Abstract
The latest revision of the bone remodeling process has now established that it involves three successive phases: (1) a short initial resorption phase by primary osteoclasts, (2) a longer reversal-resorption phase with intermixed osteoblastic reversal cells and secondary osteoclasts, and (3) a subsequent formation phase. The present chapter focuses on: (1) the histological characteristics of remodeling sites within the reversal-resorption phase, (2) a new definition of the eroded surfaces largely reflecting the reversal-resorption phase, (3) the histological characteristics of eroded surfaces arrested within the reversal-resorption phase, (4) the contribution of a prolonged reversal-resorption phase to the bone loss with age, utilizing cortical porosity as a measure of the bone loss.
The present invited review was completed and submitted to the publisher on 15-Mar-20.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Coupling
- Bone loss
- Bone remodeling cycle
- Basic multicellular unit
- Reversal phase
- Bone resorption
- Bone formation
- Osteoclasts
- Canopy
- Osteoblasts
- Bone marrow envelope
- BMU balance
- Cortical porosity
- Cortical thinning
- Aging
- Osteoporosis
- Remodeling-based bone formation
1 Histological Appearance of the Reversal-Resorption Phase
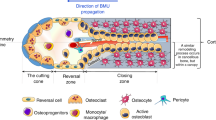
The recent revision of the successive steps of bone remodeling has provided a new perspective on this process during physiological and pathophysiological conditions, and the critical remodeling steps contributing to bone loss. We know now that bone remodeling comprises three successive phases: (1) a short initial resorption phase by primary osteoclasts, (2) a longer reversal-resorption phase with intermixed reversal cells (osteoprogenitors) and secondary osteoclasts, and (3) a subsequent formation phase with mature bone-forming osteoblasts [1], as extensively discussed in the previous chapter and illustrated in Fig. 1. In cortical bone, it is important to be aware of the fact that intracortical remodeling events not only generate new canals (type 1 remodeling), but also remodel existing canals (type 2 remodeling) [2,3,4,5,6,7]. In adults, type 2 remodeling is much more prevalent than type 1 remodeling, as the intracortical canal network is now fully developed.
Models of the sequential steps of the intracortical and trabecular bone remodeling cycle conducted by basic multicellular units (BMUs), as a function of time. Note that intracortical remodeling events may either generate a new canal (type 1) or remodel an existing canal (type2). Importantly, all type 1 events start as a type 2 event, branching of to form a new canal. Trabecular remodeling events are separated from the marrow cavity by a so-called bone remodeling compartment (BRC) canopy, which corresponds to lifted a bone marrow envelope (BME) (see previous chapter). OC, osteoclast; Rv.C, reversal cells; OB, osteoblast; BLC, bone lining cell
Histologically, the reversal-resorption phase can be observed as eroded surfaces colonized by a mixture of mononucleated reversal cells and scattered multinucleated osteoclasts, creating deeper eroded surfaces in cancellous bone or widening the eroded pores in cortical bone until bone formation is subsequently initiated [1]. Indeed, most of the bone resorption occurs in the reversal-resorption phase, as the initial resorption generating these eroded surfaces is only responsible for 17% (5–52%) of the resorption conducted by cortical remodeling events [1]. Histomorphometric studies on human bone have reported that more than 80% of the eroded surfaces are colonized by reversal cells [8,9,10,11,12]. The remaining surfaces are colonized by primary osteoclasts and in particular secondary osteoclasts of the reversal-resorption phase, supporting that more than 95% of the eroded surfaces reflect remodeling events in this phase. This renders the extent of eroded surfaces on trabecular bone and prevalence of eroded pores in cortical bone a suitable estimate of the extent surfaces/pores within the reversal-resorption phase.
2 Histomorphometry of Eroded Bone Surfaces
Eroded surfaces have been investigated by bone histomorphometry for decades, as a static morphometric parameter. Unfortunately, eroded surfaces are often misleadingly referred to as scalloped, irregular, crenated, or lacunar surfaces [12,13,14], rendering their analysis subjective and inaccurate. The same holds true for the eroded surfaces embedded in the bone matrix as cement lines [12, 14, 15]. Instead, eroded surfaces should strictly be defined as surfaces with erosions breaking the lamellae of the existing bone structural units, as a clear sign of osteoclastic resorption [2, 8]. This new definition provides a more accurate and robust definition of these surfaces in both trabecular and cortical bone (Fig. 2). The broken lamellae are clearly visible under polarized light if the specimens and sections are appropriately handled. Importantly, one should think of the erosion as a canyon, where the erosion (broken lamellae) is clearest on the canyon walls at either side, but where the complete canyon is created by erosion, although the broken lamellae are less clear on the canyon floor. The detection of eroded surfaces is not dependent on the presence of osteoclasts or neighboring osteoid surfaces. Eroded surfaces may not only reflect remodeling sites with active eroded surfaces, but also so-called arrested reversal surfaces, which previously have been defined as reversal surfaces without any neighboring osteoclasts or osteoid surface [8, 11, 16]. Moreover, the eroded surfaces may also represent remodeling sites with an insufficient remodeling-based bone formation (RBF) having a negative BMU balance [17, 18], leaving behind part of the eroded surfaces on the canyon wall without formation. In contrast, some remodeling sites may have an overflow RBF having a positive BMU balance [15, 19, 20]. In this case, the bone formation extends beyond the eroded cement lines (classically called scalloped cement lines) and onto neighboring quiescent surfaces, forming quiescent cement lines (classically called smooth cement lines) [15, 19,20,21,22]. Importantly, this strict new definition of eroded versus quiescent surfaces also applies to when these surfaces are embedded in the matrix as erosive versus quiescent cement lines (Fig. 2).
Histological appearance of eroded surfaces (ES) within active and quiescent remodeling sites in trabecular bone. Note that some ES are arrested—defined as ES with no osteoclasts or adjacent osteoid surface (OS)—due to problems with the transition into bone formation. The thickness of bone formed may not refill the erosion exactly. In some cases it either overfills (overflow remodeling-based bone formation (RBF)) upon the adjacent quiescent surface (QS) or underfills (insufficient RBF) the erosion, leaving behind part of the ES
As the classical definition of the eroded surfaces has been imprecise, many leading bone histomorphometry groups have justifiably only included eroded surfaces clearly fulfilling the classical definition. A comparison across numerous studies and groups shows that the classical definition resulted in variable median estimates of 1.0–5.3% ES/BS (eroded surface/bone surface) on trabecular bone of post-menopausal osteoporotic (PMO) women (Table 1). On the other hand, the new definition resulted in mean estimates of 14–15% ES/BS on trabecular bone of PMO women (Table 1). This highlights that the classical definition underestimates the eroded surfaces, and consequently the percentage of trabecular bone surfaces within the reversal-resorption phase. Accordingly, this may explain why its importance has been vastly overlooked.
In rodent bone, eroded surfaces are more difficult to recognize, as the erosions are quite shallow, especially in mice [14]. Moreover, the investigations are often conducted in growing rodents that have pronounced bone modeling [31], rendering it difficult to separate erosions due to modeling from remodeling activities. In rats above 6 months of age, it is considered valid to assume that the investigated eroded surfaces are mainly a remodeling-based parameter [14]. Overall, this presents a challenge for the histomorphometric investigations of remodeling-based eroded surfaces, i.e. reversal-resorption phase, in mice and young rats.
3 How to Investigate the Remodeling Events Responsible for Bone Loss?
The absence and loss of information is a major challenge when investigating the remodeling events responsible for bone loss, as we can only investigate the bone structures that remain, and not the bone structures that have been lost. This is especially a problem when studying the remodeling events responsible for the age-related loss of trabecular bone [32,33,34] and for osteoporosis [35, 36], since we do not know how much bone is lost, but only how much bone remains. Here, it is important to note that aging is associated with a reduced number of trabeculae, while the thickness of the remaining trabeculae is less affected (Fig. 3). Accordingly, the histomorphometric properties observed in bone specimens from the elderly may rather reflect why the investigated trabeculae in elderly are remaining, and not why the trabeculae are gradually lost with age. The same is the case for bone histomorphometric studies that compare the trabecular bone remodeling of osteoporotic patients with controls.
In contrast, cortical porosity gives a more direct and reliable measure of the actual age-related cortical bone loss (Fig. 3). Here, the increasing cortical porosity can be directly linked to the remodeling events contributing to the bone loss. This is done by combining measurements of the individual pore cross sectional area with detailed histomorphometry of their respective remodeling type, stage and position, as well as measurements of the quiescent osteon diameter and wall thickness [2, 3, 37]. The cortical porosity cannot apply to total bone loss, since an excessive cortical porosity leads to trabecularization reducing the cortical thickness, as also seen with age (Fig. 3). This again renders it difficult to estimate the true extent of the cortical bone loss, especially in elderly patients with a severe bone loss (cortical thinning) due to osteoporosis or similar pathological conditions [35, 36].
4 Contribution of the Reversal-Resorption Phase to Bone Loss
As a consequence of the above considerations, bone histomorphometry studies focusing on age-related increase in cortical porosity may provide the most reliable assessment of the remodeling events responsible for the age-induced bone loss. Note that this elevated porosity is the result of enlarged pores, not a higher pore density [38,39,40,41,42]. Such studies have shown that the accumulation and coalescence of enlarged eroded pores upon existing intracortical canals (type 2 remodeling) was the main contributor to age-related cortical porosity in iliac bone specimens from women [2]. Likewise, as shown in fibular bone these accumulating eroded pores led to endocortical trabecularization [37], and to enhanced cortical fragility [43]. This accumulation of eroded pores and reduced abundance of formative pores support the concept that a prolongation of the reversal-resorption phase, causing a delayed initiation of subsequent bone formation, is a major contributor to the cortical bone loss [1, 2, 37, 43]. Nevertheless, it remains an open question whether this reflects a generalized prolongation of the reversal-resorption phase in all BMUs or an accumulation of remodeling sites with uncoupled BMUs with a persistent lack of transition to subsequent bone formation (Fig. 4).
Model of the different types of trabecular and intracortical BMUs, their sequential remodeling steps and their net bone loss (−Δ). The bone loss may be the result of BMUs with a coupled resorption and formation, but where the formation underfills the erosion (negative BMU balance). In other words there is insufficient remodeling-based bone formation (RBF). The bone loss may also be the result of BMUs with an uncoupled resorption and formation, causing erosions with no or a much delayed transition to formation. These surfaces are also referred to as arrested erosion reflecting BMUs with an arrested reversal-resorption (Rv-Rs) phase. These uncoupled BMUs seem to be the main contributor to the age-related cortical bone loss in women [2]
These recent studies on iliac cortical bone question the classical concept that a negative BMU balance is the driver of cancellous and cortical age-related bone loss [18, 44,45,46,47,48,49,50]. According to this classical concept, the magnitude of bone resorption (erosion depth or osteon diameter) was reported to be followed by an insufficient magnitude of bone formation (wall thickness), resulting in remodeling sites with an insufficient RBF (Fig. 2). This would then cause a net bone loss at each remodeling cycle, which would accumulate over time and hence result in an age-related bone loss. If this was the case for cortical bone, the pore diameter of quiescent osteons should increase with age and their pore cross sectional area should be the main contributor to the cortical porosity. This was, however, not the case in the recent detailed analysis of the remodeling events contributing to the cortical porosity with age [2, 3], demonstrating that the accumulation of enlarged eroded pores (not quiescent pores) was the main contributor to the increase in cortical porosity with age.
One may question whether the conclusions drawn in cortical bone are transferable to cancellous bone. Is the prolongation of the reversal-resorption phase also contributing to cancellous bone loss during aging and osteoporosis? This may be the case, as osteoporotic patients have been reported to accumulate remodeling sites arrested in the reversal-resorption phase [8, 11]. These remodeling sites had a low density of reversal cells, i.e. osteoprogenitors [8, 11], rendering it difficult to reach the critical density of osteoprogenitors required for initiation of bone formation and thereby transitioning to the subsequent remodeling-based bone formation [1].
References
Lassen NE, Andersen TL, Ploen GG, Soe K, Hauge EM, Harving S, et al. Coupling of bone resorption and formation in real time: new knowledge gained from human Haversian BMUs. J Bone Miner Res. 2017 Jul;32(7):1395–405.
Andreasen CM, Delaisse JM, van der Eerden BCJ, van Leeuwen JP, Ding M, Andersen TL. Understanding age-induced cortical porosity in women: the accumulation and coalescence of eroded cavities upon existing intracortical canals is the main contributor. J Bone Miner Res. 2018;33:606–20.
Andreasen CM, Delaisse JM, van der Eerden BCJ, van Leeuwen JPTM, Ding M, Andersen TL. Understanding age-induced cortical porosity in women: is a negative BMU balance in quiescent osteons a major contributor? Bone. 2018;117:70–82.
Jaworski ZF, Meunier P, Frost HM. Observations on two types of resorption cavities in human lamellar cortical bone. Clin Orthop Relat Res. 1972;83:279–85.
Pankovich AM, Simmons DJ, Kulkarni VV. Zonal osteons in cortical bone. Clin Orthop Relat Res. 1974:356–63.
Tappen NC. Three-dimensional studies on resorption spaces and developing osteons. Am J Anat. 1977;149:301–17.
Maggiano IS, Maggiano CM, Clement JG, Thomas CD, Carter Y, Cooper DM. Three-dimensional reconstruction of Haversian systems in human cortical bone using synchrotron radiation-based micro-CT: morphology and quantification of branching and transverse connections across age. J Anat. 2016;228:719–32.
Andersen TL, Abdelgawad ME, Kristensen HB, Hauge EM, Rolighed L, Bollerslev J, et al. Understanding coupling between bone resorption and formation: are reversal cells the missing link? Am J Pathol. 2013;183:1–12.
Baron R, Magee S, Silverglate A, Broadus A, Lang R. Estimation of trabecular bone resorption by histomorphometry: evidence for a prolonged reversal phase with normal resorption in post-menopausal osteoporosis and coupled increase in primary hyperparathyroidism. Clin Disorders Bone Min Metab. 1983:191–5.
Baron R, Vignery A, Lang R. Reversal phase and osteopenia: defective coupling of resorption to formation in the pathogenesis of osteoporosis. In: Deluca HF, Frost HM, Jee WSS, Johnston CC, Parfitt AM, editors. Osteoporosis: recent advances in pathogenesis and treatment. Baltimore, MD: University Park Press. 1980:311–20.
Jensen PR, Andersen TL, Hauge EM, Bollerslev J, Delaisse JM. A joined role of canopy and reversal cells in bone remodeling – lessons from glucocorticoid-induced osteoporosis. Bone. 2015;73:16–23.
Balena R, Shih MS, Parfitt AM. Bone resorption and formation on the periosteal envelope of the ilium: a histomorphometric study in healthy women. J Bone Miner Res. 1992;7:1475–82.
Dempster DW, Compston JE, Drezner MK, Glorieux FH, Kanis JA, Malluche H, et al. Standardized nomenclature, symbols, and units for bone histomorphometry: a 2012 update of the report of the ASBMR histomorphometry nomenclature committee. J Bone Miner Res. 2013;28:2–17.
Erben RG, Glosmann M. Histomorphometry in rodents. Methods Mol Biol. 2019;1914:411–35.
Dempster DW, Zhou H, Recker RR, Brown JP, Recknor CP, Lewiecki EM, et al. Remodeling- and modeling-based bone formation with teriparatide versus denosumab: a longitudinal analysis from baseline to 3 months in the AVA study. J Bone Miner Res. 2018;33:298–306.
Andreasen CM, Ding M, Overgaard S, Bollen P, Andersen TL. A reversal phase arrest uncoupling the bone formation and resorption contributes to the bone loss in glucocorticoid treated ovariectomised aged sheep. Bone. 2015;75:32–9.
Riggs BL, Parfitt AM. Drugs used to treat osteoporosis: the critical need for a uniform nomenclature based on their action on bone remodeling. J Bone Miner Res. 2005;20:177–84.
Eriksen EF, Hodgson SF, Eastell R, Cedel SL, O'Fallon WM, Riggs BL. Cancellous bone remodeling in type I (postmenopausal) osteoporosis: quantitative assessment of rates of formation, resorption, and bone loss at tissue and cellular levels. J Bone Miner Res. 1990;5:311–9.
Frost M, Rahbek ET, Ejersted C, Høilund-Carlsen PF, Bygum A, Thomsen JS et al. Modeling-based bone formation transforms trabeculae to cortical bone in the sclerotic areas in Buschke-Ollendorf syndrome. A case study of two females with LEMD3 variants. Bone. 2020 Jun;135:115313.
Dempster DW, Zhou H, Ruff VA, Melby TE, Alam J, Taylor KA. Longitudinal effects of teriparatide or zoledronic acid on bone modeling- and remodeling-based formation in the SHOTZ study. J Bone Miner Res. 2018;33:627–33.
Ominsky MS, Libanati C, Niu QT, Boyce RW, Kostenuik PJ, Wagman RB, et al. Sustained modeling-based bone formation during adulthood in cynomolgus monkeys may contribute to continuous BMD gains with denosumab. J Bone Miner Res. 2015;30:1280–9.
Jee WS, Tian XY, Setterberg RB. Cancellous bone minimodeling-based formation: a Frost, Takahashi legacy. J Musculoskelet Neuronal Interact. 2007;7:232–9.
Recker RR, Delmas PD, Halse J, Reid IR, Boonen S, Garcia-Hernandez PA, et al. Effects of intravenous zoledronic acid once yearly on bone remodeling and bone structure. J Bone Miner Res. 2008;23:6–16.
Recker RR, Bare SP, Smith SY, Varela A, Miller MA, Morris SA, et al. Cancellous and cortical bone architecture and turnover at the iliac crest of postmenopausal osteoporotic women treated with parathyroid hormone 1-84. Bone. 2009;44:113–9.
Eriksen EF, Melsen F, Sod E, Barton I, Chines A. Effects of long-term risedronate on bone quality and bone turnover in women with postmenopausal osteoporosis. Bone. 2002;31:620–5.
Dempster DW, Cosman F, Kurland ES, Zhou H, Nieves J, Woelfert L, et al. Effects of daily treatment with parathyroid hormone on bone microarchitecture and turnover in patients with osteoporosis: a paired biopsy study. J Bone Miner Res. 2001;16:1846–53.
Dempster DW, Brown JP, Fahrleitner-Pammer A, Kendler D, Rizzo S, Valter I, et al. Effects of long-term denosumab on bone histomorphometry and mineralization in women with postmenopausal osteoporosis. J Clin Endocrinol Metab. 2018;103:2498–509.
Chavassieux P, Portero-Muzy N, Roux JP, Horlait S, Dempster DW, Wang A et al. Reduction of cortical bone turnover and erosion depth after 2 and 3 years of denosumab: iliac bone histomorphometry in the FREEDOM trial. J Bone Miner Res. 2019 Sep;34(9):1597–608.
Chavassieux PM, Arlot ME, Reda C, Wei L, Yates AJ, Meunier PJ. Histomorphometric assessment of the long-term effects of alendronate on bone quality and remodeling in patients with osteoporosis. J Clin Invest. 1997;100:1475–80.
Jensen PR, Andersen TL, Chavassieux PM, Roux JP, Delaisse JM. Why do bisphosphonates compromise bone formation. J Bone Miner Res. 2015;30(Suppl 1).
Erben RG. Trabecular and endocortical bone surfaces in the rat: modeling or remodeling? Anat Rec. 1996;246:39–46.
Thomsen JS, Jensen MV, Niklassen AS, Ebbesen EN, Bruel A. Age-related changes in vertebral and iliac crest 3D bone microstructure—differences and similarities. Osteoporos Int. 2015;26:219–28.
Bach-Gansmo FL, Bruel A, Jensen MV, Ebbesen EN, Birkedal H, Thomsen JS. Osteocyte lacunar properties and cortical microstructure in human iliac crest as a function of age and sex. Bone. 2016;91:11–9.
Thomsen JS, Niklassen AS, Ebbesen EN, Bruel A. Age-related changes of vertical and horizontal lumbar vertebral trabecular 3D bone microstructure is different in women and men. Bone. 2013;57:47–55.
Arnold JS. Focal excessive endosteal resorption in aging and senile osteoporosis. In: Barzel US, editor. Osteoporosis. New York: Grune & Stratton; 1970. p. 80–100.
Keshawarz NM, Recker RR. Expansion of the medullary cavity at the expense of cortex in postmenopausal osteoporosis. Metab Bone Dis Relat Res. 1984;5:223–8.
Andreasen CM, Bakalova LP, Bruel A, Hauge EM, Kiil BJ, Delaisse JM et al. The generation of enlarged eroded pores upon existing intracortical canals is a major contributor to endocortical trabecularization. Bone. 2020;130:115127.
Thompson DD. Age changes in bone mineralization, cortical thickness, and Haversian canal area. Calcif Tissue Int. 1980;31:5–11.
Stein MS, Feik SA, Thomas CD, Clement JG, Wark JD. An automated analysis of intracortical porosity in human femoral bone across age. J Bone Miner Res. 1999;14:624–32.
Thomas CD, Feik SA, Clement JG. Increase in pore area, and not pore density, is the main determinant in the development of porosity in human cortical bone. J Anat. 2006;209:219–30.
Bousson V, Meunier A, Bergot C, Vicaut E, Rocha MA, Morais MH, et al. Distribution of intracortical porosity in human midfemoral cortex by age and gender. J Bone Miner Res. 2001;16:1308–17.
Lerebours C, Thomas CD, Clement JG, Buenzli PR, Pivonka P. The relationship between porosity and specific surface in human cortical bone is subject specific. Bone. 2015;72:109–17.
Bakalova LP, Andreasen CM, Thomsen JS, Bruel A, Hauge EM, Kiil BJ, et al. Relating intracortical bone mechanics to pore morphology and remodeling characteristics in the human fibula. J Bone Miner Res. 2018;33:2177–85.
Brockstedt H, Kassem M, Eriksen EF, Mosekilde L, Melsen F. Age- and sex-related changes in iliac cortical bone mass and remodeling. Bone. 1993;14:681–91.
Broulik P, Kragstrup J, Mosekilde L, Melsen F. Osteon cross-sectional size in the iliac crest: variation in normals and patients with osteoporosis, hyperparathyroidism, acromegaly, hypothyroidism and treated epilepsia. Acta Pathol Microbiol Immunol Scand A. 1982;90:339–44.
Eriksen EF, Melsen F, Mosekilde L. Reconstruction of the resorptive site in iliac trabecular bone: a kinetic model for bone resorption in 20 normal individuals. Metab Bone Dis Relat Res. 1984;5:235–42.
Kragstrup J, Melsen F, Mosekilde L. Thickness of lamellae in normal human iliac trabecular bone. Metab Bone Dis Relat Res. 1983;4:291–5.
Lips P, Courpron P, Meunier PJ. Mean wall thickness of trabecular bone packets in the human iliac crest: changes with age. Calcif Tissue Res. 1978;26:13–7.
Vedi S, Compston JE, Webb A, Tighe JR. Histomorphometric analysis of dynamic parameters of trabecular bone formation in the iliac crest of normal British subjects. Metab Bone Dis Relat Res. 1983;5:69–74.
Compston JE, Vedi S, Kaptoge S, Seeman E. Bone remodeling rate and remodeling balance are not co-regulated in adulthood: implications for the use of activation frequency as an index of remodeling rate. J Bone Miner Res. 2007;22:1031–6.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Andersen, T.L., Delaisse, JM., Thomsen, J.S., Andreasen, C.M. (2022). Significance of Reversal-Resorption Phase in Bone Loss. In: Takahashi, H.E., Burr, D.B., Yamamoto, N. (eds) Osteoporotic Fracture and Systemic Skeletal Disorders. Springer, Singapore. https://doi.org/10.1007/978-981-16-5613-2_7
Download citation
DOI: https://doi.org/10.1007/978-981-16-5613-2_7
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-16-5612-5
Online ISBN: 978-981-16-5613-2
eBook Packages: MedicineMedicine (R0)