Abstract
Coronavirus Disease 19 (COVID-19) evolved into a pandemic to torment the world since the last 15 months and has resulted in millions of individuals getting affected. It has caused significant strain on the health care systems in developed and developing countries alike. It has killed more than two million patients globally. The potential of this virus to cause multi-organ dysfunction with associated significant mortality and morbidity has made it the most formidable enemy we have faced since the great plague. COVID-19 is becoming a mystery with its plethora of typical and atypical clinical presentations. Its ability to get attached to widely distributed human angiotensin-converting enzyme-2 (hACE2) receptors, has enabled it to cause multi-organ dysfunction and extensive disease. In this chapter, we review the pulmonary and extra-pulmonary manifestations of SARS-CoV-2 and try to elucidate organ-specific patho-physiology. Organ dysfunction leading to a myriad of cardiac dysfunction, symptoms related to gut and liver, nervous system involvement, renal and ocular injury is being discussed in this chapter. An effort to raise awareness of the potential to cause long covid syndrome is being made to identify the possible burden of morbidity we might have to experience post covid 19 pandemic.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
9.1 Introduction
SARS-CoV-2 virus pandemic has had a major impact on health care systems around the world. Patients infected with coronavirus present with a variety of symptoms varying in severity. Majority of the patients remain asymptomatic or develop mild and moderate symptoms at the most. Only some develop severe symptoms (dyspnoea, hypoxia, or > 50% lung involvement on imaging) and critical illness (acute respiratory distress syndrome, respiratory failure, shock, or multiorgan system dysfunction. The number of pre-existing/coexisting conditions is strongly associated with SARS-CoV-2 severity and mortality. The highest levels of the SARS-CoV-2 virus are detected in the respiratory tract. The other organs may also show some level. The important organs involved are the kidneys, liver, heart, brain and blood. Possible organ tropism of the virus might influence the course of the disease and may aggravate potential underlying conditions. In this chapter, we aim to discuss the possible pathogenesis of the organs involved, the acute effects and the long-term effects.
9.2 Lung Involvement
It is the primary organ involved and leads to major clinical manifestations in the form of disease or disability. The severity associated with SARS-CoV-2 infection ranges from no symptoms or mild pneumonia (in around 80%) to severe disease (characterized by hypoxia in 15%), a critical disease associated with shock, respiratory failure is only in around 5% of patients. Patients usually present with cough (initially dry), fever, expectoration, fatigue and dyspnea. The frequency of these reported symptoms varies in different studies from different geographic areas. 20–41% of all hospitalized patients go on to develop acute respiratory distress syndrome (ARDS).
SARS-CoV-2 infection causes alveolar and interstitial inflammation. This results in fever, cough and dyspnoea. Levels of pro-inflammatory cytokines and chemokines are significantly increased in patients with COVID-19 infection. The dysregulated cytokine response ‘cytokine storm’ plays a central role in the pathology of COVID-19 lung. What triggers the ‘cytokine storm’, the exact mechanisms behind it have not been identified as yet. There is evidence of extensive hemophagocytosis but it is very distinct from the classic macrophage activation syndrome (MAS). In addition to this, an immune suppression stage characterized by lymphopenia, low CD4 and CD8 T cell counts is also noted in many patients, increasing the risk of bacterial super infection.
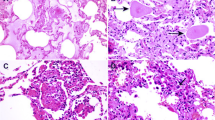
Autopsy studies have demonstrated acute interstitial pneumonia and diffuse alveolar damage (DAD). There is also significant macrophage infiltration, formation of hyaline membranes, alveolar wall oedema/thickening. These pathological changes manifest as severe hypoxemia. The microvasculature involvement with hyaline thrombosis is also noted in many studies on autopsy. Pulmonary haemorrhage, vessel wall oedema, intravascular neutrophil trapping and immune cell infiltration are other important pathological changes observed. The pulmonary vascular changes can at best be described as ‘diffuse pulmonary intravascular coagulopathy’ (PIC). These vascular pathological changes are mostly limited to the lungs. Pulmonary vasculopathy is driven by the proximity of type II pneumocytes and the pulmonary vasculature. Extensive microthrombi may lead to pulmonary infarction, haemorrhage, pulmonary hypertension and secondary right ventricular stress. Hypoxemia, high flow oxygen and alveolar trauma due to mechanical ventilation also seem to contribute to the development of PIC.
Hypoxia is one of the most important presenting features of COVID-19 lung pathology, but interestingly, it is often very well tolerated by patients. Therefore, the term ‘happy hypoxia’.
Lung compliance in covid 19 related ARDS is usually well preserved. Hypoxia-driven tachypnea allows high volumes and hypocapnia which fails to stimulate the sensation of dyspnea, a pathophysiological mechanism akin to hypobaric hypoxia observed at high altitudes.
Management is usually based on the duration of symptoms, type of lung injury and extent of lung injury. A high flow oxygen for hypoxia at early stages, simple and effective use of gravitational forces (using prone/lateral positioning), helps in effective diaphragmatic excursions, perfusion redistribution and better oxygenation. Awake proning has been incorporated in many hospital protocols and is very effective in preventing/delaying intubation and reversing hypoxemia. Patients with severe ARDS need higher PEEP volumes, prone positioning and may also need extracorporeal support in select settings.
The excessive increase in pro-inflammatory cytokines (IL-1 and, IL-6), interferon, TNF-α is an inflammatory over-reaction to SARS-CoV-2 infection. This dysregulated response ultimately leads to endothelial cell dysfunction, vascular endothelial damage, capillary leak and diffuse alveolar injury [35]. In this context, anti-IL-6 inhibitors (tocilizumab), inhibitors of JAK kinases (baricitinib), and corticosteroids have shown promising results in the management.
Not all symptoms resolve and may persist for a varying period depending on the severity of the pathology leading to Persistent or Long COVID (Fig. 9.1).
9.3 Heart and Blood Vessels
Underlying cardiovascular comorbidities (hypertension, diabetes, cardiovascular disease) are known to worsen outcomes in COVID 19 patients. Cardiovascular inflammation also complicates myocardial injury, ventricular dysfunction causing heart failure and arrhythmias responsible for sudden death in a certain group of individuals. Obesity has independently emerged as an independent risk factor for adverse cardiovascular outcomes.
Evidence of myocardial injury in patients has been a remarkable finding which persists even after clinical recovery. ACE2 expression in cardiac and vascular tissue potentiates direct damage due to viral infection. Presence of these receptors in multiple organs and their interaction with the SARS CoV2 virus could be responsible for multi-organ dysfunction characteristically seen in severe infections (Fig. 9.2). Down regulation of these receptors and resultant decrease in angiotensin 1–7 levels also compromises ventricular function leading to severe myocardial dysfunction.
Increase in inflammatory cytokines stimulates leucocyte adhesion molecule expression on the endothelial cells thereby making underlying atherosclerotic lesions vulnerable to disruption resulting in clinically pronounced acute coronary syndrome. Systemic cytokines induce dysfunction of the coronary microvasculature with resultant myocardial ischaemia/injury further compromising cardiac function (Fig. 9.3).
Mismatched myocardial oxygen demand and supply coupled with hypotension, during the cytokine storm syndrome, can reduce organ perfusion and may result in cascading effects on organ perfusion leading to multi-organ dysfunction.
Fulminant myocarditis with resultant severe hypotension/arrhythmias COVID-19 may result in severe left ventricular systolic dysfunction/cardiogenic shock.
A syndrome not unlike ‘Kawasaki-like syndrome’ has been reported too. These patients suffer due to a combination of circulatory dysfunction with macrophage activation syndrome. The manifestations are due to vascular and skin involvement. Persistent cardiovascular inflammation and residual vascular endothelial dysfunction could contribute to sudden cardiac deaths or persistent dyspnoea on exertion on follow-up.
9.4 Gastrointestinal Tract and Liver
The virus’s avid affinity for ACE2 receptors located in the ileum and colon is responsible for luminal symptoms. ACE2 receptor involvement is partially responsible for luminal inflammation, which manifests as diarrhoea in infected patients. Heavy load of virus in intestinal epithelial cells, development of diarrhea and shedding of enterocytes are possible mechanisms resulting in the virus transmission through the faecal–oral route. Only a small fraction of patients manifest gastrointestinal symptoms alone. The most common reported symptom was the loss of appetite. Nausea and vomiting are seen in the majority, nearly 70% of the patients, while diarrhoea and abdominal pain are seen in less than one-third of symptomatic patients (Fig. 9.4a).
Transient elevation of liver enzymes is commonly seen in most viral infections. Similarly in COVID-19 infected patients mild elevation of liver enzymes is described but this rise is usually less than 5 times the normal. Some authors have reported liver enzyme abnormalities in more than 50% of infected patients. Liver dysfunction is usually present in patients having the severe disease at the time of presentation to the hospital. Acute liver failure in the absence of underlying chronic liver abnormality has not been reported with Covid 19 infection. During the course of management, it becomes difficult to differentiate the individual contribution of either the infection or the other treatment modalities, such as antiviral agents or other drugs administered. The liver function abnormalities can be attributed to direct involvement of hepatocytes with the virus and resultant cytopathic effect or a result of a combination of cytokine storm, sepsis, hypoxia and/or hypotension.
In covid 19 survivors, the persistence of loss of appetite and symptoms suggestive of reflux have been reported even after 6 months of recovery. This is an important component of long COVID syndrome.
9.5 Haematological Manifestations
COVID-19 infection has a significant impact on the haematopoietic system. The most important haematological finding is lymphopenia, which has important prognostic potential. Cytokine storm occurs approximately 7–14 days from the onset of the initial symptoms with, significant lymphopenia becoming more evident. Neutrophil to lymphocyte ratio (NLR) has prognostic value in determining the severity of the illness. Cytokine activation has the potential to impair lymphocyte turnover. More than 95% of patients with severe COVID 19 patients have significant lymphocytopenia as compared to non-severe cases. Nearly two-third of severe cases have significant thrombocytopenia and leukopenia. Thrombocytopenia has been independently noted to have a significant association with the severity of the COVID-19 disease. Other important biomarkers of acute severe inflammation and infection, noted to be associated with poor prognosis, are high serum procalcitonin, high serum cortisol, high CRP and serum ferritin.
Hypercoagulability of blood, indicated by elevated D-dimer levels is common among hospitalized COVID-19 patients having severe disease. Gradual increase of D-dimer levels during the course of the disease is associated with deterioration and poor survival. Prothrombin time (PT) and activated partial thromboplastin time (aPTT) prolongation, rising fibrin degradation products, and life-threatening disseminated intravascular coagulation (DIC) are important parameters to be looked at for in severe cases. Significant endothelial dysfunction coupled with deregulated immune response can be implicated for these abnormalities. Up to 10% of COVID-19 infected patients develop clinically evident venous thromboembolism (VTE). Presence of significant underlying comorbidities like active malignancy/chronic liver disease/bedridden state/active trauma or surgical interventions, along with endothelial cell damage increases the risk of VTE. Pharmacological thrombo-prophylaxis, as in any other critically ill patient, with low molecular weight heparin, is recommended to prevent VTE. It has been noted that if during the acute episodes, therapeutic anticoagulation is administered, the risk of life-threatening bleeding increases significantly. Therefore unless indicated only thrombo-prophylaxsis, as indicated for the management of any bedridden critically ill patient, an appropriate dose should be administered.
9.6 Neurological Manifestations
Coronaviruses are neurotropic viruses and have been involved in direct CNS infection leading to neurological complications. Post-infectious, as well as para-infectious complications are increasingly being reported now with COVID 19 infection.
As many as one-third of patients have been reported to present with neurological manifestations. The commonest CNS symptoms reported are dizziness and headache. The most commonly hyped symptoms are taste impairment and anosmia but they have more psychological effects than actual association with severity. Other less frequent symptoms reported are altered consciousness, acute cerebrovascular disease, neuralgia, visual disturbances, rarely gait disturbances and seizure. Severely infected patients report a higher incidence of neurological symptoms.
Clinical manifestations might be due to direct viral infiltration or a result of hyper-inflammatory status (Fig. 9.5). Dysregulated immune response and resultant metabolic dysfunction might contribute to neurological manifestations in COVID-19 infection. Neurological manifestations of COVID-19 infection may also be a manifestation of severe systemic illness or related to it. Critically ill patients are more likely to develop encephalopathy, myopathy, autonomic neuropathy and polyneuromyopathy. SARS-COV-2 virus is postulated to enter the CNS through the retrograde neuronal route. Infection of olfactory neurons enables the virus to spread directly from the respiratory tract to the CNS. The extensive distribution of ACE2 receptors in the brain and endothelial cells may be responsible for direct neurological and skeletal muscle damage by the virus.
Severe systemic inflammatory response triggers dysregulation of the coagulation and diffuse intravascular coagulation resulting in an increased incidence of thrombotic complications in patients with COVID-19 infections. Imbalance between procoagulant and anticoagulant mechanisms may lead to cardiac dysfunction making a patient more vulnerable to cardio-embolism and subsequent complications. Hypotension or hypertension may also contribute by causing impaired cerebral perfusion or raised intracranial pressure causing cerebrovascular events.
Cerebrovascular disease related to hyper-coagulability may be associated with the COVID-19 pandemic but ischaemic stroke in covid 19 hospitalized patients have been reported denovo in less than 1% of patients. Whenever coexistent, patients with stroke and COVID-19 infection are known to have an increased risk of poor outcome and severe disability.
9.7 Anosmia and Ageusia
Olfactory (OD) and gustatory (GD) sense dysfunctions have been hyped as common symptoms of COVID-19 but the exact prevalence is not known as there could be under or over-reporting. Prevalence of smell and taste disturbances varies considerably. In a group of patients, nearly 85% were reported to have OD (anosmia or hyposmia) and GD (hypogeusia or ageusia). Women are more likely to suffer. Early olfactory recovery is a norm in the majority, while in some patients, symptoms could last even 14 days. OD and GD are not usually associated with milder disease but are present in patients with moderate to severe COVID19 infection.
Over the course of the pandemic, OD and GD have emerged as important screening tools despite the lack of a clear pathogenic mechanism explaining them. Specific viral neuro-invasivity and neuro-tropism, especially for olfactory nerves and the possibility of the temporal lobe, the amygdala, insula, limbic lobe (psycho sensorial syndrome) involvement may be a pathogenic mechanism.
9.8 Renal Involvement
Since ACE2 receptors are present in several cells in the kidney like podocytes, mesangial cells, epithelium of the Bowman’s capsule/tubules and ducts, the theoretical possibility of kidney damage is definitely there in COVID 19 infection. The most frequent abnormality is mild-to-moderate proteinuria without any pre-existing kidney damage. Several mechanisms have been postulated for this abnormality. High levels of inflammatory cytokines like IL-1β, IL-8, IFN-γ and TNF-α in these patients suggest a possibility of cytokine release syndrome (CRS). This is somewhat similar to sepsis-associated AKI, wherein uncontrolled inflammation leads to acute kidney injury. Altered haemodynamics can further add to acute renal dysfunction. Acute kidney injury (AKI) is more common in critically ill patients which may be seen in nearly one-third of patients in the intensive care unit. Angiotensin-converting enzyme 2 (ACE2), transmembrane serine protease 2 (TMPRSS2) and cathepsin L (CTSL) expressed in many kidney cells, have been implicated for inducing SARS-CoV-2 associated kidney injury. Affinity of SARS-CoV-2 to attach to receptors on renal cells leading to viral overload in the cells could be another reason for acute kidney injury. It is also being increasingly recognized now that kidney involvement is associated with worse outcomes. Most of these acute changes are reversible.
9.9 Skin Manifestations
Skin abnormalities can be observed in as many as 20% of COVID-19 infected patients. The presentation of clinical abnormalities is heterogeneous and varies from urticarial to vesicular and from purpuric to papulosquamous lesions. Clinical involvement in skin, in the form of a morbilliform rash, urticarial lesions, livedo reticularis, and vesicular, varicella-like eruptions have been commonly reported (Fig. 9.6). A severe multisystem inflammatory syndrome having significant mucocutaneous findings akin to severe Kawasaki disease has also been reported with COVID19.
It is not very clear if these skin manifestations are due to direct virus inoculation/invasion or secondary to dysregulated host immune response. Some of these lesions could be a result of vasculopathy associated with SARS COV2 infection. The nature of the association between COVID-19 and skin lesions and the systemic implications of their presence remains to be determined.
In addition, skin involvement due to mechanical/friction dermatitis, and contact dermatitis due to personal protective equipment (PPE)/sanitizers have been reported in health care workers. Although not directly related to the covid 19 virus, these skin manifestations need to be taken into account as pandemic related skin manifestations and add to the burden of clinical cases during COVID-19 infection management.
9.10 Endocrine Abnormalities
Stress of infection and dysregulated immune response leads to variety of endocrine abnormalities and may unmask pre-existing subclinical diseases. SARS-CoV2 expresses amino acids mimicking host adrenocorticotropic hormone (ACTH) and antibodies to ACTH mimics may lead to a relative cortisol insufficiency. In one study among patients, high cortisol levels were observed at presentation. This may indicate hormonal stress due to severe systemic. Very high cortisol at presentation in severely ill patients has been found to be associated with poor outcomes. Autopsy studies have shown necrosis of the adrenal gland. High load of virus in the glands may point towards damage and resultant altered cortisol dynamics in patients. The hypothalamic–pituitary–adrenal (HPA) axis may also be involved by the virus directly, leading to hypophysitis or direct hypothalamic damage. High level of ACE2 expression in these tissues may explain possible viral tropism and resultant damage.
Unmasking of Type 2 diabetes mellitus, severe illness in established poorly controlled diabetics and predisposition to infections/vascular adverse events complete the conundrum of virus related damage in diabetics. A number of patients develop ‘sick euthyroid syndrome’ especially when the critical phase of illness sets in.
9.11 Ocular Manifestations
The human conjunctiva is considered to be a potential site for SARS-CoV-2 infection and possible transmission. Currently there is no evidence that viral replicates and can be shed especially in tears. Direct viral inoculation may lead to injury and inflammation of the conjunctiva or cornea. Therefore the current recommendations necessitates the use of protective eye gear for all healthcare workers involved in the care of COVID 19 infected patients. In some initial studies, as many as 30% of patients have been noted to have ocular manifestations. The commonest manifestation is dry eye and foreign body like sensation whereas keratitis and conjunctival follicular reactions have also been observed by some. (Ocular Manifestations of COVID-19; Nasiri et al) Other common manifestations include conjunctival congestion, chemosis or epiphora. Some studies have suggested possible prolonged shedding of virus in the tears and significant retinal involvement. The exact extent and patho-physiology responsible for the ophthalmic manifestations associated with SARS-CoV-2 are not fully understood.
9.11.1 Persistent/Long COVID
Large number of investigators have shown persistent residual respiratory and cardiac dysfunction in patients recovering from severe COVID19 infections, especially the ones requiring high flow oxygen and ventilation. These lung and cardiac changes take a long time to recover. Another important complication reported is serious psychological morbidity and chronic fatigue in survivors. Nearly one-third of survivors report these chronic symptoms. Older patients and patients with multiple pre-existing comorbidities are more likely to have these symptoms. Persisting symptoms in covid survivors are likely to adversely affect the quality of life and result in significant morbidity. Persistence of these symptoms points towards the potential of COVID 19 infection to leave the world crippled.
Take Home Message
COVID-19 probably presents with a broad spectrum of clinical signs and symptoms. There may be significant involvement of deep-seated vital organs (heart, lung, CNS & Kidney) or milder involvement of superficially located organs (eyes and skin). Multisystem involvement associated with deregulated immune response leading to organ failure might lead to adverse clinical outcomes and increased morbidity/mortality. The high binding affinity to omnipresent ACE2 receptors is postulation for multi-organ involvement. The exact role of the dysregulation of ACE2 receptors expression, presence of pre-existing comorbidities, dysregulated immune response and/or direct viral damage are some questions that need to be addressed. The unpredictability of this pandemic needs more careful surveillance, customized control measure implementation, and specifically focused medical research looking at various treatment strategies to guide our management. There is a need to address the long-term effects and morbidity in COVID survivors to improve quality of life in post COVID era.
Suggested Reading
Buja LM, Wolf D, Zhao B et al (2020) Emerging spectrum of cardiopulmonary pathology of the coronavirus disease 2019 (COVID-19): report of three autopsies from Houston, Texas and review of autopsy findings from other United States Cities. Cardiovasc Pathol 48:107233
Cui J, Li F, Shi ZL (2019) Origin and evolution of pathogenic coronaviruses. Nat Rev Microbiol 17:181–192
Ding Y, He L, Zhang Q et al (2004) Organ distribution of severe acute respiratory syndrome (SARS) associated coronavirus (SARS-CoV) in SARS patients: implications for pathogenesis and virus transmission pathways. J Pathol 203:622–630
Genovese G, Moltrasio C, Berti E, Marzano AV (2021) Skin manifestation in COVID 19. Dermatology 237:1–12
Guarneri C, Rullo EV, Pavone P et al (2021) Silent COVID-19: what your skin can reveal. Lancet Infect Dis. 21(1):24–25. https://doi.org/10.1016/S1473-3099(20)30402-3
Han H, Xie L, Liu R et al (2020) Analysis of heart injury laboratory parameters in 273 COVID-19 patients in one hospital in Wuhan, China. J Med Virol. 92(7):819–823. https://doi.org/10.1002/jmv.25809
Inciardi RM, Lupi L, Zaccone G et al (2020) Cardiac involvement in a patient with coronavirus disease 2019 (COVID-19). JAMA Cardiol. 174:30–33
Keyviyarasi R, Pureti LP et al (2020) Coronavirus pathogenesis, comorbidities and multioragna damage—A review. Life Sci 255:117839
Lechien JR, Chiesa-Estomba CM, De Siati DR et al (2020) Olfactory and gustatory dysfunctions as a clinical presentation of mild-to-moderate forms of the coronavirus disease (COVID-19): a multicenter European study. Eur Arch Otorhinolaryngol 277(8):2251–2261
Li H, Liu L, Zhang D et al (2020) SARS-CoV-2 and viral sepsis: observations and hypotheses. Lancet. 395:1517–1520
Lillicrap D (2020) Disseminated intravascular coagulation in patients with 2019-nCoV pneumonia. J Thromb Haemost. 18:786–787
Lippi G, Plebani M (2020) Procalcitonin in patients with severe coronavirus disease 2019 (COVID-19): a meta-analysis. Clin Chim Acta. 505:190–191
Lippi G, Plebani M, Henry BM (2020) Thrombocytopenia is associated with severe coronavirus disease 2019 (COVID-19) infections: a meta-analysis. Clin Chim Acta. 506:145–148
Mao R, Qiu Y, He JS et al (2020) Manifestations and prognosis of gastrointestinal and liver involvement in patients with COVID-19: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol 5(7):66–678
McGonagle D, O’Donnell JS, Sharif K et al (2020) Immune mechanisms of pulmonary intravascular coagulopathy in COVID-19 pneumonia. Lancet. 2(7):e437–e445
Nath A (2020) Neurologic complications of coronavirus infections. Neurology. 94:809–810
Ntaios G, Michel P, Georgiopoulos G et al (2020) Characteristics and outcomes in patients with COVID-19 and acute ischemic stroke. The global COVID-19 stroke registry. Stroke 51(9):e254–e258. https://doi.org/10.1161/STROKEAHA.120.031208
Ong J, Young BE, Ong S (2020) COVID-19 in gastroenterology: a clinical perspective. Gut. 69:1144–1145
Pal R (2020) COVID-19, hypothalamo-pituitary-adrenal axis and clinical implications. Endocrine 28:1–2. https://doi.org/10.1007/s12020-020-02325-1
Puelles VG, Lutgehetmann M, Lindenmeyer MT et al (2020) Multiorgan and renal tropism of SARS-CoV-2. N Engl J Med. 383(6):590–592
Ragab D, Salah Eldin H, Taeimah M et al (2020) The COVID-19 cytokine storm; what we know so far. Front Immunol. 11:1446
Recalcati S (2020) Cutaneous manifestations in COVID-19: a first perspective. J Eur Acad Dermatol Venereol 34(5):e21–e213. https://doi.org/10.1111/jdv.16387
Richardson S, Hirsch JS, Narasimhan M et al (2020) Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA. 323(20):2052–2059
Seah I, Agrawal R (2020) Can the coronavirus disease 2019 (COVID-19) affect the eyes? A review of coronaviruses and ocular implications in humans and animals. Ocul Immunol Inflamm. 28:391–395
South AM, Diz DI, Chappell MC (2020) COVID-19, ACE2, and the cardiovascular consequences. Am J Physiol Heart Circ Physiol. 318:H1084–H1090
Tan T, Khoo B, Mills EG et al (2020) Association between high serum total cortisol concentrations and mortality from COVID-19. Lancet Diabetes Endocrinol. 8(8):659–660. https://doi.org/10.1016/S2213-8587(20)30216-3
Terpos E, Ntanasis-Stathopoulos I, Elalamy I et al (2020) Hematological findings and complications of COVID-19. Am J Hematol 95(7):834–847. https://doi.org/10.1002/ajh.25829
Thakur V, Ratho RK, Kumar P, Bhatia SK, Bora I, Mohi GK, Saxena SK, Devi M, Yadav D, Mehariya S (2021) Multi-organ involvement in COVID-19: beyond pulmonary manifestations. J Clin Med 10:446
Varga Z, Flammer AJ, Steiger P et al (2020) Endothelial cell infection and endotheliitis in COVID-19. Lancet. 395:1417–1418
Verdoni L, Mazza A, Gervasoni A et al (2020) An outbreak of severe Kawasaki-like disease at the Italian epicentre of the SARS-CoV-2 epidemic: an observational cohort study. Lancet 395:1771–1778. https://doi.org/10.1016/S0140-6736(20)31103-X
Wang Y, Lu X, Chen H et al (2020) Clinical course and outcomes of 344 intensive care patients with COVID-19. Am J Respir Crit Care Med. 201(11):1430–1434
Wheatland R (2004) Molecular mimicry of ACTH in SARS—implications for corticosteroid treatment and prophylaxis. Med Hypotheses. 63:855–862
Wu Z, McGoogan JM (2020) Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72,314 cases from the Chinese center for disease control and prevention. JAMA. 323(13):1239–1242
Zhou G, Chen S, Chen Z (2020) Advances in COVID-19: the virus, the pathogenesis, and evidence-based control and therapeutic strategies. Front Med. 14(2):117–125
Zubair AS, McAlpine LS, Gardin T et al (2020) Neuropathogenesis and neurologic manifestations of the coronaviruses in the age of coronavirus disease 2019: a review. JAMA Neurol 77:1018–1027
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 The Author(s), under exclusive licence to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Bhalla, A., Suri, V. (2021). Organ Involvement in COVID 19: Lung and Beyond. In: Sobti, R.C., Dhalla, N.S., Watanabe, M., Sobti, A. (eds) Delineating Health and Health System: Mechanistic Insights into Covid 19 Complications. Springer, Singapore. https://doi.org/10.1007/978-981-16-5105-2_9
Download citation
DOI: https://doi.org/10.1007/978-981-16-5105-2_9
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-16-5104-5
Online ISBN: 978-981-16-5105-2
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)