Abstract
Solitary pulmonary nodule (SPN) poses as a diagnostic challenge for clinicians due to the inherent diverse aetiology and varied risk of malignancy. The widespread adoption of chest CT for diagnosing respiratory ailments and in staging of malignancies has led to an increased diagnosis of SPN thus making it crucial for clinicians to be aware of the management of solitary pulmonary nodules. Current management algorithms can help guide the approach and strategies to be adopted in solitary pulmonary nodules. With the advent of minimally invasive surgery, these nodules can be excised with thoracoscopic approaches thus reducing the morbidity of open explorations. This chapter discusses the relevant aspects of diagnosis and management of solitary pulmonary nodules.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
Introduction
Solitary pulmonary nodule (SPN) is defined as a “focal, rounded opacity ≤3 cm in diameter, mostly surrounded by an aerated lung, including contact with the pleura, but without potentially related abnormalities in the thorax”. (potentially related abnormalities include: pleural effusion, mediastinal lymphadenopathy, atelectasis) [1].
Widespread use of multi-detector computed tomography (CT) has increased the incidental detection of such nodules. A clinician however would mainly encounter a SPN in practice during the following situations:
-
Patients being investigated with chest radiology for respiratory symptoms
-
Incidental detection on chest imaging done for other purposes
-
During screening studies for lung cancer
-
Patients with a known cancer undergoing imaging for:
-
staging
-
during surveillance scans
-
post treatment follow-up imaging
-
Solid and Subsolid Nodules
Apart from knowing what a SPN is, it is also important to understand the concept of Solid and Subsolid nodules (SSN).
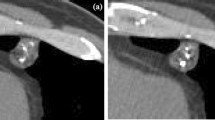
Solid nodule, the most common type of SPN, is characterised by homogenous soft tissue attenuation on CT (Fig. 1).
Subsolid nodule present as two types of focal opacities: part-solid nodule or a pure ground glass nodule.
-
Part-solid nodule (PSN): has both solid and ground glass component ≤3 cm in diameter.
-
Pure ground-glass nodule (PGGN): ground glass opacity ≤3 cm in diameter.
Solid component: the term is used to describe part of a nodule that obscures the underlying broncho-vascular structure.
Ground-glass component: it refers to opacification, which is more than the background, but visibility of underlying vascular structure is not hampered [1].
Approach to a SPN
Common societies/groups formulating guidelines and algorithms for management of lung nodules:
-
British Thoracic Society (BTS) [1].
-
Fleischner Society [2].
-
American College of Chest Physicians (ACCP) [3].
-
National Comprehensive Cancer Network [4].
Despite minor discrepancies, all proposed approaches take into consideration the following factors:
-
clinical risk factors for lung cancer
-
imaging features of the nodules
-
comparison with previous imaging studies
-
most appropriate management.
The approach in this chapter would be largely based on the recommendations of the British Thoracic Society (BTS) [1].
The approach consists of
-
1.
Risk assessment for malignancy
-
2.
Surveillance
-
3.
Management
Risk Assessment for Malignancy Based on Clinicoradiological Parameters
Assessment of a SPN in terms of ‘risk of malignancy’ is very important to guide further management, lower the risk less invasive is the adopted approach and vice versa.
The BTS guidelines published in 2015 identified risk factors consistently associated with malignancy [1]. The initial assessment is used to identify and differentiate nodules having a sufficiently low chance of malignancy which can be managed by only follow up imaging from those which need further assessment.
Clinical and radiological risk factors consistently associated with malignancy in solid nodules are summarised in Table 1.
A possible benign aetiology is predicted by the presence of a diffuse, central, laminated, or popcorn pattern of calcification and peri-fissural location. The BTS came up with an initial assessment algorithm for solid nodules on CT (Fig. 2), and as per the guidelines patients with nodules with aforementioned benign features can be discharged [1].
BTS algorithm for solid nodules on CT scan [1] (published with permission from British Thoracic Society)
Table 2 highlights the risk of malignancy in a solitary pulmonary nodule based upon the size (Dutch-Belgian CT screening trial, NELSON trial) [5]. It revealed that subjects with nodules <5 mm in the maximum transverse diameter or <100 mm3 in volume had no increased risk of developing lung cancer after 2 years than those without nodules. Based on the findings mentioned above they recommended CT surveillance for nodules <8 mm in diameter or <300 mm3 in volume.
Risk Prediction Models: Brock University Model and Herder Model
The BTS guidelines recommend the use of two risk prediction models (Brock University model and Herder model) for nodules >300 mm3 volume or >8 mm diameter [1]. These models are readily available for anyone to use in the form of a calculator-app on all smartphones (“BTS Pulmonary Nodule Risk Calculator” on App Store or Google Play Store). The examples of malignancy risk prediction by Brock model and Herder model can be seen in Fig. 3 and Fig. 4 respectively.
Whereas the Brock model is primarily based on clinical parameters supplemented with CT findings, PET CT is an additional modality of assessment in the Herder model. CT surveillance is recommended for patients with nodules that have <10% risk of malignancy as assessed by the Brock University model, whereas PET CT is advisable for patients with higher risk who are further re-evaluated with the Herder model [6]. The Herder model further classifies FDG (fluorodeoxyglucose) uptake in PET as absent, faint, moderate or intense [7].
Following reassessment with the Herder model consider the following:
-
Risk of malignancy <10%—CT surveillance
-
Risk is 10–70%—Image guided biopsy
-
Risk is >70%—Surgical resection (or nonsurgical treatment for those who are not fit)
Surviellance of Solid SPN
The aim during surveillance is to assess nodule growth to discriminate between benign and malignant nodules. Assessment of a nodule size has traditionally been done by measuring the largest transverse cross-sectional diameter. However, over the last decade volumetric analysis (manual or semiautomated/automated) has been increasingly reported as an alternative and better tool to assess nodule growth.
The volume-doubling time (VDT) of a nodule utilizes a simple exponential growth model that assumes uniform 3-dimensional tumour growth and is calculated based upon estimate of the difference in the diameter at baseline and follow-up CT and the time interval between the two scans [8,9,10]. Using automated volumetry, growth of nodule, defined as increase in volume >25%, can be reliably predicted at a 3 month interval CT. Another advantage of a 3-month CT is that most nodules which resolve do so in 3 months interval. On the other hand, for small nodules, 5–6 mm size, the CT surveillance interval may be extended to 12 months to detect appreciable growth. Stable disease at 1 year is indicative of benign aetiology [8].
Key BTS Recommendations on Surveillance [1, 8]
-
1.
If initial risk stratification assigns a nodule a chance of malignancy of <10%, assess growth rate using automated volumetric analysis.
-
2.
Assess growth for nodules of ≥80 mm3 in volume (or ≥6 mm in diameter) by calculating VDT using CT scan at 3 months and 1 year.
-
3.
Significant growth is defined as ≥25% change in volume
-
4.
For nodules showing clear growth or a VDT <400 days, offer further diagnostic workup (biopsy, imaging, or resection)
-
5.
For nodules that have a VDT of 400–600 days, consider ongoing yearly surveillance or biopsy as per the patient’s preference.
-
6.
Discharge patients with solid nodules that show stability (<25% volume change) on CT at 1 year.
-
7.
If 2-dimensional nodule diameter parameters are used to assess growth, follow up with CT for a total of 2 years.
Management of Subsolid Nodules (SSNs)
SSNs are often benign, preinvasive or early invasive malignant lesions with an indolent disease which carry a better prognosis and calls for a different management strategy than solid nodules (Fig. 5). The pathological correlates [11,12,13] of a SSN are tabulated in Table 3:
Thus, SSNs may represent both preinvasive and invasive lesions. Clinical and morphological variables that are more likely to be associated with malignancy in SSNs are:
-
advanced age,
-
prior history of lung cancer,
-
size,
-
part-solid nature (independent predictor of malignancy)
-
pleural retraction
-
indentation
-
bubble-like appearance in a pGGN
Approximately 25% of SSNs resolve after 3 months.
Figure 5 highlights the BTS algorithm for management of SSN.
Key BTS Recommendations for Management of SSNs [1, 8]
-
1.
All SSNs should be revaluated with a repeat thin-section CT at 3 months.
-
2.
For SSNs of ≥5 mm in diameter that is stable at 3 months, use the Brock prediction model to calculate the risk of malignancy
-
3.
Other characteristics such as morphological features (size of solid component, bubble- -like appearance, and pleural indentation), smoking status, peripheral eosinophilia, history of lung cancer etc. should be considered in estimation of risk of malignancy
-
4.
Consider resection/nonsurgical treatment or observation for pGGN that increase in size ≥2 mm in the maximum diameter and if observation is planned then repeat CT after a maximum of 6 months.
-
5.
PSN that show enlargement of the solid component or for pGGN which develop a solid component, consider resection / nonsurgical treatment over observation the options being considered based upon the patient’s choice, age, comorbidities, and risk of surgery.
Biopsy Techniques
It is important to establish tissue diagnosis of a solitary pulmonary nodule for further management when there is diagnostic uncertainty. There are two approaches of performing a biopsy:
-
Nonsurgical
-
Surgical
Nonsurgical biopsy: Preferred when there is sufficient ambiguity pertaining to the diagnosis which precludes a definitive management and can be either CT or bronchoscopic aided.
CT-guided percutaneous transthoracic biopsy: It is the preferred technique of minimally invasive biopsy. The reported yields are high (pooled estimate of 91%) with limitations of a high incidence of pneumothorax (6.6% requiring chest drain in the largest series) with the procedure [14,15,16,17].
Bronchoscopic aided biopsy: The yield of standard bronchoscopy is low but can be augmented with innovative techniques such as
-
fluoroscopy
-
EBUS, radial endobronchial ultrasound
-
ENB, electromagnetic navigation bronchoscopy.
A 65%–84% yield with ENB and 46%–77% with radial EBUS has been reported [18].
The relative draw backs of bronchoscopic guided technique are:
-
access limitations of small lesions <2 cm located in peripheral third of the lung
-
time consuming
-
lack of wide availability (particularly EBUS AND ENB)
Surgical biopsy: Excision biopsy for SPN may be performed in the following situations:
-
high clinical suspicion of malignancy despite histological indications of benign or indeterminate pathological characteristics
-
risk of malignancy is high enough to warrant excision without preoperative biopsy
Thoracoscopic wedge resection remains the gold standard for surgical lung biopsy (Figs. 6 and 7). The morbidity and mortality of the surgical procedure should be weighed in contrast to the possibility of progression during radiological surveillance when considering surgical resection as an option. A 0.4% mortality for wedge resection/ segmentectomy has been reported by the UK and Ireland Society of Cardio-Thoracic Surgeons. A 30-day mortality of 2.1% and a 90-day mortality of 4.2% has been reported in patients undergoing wedge resection or segmentectomy (The English National Lung Cancer Audit) [19].
Surgical and Non-Surgical Treatment
Once a decision to resect a SPN has been taken, few issues regarding the optimal surgical treatment need to be addressed. The two major considerations are the approach and the extent of resection.
Surgical Approach: Thoracotomy Vs Thoracoscopy Vs Robotics
Before the advent of Video assisted thoracoscopic surgery (VATS), a thoracotomy was the gold standard procedure for lung resection of any form. Over the years VATS has proven to be an excellent alternative approach for pulmonary resections. Traditional multi-port VATS as well as single port VATS is being widely performed across the globe. It is better than traditional thoracotomy in terms of better cosmesis, less pain and early recovery. The long-term oncological equality or superiority still needs to be proven however.
Robotic assisted thoracoscopic surgery (RATS) is another new entrant. The excellent dexterity, 7-degrees freedom of movement, true 3-dimensional binocular vision and filtering of tremors makes it an excellent choice. However, cost concerns and availability are important limitations.
Extent of Resection- Lobar Vs Sub-Lobar Resection
Issues in consideration for extent of resection
-
location of the nodule
-
need for lung sparing surgery
-
sub-lobar versus lobar resection
-
non-anatomical resection (wedge resection) versus an anatomical segmentectomy.
Lobectomy has been found to be superior to sub-lobar resection (segmentectomy/wedge resection) as regards local recurrence in early lung cancer in a prospective randomized trial [20, 21]. However, sub lobar resections may be equivalent to lobar resections in small tumor size <2 cm, elderly or those who may not tolerate a lobectomy [8, 20, 22, 23].
Key BTS Recommendations on Surgery (Fig. 8) [1, 8]
-
1.
VATS rather than an open surgery should be the preferred surgical approach in SPN
-
2.
In the context of a pulmonary nodule biopsy confirmed as lung cancer preoperatively or following a wedge resection with intraoperative frozen section analysis, lobectomy should be offered as definitive management which may be attempted at the same anaesthetic setting.
-
3.
In a patient where preservation of functioning lung tissue is desirable, anatomical segmentectomy can minimize operative risk as also improve physiological outcome
-
4.
For nodules less than 2 cm in diameter without evidence of nodal disease a diagnostic anatomical segmentectomy may be considered if no pathological confirmation and frozen section is possible
Localization Techniques for Nodules (Table 4)
Bi-digital palpation in thoracotomy makes it easy to localize and resect even deep-seated small lesions, if a limited resection is planned. Certain characteristics of the nodules make it difficult to locate in thoracoscopy viz.: small size, located deep to the visceral pleura and nodules exhibiting a ground-glass morphology.
Several preoperative marking techniques have been developed to facilitate localization of these nodules, their use is subject to availability of the facility and expertise (Table 4) [24,25,26,27,28,29].
Nonsurgical Treatment Without Pathological Confirmation
Patients with SPN who are deemed unfit for surgical treatment and patients who choose to opt out of surgical treatment can be offered non-surgical treatment options. An attempt should be made to obtain a histological confirmation, however if such is not possible, treatment may proceed without biopsy, provided the risk of malignancy is higher than 70%. The main alternatives to surgery are:
-
Stereotactic body radiotherapy (SABR)
-
Radiofrequency ablation (RFA)
-
Radical radiotherapy
Key BTS Recommendations for Non-surgical Treatment [1, 8]
-
1.
Stereotactic body radiotherapy or Radiofrequency ablation is a viable option for patients who are unfit for surgery, with pulmonary nodule(s) which are at a high risk of malignancy, and where biopsy is nondiagnostic or not possible.
-
2.
Conventional radical radiotherapy can be considered for patients who have pulmonary nodule(s) with high probability of malignancy, who are unfit for surgery, and in whom SABR or RFA is not feasible
Key Clinical Points
-
1.
SPN are classified into two types: Solid and Subsolid nodules, subsolid nodules are further categorized as PSN and PGGN types
-
2.
Management of solitary pulmonary nodules should be carried out by a multidisciplinary team (surgeon, oncologist, pulmonologist, radiologist, pathologist, radiation oncologist)
-
3.
Initial assessment is used to identify and differentiate nodules having a sufficiently low chance of malignancy which can be managed by only follow up imaging from those which need further assessment.
-
4.
CECT of the thorax and PET CT are useful diagnostic imaging modalities for characterization of SPN
-
5.
There is enough evidence to support the use of two malignancy prediction calculators (Brock & Herder) for better characterization of the risk of malignancy, and a higher nodule size threshold for follow-up (≥5 mm or ≥80 mm3) of solid pulmonary nodules.
-
6.
For management of nodules with extended volume-doubling times CT based volumetry should be preferred
-
7.
The BTS algorithms can guide the diagnostic and management strategies of SPN
-
8.
Subsolid nodules have a good prognosis and therefore a less aggressive approach may be adopted in their treatment.
References
Callister ME, Baldwin DR, Akram AR, Barnard S, Cane P, Draffan J, Franks K, Gleeson F, Graham R, Malhotra P, Prokop M, Rodger K, Subesinghe M, Waller D, Woolhouse I, British Thoracic Society Pulmonary Nodule Guideline Development Group, British Thoracic Society Standards of Care Committee. British Thoracic Society guidelines for the investigation and management of pulmonary nodules. Thorax. 2015;70 Suppl 2:ii1–54. Erratum in: Thorax. 2015 Dec;70(12):1188. https://doi.org/10.1136/thoraxjnl-2015-207168.
MacMahon H, Naidich DP, Goo JM, Lee KS, Leung ANC, Mayo JR, Mehta AC, Ohno Y, Powell CA, Prokop M, Rubin GD, Schaefer-Prokop CM, Travis WD, Van Schil PE, Bankier AA. Guidelines for management of incidental pulmonary nodules detected on CT images: from the Fleischner Society 2017. Radiology. 2017;284(1):228–43. https://doi.org/10.1148/radiol.2017161659.
Gould MK, Donington J, Lynch WR, Mazzone PJ, Midthun DE, Naidich DP, Wiener RS. Evaluation of individuals with pulmonary nodules: when is it lung cancer? Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2013;143(5 Suppl):e93S–e120S. https://doi.org/10.1378/chest.12-2351.
NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) Non-small cell lung cancer version 2.2019; 21 November, 2018.
Horeweg N, van Rosmalen J, Heuvelmans MA, van der Aalst CM, Vliegenthart R, Scholten ET, ten Haaf K, Nackaerts K, Lammers JW, Weenink C, Groen HJ, van Ooijen P, de Jong PA, de Bock GH, Mali W, de Koning HJ, Oudkerk M. Lung cancer probability in patients with CT-detected pulmonary nodules: a prespecified analysis of data from the NELSON trial of low-dose CT screening. Lancet Oncol. 2014;15(12):1332–41. https://doi.org/10.1016/S1470-2045(14)70389-4.
McWilliams A, Tammemagi MC, Mayo JR, Roberts H, Liu G, Soghrati K, Yasufuku K, Martel S, Laberge F, Gingras M, Atkar-Khattra S, Berg CD, Evans K, Finley R, Yee J, English J, Nasute P, Goffin J, Puksa S, Stewart L, Tsai S, Johnston MR, Manos D, Nicholas G, Goss GD, Seely JM, Amjadi K, Tremblay A, Burrowes P, MacEachern P, Bhatia R, Tsao MS, Lam S. Probability of cancer in pulmonary nodules detected on first screening CT. N Engl J Med. 2013;369(10):910–9. https://doi.org/10.1056/NEJMoa1214726.
Herder GJ, van Tinteren H, Golding RP, Kostense PJ, Comans EF, Smit EF, Hoekstra OS. Clinical prediction model to characterize pulmonary nodules: validation and added value of 18F-fluorodeoxyglucose positron emission tomography. Chest. 2005;128(4):2490–6. https://doi.org/10.1378/chest.128.4.2490.
Baldwin DR. Management of pulmonary nodules according to the 2015 British Thoracic Society guidelines. Key messages for clinical practice. Pol Arch Med Wewn. 2016;126(4):262–74. https://doi.org/10.20452/pamw.3379.
Korst RJ, Lee BE, Krinsky GA, Rutledge JR. The utility of automated volumetric growth analysis in a dedicated pulmonary nodule clinic. J Thorac Cardiovasc Surg. 2011;142(2):372–7. https://doi.org/10.1016/j.jtcvs.2011.04.015.
Ko JP, Berman EJ, Kaur M, Babb JS, Bomsztyk E, Greenberg AK, Naidich DP, Rusinek H. Pulmonary Nodules: growth rate assessment in patients by using serial CT and three-dimensional volumetry. Radiology. 2012;262(2):662–71. https://doi.org/10.1148/radiol.11100878.
Travis WD, Brambilla E, Noguchi M, Nicholson AG, Geisinger KR, Yatabe Y, Beer DG, Powell CA, Riely GJ, Van Schil PE, Garg K, Austin JH, Asamura H, Rusch VW, Hirsch FR, Scagliotti G, Mitsudomi T, Huber RM, Ishikawa Y, Jett J, Sanchez-Cespedes M, Sculier JP, Takahashi T, Tsuboi M, Vansteenkiste J, Wistuba I, Yang PC, Aberle D, Brambilla C, Flieder D, Franklin W, Gazdar A, Gould M, Hasleton P, Henderson D, Johnson B, Johnson D, Kerr K, Kuriyama K, Lee JS, Miller VA, Petersen I, Roggli V, Rosell R, Saijo N, Thunnissen E, Tsao M, Yankelewitz D. International association for the study of lung cancer/american thoracic society/european respiratory society international multidisciplinary classification of lung adenocarcinoma. J Thorac Oncol. 2011;6(2):244–85. https://doi.org/10.1097/JTO.0b013e318206a221.
Ichinose J, Kohno T, Fujimori S, Harano T, Suzuki S, Fujii T. Invasiveness and malignant potential of pulmonary lesions presenting as pure ground-glass opacities. Ann Thorac Cardiovasc Surg. 2014;20(5):347–52. https://doi.org/10.5761/atcs.oa.13-00005.
Matsuguma H, Mori K, Nakahara R, Suzuki H, Kasai T, Kamiyama Y, Igarashi S, Kodama T, Yokoi K. Characteristics of subsolid pulmonary nodules showing growth during follow-up with CT scanning. Chest. 2013;143(2):436–43. https://doi.org/10.1378/chest.11-3306.
Baldwin DR, Eaton T, Kolbe J, Christmas T, Milne D, Mercer J, Steele E, Garrett J, Wilsher ML, Wells AU. Management of solitary pulmonary nodules: how do thoracic computed tomography and guided fine needle biopsy influence clinical decisions? Thorax. 2002;57(9):817–22. https://doi.org/10.1136/thorax.57.9.817.
Gupta S, Krishnamurthy S, Broemeling LD, Morello FA Jr, Wallace MJ, Ahrar K, Madoff DC, Murthy R, Hicks ME. Small (≤2-cm) sub pleural pulmonary lesions: short-versus long-needle-path CT-guided biopsy: comparison of diagnostic yields and complications. Radiology. 2005;234(2):631–7. https://doi.org/10.1148/radiol.2342031423.
Wagnetz U, Menezes RJ, Boerner S, Paul NS, Wagnetz D, Keshavjee S, Roberts HC. CT screening for lung cancer: implication of lung biopsy recommendations. AJR Am J Roentgenol. 2012;198(2):351–8. https://doi.org/10.2214/AJR.11.6726.
Fontaine-Delaruelle C, Souquet PJ, Gamondes D, Pradat E, De Leusse A, Ferretti GR, Couraud S. Negative predictive value of transthoracic core-needle biopsy: a multicenter study. Chest. 2015;148(2):472–80. https://doi.org/10.1378/chest.14-1907.
Baaklini WA, Reinoso MA, Gorin AB, Sharafkaneh A, Manian P. Diagnostic yield of fiberoptic bronchoscopy in evaluating solitary pulmonary nodules. Chest. 2000;117(4):1049–54. https://doi.org/10.1378/chest.117.4.1049.
Powell HA, Tata LJ, Baldwin DR, Stanley RA, Khakwani A, Hubbard RB. Early mortality after surgical resection for lung cancer: an analysis of the English National Lung cancer audit. Thorax. 2013;68(9):826–34. https://doi.org/10.1136/thoraxjnl-2012-203123.
Ginsberg RJ, Rubinstein LV. Randomized trial of lobectomy versus limited resection for T1 N0 non-small cell lung cancer. Lung Cancer Study Group. Ann Thorac Surg. 1995;60(3):615–22; discussion 622–3. https://doi.org/10.1016/0003-4975(95)00537-u.
Detterbeck FC. Lobectomy versus limited resection in T1N0 lung cancer. Ann Thorac Surg. 2013;96(2):742–4. https://doi.org/10.1016/j.athoracsur.2013.03.074.
Billmeier SE, Ayanian JZ, Zaslavsky AM, Nerenz DR, Jaklitsch MT, Rogers SO. Predictors and outcomes of limited resection for early-stage non-small cell lung cancer. J Natl Cancer Inst. 2011;103(21):1621–9. https://doi.org/10.1093/jnci/djr387.
Okami J, Ito Y, Higashiyama M, Nakayama T, Tokunaga T, Maeda J, Kodama K. Sublobar resection provides an equivalent survival after lobectomy in elderly patients with early lung cancer. Ann Thorac Surg. 2010;90(5):1651–6. https://doi.org/10.1016/j.athoracsur.2010.06.090.
Miyoshi K, Toyooka S, Gobara H, Oto T, Mimura H, Sano Y, Kanazawa S, Date H. Clinical outcomes of short hook wire and suture marking system in thoracoscopic resection for pulmonary nodules. Eur J Cardiothorac Surg. 2009;36(2):378–82. https://doi.org/10.1016/j.ejcts.2009.03.039.
Koyama H, Noma S, Tamaki Y, Goto K, Kitamura E, Maeda T, Matsumoto S, Sano A, Sugimura K. CT localisation of small pulmonary nodules prior to thorascopic resection: evaluation of a point marker system. Eur J Radiol. 2008;65(3):468–72. https://doi.org/10.1016/j.ejrad.2007.04.019.
Mayo JR, Clifton JC, Powell TI, English JC, Evans KG, Yee J, McWilliams AM, Lam SC, Finley RJ. Lung nodules: CT-guided placement of microcoils to direct video-assisted thoracoscopic surgical resection. Radiology. 2009;250(2):576–85. https://doi.org/10.1148/radiol.2502080442.
Watanabe K, Nomori H, Ohtsuka T, Kaji M, Naruke T, Suemasu K. Usefulness and complications of computed tomography-guided lipiodol marking for fluoroscopy-assisted thoracoscopic resection of small pulmonary nodules: experience with 174 nodules. J Thorac Cardiovasc Surg. 2006;132(2):320–4. https://doi.org/10.1016/j.jtcvs.2006.04.012.
Vandoni RE, Cuttat JF, Wicky S, Suter M. CT-guided methylene-blue labelling before thoracoscopic resection of pulmonary nodules. Eur J Cardiothorac Surg. 1998;14(3):265–70. https://doi.org/10.1016/s1010-7940(98)00160-2.
Ambrogi MC, Melfi F, Zirafa C, Lucchi M, De Liperi A, Mariani G, Fanucchi O, Mussi A. Radio-guided thoracoscopic surgery (RGTS) of small pulmonary nodules. Surg Endosc. 2012;26(4):914–9. https://doi.org/10.1007/s00464-011-1967-8.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Editor’s Note
References: Main chapter references are included after the “References Editor’s Note” section.
Editor’s Note
Preoperative localization of solitary pulmonary nodules is crucial for excision by minimally invasive techniques. Indications for preoperative assisted localization include: (1) solitary or multiple small nodules i.e. <15 mm or nodules located in depth >10 mm from visceral pleura, (2) pure ground-glass or subsolid nodule, and (3) difficult to localize nodules via palpation intraoperative.
The localization techniques can be (a) CT guided (b) bronchoscopic guided (c) Virtual 3D assisted CT localization. The techniques employed in CT guided preoperative localization include: (1) percutaneous hookwire localization (2) percutaneous coil localization and (3) localization by percutaneous liquid material injection (lipoidal, methylene blue, indocyanine green etc).
Hookwire localization is the most commonly performed technique in which a hookwire trocar is inserted in a target area near the lesion under radiologic guidance with care taken to avoid puncturing the lesion. The recommended distance between the target area and nodule should be <1 cm. A post localization CT is done to confirm position of the hookwire and look for complications like pneumothorax. The percutaneous coil localization is similar to the hookwire localization, but entails use of coil instead of hookwire. Unlike the hookwire localization where the end of the hook is fixed to the skin, percutaneous coil localization is of two types in one type the percutaneous coil is positioned in the lung and the other the tail is positioned outside the visceral pleura.
Localization by liquid material injection such as lipoidal, methylene blue and indocyanine green have been used as alternative to hookwire and coil localization. In lipoidal injection the site turns into a mass and this enables identification in subsequent surgery. An inherent advantage with percutaneous lipoidal injection for localization is that surgery need not be immediately undertaken and can be done after 1–2 days as opposed to 1–2 h for percutaneous hook wire localization. Localization with methylene blue has limitations in a pigmented lung and also because of early diffusion thus calls for early operative intervention preferably in 1–2 h after injection. Indocyanine green injection and localization with use of intraoperative near infra-red technology is a promising approach and can also be used with preoperative 3D assisted printing localization technology.
The two main techniques employed for bronchoscopic localization are: (1) Localization under electromagnetic navigation bronchoscopy (2) virtual bronchoscopic navigation localization technology. In electromagnetic navigation bronchoscopy a probe with a sensor is guided into position with the help of an extracorporeal magnetic positioning plate. In virtual bronchoscopic navigation technology fluorescent dye is injected into the nodule aided by bronchoscope and subsequent computer based 3D mapping is used.
The operation for resection of the nodule entails excision of puncture site and tract as also area in which liquid material is injected in addition to ensuring a clear resection margin. Therefore. a site on visceral pleura as close to the nodule as possible should be selected for the puncture site. Some limitations of percutaneous localization techniques enlisted are: (1) tumor to visceral pleura distance >4 cm results in increased complications (2) location of tumor close to heart/ great vessel (3) accessibility of localization hampered by scapula or ribs. There are less chances of complications like pneumothorax and bleeding in bronchoscopic localization however the procedure is complex and has higher costs as well as limited accuracy.
References for Editor’s note
-
1.
Liu B, Gu C. Expert consensus workshop report: guidelines for preoperative assisted localization of small pulmonary nodules. J Can Res Ther. 2020;16:967–73.
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Ali, K., Bal, S. (2022). Management of Solitary Pulmonary Nodule. In: Sharma, D., Hazrah, P. (eds) Recent Concepts in Minimal Access Surgery. Springer, Singapore. https://doi.org/10.1007/978-981-16-5473-2_18
Download citation
DOI: https://doi.org/10.1007/978-981-16-5473-2_18
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-16-5472-5
Online ISBN: 978-981-16-5473-2
eBook Packages: MedicineMedicine (R0)