immune cells within the body, and dysfunction in these cells may have an adverse consequence for GI function. About 75% of autistic people presented with some GI problems in relation to alternate GM such as digestive problems especially allergy or gluten sensitivity. The most popular bacterial species with ASD patients called Bacteroides fragilis that has been detected in low levels between such patients. The administration of B. fragilis on autism mice models indicated good behaviours, less anxious with low repetitive behaviour and high communication levels. Different types of the colonic microbiota can be altered by antibiotics consumptions that can cause several neurological disorders, i.e., depressed illness, ASD, and Parkinson diseases (PD) (Diaz Heijtz et al. 2011; van De Sande and van Buul 2014). Additionally, poorer performance in animal models was related to higher levels of the Clostridia (Bercik et al. 2011a, b). Another study used calm and anxious mice, where the scientists have transferred colonic bacterial samples from one model to the other, where the mice was originally calm, later becoming anxious (Persico and Napolioni 2013; Bercik et al. 2011a, b).
In addition, similar demonstration observed where intestinal microbiota transplanted from anxious volunteers. That data suggested correlation to the production of neurotransmitters, i.e., serotonin and dopamine. Both are key factors for modulating mood and emotion which can therefore cause anxiety. Also, a possible mechanism of microbiota–brain communication could be the biologically active nanoparticles molecules that are delivered to all different organs in the body especially to the brain, after being inside the systemic circulation, causing different immunological and metabolic responses. Another mechanism could be the permeability of the blood–brain barrier that controls the in/out of molecules and nutrients in the brain. Taken these effects together on the development and maturation of the nervous system, it is strongly indicated that the colonic microbiota has an impact on the development of the brain health and responses with different cognitive functions.
4.2 How Can the Brain Influence the Gut Microbiota?
The brain has different ways to affect and control the gut through the vagus nerve directly and/or indirectly. The brain and the gut communicated well via the neurons in addition to the blood-borne chemicals and hormones that have been used as the signals for hunger and stress (van De Sande and van Buul 2014). Also, the direct influence can be through neurotransmitters and other molecules that are released in the gut (Montiel-Castro et al. 2013). The second indirect way is by any alteration in the gut by micronutrients available or local environmental changes. The brain is being able to affect the environment in the gut by sending signals back and forwards. Both ways are reversible, from the gut to the brain with reversible neurotransmitters, hormones, and different chemical and information. Both ways could be modulated by different epithelial cell function, mucus secretion, and changes in the gastrointestinal (GI) motility (Varghese et al. 2006; Ghia et al. 2008). For instance, norepinephrine released and passed to the stressed GI tract has stimulated the growth of especial bacterial species, adhere to the mucosa (Green et al. 2003; Chen et al. 2003; Freestone et al. 2002). GI functions were able to be ameliorated by dietary probiotics consumptions (Eutamene and Bueno 2007) in addition to the GM changes, mainly declined levels of lactobacilli (Gareau et al. 2007). Another study indicated that inflammatory markers (cytokines) and corticosterone levels have been amplified in association to the changes of the intestinal microbiota in stressed animal models where offspring are separated from their mothers (O’Mahony et al. 2009). Additionally, a similar study has demonstrated high levels of stress responses with the gut permeability in addition to GM compositions (Varghese et al. 2006).
5 Neurodevelopmental Disorder
Neurodevelopmental disorders such as intellectual disability, autism, and learning disabilities are reflected in many disabilities to communicate, move, or behave which usually change with age, although some children may develop permanent disabilities. Recently, it has been published that the intestinal microbiota influences the nervous system with different neurodevelopmental disorders mainly brain development and behavioural phenotypes (Collins et al. 2012; El-Metwally et al. 2019). Depressed and autistic patients/models show alternated numbers and compositions of their GM profile (Montiel-Castro et al. 2013). Therefore, it might be implicated in shaping neurodevelopmental by influencing a variety of complex procedures such as cognition, personality, mood, sleep, and eating behaviour, due to some neurotoxins. Understanding these aspects could help in early diagnosis, treatment, diseases prevention or to suggest potential therapeutic strategies. A study reported abnormal excreted hydrogen levels, but later showed an improvement after eliminating fructose from their diets (Ledochowski et al. 2000a; Ledochowski et al. 2000b). Furthermore, the level of tryptophan in blood declined with such treatments that promoted the GIT motility, mucosal biofilm, and the GM composition, through SCFA metabolism (Ledochowski et al. 2001; Gibson et al. 2007). In a different study, the blood tryptophan levels have been increased after Bifidobacterium infantis supplementations, which helped the recovery process of the tryptophan metabolism (Desbonnet et al. 2008). Therefore, it shows that fructose consumptions are associated with the depressive condition in correlation to tryptophan levels. The patients with ASD show changes of their colonic profile with reference to clostridial species (Finegold et al. 2002; Song et al. 2004). One study indicated that the number of Clostridium spp. were ten-fold more than the healthy subjects (El-Ansary et al. 2013).
6 Parkinson Disease
Parkinson disease (PD) affects approximately seven million people worldwide, the elderly especially groups aged 50 years and over (El-Metwally et al. 2019). It is a degenerative disorder of the central nervous system resulting from the death of dopamine-generating cells in the substantial nigra in midbrain (Shulman et al. 2011; Abou-Donia et al. 2013). Early symptoms of PD include shaking, rigidity, slowness of movement, and difficulty with walking. Later, thinking and behavioural problems may occur, with dementia and depression arise in the advanced stages (Galpern and Lang 2006; Aarsland et al. 2009; van De Sande and van Buul 2014). Other symptoms include sensory, sleep, and emotional problems, where most cases of PD occurring more common among men than women (El-Metwally et al. 2019). Although most cases of PD are idiopathic, a small proportion of cases can be attributed to known genetic and others to environmental factors, mainly the diets (Davie 2008; Barnett-Cowan et al. 2010). It has been suggested recently that PD may be less prevalent in those of African and Asian men (de Lau and Breteler 2006; van De Sande and van Buul 2014) that could be related to their consumed diets. Using probiotics and/or antimicrobials between animal models shows an alteration in memory, anxiety, and amygdala levels of brain-derived neurotrophic factor (Collins et al. 2012; Davari et al. 2013; Hsiao et al. 2013; El-Metwally et al. 2019). Recently, it appears that protein aggregates that are the mainstay for PD pathology depend on toxic forms of these proteins, i.e., oligomeric α-synuclein and oligomeric tau, for diagnosis and therapeutic intervention (Sengupta et al. 2015). Furthermore, muscles and nerves that control the digestive process may be affected by PD, resulting in constipation and gastroparesis where food remains in the stomach for a longer period than normal (Barichella et al. 2009).
Interestingly, gut microbes may be anticipated in the aetiology of PD (van De Sande and van Buul 2014). A study conducted previously illustrated that GM plays an important role with PD patients especially because of their gastrointestinal immune responses (Montiel-Castro et al. 2013). Indeed, unbalanced gut microbiota has been recognized with such patients causing inflammatory markers which might lead to PD pathology as the mechanism of neuroinflammation. A study shows that poorer performance between animal models is related to higher levels of the microbiota with special reference to clostridia (Magnusson et al. 2015). Collected previous data in comparison between healthy control subjects and people diagnosed with PD illustrated that mucosal biopsy in correlation to faecal microbiota compositions and activities was correlated well with the clinical evaluations of PD (van De Sande and van Buul 2014). They show that the collected faecal samples from healthy peoples have more anti-inflammatory species significantly, especially the butyric acid producers than the faecal samples obtained from PD patients. For example, Faecalibacterium was significantly more abundant in healthy subjects than PD patients. However, genus Ralstonia that are the pro-inflammatory species were more abundant in collected PD faecal samples than the control ones. Little data is known about the amount of each micronutrient required in such mood disorders additionally to the colonic microbiota that interacted well with brain diseases.
7 Conclusion
Nutritional therapy is necessary for brain disorders as to patients having malnutrition diseases caused by the decrease of food intake, malabsorption, accelerated nutrient loss, and increase of nutritional requirements in association with the gut microbiota, the gut–brain axis. It could improve nutritional states but also promote the reconstruction of mucosa and regulate the immune function. Thus, gut–brain microbiome alterations and their role in the psychiatric treatment of anxiety and depression provide potential therapeutic suggestions in the aetiology of mood disorders. More studies regarding dietary factors effecting and gut–brain microbiome interaction, e.g., composition and activity, are needed within the use of probiotics, prebiotics and functional food providing essential micronutrients.
References
Aarsland D, Londos E, Ballard C (2009) Parkinson’s disease dementia and dementia with Lewy bodies: different aspects of one entity. Int Psychogeriatr 21(2):216–219. https://doi.org/10.1017/S1041610208008612
Abou-Donia MB, Abou-Donia MM, ElMasry EM, Monro JA, Mulder MF (2013) Autoantibodies to nervous system-specific proteins are elevated in sera of flight crew members: biomarkers for nervous system injury. J Toxicol Environ Health A 76(6):363–380. https://doi.org/10.1080/15287394.2013.765369
Ahern PP, Maloy KJ (2020) Understanding immune-microbiota interactions in the intestine. Immunology 159(1):4–14. https://doi.org/10.1111/imm.13150
Albugami M, Qadi N, Almugbel F, Mohammed A, Alttas A, Elamin A et al (2018) The demographic characteristics and the risk factors of dementia in Saudi elderly. Am J Psychiatry Neurosci 6(1):1–8. https://doi.org/10.11648/j.ajpn.20180601.11
Barichella M, Cereda E, Pezzoli G (2009) Major nutritional issues in the management of Parkinson’s disease. Mov Disord 24(13):1881–1892. https://doi.org/10.1002/mds.22705
Barnett-Cowan M, Dyde RT, Fox SH, Moro E, Hutchison WD, Harris LR (2010) Multisensory determinants of orientation perception in Parkinson’s disease. Neuroscience 167(4):1138–1150. https://doi.org/10.1016/j.neuroscience.2010.02.065
Bercik P, Denou E, Collins J, Jackson W, Lu J, Jury J et al (2011a) The intestinal microbiota affect central levels of brain-derived neurotropic factor and behavior in mice. Gastroenterology 141(2):599–609. https://doi.org/10.1053/j.gastro.2011.04.052
Bercik P, Park AJ, Sinclair D, Khoshdel A, Lu J, Huang X et al (2011b) The anxiolytic effect of Bifidobacterium longum NCC3001 involves vagal pathways for gut-brain communication. Neurogastroenterol Motil 23(12):1132–1139. https://doi.org/10.1111/j.1365-2982.2011.01796.x
Blumberg R, Powrie F (2012) Microbiota, disease, and back to health: a metastable journey. Sci Transl Med 4:137. https://doi.org/10.1126/scitranslmed.3004184
Boge T, Rémigy M, Vaudaine S, Tanguy J, Bourdet-Sicard R, Werf SV (2009) A probiotic fermented dairy drink improves antibody response to influenza vaccination in the elderly in two randomised controlled trials. Vaccine 27(41):5677–5684. https://doi.org/10.1016/j.vaccine.2009.06.094
Calder PC (2020) Nutrition, immunity and COVID-19. BMJ Nutr Prev Health 3:74–92. https://doi.org/10.1136/bmjnph-2020-000085
Canitano R, Scandurra V (2008) Risperidone in the treatment of behavioral disorders associated with autism in children and adolescents. Neuropsychiatr Dis Treat 4(4):723–730. https://doi.org/10.2147/ndt.s1450
Carabotti M, Scirocco A, Maselli MA, Severi C (2015) The gut-brain axis: interactions between enteric microbiota, central and enteric nervous systems. Ann Gastroenterol 28(2):203–209
Chen C, Brown DR, Xie Y, Green BT, Lyte M (2003) Catecholamines modulate Escherichia coli O157:H7 adherence to murine cecal mucosa. Shock 20(2):183–188. https://doi.org/10.1097/01.shk.0000073867.66587.e0
Collins SM, Surette M, Bercik P (2012) The interplay between the intestinal microbiota and the brain. Nat Rev Microbiol 10(11):735–742. https://doi.org/10.1038/nrmicro2876
Davari S, Talaei SA, Alaei H, Salami M (2013) Probiotics treatment improves diabetes-induced impairment of synaptic activity and cognitive function: behavioral and electrophysiological proofs for microbiome-gut-brain axis. Neuroscience 240:287–296. https://doi.org/10.1016/j.neuroscience.2013.02.055
Davie CA (2008) A review of Parkinson's disease. Br Med Bull 86:109–127. https://doi.org/10.1093/bmb/ldn013
van De Sande MM, van Buul VJ (2014) Autism and nutrition: the role of the gut–brain axis. Nutr Res Rev 27(2):199–214. https://doi.org/10.1017/S0954422414000110
Desbonnet L, Garrett L, Clarke G, Bienenstock J, Dinan TG (2008) The probiotic Bifidobacteria infantis: an assessment of potential antidepressant properties in the rat. J Psychiatr Res 43(2):164–174. https://doi.org/10.1016/j.jpsychires.2008.03.009
Diaz Heijtz R, Wang S, Anuar F, Qian Y, Björkholm B, Samuelsson A et al (2011) Normal gut microbiota modulates brain development and behavior. Proc Natl Acad Sci U S A 108(7):3047–3052. https://doi.org/10.1073/pnas.1010529108
El-Ansary A, Shaker GH, Rizk MZ (2013) Role of gut-brain axis in the aetiology of neurodevelopmental disorders with reference to autism. J Clin Toxicol S6:005. https://doi.org/10.4172/2161-0495.S6-005
El-Metwally A, Toivola P, Al-Rashidi M, Nooruddin S, Jawed M, AlKanhal R et al (2019) Epidemiology of Alzheimer’s disease and dementia in Arab countries: a systematic review. Behav Neurol 2019:3935943. https://doi.org/10.1155/2019/3935943
Eutamene H, Bueno L (2007) Role of probiotics in correcting abnormalities of colonic flora induced by stress. Gut 56(11):1495–1497. https://doi.org/10.1136/gut.2007.124040
Finegold SM, Molitoris D, Song Y, Liu C, Vaisanen M-L, Bolte E et al (2002) Gastrointestinal microflora studies in late-onset autism. Clin Infect Dis 35(Suppl 1):S6–S16. https://doi.org/10.1086/341914
Freestone PP, Williams PH, Haigh RD, Maggs AF, Neal CP, Lyte M (2002) Growth stimulation of intestinal commensal Escherichia coli by catecholamines: a possible contributory factor in trauma-induced sepsis. Shock 18(5):465–470. https://doi.org/10.1097/00024382-200211000-00014
Galpern WR, Lang AE (2006) Interface between tauopathies and synucleinopathies: a tale of two proteins. Ann Neurol 59(3):449–458. https://doi.org/10.1002/ana.20819
Gareau MG, Jury J, MacQueen G, Sherman PM, Perdue MH (2007) Probiotic treatment of rat pups normalises corticosterone release and ameliorates colonic dysfunction induced by maternal separation. Gut 56(11):1522–1528. https://doi.org/10.1136/gut.2006.117176
Gentile CL, Weir TL (2018) The gut microbiota at the intersection of diet and human health. Science 362(6416):776–780. https://doi.org/10.1126/science.aau5812
Ghia J-E, Blennerhassett P, Collins SM (2008) Impaired parasympathetic function increases susceptibility to inflammatory bowel disease in a mouse model of depression. J Clin Invest 118(6):2209–2018. https://doi.org/10.1172/JCI32849
Gibson PR, Newnham E, Barrett JS, Shepherd SJ, Muir JG (2007) Review article: fructose malabsorption and the bigger picture. Aliment Pharmacol Ther 25(4):349–363. https://doi.org/10.1111/j.1365-2036.2006.03186.x
Green BT, Lyte M, Kulkarni-Narla, & R, B. D. (2003) Neuromodulation of enteropathogen internalization in Peyer’s patches from porcine jejunum. J Neuroimmunol 141(1–2):74–82. https://doi.org/10.1016/s0165-5728(03)00225-x
Hemarajata P, Versalovic J (2013) Effects of probiotics on gut microbiota: mechanisms of intestinal immunomodulation and neuromodulation. Ther Adv Gastroenterol 6(1):39–51. https://doi.org/10.1177/1756283X12459294
Hill C, Guarner F, Reid G, Gibson GR, Merenstein DJ, Pot B et al (2014) The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat Rev Gastroenterol Hepatol 11:506–514. https://doi.org/10.1038/nrgastro.2014.66
Hsiao EY, McBride SW, Hsien S, Sharon G, Hyde ER, McCue T et al (2013) Microbiota modulate behavioral and physiological abnormalities associated with neurodevelopmental disorders. Cell 155(7):1451–1463. https://doi.org/10.1016/j.cell.2013.11.024
Kang D-W, Park JG, Ilhan ZE, Wallstrom G, Labaer J, Adams JB, Krajmalnik-Brown R (2013) Reduced incidence of Prevotella and other fermenters in intestinal microflora of autistic children. PLoS One 8(7):e68322. https://doi.org/10.1371/journal.pone.0068322
Khalil NA, Walton GE, Gibson GR, Tuohy KM, Andrews SC (2013) In vitro batch cultures of gut microbiota from healthy and ulcerative colitis (UC) subjects suggest that sulphate-reducing bacteria levels are raised in UC and by a protein-rich diet. Int J Food Sci Nutr 65(1):1–10. https://doi.org/10.3109/09637486.2013.825700
Kohane IS, McMurry A, Weber G, MacFadden D, Rappaport L, Kunkel L et al (2012) The co-morbidity burden of children and young adults with autism spectrum disorders. PLoS One 7(4):e33224. https://doi.org/10.1371/journal.pone.0033224
Kolida S, Gibson GR (2007) Prebiotic capacity of inulin-type fructans. J Nutr 137(11 Suppl):2503S–2506S. https://doi.org/10.1093/jn/137.11.2503S
de Lau LM, Breteler MM (2006) Epidemiology of Parkinson’s disease. Lancet Neurol 5(6):525–535. https://doi.org/10.1016/S1474-4422(06)70471-9
Ledochowski M, Widner B, Bair H, Probst T, Fuchs D (2000a) Fructose- and sorbitol-reduced diet improves mood and gastrointestinal disturbances in fructose malabsorbers. Scand J Gastroenterol 35(10):1048–1052. https://doi.org/10.1080/003655200451162
Ledochowski M, Widner B, Sperner-Unterweger B, Propst T, Vogel W, Fuchs D (2000b) Carbohydrate malabsorption syndromes and early signs of mental depression in females. Dig Dis Sci 45(7):1255–1259. https://doi.org/10.1023/a:1005527230346
Ledochowski M, Widner B, Murr C, Sperner-Unterweger B, Fuchs D (2001) Fructose malabsorption is associated with decreased plasma tryptophan. Scand J Gastroenterol 36(4):367–371. https://doi.org/10.1080/003655201300051135
Lomax AR, Calder PC (2009) Probiotics, immune function, infection and inflammation: a review of the evidence from studies conducted in humans. Curr Pharm Des 15(13):1428–1518. https://doi.org/10.2174/138161209788168155
Macfarlane GT, Gibson GR, Cummings JH (1992) Comparison of fermentation reactions in different regions of the human colon. J Appl Bacteriol 72(1):57–64. https://doi.org/10.1111/j.1365-2672.1992.tb04882.x
Magnusson KR, Hauck L, Jeffrey BM, Elias V, Humphrey A, Nath R et al (2015) Relationships between diet-related changes in the gut microbiome and cognitive flexibility. Neuroscience 300:128–140. https://doi.org/10.1016/j.neuroscience.2015.05.016
Montiel-Castro AJ, González-Cervantes RM, Bravo-Ruiseco G, Pacheco-López G (2013) The microbiota–gut–brain axis: neurobehavioral correlates, health and sociality. Front Integr Neurosci 7:70. https://doi.org/10.3389/fnint.2013.00070
O’Mahony SM, Marchesi JR, Scully P, Codling C, Ceolho A-M, Quigley EM et al (2009) Early life stress alters behavior, immunity, and microbiota in rats: implications for irritable bowel syndrome and psychiatric illnesses. Biol Psychiatry 65(3):263–267. https://doi.org/10.1016/j.biopsych.2008.06.026
Peñagarikano O, Abrahams BS, Herman EI, Winden KD, Gdalyahu A, Dong H et al (2011) Absence of CNTNAP2 leads to epilepsy, neuronal migration abnormalities, and core autism-related deficits. Cell 147(1):235–246. https://doi.org/10.1016/j.cell.2011.08.040
Persico AM, Napolioni V (2013) Urinary p-cresol in autism spectrum disorder. Neurotoxicol Teratol 36:82–90. https://doi.org/10.1016/j.ntt.2012.09.002
Pickard JM, Zeng MY, Caruso R, Núñez G (2017) Gut microbiota: role in pathogen colonization, immune responses, and inflammatory disease. Immunol Rev 279(1):70–89. https://doi.org/10.1111/imr.12567
Rajeh SA, Bademosi O, Ismail H, Awada A, Dawodu A, Al-Freihi H et al (1993) A community survey of neurological disorders in Saudi Arabia: the Thugbah study. Neuroepidemiology 12(3):164–178. https://doi.org/10.1159/000110316
Round JL, Mazmanian SK (2010) Inducible Foxp3+ regulatory T-cell development by a commensal bacterium of the intestinal microbiota. Proc Natl Acad Sci U S A 107(27):12204–12209. https://doi.org/10.1073/pnas.0909122107
Rowland I, Gibson G, Heinken A, Scott K, Swann J, Thiele I, Tuohy K (2018) Gut microbiota functions: metabolism of nutrients and other food components. Eur J Nutr 57(1):1–24. https://doi.org/10.1007/s00394-017-1445-8
Salminen SJ, Gueimonde M, Isolauri E (2005) Probiotics that modify disease risk. J Nutr 135(5):1294–1298. https://doi.org/10.1093/jn/135.5.1294
Scott KP, Gratz SW, Sheridan PO, Flint HJ, Duncan SH (2013) The influence of diet on the gut microbiota. Pharmacol Res 69(1):52–60. https://doi.org/10.1016/j.phrs.2012.10.020
Sekirov I, Russel SL, L. C., & Finlay, B. B. (2010) Gut microbiota in health and disease. Physiol Rev 90(3):859–904. https://doi.org/10.1152/physrev.00045.2009
Sender R, Fuchs S, Milo R (2016) Revised estimates for the number of human and bacteria cells in the body. PLoS Biol 14(8):1002533. https://doi.org/10.1371/journal.pbio.1002533
Sengupta U, Guerrero-Muñoz MJ, Castillo-Carranza DL, Lasagna-Reeves CA, Gerson JE, Paulucci-Holthauzen AA et al (2015) Pathological interface between oligomeric alpha-synuclein and tau in synucleinopathies. Biol Psychiatry 78(10):672–683. https://doi.org/10.1016/j.biopsych.2014.12.019
Sharon G, Cruz NJ, Kang D-W, Gandal MJ, Wang B, Kim Y-M et al (2019) Human gut microbiota from autism spectrum disorder promote behavioral symptoms in mice. Cell 177(6):1600–1618. https://doi.org/10.1016/j.cell.2019.05.004
Shulman JM, Jager PL, Feany MB (2011) Parkinson's disease: genetics and pathogenesis. Annu Rev Pathol 6:193–222. https://doi.org/10.1146/annurev-pathol-011110-130242
Singh RK, Chang H-W, Yan D, Lee KM, Ucmak D, Wong K et al (2017) Influence of diet on the gut microbiome and implications for human health. J Transl Med 15:73. https://doi.org/10.1186/s12967-017-1175-y
Song Y, Liu C, Finegold SM (2004) Real-time PCR quantitation of clostridia in feces of autistic children. Appl Environ Microbiol 70(11):6459–6465. https://doi.org/10.1128/AEM.70.11.6459-6465.2004
Steliou K, Boosalis MS, Perrine SP, Sangerman J, Faller DV (2012) Butyrate histone deacetylase inhibitors. BioRes Open Access 1(4):192–198. https://doi.org/10.1089/biores.2012.0223
de Theije CG, Wu J, Silva SL, Kamphuis PJ, Garssen J, Korte SM, Kraneveld AD (2011) Pathways underlying the gut-to-brain connection in autism spectrum disorders as future targets for disease management. Eur J Pharmacol 668(Suppl 1):S70–S80. https://doi.org/10.1016/j.ejphar.2011.07.013
Thomas CM, Versalovic J (2010) Probiotics-host communication: modulation of signaling pathways in the intestine. Gut Microbes 1(3):148–163. https://doi.org/10.4161/gmic.1.3.11712
Valdes AM, Walter J, Segal E, Spector TD (2018) Role of the gut microbiota in nutrition and health. BMJ 361:k2179. https://doi.org/10.1136/bmj.k2179
Varghese AK, Verdú EF, Bercik P, Khan WI, Blennerhassett PA, Szechtman H, Collins SM (2006) Antidepressants attenuate increased susceptibility to colitis in a murine model of depression. Gastroenterology 130(6):1743–1753. https://doi.org/10.1053/j.gastro.2006.02.007
Wakefield AJ (2002) The gut-brain axis in childhood developmental disorders. J Pediatr Gastroenterol Nutr 34(Suppl 1):S14–S17. https://doi.org/10.1097/00005176-200205001-00004
Willson K, Situ C (2017) Systematic review on effects of diet on gut microbiota in relation to metabolic syndromes. J Clin Nutr Metab 1:2
Wu GD, Chen J, Hoffmann C, Bittinger K, Chen Y-Y, Keilbaugh SA et al (2011) Linking long-term dietary patterns with gut microbial enterotypes. Science 334(6052):105–108. https://doi.org/10.1126/science.1208344
Young VB (2012) The intestinal microbiota in health and disease. Curr Opin Gastroenterol 28(1):63–69. https://doi.org/10.1097/MOG.0b013e32834d61e9
Zhang Q, Raoof M, Chen Y, Sumi Y, Sursal T, Junger W et al (2010) Circulating mitochondrial DAMPs cause inflammatory responses to injury. Nature 464(7285):104–107. https://doi.org/10.1038/nature08780
Zmora N, Suez J, Elinav E (2019) You are what you eat: diet, health and the gut microbiota. Nat Rev Gastroenterol Hepatol 16(1):35–56. https://doi.org/10.1038/s41575-018-0061-2
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Khalil, N.A., Sarbini, S.R. (2022). Gut–Brain Cross Talk: Microbiome and Micronutrients. In: Mohamed, W., Yamashita, T. (eds) Role of Micronutrients in Brain Health. Nutritional Neurosciences. Springer, Singapore. https://doi.org/10.1007/978-981-16-6467-0_3
Download citation
DOI: https://doi.org/10.1007/978-981-16-6467-0_3
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-16-6466-3
Online ISBN: 978-981-16-6467-0
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)