Abstract
Heavy metals (HMs) are environmental and food chain contaminants having chronic and epidemic effects on human health. Introduction of HMs in the food chain takes place by their excessive uptake from soil through the crop plants, making it a global issue of concern to take necessary steps to counteract the problem. The HMs also cause toxicities to plants by affecting their growth and productivity. With the continuously changing global climatic conditions, the HM contamination in the soil is exaggerating, thereby resulting in the considerable yield reduction of major crop species. Furthermore, HM-induced soil pollution associated with the improper fertilization practices appears as a serious threat to the sustainable agriculture. It is therefore, a serious worldwide concern to minimize the HM toxicity in crop plants. Phytoremediation is a promising plant-based, cost-effective, and eco-friendly approach for the effective removal of the HMs from the environment. Several plants known as metallophytes accumulate higher level of HMs without having any toxic effects and, therefore, can be used to remove large amounts of HMs from the soil. The present chapter summarizes the mechanisms of HM uptake, translocation, and detoxification in plants. The mechanism adopted by the metallophytes in HM hyperaccumulation and their role in ameliorating the HM toxicity has also been discussed.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
19.1 Introduction
Progressive industrialization, modern agricultural practices, and increased anthropogenic activities due to urbanization are emerging as potential causes for heavy metal (HM) contamination in the environment (Singh et al. 2016). The HM contamination leading to toxicity in animals and humans has become a major concern in the last few decades. Unrestricted usage of pesticides and chemical fertilizers in agriculture, compost wastes, smelting industries, and metal mining are increasing the HM contamination throughout the world. HMs cause toxicity to the plants with significant negative influence on their growth and productivity (Arif et al. 2016). HMs are metallic chemical elements with high densities, atomic weights and numbers (Nagajyoti et al. 2010; Kamal et al. 2010). They are the natural components of the Earth’s crust. HMs are nondegradable and create toxic effect even at very low concentrations. Examples of some common HMs are arsenic (As), cadmium (Cd), chromium (Cr), lead (Pb), mercury (Hg), and thallium (Tl). Alternatively, some HMs, such as copper (Cu), selenium (Se), and zinc (Zn), are considered as the trace elements playing a crucial role in the metabolic processes of plants and animals. However, these trace elements can lead to poisoning at higher concentrations. The HM contamination affects the soil microbial communities, influences the biogeochemical cycles, and directly impacts the ecological niche and diversity of soil bacterial communities (Thakare et al. 2021). It is therefore, a serious worldwide concern to take necessary steps to counteract the problem of HM toxicity in the environment.
Phytoremediation is a cost-effective and mutualistic eco-friendly approach with direct associations of the HM-tolerant plants and the HM-polluted soils (Muthusaravanan et al. 2018; Sarma et al. 2021; Sonowal et al. 2022). It is a plant-based technique, which involves the use of plants to extract and remove the HM pollutants or lower their bioavailability in the soil (Marques et al. 2009). Plants have the ability to absorb ionic compounds from the soil even at low concentrations through their root systems. They extend their root system into the soil matrix and establish the rhizosphere ecosystem to accumulate the HMs and modulate their bioavailability, through which they reclaim the polluted soil and stabilize the soil fertility. Phytoremediation is an autotrophic system powered by solar energy, therefore, simple to manage, and the cost of installation and maintenance is low. Being environment-friendly, it can reduce the HM pollutants from the ecosystem. Additionally, it can be applied over a large-scale field and can also easily be disposed. It prevents the erosion and metal leaching by stabilizing the HMs and reduces the risk of spreading the HM contaminants. It also improves the soil fertility by releasing various organic matters to the soil (Yan et al. 2020). Several studies have unraveled the molecular mechanisms underlying the HM tolerance in plants and have developed techniques to improve the phytoremediation efficiency of plants. The present chapter highlights the mechanisms of HM uptake, translocation, and detoxification in plants. The strategies adopted by the plants to improve the HM bioavailability, accumulation, and tolerance have also been discussed in this chapter, which may contribute in developing phytoremediation techniques to eliminate HM toxicity.
19.2 Plants’ Responses to Heavy Metal Toxicity
Mineral nutrients are one of the key regulators of plant growth and productivity. A number of metals are important for the growth of plants. However, their essentiality depends on their concentrations in plant (different stages) and environment. Furthermore, some trace elements (mainly Fe, Zn, Cu, Ni, Co, and Mo) are essential for plant and cellular biochemistry being involved in cell protection, gene regulation, and signal transduction. However, excess concentrations of these elements than their optimum levels may cause toxicities to plants by retarding plant growth and yield. Other heavy metals (As, Cd, Hg, Pb, and Cr) are biologically nonessential and show toxicity even at low concentrations (DalCorso et al. 2019).
Heavy metals interfere with metabolic reactions in plant systems. HM toxicity reduces the plant growth, photosynthetic activities, mineral nutrition, and activity of essential enzymes. They are cytotoxic and carcinogenic to humans at low concentrations. HMs induce the production of reactive oxygen species (ROS) causing oxidative stress in plants. The ROS causes oxidation of DNA, proteins, and lipids leading to the cell death (Ojuederie and Babalola 2017). HM tolerance in plants is mediated by the vacuolar compartmentalization and sequestration of the HMs within the plant cells. Plants also develop antioxidant defense system which protects cells through effective scavenging of ROS. Understanding the mechanisms of HM detoxification is essential for searching the potential HM-tolerant plant species which can be used for the removal of HM from the contaminated sites.
19.3 Mechanism of Uptake, Translocation, and Detoxification of Heavy Metals in Plants
The HMs are usually present as insoluble forms in the soil. The uptake of HM in plants is governed by different factors, including the water content, pH, and organic substances. Higher water content increases the solubility of the HMs consequently, increasing their bioavailability. Wang et al. (2015) have reported the flooded conditions to intensify the bioavailability of arsenic (As) resulting in the efficient As uptake in rice. High soil temperature also increases the solubility and bioavailability of the HMs, thereby increasing its uptake in plants (Arao et al. 2018). The pH also enhances the dissolution of HMs by acidifying the rhizosphere by increasing the proton secretion from the roots (Peng et al. 2005). The organic substances’ exude from the roots also increases the bioavailability of HMs to the plants. Cieslinski et al. (1998) have revealed the presence of organic acids in the rhizosphere to increase the solubility and availability of cadmium (Cd). Additionally, root proliferation enhances the HM uptake in plants (Whiting et al. 2000).
19.3.1 Heavy Metal Uptake and Translocation
The bioavailable HMs are absorbed by the root hairs and driven across the plasma membrane of the root epidermal cells. Different HMs employ various ways to enter the plant roots. The HM uptake in roots generally occurs through apoplastic or symplastic route (Yan et al. 2020). The apoplastic pathway mediates the movement of HMs through the cell wall and intercellular spaces. Kidwai et al. (2019) have revealed that increased lignification in the roots act as an apoplastic barrier for the As entry in root cells resulting in the reduced As accumulation. On the other hand, the symplastic pathway includes plasma membrane-localized nonspecific ion channels or transporters. Arsenate As(V), the major form of As enters the plant root tissue via the phosphate (Pi) transporters (Shi et al. 2019). The Cd2+ uptake in plants takes place through the transporters involved in the Mg2+, Ca2+, Fe2+, Zn2+, and Cu2+ uptake (Ismael et al. 2019). The lead (Pb) uptake occurs via the Ca2+ permeable channels on roots (Pourrut et al. 2011). After entering the root cells, HMs are translocated to the aerial parts of the plants through xylem vessels.
19.3.2 Heavy Metal Detoxification
Detoxification of HMs is an important requirement for employing the phytoremediation approach (Viehweger 2014). The improved HM detoxification process in the hyperaccumulators allows them to persist under the HM-contaminated sites without having any toxic effect. Plants adopt avoidance or tolerance strategies to cope with the HM toxicity through HM homeostasis. Avoidance is the simplest strategy, which acts as the first line of defense at extracellular level to restrict HM uptake from the soil and prevent their translocation into aerial tissues (Dalvi and Bhalerao 2013). It comprises of different strategies—root sorption, ion precipitation, and exclusion of the HMs (Yan et al. 2020). Root sorption is the first step of HM avoidance, where HMs are immobilized in the rhizosphere by forming HM complex with different ligands (e.g., amino acid, organic acid). The precipitation of HM ions occurs by the alteration of the rhizosphere pH due to root exudates. Exclusion of the HMs is mediated by the barrier between the root and the shoot systems that restrict the aerial translocation of HMs. Arbuscular mycorrhizal (AM) fungi immobilize the HMs by binding with insoluble glycoprotein (glomalin) produced by AM hyphae, thereby inhibiting HM entry in plants (Basu and Kumar 2020a, 2021a). Presence of cell wall polysaccharide-derived functional groups (e.g., carboxyl, hydroxyl) favors ion-exchange with the wall counter-ions leading to increased HM binding capacity, which reduces the HM entry in the protoplast (Parrotta et al. 2015). HMs are also immobilized by reacting with the polygalacturonic acid present in the pectin of cell wall, consequently preventing their entry into the root cells.
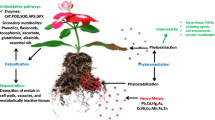
The tolerance strategy acts as the second line of defense at intracellular level through inactivation, chelation, and vacuolar partitioning of the HMs (Fig. 19.1). Detoxification of HMs within the cytosol occurs by their chelation with inorganic or organic ligands. The organic ligands include amino acids, organic acids, phytochelatins (PCs), metallothioneins (MTs), and polyphenols, proteins, or pectins of cell wall. Arsenic detoxification in plants is associated with the binding of arsenite with the phytochelatins (PC) or glutathione followed by their vacuolar sequestration (Aborode et al. 2016). The MTs also play an important role in the HM detoxification (Kumar et al. 2012). HMs also induce the ROS production causing oxidative stress in plants. Detoxification of the ROS is mediated by the antioxidant enzymes, including superoxide dismutase, peroxidase, and ascorbate-glutathione cycle enzymes (Basu et al. 2017, 2021a, b). Different nonenzymatic antioxidants have also been revealed to contribute in the ROS scavenging in plants (Basu et al. 2020). Enhanced antioxidant enzyme activities decline the membrane lipid peroxidation in plants, thereby improving the plant growth under HM toxicity (Kumar et al. 2021a).
Mechanism of heavy metal (HM) detoxification in metallophytes (hyperaccumulators). Detoxification of HMs within the cytosol occurs by their chelation with inorganic or organic ligands (amino acids, organic acids, phytochelatins, metallothioneins). Detoxification of HM-induced reactive oxygen species is mediated by the antioxidant enzymes (superoxide dismutase, peroxidase, ascorbate peroxidase). Metal homeostasis and tolerance in the hyperaccumulators is mediated by the vacuolar sequestration of the HMs within the plant cells
19.3.3 Transporters for Heavy Metal Uptake, Translocation, and Detoxification
The HM uptake and translocation in plants is facilitated by the metal ion transporters and complexing agents. The root cell’s plasma membrane-localized channels or H+-coupled carrier proteins play an important role in the HM uptake from the soil (Yan et al. 2020). They are also involved in the influx or efflux of the HM ions, thereby facilitating the root-to-shoot translocation (Komal et al. 2015). The plasma membrane and tonoplast localized transporters belong to the zinc-regulated, iron-regulated transporter protein (ZIP), heavy metal-transporting ATPase (P1B-ATPase), natural resistance-associated macrophage proteins (NRAMP), cation diffusion facilitator (CDF) or metal tolerance protein (MTP), and multidrug and toxin extrusion (MATE) protein families are involved in the HM uptake, translocation, and cellular homeostasis.
The ZIP family transporters mediate the uptake and transport of cations (Zn, Mn, and Fe) to the aerial parts of the plants (Guerinot 2000). Assuncao et al. (2001) have revealed the overexpression of the ZIP family transporter-related genes to increase the Zn uptake in the Zn hyperaccumulator Thlaspi caerulescens (ZNT1 and ZNT2) and Arabidopsis halleri (ZIP6 and ZIP9).
The P1B-type ATPases belong to the heavy metal transporting ATPases (HMAs) transporter family, which are involved in the transport of HMs (Cd, Co, Pb, and Zn) to the plasma membrane or the vacuolar sequestration of the HMs and play a vital role in metal homeostasis and tolerance (Hanikenne and Baurain 2014). The HMA3 (P1B-ATPase) localized on the tonoplast is responsible for the vacuolar compartmentation of HMs (Liu et al. 2017); whereas, the HMA4 carries out the aerial translocation of Cd and Zn (Wang et al. 2019). The overexpression of the BjHMA4 (from Brassica juncea) has been shown to promote the HM tolerance in rice and wheat by inducing the efflux of Cd and Zn from the root cytoplasm into the xylem vessels (Wang et al. 2019).
The NRAMPs are a ubiquitous family of metal transporters responsible for the uptake and transport of various HMs (As, Cd, Co, Cu, Fe, and Mn) in different plant species (Nevo and Nelson 2006). Cailliatte et al. (2009) reported the NRAMP6 to contribute in the Cd transport. Later, Cailliatte et al. (2010) also revealed the plasma membrane-localized AtNRAMP1 to mediate the transport of Fe and Mn in Arabidopsis. Tiwari et al. (2014) reported the plasma membrane-localized OsNRAMP1 to facilitate the arsenite (AsIII) mobilization to the aerial parts of rice through xylem loading. Bastow et al. (2018) showed the tonoplast-localized NRAMP3 and NRAMP4 to mediate the mobilization of vacuolar Fe in germinating seed.
The CDF or MTP transporter family is involved in the regulation of HM (Cd, Co, Mn, Ni, and Zn) homeostasis through the vacuolar sequestration or transport to the extracellular space (Ricachenevsky et al. 2013). The tonoplast-localized MTP1 and MTP4 have been reported to be the Zn2+/H+ and Cd2+/H+ antiporters involved in the vacuolar Zn and Cd sequestration in cucumber (Migocka et al. 2014). Comparative analyses of A. thaliana and Zn hyperaccumulators A. halleri and T. caerulescens have revealed higher expressions of MTP1, MTP8, and MTP11 in the hyperaccumulator species with enhanced Zn homeostasis (van de Mortel et al. 2006).
The MATE transporters also play crucial role in translocation of HMs (Al, Mn, and Zn). Dong et al. (2019) revealed the CcMATE4 and CcMATE34 (from Cajanus cajan) to be upregulated in the roots of pigeon pea under the Al, Mn, and Zn stresses. Ma et al. (2018) showed the GsMATE (from Glycine soja) overexpression to cause increased Al tolerance in A. thaliana.
19.4 About Phytoremediation
Phytoremediation includes several strategies for the detoxification of the HM-contaminated soils (Fig. 19.2). Various plant species used for different phytoremediation strategies for removal of the HM contaminants are presented in Table 19.1.
Schematic diagram illustrating different mechanisms of phytoremediation for removal of heavy metals (HMs) from the contaminated sites. Phytoextraction mediates the extraction and removal of HMs from contaminated soil and water, phytostabilization mediates the reduction of HM bioavailability through belowground immobilization, phytovolatilization mediates the conversion of toxic HMs into less-toxic forms and releases them as volatile compounds into atmosphere, and phytodegradation mediates breakdown of toxic HMs into less-toxic forms
19.4.1 Phytoextraction
Phytoextraction (or phytoaccumulation) is the method where plants are used to remove the HM from the polluted soil and water through their uptake and accumulation into the harvestable plant parts (Suman et al. 2018). In this process, plants absorb the contaminants from soil or water together with other necessary nutrients required for plants’ growth. The absorbed contaminants are translocated and accumulated in the aboveground plant tissues but are not destroyed (Rashid et al. 2014). Phytoextraction of HMs includes different steps: (1) HM mobilization in rhizosphere, (2) HM uptake by plant roots, (3) root-to-shoot translocation of HMs, and (4) vacuolar sequestration of HMs (Ali et al. 2013).
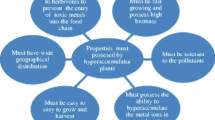
Phytoextraction is the most important phytoremediation procedure for HM removal from the contaminated soil. Selection of suitable plant species is crucial for the efficacious phytoextraction. The plant species should possess (1) extraordinary tolerance to the HM toxicity, (2) enhanced extractability and HM accumulation capacity in aboveground tissues, (3) high growth rate and high biomass, (4) extensive root and sufficient shoot system, (5) easily cultivable and environmental stress resistant, and (6) pathogen and pest resistance, herbivore repulsive to avoid HM flow into the food chain (Ali et al. 2013). Therefore, hyperaccumulator plants having higher HM accumulation ability in the aboveground tissues and higher biomass production are appropriate for the phytoremediation of HM-contaminated sites. A number of edible crops accumulate high quantity of HM. However, edible crops are not recommended for phytoremediation as the HMs accumulated in their edible parts may contaminate the food chain. Therefore, nonedible hyperaccumulators should be selected for safe and effective phytoremediation of HMs.
Phytoextraction technique is extensively employed to remove radioactive (Shahandeh and Hossner 2002) and metallic wastes (Kamal et al. 2004). Aquatic macrophytes like Centella asiatica and Eichhornia crassipes have been found to remove copper 99.6 and 97.3%, respectively (Mokhtar et al. 2011). Accumulation of HMs by the hyperaccumulators depends on the HM bioavailability within the rhizosphere, HM uptake rate by roots, proportion of fixed HM within the roots, rate of xylem loading/translocation to shoots, and cellular HM tolerance (Etim 2012).
Phytoextraction is performed with or without addition of chelate complexant for removal of HMs that remain sorbed to the solid soil components (Yan et al. 2020). Addition of chelating agents induces the formation of HM–chelate complexes preventing their sorption and precipitation. Thus the chelating agents maintain the bioavailability of HM for uptake by the mettalophytes. Chelating agents having strong affinity for the targeted HM enhance the phytoaccumulation ability of hyperaccumulators. However, the used chelate must be biodegradable for its rapid removal from the polluted site.
19.4.2 Phytostabilization
Phytostabilization is the method of phytoremediation where HM-tolerant plants are used to immobilize HMs belowground through their accumulation into plant roots (Mendez and Maier 2008). This process can also occur through precipitation of the HMs within the rhizosphere, adsorption onto the root surface, absorption, and vacuolar sequestration inside the root cells (Gerhardt et al. 2017). Phytostabilization decreases the bioavailability of the HMs by inhibiting their migration into the ecosystem and preventing their entry into the food chain. This process also serves as a filtration barrier against the root-to-shoot translocation of HM and is advantageous over phytoextraction as it does not require the disposal of the hazardous biomass (Lorestani et al. 2013).
Phytostabilization requires the selection of excessive HM-tolerant plant species (Yan et al. 2020). Plants should be easily maintainable under field conditions with fast growth rate and production of profuse biomass to cover the HM-contaminated site. Phytostabilization also requires dense rooting systems with increased root surface and depth for the stabilization of soil structure, and prevention of soil erosion through the HM immobilization. Improvement of the phytostabilization efficiency also requires the addition of the inorganic or organic amendments, which can improve the contaminated soil quality by enhancing the organic matter and essential nutrient contents consequently promoting plant colonization and water-holding capacity. This also alters the soil pH and redox state, thereby reducing the solubility and bioavailability of HM and also changing the HM speciation (Burges et al. 2018). For instance, application of Gliricidia sepium biomass as soil amendment elevated the phytostabilizing nature of Zea mays thereby remediating the Pb-contaminated soil (Muthusaravanan et al. 2018). Phytostabilization can also be promoted by the soil microbes including plant growth-promoting rhizobacteria (PGPR) and AM fungi. Dual application of silicon along with AM fungi has also been found to mitigate HM stress in crop plants (Basu and Kumar 2021b). These improve HM immobilization efficiency through production of chelators and adsorption of HM on cell walls, thereby stimulating the processes of precipitation (Ma et al. 2011). Madhaiyan et al. (2007) revealed HM-tolerant methylotrophic bacteria Burkholderia sp. and Magnaporthe oryzae to reduce Cd and Ni toxicity in tomato plants. Tamburini et al. (2017) examined the phytostabilization potential of strains belonging to Amycolatopsis, Novosphingobium, Pseudomonas, Streptomyces, and Variovorax, among which the Variovorax strain was found to be useful in the process of bioaugmentation in the mine areas, thereby promoting germination and plant growth.
19.4.3 Phytovolatilization
Phytovolatilization is the approach of phytoremediation where plants are used for uptake and conversion of toxic soil HMs into relatively less-toxic form subsequently releasing them into the atmosphere through transpiration as volatile compounds (Moreno et al. 2004). Detoxification of organic pollutants and HMs (As, Hg, and Se) is accomplished with this process (Mahar et al. 2016). Several studies revealed Chara canescens, Brassica juncea (Banuelos and Meek 1990), and aquatic plant Typha latifolia (LeDuc and Terry 2005) to be potential volatilizers of selenium (Se). Through the process of phytovolatilization, inorganic Se is converted into less-toxic volatile dimethyl selenide (DMSe) that can be dispersed into the air. Similarly, toxic Hg is converted to less-toxic volatile mercuric oxide and evaporated into the atmosphere (Bizily et al. 2000). Plant species T. latifolia has also been revealed to volatilize As, Cd, Co, Cr, Mn, Ni, and Zn (Varun et al. 2011). Phytovolatilization is advantageous over the other phytoremediation techniques as there is no need for the harvesting or disposal of the HM hyperaccumulating plants. Therefore, it is considered as a permanent solution for the HM removal as the volatilized products usually do not redeposit at the contaminated site.
19.4.4 Phytofiltration
Phytofiltration is the approach of phytoremediation where plants are used to remove HMs from contaminated groundwater or waste waters. This process can be of different types based on the plant parts used for the remediation practice—rhizofiltration (root), caulofiltration (shoot), and blastofiltration (seedling). Rhizofiltration includes the exudation from the roots that alters the rhizosphere pH leading to the HM precipitation on plant roots, thereby restricting the HMs to contaminate the underground water (Yan et al. 2020). During this process, HMs are adsorbed onto the root surface or absorbed by the roots. Therefore, the metallophytes used for this process contain dense root systems and huge biomass. They are initially grown hydroponically in clear water for developing the huge root system. Following the initial development, the plants are acclimatized to the HM-polluted environment by substituting the clear water with polluted water and subsequently transferred to the contaminated water for the HM removal. The hyperaccumulators are harvested and disposed after their roots become saturated with HMs.
Aquatic macrophytes like azolla, cattail, water hyacinth, poplar, and duckweed are usually used for remediation of the wetlands (Rezania et al. 2016). Arsenic-hyperaccumulating ferns Pteris vittata and P. cretica have been found to remove As from drinking water through phytofiltration. These plants have higher HM tolerance and HM accumulating capacity, rapid growth rate, and high biomass production. Several terrestrial plant species including B. juncea and Helianthus annuus have also been found to be used for rhizofiltration due to their longer and hairy root systems (Tome et al. 2008).
19.4.5 Phytodegradation
Phytodegradation is the approach of phytoremediation where plants are used to breakdown the toxic HMs to simpler less-toxic forms either through the plants’ metabolic process inside or the enzymes produced by plants (Muthusaravanan et al. 2018). This process facilitates the degradation of pesticides, chlorinated solvents, and several inorganic/organic compounds. Moderately hydrophobic organic pollutants including short-chain aliphatic hydrocarbons, chlorinated solvents, benzene, ethyl benzene, toluene, and xylene at shallow depths are efficiently removed by this process. Phytodegradation is affected by few factors, such as concentration of HMs present in the soil, HM uptake efficiency, and the amount of water present in the ground. Phytodegradation is mediated by a number of the enzymes like nitroreductase, nitrilase, dehalogenase, laccase, and peroxidase. Rajakaruna et al. (2006) have revealed the aquatic plant species Myriophyllum aquaticum to produce nitroreductase enzyme that facilitates the reduction of trinitrotoluene (TNT).
19.5 Phytoremediation of Different Heavy Metals
Metallophytes are the HM hyperaccumulators having the natural ability to accumulate large amounts of toxic HMs from the contaminated soil, making them exclusive to be exploited in phytoremediation to clean up the environment. The hyperaccumulators possess unique HM tolerance strategies than the non-hyperaccumulators, which make them suitable for the phytoremediation. The metallophytes have higher proficiency of HM uptake, root-to-shoot translocation, and detoxification. Abundant studies have been performed on metallophytes for understanding their strategies of HM tolerance. Approximately 450 plant species across 45 angiosperm families (e.g., Asterraceae, Brassicaceae, Euphorbiaceae, Fabaceae, Lamiaceae, and Scrophulariaceae) have been recognized as hyperaccumulators (Suman et al. 2018). Some metallophytes can accumulate more than two elements. For instance, Sedum alfredii can accumulate Cd, Pb, and Zn (Yan et al. 2020). Details of different heavy metal hyperaccumulating plants are summarized in Table 19.2.
19.5.1 Aluminum
Aluminum (Al) being one of the most abundant elements is very toxic for plants and animals. Chronic Al intoxication causes osteomalacia fractures, encephalopathy, chronic renal failure, Parkinsonism dementia, and Alzheimer’s disease in human (Exley 2016). It is also carcinogenic. Elevated mobile Al concentration is the main reason for phytotoxicity of acid soils resulting in the inhibition of plant growth, nutrient uptake, and productivity. The mechanisms of Al tolerance have been studied in barley, wheat, soybean, maize, and Arabidopsis, which include exudation of organic acids and H+ ions from roots and secretion of mucilage to immobilize Al in the rhizosphere (Kochian et al. 2015; Belimov et al. 2020). Internal Al detoxification in plants involves induction of antioxidant activities, efflux of Al from the root tissues, and vacuolar sequestration of Al.
Symbiotic microorganisms, including PGPR, play an important role in counteracting Al toxicity on plants. Inoculation of maize plants grown in acid soil with P-solubilizing Burkholderia sp. has been revealed to decrease the Al accumulation in roots, promoting root elongation, and thereby combating the Al toxicity (Arora et al. 2017). Negative effects of Al toxicity on nodule initiation and inhibition of nitrogen fixation have been reported in pea and soybean (Jaiswal et al. 2018; Basu and Kumar 2020b). Rhizobium sp. isolated from nodule of chick pea has been found to be able to bind Al3+ due to production of siderophores, suggesting capability of this bacterium to protect the plant against Al toxicity (Sujkowska-Rybkowska and Borucki 2015). On the other hand, Al-tolerant symbiotic AM fungi present in the acid soils have also been found to alleviate Al toxicity in plants (Seguel et al. 2013).
19.5.2 Arsenic
Arsenic (As), a major environmental and food chain contaminant, is a major concern since the last few decades (Zhao et al. 2010). Introduction of As in the food chain takes place by its excessive uptake from soil by crop plants or the irrigation of plants with As-contaminated water. The toxic metalloid has been reported to be carcinogenic even at low levels. Consumption of the As-contaminated food or groundwater over a long period leads to the As poisoning or the arsenicosis, which has become a major threat to the public health. Arsenic exposure affects different morphophysiological processes in plants leading to decrease in plant height, leaf number, biomass, photosynthetic activities, and productivity (Farooq et al. 2016). The As-induced ROS accumulation causes oxidation of lipids and proteins resulting in the cell death (Chen et al. 2017). Arsenic toxicity in the soil leads to straight head disease in rice plants (Rahman et al. 2008). It is therefore, a serious concern to take necessary steps to counteract the problem of As toxicity in plants.
Aquatic macrophytes, including Azolla pinnata, Hydrilla verticillata, and Lemna minor, have been shown to have competencies for removal of As from contaminated water (Mishra et al. 2008). Srivastava et al. (2014) have also revealed the aquatic macrophytes H. verticillata, L. minor, Ceratophyllum demersum, Wolffia globosa, and Eichhornia crassipes to be capable in As accumulation from the contaminated water. In this study, the combination of C. demersum and L. minor was found to have the highest potential for As removal than the plants used in other combinations or the single plants. Among ferns, Pteris longifolia, P. cretica, (Zhao et al. 2002) P. ryukyuensis, P. biaurita, P. quadriaurita (Srivastava et al. 2006), and Pityrogramma calomelanos (Francesconi et al. 2002) have been found to have greater potential for As hyperaccumulation from contaminated soil. Higher plants including Eclipta alba (Dwivedi et al. 2008), Isatis cappadocia (Karimi et al. 2009), and Sesuvium portulacastrum (Lokhande et al. 2011) have also been identified to be potential As hyperaccumulators.
19.5.3 Cadmium
Cadmium (Cd) is a tremendously toxic environmental pollutant causing lethal effects to animals and plants. It is classified as a human class I carcinogen. Chronic Cd poisoning due to prolonged oral Cd ingestion causes itai-itai disease in human (Genchi et al. 2020). In plants, Cd stress reduces growth, leaf area, dry matter, and yield (Shanying et al. 2017). It also affects photosynthesis and respiration, induces oxidative damage, and decreases nutrient uptake ability in plants. It is therefore, a serious concern to take necessary steps to remove Cd toxicity from the environment (Kapoor et al. 2021).
The process of phytoextraction of Cd has been mediated by several potential hyperaccumulating plant species, including Cassia alata, Celosia argentea, Kummerowia striata, Nicotiana tabacum, Momordica charantia, Solanum melonaena, Swietenia macrophylla, Salix mucronata, and Vigna unguiculata (Raza et al. 2020). Several other plant species like Abelmoschus manihot, Atriplex halimus, Brassica chinensis, B. juncea, B. napus, Glycine max, Lolium perenne, Macleaya cordata, Oryza sativa, Paspalum scrobiculatum, Petroselinum hortense, Quercus robur, Sedum alfredii, Solanum lycopersicum, S. tuberosum, and Triticum aestivum have also been shown the potential of Cd tolerance and phytoremediation through their enhanced antioxidant defense system. Few microorganisms like Aspergillus niger (fungus) (Ren et al. 2009), Aspergillus versicolor (Fazli et al. 2015), Pleurotus ostreatus (Kapahi and Sachdeva 2017), Pseudomonas aeruginosa, Streptomyces sp., Fomitopsis pinicola, and Bacillus sp. (Bagot et al. 2006) have also been reported to play a crucial role in removal of Cd from the contaminated soil. Salinity has been shown to be a key factor in the translocation of Cd from the roots to the shoots in Aster tripolium, Temnothorax smyrnensis, and Potamogeton pectinatus (Manousaki et al. 2008). Salinity increased the Cd concentration of the shoots as well as that of the whole plants.
19.5.4 Chromium
Chromium (Cr) is the most widespread toxic trace elements that adversely affect crop productivity throughout the world. The Cr contamination is caused by natural (weathering of rocks) or anthropogenic activities (used in various industries like chrome plating, alloys, paints, use of excess fertilizers). The Cr is found in many forms but Cr(0), Cr(III), and Cr(VI) are the most stable and common forms. Among these forms, the hexavalent chromium Cr(VI) is highly toxic to animals and plants. The Cr (VI) and its compounds have carcinogenic effects when inhaled or ingested. Being more soluble in water, Cr(VI) has greater availability for plants and it has higher ability of penetrating plant roots (Shanker et al. 2005).
Uptake of Cr(VI) in plants occurs through sulfur transporters (Kovacik et al. 2013). Toxic levels of Cr in the soil decrease plant height, roots and shoot biomass, chlorophyll content, transpiration rate, stomatal conductance, net photosynthesis, and water use efficiency of plants (Sharma et al. 2020). It also induces the ROS overproduction causing oxidative stress and damage to lipids and proteins. The Cr(VI) also causes alterations in nutrient assimilation, hormonal homeostasis, and genotoxicity in plants, thereby inhibiting plant growth and development. Furthermore, Cr(VI) causes reduced tissue density of roots due to enhanced cellular accumulation of Cr which leads to the cell death.
Plants develop antioxidant defense system to alleviate Cr toxicity through effective ROS detoxification (Maiti et al. 2012). In a recent study, Cr toxicity has been revealed to be ameliorated by increased superoxide dismutase (SOD), peroxidase (POD), catalase (CAT), and ascorbate peroxidase (APX) activities in Brassica napus L. (Zaheer et al. 2020). Increased glutathione production has also been reported to detoxify Cr toxicity in Oryza sativa, Actinidia deliciosa, Pistia stratiotes, B. napus, Salvinia natans, S. rotundifolia, and S. minima (Shahid et al. 2017).
19.5.5 Copper
Copper (Cu) is one of the important nutrients for plants and humans. The Cu toxicity has detrimental effect on human health leading to liver disorder and Alzheimer’s disease. In plants, Cu toxicity causes chlorosis and necrosis, leaf discoloration stunting, and root growth inhibition (Kumar et al. 2021b). The Cu toxicity also has detrimental effects on morphophysiology and nutrient uptake in plants. High Cu concentration induces DNA damage, decreased photosynthetic rate, loss of cell membrane integrity, decreased enzyme activity, and respiration leading to growth reduction in plants.
Several wild plant species having Cu phytoremediation potential growing in the mine polluted areas include Hypericum perforatum, Phleum pretense, Thymus kotschyanus, and Teucrium orientale (Ghazaryan et al. 2019). In a study, Lu et al. (2018) have shown aquatic plant species Acorus calamus, Arundina graminifolia, Eichhornia crassipes, Echinodorus major, Juncus effusus, Nymphaea tetragona, Pistia stratiotes, and Sagittaria sagittifolia to exhibit exceptional potential for remediation of Cu pollution. Another recent study by Covre et al. (2020) revealed Cedrela fissilis and Khaya ivorensis to have good potential for Cu accumulation. Another plant species Vetiveria zizanioides has been reported to have potential for Cu phytostabilization in Cu-mine tailing (Chu et al. 2020).
19.5.6 Lead
Lead (Pb) is a persistent toxic pollutant of concern that is produced due to increasing anthropogenic pressure on the environment. The Pb is absorbed by the plant roots via the Ca2+-permeable channels or the apoplastic pathway. Excessive Pb accumulation in plants directly or indirectly impairs the morphophysiological and biochemical functions, thereby inducing various deleterious effects. The Pb toxicity causes swollen, bent, short, and stubby roots with increased number of secondary roots per unit root length. Severe Pb toxicity in plants results in growth inhibition with fewer, smaller, and more brittle leaves having dark purplish abaxial surfaces. Plant growth retardation from Pb exposure may be attributed to nutrient metabolic disturbances and disturbed photosynthesis. Exposure of Allium sativum roots to toxic concentration of Pb leads to mitochondrial swelling, loss of cristae, vacuolization of endoplasmic reticulum and dictyosomes, and injured plasma membrane. In most cases, the toxic effect of Pb on plant growth is time- and dose-dependent. Moreover, the effects of Pb toxicity vary with plant species, i.e., hyperaccumulators naturally tolerate more Pb toxicity than the sensitive plants (Pourrut et al. 2011).
The Pb adsorption onto roots has been documented to occur in several plant species like Vigna unguiculata, Festuca rubra, Brassica juncea, Lactuca sativa, and Funaria hygrometrica. For most plant species, the majority of absorbed Pb (approximately 95% or more) is accumulated in the roots, and only a small fraction is translocated to the aerial plant parts, as reported in Avicennia marina, Phaseolus vulgaris, Pisum sativum, Vicia faba, Vigna unguiculata, Lathyrus sativus, Nicotiana tabacum, and Zea mays. Several hyperaccumulator plant species, such as Brassica pekinensis and Pelargonium sp., are capable of translocating higher concentrations of Pb to aerial plant parts, without incurring damage to their basic metabolic functions.
19.5.7 Lithium
The essentiality and toxicity of lithium (Li) on higher plants are not clear till date. Previous studies indicated Li salts to be highly toxic inducing the formation of necrosis in plants. It also causes considerable reduction in plant growth. Other Li toxic effects include altered rhythmic movement of petals and disrupted pollen development. Plant root is the first organ that comes in contact with Li in soil, and Li in excess alters gravitropic growth of maize roots. Furthermore, Li toxicity affects cold-induced dephosphorylation of microtubules in mesophyll cells of spinach. In the Li-rich soils, damage of root tips and chlorotic and necrotic spots on leaves have been observed in corn. Nonetheless, different plant species showed plastic behavior in sensitivity and tolerance to and noted that plants belonging to the families Asteraceae and Solanaceae show tolerance against Li toxicity and sustain normal plant growth.
Some plants, notably Cirsium arvense and Solanum dulcamara, accumulate higher concentration of Li. Halophilic plants like Apocynum pictum, Carduus arvense, and Holoschoenus vulgaris may reach up to 99.6–226.4 g kg−1 Li contents. Apocynum venetum is a potentially rich target of Li biofortification owing to its ability to accumulate Li in natural habitat. However, the medicinal effects (existence of various flavonoid compounds in leaves) of A. venetum might also be attributed to the existence of high level of Li and evaluate the feasibility of A. venetum for the Li bio-enrichment (Jiang et al. 2019).
19.5.8 Mercury
Mercury (Hg) is a naturally occurring persistent environmental pollutant generated from minings, petrochemicals, paintings, industries and agricultural sources like fertilizers, fungicidal sprays, and bioaccumulated in fish, animals, and human beings. Severe Hg poisoning (methylmercury) in human causes neurological disease known as Minamata or Chisso-Minamata (Yorifuji and Tsuda 2014). The Hg also affects growth and productivity of different plant species. Sahu et al. (2012) have shown the Hg stress to limit plant growth and nutrition and cause oxidative damage in wheat. High concentration soil Hg affects the roots of Aeschynomene fluminensis and Polygonum acuminatum (Mariano et al. 2020). The Hg contamination reduces the germination rate, stem height, and fruit yield and causes chlorosis in tomatoes (Shekar et al. 2011). Likewise, in rice, Hg stress impedes the tiller and panicle formation, leading to the decrease in stem height and yield (Basri et al. 2020). Chen and Yang (2012) showed the Hg to interfere with the electron transport chain in the chloroplasts and mitochondria consequently, affecting the photosynthesis and oxidative metabolism in plants. It also reduces water uptake in plants by hindering the activities of the aquaporins.
A number of plant species, including Zea mays, Ceratophyllum demersum, Anodonta grandix, Victoria amazonica, Sphagnum girgensohnii, Convolvulus sp., Cyrtomium macrophyllum, and Eichhornia crassipes have been reported to be the hyperaccumulators of Hg (Kumar et al. 2017). The leaf tissue of Cyrtomium macrophyllum shows high resistance to Hg stress (Xun et al. 2017). In a field study conducted by Fernandez et al. (2017), some native plant species (Festuca rubra L., Leontodon taraxacoides, Equisetum telmateia) were used for the phytoextraction of Hg from the mining area, where higher concentrations Hg were accumulated mainly in the leaves of the plants.
19.5.9 Zinc
Zinc (Zn) is an important component of thousands of proteins and is essential for mineral nutrition. However, increased concentration of Zn can become toxic in both plants and animals. Chronic ingestion of Zn leads to sideroblastic anemia, granulocytopenia, and myelodysplastic syndrome in human (Irving et al. 2003). In plants, Zn toxicity causes stunted plant growth, defective chlorophyll biosynthesis, chloroplast degradation, reduced Mg, Mn, and P uptake, and reduced yields (Broadley et al. 2007). Crops differ in their susceptibility to Zn toxicity. Dicots are more sensitive to the Zn toxicity in acidic soils; whereas, Graminaceous plants show more sensitivity in alkaline soil.
Numerous metallophytes belong to the families Amaranthaceae, Brassicaceae, Caryophyllaceae, Lamiaceae, Rubiaceae, Polygonaceae, and Poaceae are known to be the Zn hyperaccumulators. The Zn hypertolerance has been reported in Agrostis stolonifera, A. capillaris, Arabidopsis halleri, A. arenosa, Arenaria patula, Avicenna marina, Betula pendula, Mimulus guttatus, Mirabilis jalapa, Silene vulgaris, S. dioica, Thlaspi alpestre, T. caerulescens, and Thlaspiceras oxyceras. Some of the Zn hyperaccumulator species including Acer pseudoplatanus, Biscutella laevigata, Dianthus sp., Festuca rubra, Galium mollugo, Minuartia verna, Polycarpaea synandra, and Rumex acetosa accumulate up to 3000 μg Zn g−1 DW in their shoots.
19.6 Improvement of Phytoremediation Ability of Plants
Effective phytoremediation requires the improvement of certain traits and minimization of limitations to enhance the plants’ ability for the HM removal from the environment. To improve the growth rate and biomass of the hyperaccumulator plant species or to introduce the hyperaccumulation traits, traditional plant breeding or genetic engineering may be employed (DalCorso et al. 2019).
Traditional plant breeding includes somatic hybridization technique to transfer the HM hyperaccumulation trait to the plants having high biomass. Protoplasts isolated from the Zn hyperaccumulator T. caerulescens and higher biomass producing B. napus were fused using the electrofusion (Brewer et al. 1999). The somatic hybrids showed ability to accumulate enhanced Cd and Zn. Likewise, sunflower giant mutant has been developed by using chemical mutagen ethyl methanesulfonate (EMS). The mutants showed increased ability for extraction of Cd, Pb, and Zn (7.5, 8.2, and 9.2 times more accumulation, respectively, than control plants) (Nehnevajova et al. 2007).
Genetic engineering has also been proved to be a prospective method for enhancing the phytoremediation abilities of the plants (Sarma et al. 2021; Sonowal et al. 2022). This technique takes less time to introduce the desirable traits for phytoremediation in plants than the traditional breeding. Sexually incompatible plant species can also be improved through the genetic engineering by transferring the desirable genes from the HM hyperaccumulators. Improvement of HM removal capacity in plants can be achieved by overexpressing the candidate genes for the HM uptake (ZIP, HMA, MATE, MTP), translocation, and sequestration in the hyperaccumulators (Das et al. 2016). Chelators improve the bioavailability of HMs by acting as metal-binding ligands. Therefore, genes encoding the natural chelators can also be overexpressed for the improvement of the HM uptake and translocation in plants (Yan et al. 2020).
19.7 Conclusion
HMs are environmental and food chain contaminants posing serious threat to sustainable agricultural production. Therefore, alleviation of the HM contamination from the soil and water has become an essential requirement to encounter the food security. Phytoremediation has been established as a novel and promising technique to clean-up the HM-contaminated soil and water. Application of HM hyperaccumulating metallophytes is the most candid tactic for the phytoremediation. Additionally, application of genetic engineering in improvement of the metallophytes’ performance may further be advantageous for the efficacious phytoremediation. Comprehensive understanding of the physiological as well as the molecular mechanisms of the HM uptake, translocation, and detoxification in plants may be beneficial in improving the phytoremediation potential of the metallophytes through genetic engineering.
References
Abdel Latef AAH, Zaid A, Abo-Baker AA, Salem W, Abu Alhmad MF (2020) Mitigation of copper stress in maize by inoculation with Paenibacillus polymyxa and Bacillus circulans. Plants (Basel) 9(11):1513
Aborode FA, Raab A, Voigt M, Costa LM, Krupp EM, Feldmann J (2016) The importance of glutathione and phytochelatins on the selenite and arsenate detoxification in Arabidopsis thaliana. J Environ Sci 49:150–161
Ali H, Khan E, Sajad MA (2013) Phytoremediation of heavy metals—concepts and applications. Chemosphere 91:869–881
Anjum SA, Tanveer M, Hussain S, Ashraf U, Khan I, Wang L (2017) Alteration in growth, leaf gas exchange, and photosynthetic pigments of maize plants under combined cadmium and arsenic stress. Water Air Soil Pollut 228:13
Arao T, Makino T, Kawasaki A, Akahane I, Kiho N (2018) Effect of air temperature after heading of rice on the arsenic concentration of grain. Soil Sci Plant Nutr 64(3):433–437
Arias JA, Peralta-Videa JR, Ellzey JT, Viveros MN, Ren M, Mokgalaka-Matlala NS, Castillo-Michel H, Gardea-Torresdey JL (2010) Plant growth and metal distribution in tissues of Prosopis juliflora-velutina grown on chromium contaminated soil in the presence of Glomus deserticola. Environ Sci Technol 44(19):7272–7279
Arif N, Yadav V, Singh S, Singh S, Ahmad P, Mishra RK, Sharma S, Tripathi DK, Dubey NK, Chauhan DK (2016) Influence of high and low levels of plant-beneficial heavy metal ions on plant growth and development. Front Environ Sci 4:69
Arora P, Singh G, Tiwari A (2017) Effect of microbial inoculation in combating the aluminium toxicity effect on growth of Zea mays. Cell Mol Biol (Noisy-le-Grand) 63:79–82
Assuncao AG, Da Costa MP, De Folter S, Vooijs R, Schat H, Aarts MGM (2001) Elevated expression of metal transporter genes in three accessions of the metal hyperaccumulator Thlaspi caerulescens. Plant Cell Environ 24:217–226
Bagot D, Lebeau T, Jezequel K (2006) Microorganisms for remediation of cadmium-contaminated soils. Environ Chem Lett 4:207–211
Banuelos G, Meek D (1990) Accumulation of selenium in plants grown on selenium-treated soil. J Environ Qual 19:772–777
Basri, Sakakibara M, Sera K (2020) Mercury in soil and forage plants from artisanal and small-scale gold mining in the bombana area, Indonesia. Toxics 8:15
Bastow EL, Garcia De La Torre VS, Maclean AE, Green RT, Merlot S, Thomine S, Balk J (2018) Vacuolar iron stores gated by NRAMP3 and NRAMP4 are the primary source of iron in germinating seeds. Plant Physiol 177:1267–1276
Basu S, Kumar G (2020a) Stress signalling in the phytomicrobiome: breadth and potential. In: Kumar M, Kumar V, Prasad R (eds) Phyto-Microbiome in stress regulation. environmental and microbial biotechnology. Springer, Singapore, pp 245–268
Basu S, Kumar G (2020b) Nitrogen fixation in a legume-rhizobium symbiosis: the roots of a success story. In: Varma A, Tripathi S, Prasad R (eds) Plant microbe symbiosis. Springer, Singapore, pp 35–53
Basu S, Kumar G (2021a) Plant microbe interaction for changing endophytic colonization to improve plant productivity. In: Verma JP, Macdonald CA, Gupta VK, Podile AR (eds) New and future developments in microbial biotechnology and bioengineering. Elsevier, Amsterdam, pp 137–147
Basu S, Kumar G (2021b) Exploring the significant contribution of silicon in regulation of cellular redox homeostasis for conferring stress tolerance in plants. Plant Physiol Biochem 166:393–404
Basu S, Giri RK, Benazir I, Kumar S, Rajwanshi R, Dwivedi SK, Kumar G (2017) Comprehensive physiological analyses and reactive oxygen species profiling in drought tolerant rice genotypes under salinity stress. Physiol Mol Biol Plants 23:837–850
Basu S, Kumar G, Kumari N, Kumari S, Shekhar S, Kumar S, Rajwanshi R (2020) Reactive oxygen species and reactive nitrogen species induce lysigenous aerenchyma formation through programmed cell death in rice roots under submergence. Environ Exp Bot 177:104118
Basu S, Kumari S, Kumar P, Kumar G, Rajwanshi R (2021a) Redox imbalance impedes photosynthetic activity in rice by disrupting cellular membrane integrity and induces programmed cell death under submergence. Physiol Plant 172(3):1764–1778
Basu S, Kumari S, Kumar A, Shahid R, Kumar S, Kumar G (2021b) Nitro-oxidative stress induces the formation of roots' cortical aerenchyma in rice under osmotic stress. Physiol Plant 172(2):963–975
Belimov AA, Shaposhnikov AI, Syrova DS, Kichko AA, Guro PV, Yuzikhin OS, Azarova TS, Sazanova AL, Sekste EA, Litvinskiy VA, Nosikov VV, Zavalin AA, Andronov EE, Safronova VI (2020) The role of symbiotic microorganisms, nutrient uptake and rhizosphere bacterial community in response of pea (Pisum sativum L.) genotypes to elevated Al concentrations in soil. Plan Theory 9(12):1–25
Bizily SP, Rugh CL, Meagher RB (2000) Phytodetoxification of hazardous organomercurials by genetically engineered plants. Nat Biotechnol 18:213–217
Brewer EP, Saunders JA, Angle JS, Chaney RL, Mcintosh MS (1999) Somatic hybridization between the zinc accumulator Thlaspi caerulescens and Brassica napus. Theor Appl Genet 99:761–771
Broadley MR, White PJ, Hammond JP, Zelko I, Lux A (2007) Zinc in plants. New Phytol 173(4):677–702
Burges A, Alkorta I, Epelde L, Garbisu C (2018) From phytoremediation of soil contaminants to phytomanagement of ecosystem services in metal contaminated sites. Int J Phytoremediation 20:384–397
Cailliatte R, Lapeyre B, Briat JF, Mari S, Curie C (2009) The NRAMP6 metal transporter contributes to cadmium toxicity. Biochem J 422:217–228
Cailliatte R, Schikora A, Briat JF, Mari S, Curie C (2010) High-affinity manganese uptake by the metal transporter NRAMP1 is essential for Arabidopsis growth in low manganese conditions. Plant Cell 22:904–917
Chen J, Yang ZM (2012) Mercury toxicity, molecular response and tolerance in higher plants. Biometals 25(5):847–857
Chen Y, Han Y-H, Cao Y, Zhu Y-G, Rathinasabapathi B, Ma LQ (2017) Arsenic transport in rice and biological solutions to reduce arsenic risk from rice. Front Plant Sci 8:268
Choudhary SP, Kanwar M, Bhardwaj R, Yu J-Q, Tran L-SP (2012) Chromium stress mitigation by polyaminebrassinosteroid application involves phytohormonal and physiological strategies in Raphanus sativus L. PLoS One 7(3):e33210
Chu Z, Wang X, Wang Y, Zha F, Dong Z, Fan T, Xu X (2020) Influence of coal gangue aided phytostabilization on metal availability and mobility in copper mine tailings. Environ Earth Sci 79(3):68
Ci X-K, Liu H-L, Hao Y-B, Zhang J-W, Liu P, Dong S-T (2012) Arsenic distribution, species, and its effect on maize growth treated with arsenate. J Integr Agric 11(3):416–423
Cieslinski G, Van Rees K, Szmigielska A, Krishnamurti G, Huang P (1998) Low-molecular-weight organic acids in rhizosphere soils of durum wheat and their effect on cadmium bioaccumulation. Plant and Soil 203:109–117
Covre WP, da Silveira Pereira WV, Goncalves DAM, Teixeira OMM, do Amarante CB, Fernandes AR (2020) Phytoremediation potential of Khaya ivorensis and Cedrela fissilis in copper contaminated soil. J Environ Manage 268:110733
Dai B, Chen C, Liu Y, Liu L, Qaseem MF, Wang J, Li H, Wu A-M (2020) Physiological, biochemical, and transcriptomic responses of Neolamarckia cadamba to aluminum stress. Int J Mol Sci 21(24):9624
DalCorso G, Fasani E, Manara A, Visioli G, Furini A (2019) Heavy metal pollutions: state of the art and innovation in phytoremediation. Int J Mol Sci 20:1–17
Dalvi AA, Bhalerao SA (2013) Response of plants towards heavy metal toxicity: an overview of avoidance, tolerance and uptake mechanism. Ann Plant Sci 2:362–368
Das N, Bhattacharya S, Maiti MK (2016) Enhanced cadmium accumulation and tolerance in transgenic tobacco overexpressing rice metal tolerance protein gene OsMTP1 is promising for phytoremediation. Plant Physiol Biochem 105:297–309
de Souza Costa ET, Guilherme LR, de Melo EE, Ribeiro BT, Dos Santos B, Inacio E, da Costa Severiano E, Faquin V, Hale BA (2012) Assessing the tolerance of castor bean to Cd and Pb for phytoremediation purposes. Biol Trace Elem Res 145(1):93–100
Dheeba B, Sampathkumar P, Kannan K (2014) growth, chromium accumulation potential and biochemical changes of Vigna radiata and Zea mays grown with effective microbes. Proc Natl Acad Sci, India, Sect B Biol Sci 84:381–387
Dong B, Niu L, Meng D, Song Z, Wang L, Jian Y, Fan X, Dong M, Yang Q, Fu Y (2019) Genome-wide analysis of MATE transporters and response to metal stress in Cajanus cajan. J Plant Interact 14(1):265–275
Dwivedi S, Srivastava S, Mishra S, Dixit B, Kumar A, Tripathi RD (2008) Screening of native plants and algae growing on fly-ash affected areas near National Thermal Power Corporation, Tanda, Uttar Pradesh, India for accumulation of toxic heavy metals. J Hazard Mater 158:359–365
Etim EE (2012) Phytoremediation and its mechanisms: a review. Int J Environ Bioenergy 2:120–136
Exley C (2016) The toxicity of aluminium in humans. Morphologie 100(329):51–55
Farooq MA, Islam F, Ali B, Najeeb U, Mao B, Gill RA, Yan G, Siddique KHM, Zhou W (2016) Arsenic toxicity in plants: cellular and molecular mechanisms of its transport and metabolism. Environ Exp Bot 132:42–52
Fazli MM, Soleimani N, Mehrasbi M, Darabian S, Mohammadi J, Ramazani A (2015) Highly cadmium tolerant fungi: their tolerance and removal potential. J Environ Health Sci Eng 13:19
Fernandez S, Poschenrieder C, Marceno C, Gallego JR, Jimenez-Gamez D, Bueno A, Afif E (2017) Phytoremediation capability of native plant species living on Pb-Zn and hg-as mining wastes in the Cantabrian range, north of Spain. J Geochem Explor 174:10–20
Francesconi K, Visoottiviseth P, Sridokchan W, Goessler W (2002) Arsenic species in an arsenic hyperaccumulating fern, Pityrogramma calomelanos: a potential phytoremediator of arsenic-contaminated soils. Sci Total Environ 284:27–35
Genchi G, Sinicropi MS, Lauria G, Carocci A, Catalano A (2020) The effects of cadmium toxicity. Int J Environ Res Publ Health 17:3782
Gerhardt KE, Gerwing PD, Greenberg BM (2017) Opinion: taking phytoremediation from proven technology to accepted practice. Plant Sci 256:170–185
Ghazaryan K, Movsesyan H, Ghazaryan N, Watts BA (2019) Copper phytoremediation potential of wild plant species growing in the mine polluted areas of Armenia. Environ Pollut 249:491–501
Guerinot ML (2000) The ZIP family of metal transporters. Biochim Biophys Acta 1465:190–198
Hanikenne M, Baurain D (2014) Origin and evolution of metal P-type ATPases in Plantae (Archaeplastida). Front Plant Sci 4:544
Irving JA, Mattman A, Lockitch G, Farrell K, Wadsworth LD (2003) Element of caution: a case of reversible cytopenias associated with excessive zinc supplementation. CMAJ 169(2):129–131
Ismael MA, Elyamine AM, Moussa MG, Cai M, Zhao X, Hu C (2019) Cadmium in plants: uptake, toxicity, and its interactions with selenium fertilizers. Metallomics 11(2):255–277
Jaiswal SK, Naamala J, Dakora FD (2018) Nature and mechanisms of aluminium toxicity, tolerance and amelioration in symbiotic legumes and rhizobia. Biol Fertil Soils 54:309–318
Jiang L, Wang L, Tanveer M, Tian C (2019) Lithium biofortification of medicinal tea Apocynum venetum. Sci Rep 9:8182
Kamal M, Ghaly AE, Mahmoud N, CoteCote R (2004) Phytoaccumulation of heavy metals by aquatic plants. Environ Int 29:1029–1039
Kamal S, Prasad R, Varma A (2010) Soil microbial diversity in relation to heavy metals. In: Sherameti I, Varma A (eds) Soil heavy metals, vol 19. Springer-Verlag, Berlin, Heidelberg, pp 31–64
Kapahi M, Sachdeva S (2017) Mycoremediation potential of Pleurotus species for heavy metals: a review. Bioresour Bioprocess 4:32
Kapoor D, Singh S, Ramamurthy PC, Jan S, Bhardwaj S, Gill SS, Prasad R, Singh J (2021) Molecular consequences of cadmium toxicity and its regulatory networks in plants. Plant Gene. https://doi.org/10.1016/j.plgene.2021.100342
Karimi N, Ghaderian SM, Raab A, Feldmann J, Meharg AA (2009) An arsenic-accumulating, hypertolerant Brassica, Isatis capadoica. New Phytol 184:41–47
Karthik C, Oves M, Thangabalu R, Sharma R, Santhosh SB, Arulselvi PI (2016) Cellulosimicrobium funkei-like enhances the growth of Phaseolus vulgaris by modulating oxidative damage under Chromium(VI) toxicity. J Adv Res 7(6):839–850
Kidwai M, Dhar YV, Gautam N, Tiwari M, Ahmad IZ, Asif MH, Chakrabarty D (2019) Oryza sativa class III peroxidase (OsPRX38) overexpression in Arabidopsis thaliana reduces arsenic accumulation due to apoplastic lignification. J Hazard Mater 362:383–393
Kochian LV, Pineros MA, Liu J, Magalhaes JV (2015) Plant adaptation to acid soils: the molecular basis for crop aluminum resistance. Annu Rev Plant Biol 66:571–598
Komal T, Mustafa M, Ali Z, Kazi AG (2015) Heavy metal uptake and transport in plants. In: Sherameti I, Varma A (eds) Heavy metal contamination of soils. Springer, Cham
Kovacik J, Babula P, Klejdus B (2013) Chromium uptake and consequences for metabolism and oxidative stress in chamomile plants. J Agric Food Chem 61:7864–7873
Kovacik J, Babula P, Hedbavny J, Klejdus B (2014) Hexavalent chromium damages chamomile plants by alteration of antioxidants and its uptake is prevented by calcium. J Hazard Mater 273:110–117
Kumar G, Kushwaha HR, Panjabi-Sabharwal V, Kumari S, Joshi R, Karan R, Mittal S, Singla-Pareek SL, Pareek A (2012) Clustered metallothionein genes are co-regulated in rice and ectopic expression of OsMT1e-P confers multiple abiotic stress tolerance in tobacco via ROS scavenging. BMC Plant Biol 12:107
Kumar B, Smita K, Flores LC (2017) Plant mediated detoxification of mercury and lead. Arab J Chem 10(2):S2335–S2342
Kumar A, Basu S, Kumar G (2021a) Evaluating the effect of seed-priming for improving arsenic tolerance in rice. J Plant Biochem Biotechnol. https://doi.org/10.1007/s13562-021-00666-0
Kumar V, Pandit S, Sidhu GPS, Sharma A, Khanna K, Kaur P, Bali AS, Setia R (2021b) Copper bioavailability, uptake, toxicity and tolerance in plants: a comprehensive review. Chemosphere 262:127810
LeDuc DL, Terry N (2005) Phytoremediation of toxic trace elements in soil and water. J Ind Microbiol Biotechnol 32:514–520
Liu H, Zhao H, Wu L, Liu A, Zhao F-J, Xu W (2017) Heavy metal ATPase 3 (HMA3) confers cadmium hypertolerance on the cadmium/zinc hyperaccumulator Sedum plumbizincicola. New Phytol 215(2):687–698
Lokhande VH, Srivastava AK, Srivastava S, Nikam TD, Suprasanna P (2011) Regulated alterations in redox and energetic status are the key mediators of salinity tolerance in the halophyte Sesuvium portulacastrum (L.) L. Plant Growth Regul 65:287–298
Lorestani B, Yousefi N, Cheraghi M, Farmany A (2013) Phytoextraction and phytostabilization potential of plants grown in the vicinity of heavy metal-contaminated soils: a case study at an industrial town site. Environ Monit Assess 185:10217–10223
Lu D, Huang Q, Deng C, Zheng Y (2018) Phytoremediation of copper pollution by eight aquatic plants. Pol J Environ Stud 2(1):175–181
Ma Y, Prasad M, Rajkumar M, Freitas H (2011) Plant growth promoting rhizobacteria and endophytes accelerate phytoremediation of metalliferous soils. Biotechnol Adv 29:248–258
Ma J, Lv C, Xu M, Chen G, Lv C, Gao Z (2016) Photosynthesis performance, antioxidant enzymes, and ultrastructural analyses of rice seedlings under chromium stress. Environ Sci Pollut Res Int 23(2):1768–1778
Ma Q, Yi R, Li L, Liang Z, Zeng T, Zhang Y, Huang H, Zhang X, Yin X, Cai Z, Mu Y, Cheng Y, Zeng Q, Li X, Nian H (2018) GsMATE encoding a multidrug and toxic compound extrusion transporter enhances aluminum tolerance in Arabidopsis thaliana. BMC Plant Biol 18:212
Madhaiyan M, Poonguzhali S, Sa T (2007) Metal tolerating methylotrophic bacteria reduces nickel and cadmium toxicity and promotes plant growth of tomato (Lycopersicon esculentum L.). Chemosphere 69:220–228
Mahar A, Wang P, Ali A, Awasthi MK, Lahori AH, Wang Q, Li R, Zhang Z (2016) Challenges and opportunities in the phytoremediation of heavy metals contaminated soils: a review. Ecotox Environ Safe 126:111–121
Maiti S, Ghosh N, Mandal C, Das K, Dey N, Adak MK (2012) Responses of the maize plant to chromium stress with reference to antioxidation activity. Braz J Plant Phsiol 24(3):203–212
Manousaki E, Kalogerakis N (2009) Phytoextraction of Pb and Cd by the Mediterranean saltbush (Atriplex halimus L.): metal uptake in relation to salinity. Environ Sci Pollut Res Int 16(7):844–854
Manousaki E, Kadukova J, Papadantonakis N, Kalogerakis N (2008) Phytoextraction and phytoexcretion of cd by the leaves of Tamarix smyrnensis growing on contaminated non-saline and saline soils. Environ Res 106(3):326–332
Mariano C, Mello IS, Barros BM, da Silva GF, Terezo AJ, Soares MA (2020) Mercury alters the rhizobacterial community in Brazilian wetlands and it can be bioremediated by the plant-bacteria association. Environ Sci Pollut Res 27:13550–13564
Marques APGC, Rangel AOSS, Castro PML (2009) Remediation of heavy metal contaminated soils: phytoremediation as a potentially promising clean-up technology. Crit Rev Environ Sci Technol 39(8):622–654
Mascher R, Lippmann B, Holzinger S, Bergmann H (2002) Arsenate toxicity: effects on oxidative stress response molecules and enzymes in red clover plants. Plant Sci 163(5):961–969
Mendez MO, Maier RM (2008) Phytostabilization of mine tailings in arid and semiarid environments—an emerging remediation technology. Environ Health Perspect 116:278–283
Migocka M, Kosieradzka A, Papierniak A, Maciaszczyk-Dziubinska E, Posyniak E, Garbiec A, Filleur S (2014) Two metal-tolerance proteins, MTP1 and MTP4, are involved in Zn homeostasis and cd sequestration in cucumber cells. J Exp Bot 66(3):1001–1015
Mishra B, McDonald LM, Roy M, Lanzirotti A, Myneni SCB (2020) Uptake and speciation of zinc in edible plants grown in smelter contaminated soils. PLoS One 15(4):e0226180
Mishra VK, Upadhyay AR, Pathak V, Tripathi BD (2008) Phytoremediation of mercury and arsenic from tropical opencast coalmine effluent through naturally occurring aquatic macrophytes. Water Air Soil Pollut 192:303–314
Mokhtar H, Morad N, Fizri FFA (2011) Phytoaccumulation of copper from aqueous solutions using Eichhornia crassipes and Centella asiatica. Int J Environ Sci Dev 2:205–210
Moreno FN, Anderson CWN, Stewart RB, Robinson BH (2004) Mercury phytoextraction and phytovolatilisation from hg-contaminated artisanal mine sites. In: Phytoremediation of mercury-contaminated mine wastes, pp 147–159
Muthusaravanan S, Sivarajasekar N, Vivek JS, Paramasivan T, Naushad M, Prakashmaran J, Gayathri V, Al-Duaij OK (2018) Phytoremediation of heavy metals: mechanisms, methods and enhancements. Environ Chem Lett 16:1339–1359
Nagajyoti PC, Lee KD, Sreekanth TVM (2010) Heavy metals, occurrence and toxicity for plants: a review. Environ Chem Lett 8:199–216
Nehnevajova E, Herzig R, Federer G, Erismann K-H, Schwitzguebel J-P (2007) Chemical mutagenesis—a promising technique to increase metal concentration and extraction in sunflowers. Int J Phytoremediation 9:149–165
Nevo Y, Nelson N (2006) The NRAMP family of metal-ion transporters. BBA-Mol Cell Res 1763(7):609–620
Ojuederie OM, Babalola OO (2017) Microbial and plant-assisted bioremediation of heavy metal polluted environments: a review. Int J Env Res Publ Health 14:1–26
Paiva LB, Correa SF, Santa Catarina C, Floh EIS, Silva MGD, Vitoria AP (2014) Ecophysiological and biochemical parameters for assessing Cr+6 stress conditions in Pterogyne nitens Tul.: New and usual methods for the management and restoration of degraded areas. Environ Eng Manag J 13:3073–3081
Parrotta L, Guerriero G, Sergeant K, Cai G, Hausman J-F (2015) Target or barrier? The cell wall of early- and later-diverging plants vs cadmium toxicity: differences in the response mechanisms. Front Plant Sci 6:133
Peng HY, Yang XE, Jiang LY (2005) Copper phytoavailability and uptake by Elsholtzi asplendens from contaminated soil as affected by soil amendments. J Environ Sci Health 40:839–856
Pourrut B, Shahid M, Dumat C, Winterton P, Pinelli E (2011) Lead uptake, toxicity, and detoxification in plants. Rev Environ Contam Toxicol 213:113–136
Rahman MA, Hasegawa H, Rahman MM, Miah MAM, Tasmin A (2008) Straight head disease of rice (Oryza sativa L.) induced by arsenic toxicity. Environ Exp Bot 62:54–59
Rai V, Vajpayee P, Singh SN, Mehrotra S (2004) Effect of chromium accumulation on photosynthetic pigments, oxidative stress defense system, nitrate reduction, proline level and eugenol content of Ocimum tenuiflorum L. Plant Sci 167(5):1159–1169
Rajakaruna N, Tompkins KM, Pavicevic PG (2006) Phytoremediation: an affordable green technology for the clean-up of metalcontaminated sites in Sri Lanka. Cey J Sci (Bio Sci) 35:25–39
Rashid A, Mahmood T, Mehmood F, Khalid A, Saba B, Batool A, Riaz A (2014) Phytoaccumulation, competitive adsorption and evaluation of chelators-metal interaction in lettuce plant. Environ Eng Manag J 13:2583–2592
Raza A, Habib M, Kakavand SN, Zahid Z, Zahra N, Sharif R, Hasanuzzaman M (2020) Phytoremediation of cadmium: physiological, biochemical, and molecular mechanisms. Biology (Basel) 9(7):177
Ren W-X, Li P-J, Geng Y, Li X-J (2009) Biological leaching of heavy metals from a contaminated soil by aspergillus Niger. J Hazard Mater 167:164–169
Rezania S, Taib SM, Md Din MF, Dahalan FA, Kamyab H (2016) Comprehensive review on phytotechnology: heavy metals removal by diverse aquatic plants species from wastewater. J Hazard Mater 318:587–599
Ricachenevsky FK, Menguer PK, Sperotto RA, Williams LE, Fett JP (2013) Roles of plant metal tolerance proteins (MTP) in metal storage and potential use in biofortification strategies. Front Plant Sci 4:144
Sahu GK, Upadhyay S, Sahoo BB (2012) Mercury induced phytotoxicity and oxidative stress in wheat (Triticum aestivum L.) plants. Physiol Mol Biol Plants 18(1):21–31
Sarma H, Forid N, Prasad R, Prasad MNV, Ma LQ, Rinklebe J (2021) Enhancing phytoremediation of hazardous metal(loid)s using genome engineering CRISPR–Cas9 technology. J Hazard Mater. https://doi.org/10.1016/j.jhazmat.2021.125493
Seguel A, Cumming JR, Klugh-Stewart K, Cornejo P, Borie F (2013) The role of arbuscular mycorrhizas in decreasing aluminium phytotoxicity in acidic soils: a review. Mycorrhiza 23:167–183
Shahandeh H, Hossner LR (2002) Role of soil properties in phytoaccumulation of uranium. Water Air Soil Pollut 141:165–180
Shahzad B, Tanveer M, Hassan W, Shah AN, Anjum SA, Cheema SA, Ali I (2016) Lithium toxicity in plants: reasons, mechanisms and remediation possibilities—a review. Plant Physiol Biochem 107:104–115
Shahid M, Shamshad S, Rafiq M, Khalid S, Bibi I, Niazi NK, Dumat C, Rashid MI (2017) Chromium speciation, bioavailability, uptake, toxicity and detoxification in soil-plant system: a review. Chemosphere 178:513–533
Shanker AK, Cervantes C, Loza-Tavera H, Avudainayagam S (2005) Chromium toxicity in plants. Environ Int 31(5):739–753
Shanying H, Xiaoe Y, Zhenli H, Baligar VC (2017) Morphological and physiological responses of plants to cadmium toxicity: a review. Pedosphere 27:421–438
Sharma A, Kapoor D, Wang J, Shahzad B, Kumar V, Bali AS, Jasrotia S, Zheng B, Yuan H, Yan D (2020) Chromium bioaccumulation and its impacts on plants: an overview. Plants (Basel) 9(1):100
Shekar CC, Sammaiah D, Shasthree T, Reddy KJ (2011) Effect of mercury on tomato growth and yield attributes. Int J Pharma Biol Sci 2(2):B358–B364
Shi G, Ma H, Chen Y, Liu H, Song G, Cai Q, Lou L, Rengel Z (2019) Low arsenate influx rate and high phosphorus concentration in wheat (Triticum aestivum L.): a mechanism for arsenate tolerance in wheat plants. Chemosphere 214:94–102
Singh S, Parihar P, Singh R, Singh VP, Prasad SM (2016) Heavy metal tolerance in plants: role of transcriptomics, proteomics, metabolomics, and ionomics. Front Plant Sci 6:1143
Sonowal S, Nava AR, Joshi SJ, Borah SN, Islam NF, Pandit S, Prasad R, Sarma H (2022) Biosurfactants assisted heavy metals phytoremediation: green technology for the United Nations sustainable development goals. Pedosphere 2(1):198–210. https://doi.org/10.1016/S1002-0160(21)60067-X
Srivastava M, Ma LQ, Santos JAG (2006) Three new arsenic hyperaccumulating ferns. Sci Total Environ 364:1–3
Srivastava S, Sinha P, Sharma YK (2017) Status of photosynthetic pigments, lipid peroxidation and anti-oxidative enzymes in Vigna mungo in presence of arsenic. J Plant Nutr 40:298–306
Srivastava S, Sounderajan S, Udas A, Suprasanna P (2014) Effect of combinations of aquatic plants (Hydrilla, Ceratophyllum, Eichhornia, Lemna and Wolffia) on arsenic removal in field conditions. Ecol Eng 73:297–301
Sujkowska-Rybkowska M, Borucki W (2015) Pectins esterification in the apoplast of aluminum-treated pea root nodules. J Plant Physiol 184:1–7
Suman J, Uhlik O, Viktorova J, Macek T (2018) Phytoextraction of heavy metals: a promising tool for clean-up of polluted environment? Front Plant Sci 9:1476
Tamburini E, Sergi S, Serreli L, Bacchetta G, Milia S, Cappai G, Carucci A (2017) Bioaugmentation-assisted phytostabilisation of abandoned mine sites in south West Sardinia. Bull Environ Contam Toxicol 98:310–316
Tang J, Xu J, Wu Y, Li Y, Tang Q (2012) Effects of high concentration of chromium stress on physiological and biochemical characters and accumulation of chromium in tea plant (Camellia sinensis L.). Afr J Biotechnol 11:2248–2255
Thakare M, Sarma H, Datar S, Roy A, Pawar P, Gupta K, Pandit S, Prasad R (2021) Understanding the holistic approach to plant-microbe remediation technologies for removing heavy metals and radionuclides from soil. Curr Res Biotechnol 3:84–98. https://doi.org/10.1016/j.crbiot.2021.02.004
Tiwari M, Sharma D, Dwivedi S, Singh M, Tripathi RD, Trivedi PK (2014) Expression in Arabidopsis and cellular localization reveal involvement of rice NRAMP, OsNRAMP1, in arsenic transport and tolerance. Plant Cell Environ 37:140–152
Tome FV, Rodriguez PB, Lozano J (2008) Elimination of natural uranium and 226Ra from contaminated waters by rhizofiltration using Helianthus annuus L. Sci Total Environ 393:351–357
Tripathi P, Singh PC, Mishra A, Srivastava S, Chauhan R, Awasthi S, Mishra S, Dwivedi S, Tripathi P, Kalra A, Tripathi RD, Nautiyal CS (2017) Arsenic tolerant Trichoderma sp. reduces arsenic induced stress in chickpea (Cicer arietinum). Environ Pollut 223:137–145
van de Mortel JE, Villanueva LA, Schat H, Kwekkeboom J, Coughlan S, Moerland PD et al (2006) Large expression differences in genes for iron and zinc homeostasis, stress response, and lignin biosynthesis distinguish roots of Arabidopsis thaliana and the related metal hyperaccumulator Thlaspi caerulescens. Plant Physiol 142:1127–1134
Varun M, D’Souza RD, Pratas J, Paul MS (2011) Evaluation of phytostabilization, a green technology to remove heavy metals from industrial sludge using Typha latifolia L. experimental design. Biotechnol Bioinformatics Bioeng 1:137–145
Viehweger K (2014) How plants cope with heavy metals. Bot Stud 55:35
Wang X, Peng B, Tan C, Ma L, Rathinasabapathi B (2015) Recent advances in arsenic bioavailability, transport, and speciation in rice. Environ Sci Pollut Res Int 22:5742–5750
Wang J, Liang S, Xiang W, Dai H, Duan Y, Kang F, Chai T (2019) A repeat region from the Brassica juncea HMA4 gene BjHMA4R is specifically involved in Cd2+ binding in the cytosol under low heavy metal concentrations. BMC Plant Biol 19:89
Whiting SN, Leake JR, McGrath SP, Baker AJM (2000) Positive responses to zinc and cadmium by roots of the hyperaccumulator Thlaspi caerulescens. New Phytol 145:199–210
Xun Y, Feng L, Li Y, Dong H (2017) Mercury accumulation plant Cyrtomium macrophyllum and its potential for phytoremediation of mercury polluted sites. Chemosphere 189:161–170
Yan A, Wang Y, Tan SN, Mohd Yusof ML, Ghosh S, Chen Z (2020) Phytoremediation: a promising approach for revegetation of heavy metal-polluted land. Front Plant Sci 11:359
Yorifuji T, Tsuda T (2014) Minamata. In: Wexler P (ed) Encyclopedia of toxicology, 3rd edn. Academic Press, London, pp 340–344
Yusefi-Tanha E, Fallah S, Rostamnejadi A, Pokhrel LR (2020) Root system architecture, copper uptake and tissue distribution in soybean (Glycine max (L.) Merr.) grown in copper oxide nanoparticle (cuonp)-amended soil and implications for human nutrition. Plan Theory 9(10):1326
Zaheer IE, Ali S, Saleem MH, Imran M, Alnusairi GSH, Alharbi MB, Riaz M, Rizwan M, Soliman MH (2020) Role of iron-lysine on morpho-physiological traits and combating chromium toxicity in rapeseed (Brassica napus L.) plants irrigated with different levels of tannery wastewater. Plant Physiol Biochem 155:70–84
Zhang X, Zhang S, Xua X, Li T, Gong G, Jiaa Y, Li Y, Denga L (2010) Tolerance and accumulation characteristics of cadmium in Amaranthus hybridus L. J Hazard Mater 180:303–308
Zhao FJ, Dunham SJ, McGrath SP (2002) Arsenic hyperaccumulation by different fern species. New Phytol 15:27–31
Zhao FJ, McGrath SP, Meharg AA (2010) Arsenic as a food chain contaminant: mechanisms of plant uptake and metabolism and mitigation strategies. Annu Rev Plant Biol 61:535–559
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Sarkar, S., Basu, S., Prasad, R., Kumar, G. (2021). Phytoremediation: Mechanistic Approach for Eliminating Heavy Metal Toxicity from Environment. In: Prasad, R. (eds) Phytoremediation for Environmental Sustainability. Springer, Singapore. https://doi.org/10.1007/978-981-16-5621-7_19
Download citation
DOI: https://doi.org/10.1007/978-981-16-5621-7_19
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-16-5620-0
Online ISBN: 978-981-16-5621-7
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)