Abstract
Throughout the world, billions of people are infected with various diseases and continue to suffer from them despite various treatments. Vaccination is commonly regarded as one of the most advanced approaches to disease prevention. RNA-based innovations have sparked widespread interest in the production of prophylactic and therapeutic vaccines over the last two decades. Because of their high efficacy, safe administration, and low manufacturing cost, mRNA vaccines have emerged as a promising tool for disease prevention. In animal models and humans, mRNA vaccines can induce a healthy, long-lasting cellular and humoral immune response. Furthermore, mRNA is an intrinsically secure vector that is just a transient carrier of information that does not interfere with the genome and provides full production versatility. Following the outbreak of COVID-19 in December 2020, mRNA-based vaccines made headlines in 2020. This chapter covers mRNA vaccines (both traditional and alternative), their delivery, immune responses elicited by them, and mRNA vaccines for infectious disease prevention.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
15.1 Introduction
Vaccination is a powerful method to prevent disease progression every year or to reduce the occurrence of diseases like measles, smallpox, polio, hepatitis, mumps, rubella, classic swine fever, and cattle plague. Attenuated vaccines, inactivated vaccines, and subunit vaccines developed as conventional vaccines provide adequate defense against various infectious diseases. It is difficult to develop a vaccine against such pathogens which evade the host immune response. So, there is a need of more powerful and scalable platform to develop a vaccine. In the nineteenth century, nucleic acid therapeutics were seen as potential alternatives to conventional vaccines. Transcribed mRNA (IVT) and vasopressin-encoding mRNA were used in mice and rat but the findings were not promising (Wolff et al. 1990; Jirikowski et al. 1992). Thanks to substantial technological innovation and research support over the last decade, mRNA has emerged as a possible therapeutic mode in vaccine development. In comparison to DNA vaccines, subunit vaccines, and killed and live attenuated vaccines, mRNA vaccines are noninfectious, safe, more robust, and highly translatable. These vaccines have a distinct genetic vector and can be used several times. mRNA vaccines have the potential for quick, low-cost, and efficient production due to the high yields of in vitro transcription reactions (Karikó et al. 2008; Thess et al. 2015). Any protein can be encoded and expressed by mRNA, allowing for the development of prophylactic and therapeutic vaccines against any disease. Since changes to the encoded protein only change the sequence of the RNA molecule, its physicochemical properties remain relatively static, and a variety of products can be produced using the same existing manufacturing method, saving time and money compared to other vaccine platforms.
15.2 Advantages of mRNA Vaccines
mRNA-based vaccines have several advantages:
-
1.
Many features of natural infections can be mimicked. mRNA enters in cells and produces antigen proteins within the cell through posttranslational modifications. mRNA vaccines have the ability to produce more viral proteins within a cell, and to increase immune responses, including B- and T-cell responses.
-
2.
Multiple mRNA vaccines encoding multiple virus proteins allow the production of complex multimeric antigens. CMV vaccine, for example (mRNA-1647), includes six mRNAs, five of them coating five distinct proteins that form an immunologically critical CMV protein complex.
-
3.
In silico designing of antigens and rapid production and testing of antigens cause advancement of mRNA vaccine program.
-
4.
mRNA vaccine triggers a very strong T-cell response.
-
5.
mRNA vaccine production does not require product-devoted production practices, facilities, and purification steps. mRNA production is an easy, cost-effective process that makes vaccines available to low-income people.
-
6.
RNA is associated with RNases; mRNA produced in sterile conditions can be stored at −80 °C for months or even years.
-
7.
In safety terms, mRNA is safe because of the transient nature of mRNA and the typical low doses of vaccination.
15.3 Concept and Form of mRNA Vaccines
The protein-encoding DNA is transcribed into mRNA, which is then translated into proteins. The production and delivery of RNA vaccines vary depending on the type of mRNA vaccines. The production of therapeutically useful in vitro-transcribed mRNA is optimally translated. In vitro-transcribed mRNA is synthesized from the linear DNA molecule, having an open reading frame consisting of the protein of interest, flanking UTRs, a 5′ cap, and a poly (A) tail. Further, it is processed into mature mRNA molecules in the cytoplasm of eukaryotic cells. In cytoplasm there are various extracellular RNases that degrade the mRNA. The cellular translation machinery transforms the transported mRNA into protein which then undergoes posttranslational modifications, resulting in a fully functioning protein (Weissman 2015).
The principle for developing an mRNA vaccine is surprisingly simple. The gene sequence of selected antigen from the targeted pathogen is synthesized and cloned into the plasmid DNA. In vitro-transcribed mRNA vaccine is administrated to the subjects which elicit potent humoral and cellular immune responses by using the host cell machinery. The signal peptide and transmembrane domain help in the transport of antigens to their cellular location or to the correct cellular compartment. As a consequence, the antigen may be expressed as a protein that is intracellular, secreted, or membrane bound (Maruggi et al. 2017). When the host’s innate immune system recognizes any RNA sequences of viral origin, mRNA vaccines induce robust innate responses, which recruit chemokines and cytokines such as interleukin-12 (IL-12) and tumor necrosis factor (TNF-α), at the injection site (Pepini et al. 2017; Edwards et al. 2017). Currently, two types of mRNA vaccines are used: traditional mRNA/non-replicating mRNA with 5′ and 3′ untranslated regions (UTRs) flanking the antigen of interest, and self-amplifying mRNA derived from positive-stranded RNA virus genomes, encodes both the antigen and the viral replication machinery (Maruggi et al. 2019). Currently, there are two types of mRNA vaccines used:
15.3.1 Non-replicating mRNA/Conventional mRNA Vaccines
These vaccines carry ORFs having antigen-coding sequences flanked with 5′ and 3′ UTRs, cap, and poly (A) tail through which mRNA interacts with the translational machinery for the expression of antigen (Jansen 2001). Additionally, the codon substitution procedure has been employed in DNA, RNA, and viral vector vaccines to enhance protein expression by replacing uncommon codons with widely used compatible codons. However, a recent review suggests that codon optimization does not increase the production of proteins for mRNA therapeutics (Mauro and Chappell 2014). The uniformity and small size of the RNA molecule are advantages of the traditional mRNA vaccine approach (Fig. 15.1a).
(a) Convention mRNA construction with supporting coding sequences, (b) self-amplifying mRNA construct (source: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6963228/)
Expression of the mRNA is limited due to its stability and activity under in vivo conditions. However, antigen expression and tenability can be improved by remodeling the structure of mRNA (Ross 1995). To elicit an antigen-specific immune response, mRNA vaccines must be expressed in situ; thus, vaccine optimization is important to understand the durability and consequences of antigenic expression following mRNA injections. mRNA formulation, route of administration, nucleoside modifications, and sequence optimization can influence kinetics and translational magnitude (Pardi et al. 2015). Intramuscular vaccination of unmodified mRNA induced protein expression in vivo, which lasted at least 6 days (Vogel et al. 2018).
Nucleoside base modification is an emerging fast modification approach for the production of modified mRNA that improves mRNA potency by reducing pattern recognition receptor (PRR) activation and dsRNA contaminant removal. Nucleoside modification enhanced protective immune response in mice and nonhuman primates (NHPs) against influenza and Zika viruses (Maruggi et al. 2019). Sequence-optimized mRNA (high GC content and optimized UTRs) formulated with lipid nanoparticles (LNPs) elicited robust effective antibody titers against rabies and influenza antigens in rodents (Thess et al. 2015). Another technology called RNActive technology enhances the adjuvant properties that can be beneficial for vaccination. A sequence-optimized, unmodified mRNA and a protamine-containing noncoding RNA complex serve as carriers in such adjusted vaccines (Rauch et al. 2017). In brief, the most advanced traditional mRNA vaccines (sequence-optimized mRNA, nucleoside-modified mRNA, and mRNA adjuvanted with protamine-complexed noncoding RNA) have been currently studied in preclinical and clinical trials.
15.3.2 Self-Amplifying mRNA Vaccines
Positive-sense ssRNA viruses such as picornaviruses, flaviviruses, and alphaviruses have engineered RNA genomes that can be used to make self-amplifying mRNA vaccines. Venezuelan equine encephalitis viruses (VEEVs), SINV, Semliki Forest virus (SFV), and Sindbis virus are some of the well-studied self-amplified mRNA molecules. Self-amplifying mRNA replicons are made by substituting the viral structural genes with the interested antigen gene, and then delivering them to target cells’ cytoplasm, where they can undergo RNA amplification and express the desired antigen in large amount (Fig. 15.1b). Due to the lack of endogenous viral structural genes, these replicons develop contagious virions or viruslike particles at the vaccination site, significantly diminishing the safety issues linked with a live attenuated virus vaccine. Self-amplifying mRNA could be generated in vitro or by delivering plasmid DNA containing the RDRP complex and the antigen of interest (Ljungberg and Liljeström 2015). After injecting self-amplifying mRNA or unmodified traditional mRNA into mice, the kinetics of antigen expression, immune responses, and protective efficacy were studied. They observed that the self-amplifying mRNA vaccine produced more potent immune responses and released much higher levels of the protein over a longer period of time than the standard mRNA vaccine (Vogel et al. 2018). The dsRNA amplification intermediates, as well as the magnitude and length of antigen expression parameters of self-amplifying mRNA, all lead to an increased immune response, which activates innate immunity, triggers host-sensing machinery, and confers an adjuvant effect (Magini et al. 2016).
The ability to encode several antigens in the same replica is another aspect of the self-enhancing mRNA vaccine platform. This may allow the development of vaccine expressing both a target antigen and an immunomodulating biological molecule for enhanced efficacy, vaccine encoding B-cell and T-cell antigen, single combo vaccine targeting multiple pathogens, or vaccine targeting multi-subunit complex antigens (Maruggi et al. 2019). Self-amplifying mRNA replicon expressing five full-length subunits (gH, gL, UL128, UL130, and UL131A) of the human cytomegalovirus (HCMV) pentameric complex elicits potent and broadly neutralizing antibodies in mice (Wen et al. 2014). The self-amplifying mRNA vaccine encoding HCMV gH/gL formulated with LNPs induced antibodies and CD4+ T cells. Similarly, self-amplifying mRNA vaccine (0.1–0.2 mg) encoding influenza M1 and/or NP elicits potent antigen-specific T-cell responses and protection from viral challenge in mice (Magini et al. 2016).
In summary, self-amplifying mRNA is a flexible technology that can be used to produce single- or multi-antigen vaccines with low doses, high antigen expression, and a powerful, intrinsic adjuvant effect. The rational design of improved self-amplifying mRNA vaccines will be possible with self-amplifying mRNA replicon with innate immunity and consequent modulation of antigen-specific immediate response.
15.4 Delivery System for mRNA Vaccines
In order to achieve their target and perform well, mRNA vaccines need a delivery system because of the nuclease degradation and the large and negative burden of the naked RNA impedes the crossing of the cell membrane. Vehicles or delivery systems or methods for transporting RNA into cells are necessary. The vehicle (endosome) should cross the cell membrane of target cell after endocytosis and released and the mRNA cargo into the cytosol, where it is translated (Stanton 2018). Several major delivery vehicles are currently being investigated, some of which encapsulate mRNA molecules in particles and some polymers bind to RNA through charge interaction (Houseley and Tollervey 2009). For delivery of nonviral RNA, formulated lipid nanoparticles (LNPs) are commonly used as they increase the fusogenicity and endosomal escape. In addition, cationic ionizing amino lipids which condense with nucleic acid, poly(ethylene) glycol (PEG) lipids which provide steric stabilization, and cholesterol that enables vesicle stability in vivo are also used for RNA delivery (Cullis and Hope 2017). Several amino lipids were developed for siRNA delivery and the first therapeutic siRNA delivered by LNP was approved by the FDA (United States Food and Drug Administration) in 2018 (Alnylam Pharmaceuticals 2018) (http://investors.alnylam.com/news-releases/news-release-details/alnylam-announces-first-everfda-approval-rnai-therapeutic). Self-replicating mRNA vaccines delivered via polymer have shown a lot of considerable results. mRNA comprises high-amine-density molecule with branching structures eliciting CD8+ T-cell and antibody responses that protect mice from lethal challenge by Ebola virus, H1N1 influenza, and Zika viruses (Chahal et al. 2016; Chahal et al. 2017). The two-vial method was developed where the delivery formula can be produced and stored separately from the target mRNA. A delivery vehicle based on emulsion was used to bind with self-amplifying mRNA to the surface of the nanoparticle before immunization.
In mice, rats, and rabbits, self-amplifying mRNA delivered through cationic nanoemulsion elicited potent immune responses against viral, bacterial, and parasite infections (Bogers et al. 2015; Baeza Garcia et al. 2018). The effectiveness of mRNA is also influenced by the route of administration, which is a significant step in RNA expression. Vaccines are usually given as i.d., i.m., or s.c. injections into dendritic cell-rich tissue such as the skin and skeletal muscles. Optimized cationic lipids and lipoplex formation have been developed to enhance DC targeting by increasing the absorption, release, and translation of a self-amplifying mRNA molecule (Englezou et al. 2018). Vaccination of lipoplex-formulated self-amplifying mRNA-encoded influenza NP antigen elicited pro-inflammatory cytokines, humoral responses, and T cellular responses in mice. Vaccine thermal stability is another issue for storage and delivery in countries with inadequate cold-chain infrastructure.
15.5 Immune Response by mRNA Vaccines
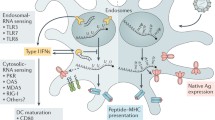
Endosomal Toll-like receptors (TLRs) and retinoic acid-inducible gene-I (RIG-I) receptors are the two major RNA receptors involved in the activation of the innate immune system after mRNA recognition. TLRs (TLR-3, TLR7, TLR8, and TLR9) are located in the endosomal compartment of antigen-presenting cells (APCs), such as DCs, macrophages, and monocytes (Lee and Barton 2014). TLR3 detects dsRNA while TLR7 and TLR8 both detect ssRNA. TLR7 acts through the NF-kB pathway, whereas TLR7 and TLR8 lead to production of type I interferons (IFNα/β) and stimulation of B-cell response TLR3 recognizes dsRNA, while TLR7 and TLR8 recognize ssRNA. TLR7 activates the NF-kB pathway, while TLR7 and TLR8 activate the B-cell response by generating type I interferons (IFN/) (Takeuchi and Akira 2010; Ablasser et al. 2009). The RIG-I-like receptor family (RLR), which is found in the cytoplasm, recognizes single- and double-stranded RNA and stimulates IFN synthesis by recognizing ssRNA and dsRNA having a 5′ triphosphate (Sabbah et al. 2009). Type I interferons are primarily produced following the administration of an mRNA vaccine, which modulates antigen expression, APC activity, and T-cell differentiation (De Beuckelaer et al. 2017).
TCR elicited the desired T-cell response, including CD8 T-cell differentiation and proliferation into cytotoxic T cells, when they received the signals (Agarwal et al. 2009). STAT1 is triggered and anti-proliferation and pro-apoptosis events are initiated when IFN signaling occurs before the TCR is activated (Tanabe et al. 2005). As a result, it is proposed that the interferon type I response be controlled in order to enhance mRNA vaccine candidate translation and, as a result, vaccine potency (Pepini et al. 2017).
During mRNA processing, APCs present the mRNA-derived peptide through major histocompatibility complex (MHC) class I or II for activation and differentiation of T cell from naive T cells to effector cells. T-cell activation and differentiation also depend on the binding of co-stimulatory molecules, such as CD80 and CD86, by CD28 to T cells and secreted cytokines. The humoral response is recognized by antibodies secreted from B cells. mRNA vaccine-derived antigens bind to the B-cell receptors (BCR) and induce the production of antigen-specific antibodies (Charles et al. 2001; Lindgren et al. 2017). The accessibility of extracellular protein for B-cell recognition can be increased by using a secretion of signal peptide in the RNA sequence or by adding an MHC class II targeting sequence of a lysosomal or endosomal protein, such as lysosomal associated membrane protein (LAMP), in mRNA vaccines. mRNA-derived peptides are displayed on the B cell through MHC class II which bind to receptors of dendritic cell displaying the MHC II/peptide and release activation signals and cytokines. B cells proliferate and differentiate into memory B cells and antibody-secreting plasma cells (Lindgren et al. 2017). In conclusion, mRNA stimulates antigen-specific T-cell responses and increases the number of B cells, resulting in long-lasting antibodies (Pardi et al. 2018) (Fig. 15.2).
Messenger RNA-encoded vaccine antigen processing in antigen-presenting cells (APCs) (source: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6963228/)
15.6 mRNA Vaccines for Infectious Disease Prevention
mRNA vaccines have been studied extensively for the prevention of infectious diseases. mRNA vaccines against infectious diseases could be developed as prophylactic or therapeutic. T-cell and humoral immune responses are both induced when mRNA vaccines expressing antigens of infectious pathogens are used (Iavarone et al. 2017). When compared to whole microbe, live attenuated, and subunit vaccines, the development of mRNA vaccines is fully cell free, simple, and fast. Because of its pace and simplicity, mRNA is a promising product that may be able to bridge the gap between emerging infectious diseases and the urgent need for successful vaccines (Zhang et al. 2019).
15.6.1 mRNA Vaccines for Influenza Virus Infection
The most commonly used and FDA-approved mRNA vaccines against influenza virus were developed using conventional methods and showed promising results in a mouse model. During an outbreak of a deadly strain of H7N9 influenza in China in 2013, a self-amplifying mRNA vaccine encoding influenza HA antigen showed promising results (Krammer and Palese 2015). Unmodified conventional mRNA encoding various influenza virus antigens in combination with a protamine-complexed RNA adjuvant was found to be immunogenic and offered full defense against influenza virus in mice, ferrets, and domestic pigs (Petsch et al. 2012). T- and B-cell immune responses are induced by conventional or self-amplifying mRNA-based influenza vaccines formulated with LNP or CNE. Antiviral activity against influenza has also been found effective for chitosan, polyethylenimine (PEI), and dendrimer-based self-amplifying mRNA (Démoulins et al. 2016; Chahal et al. 2016). After i.d. and i.m. vaccination in humans, LNP-formulated N1-methyl-pseudouridine-modified traditional mRNA encoding HA of the H10N8 pandemic influenza strain displayed H10-specific memory B cells as well as a temporary presence of plasmablasts (Suan et al. 2017; Pilkinton et al. 2017). The first human trial of an mRNA-based influenza vaccine was recently released, combining nucleoside-modified traditional mRNA encoding an H10N8 HA antigen with LNP formulation (Bahl et al. 2017).
15.6.2 mRNA Vaccines for Zika Virus Infection
LNP-formulated nucleoside-modified traditional mRNA developed impressive neutralizing titers and provided protection against Zika virus challenge in mice (Pardi et al. 2017). Another study found that an mRNA vaccine encoding a modified Zika prM-E antigen with a mutated conserved fusion-loop epitope in domain II of the E protein protects the host from Zika virus infection while also increasing vaccine protection. Furthermore, this vaccine decreased antibody development, increasing dengue virus infection in cells or mice (Richner et al. 2017). A single i.m. vaccination with a Zika self-amplifying mRNA vaccine formulated with nanostructured lipid carriers was found to be adequate to improve immune responses and fully protect mice against a Zika virus challenge (Erasmus et al. 2018).
15.6.3 mRNA Vaccines for Rabies Virus Infection
A protamine-formulated and sequence-optimized traditional mRNA vaccine encoding rabies virus glycoprotein (RABV-G) induces protective immunity in mice and produces neutralizing antibody response in pigs (Schnee et al. 2016). Humans vaccinated with the rabies mRNA vaccine had a positive response with some side effects such as mild-to-moderate injection-site reactions, fever, fatigue, and pain. The delivery mechanism for the rabies mRNA vaccine was optimized to achieve a long-term immune response (Lutz et al. 2017). The neutralization titer of the LNP-formulated rabies mRNA vaccine was found to be higher. A phase I clinical trial to test LNP-formulated sequence-optimized mRNA vaccine (ClinicalTrials.gov. NCT04380701 2020) has recently begun (CureVac 2018).
15.6.4 mRNA Vaccines for Cancer
Designing of cancer vaccines is mainly based on the tumor-associated gene of the target cell. Most of the vaccines developed till date are therapeutic vaccines that stimulate cell-mediated responses. For example cellular responses generated from CTLs reduce the tumor burden (122). Dendritic cells present the antigens that play a central role in generating antigen-specific immune responses. In 1996, Boczkowski and colleagues reported that electroporated DCs with mRNA induce robust immune responses against tumor antigens (124). Various immune-regulatory proteins have been discovered that enhance the strength of cancer vaccines. The immune-stimulatory function of DCs was significantly increased via production on IL-12 after electroporation with mRNAs encoding co-stimulatory molecules such as CD83, tumor necrosis factor receptor superfamily member 4 (TNFRSF4, also known as OX40), and 4-1BB ligand (4-1BBL) (125–128). Cocktail of mRNA encoding adjuvants CD70, TLR4, and CD40L enhances the DC activation and transposition of CD4+ T cells in T helper cells (Th1) (132–136). DC vaccines have also been used in several clinical trials to treat metastatic prostate cancer, metastatic lung cancer, renal cell carcinoma, brain cancers, melanoma, acute myeloid leukemia, and pancreatic cancer. The outcomes of mRNA vaccines depend on the route of administration (intradermal, intramuscular, subcutaneous, or intranasal) and some unconventional routes of vaccination (intranodal, intravenous, intrasplenic, or intratumoral). Intranodal vaccine administration involves the injection of mRNA directly into secondary lymphoid tissue directly at the site of T-cell activation that elicits potent prophylactic or therapeutic antitumor T-cell responses (62, 66). According to a more recent report, intranodal injection of mRNA encoding the E7 protein of the human papillomavirus (HPV) 16 with TriMix increased the number of tumor-infiltrating CD8+ T cells and inhibited the growth of an E7-expressing tumor model in mice (67). Intranasal vaccine administration of mRNA vaccine enables rapid antigen uptake by DCs. mRNA complexed with Stemfect LNPs delivered intranasally resulted in detained tumor onset and increased survival in prophylactic and therapeutic mouse tumor models (145). Intratumoral mRNA vaccination is another approach in which mRNAs encoding tumor-associated antigens are not introduced into cells rather than tumor-specific immunity is activated through immune-stimulatory molecules. In a more recent research, mice with OVA-expressing lymphoma or lung carcinoma received intratumoral delivery of mRNA encoding an engineered cytokine IFN-β fused with TGF-β, which inhibited tumor development (147). Intratumoral administration of TriMix mRNA stimulates CD8+ DCs and tumor-specific T cells, causing tumor growth to be delayed in mice (148).
15.6.5 mRNA Vaccines for Parasitic Infection
Plasmodium macrophage migration inhibitory factor (PMIF) is an orthologue of mammalian macrophage migration inhibitory factor (MIF), and secretion of PMIF from plasmodium attenuates the immune response of the host. The self-amplifying mRNA vaccine can provide protective immunity against malaria infection by neutralizing the plasmodium macrophage migration inhibitory factor (PMIF). Mice vaccinated twice with a self-amplifying mRNA replicon encoding PMIF had higher PMIF-specific CD4+ cells and anti-PMIF IgG titer, suggesting that the parasites were controlled and reinfection was prevented (Baeza Garcia et al. 2018). Vaccination with F2-RNA alone resulted in low antigen-specific Th1 responses and quite low IgG responses, while vaccination with mRNA replicon encoding for the LEISH-F2 gene formulated with glucopyranosyl lipid A in a stable oil-in-water emulsion induced very high IFN-y secretions and antigen-specific Th1 responses that significantly reduced the parasite burden in the liver of mice (Johanning et al. 1995). A lipid nanoparticle (LNP)-based self-replicating RNA encoding T. gondii nucleoside triphosphate hydrolase-II (NTPase-II) induces high specific immunoglobulin (IgG) titers and IFN production, resulting in increased survival time and rate of mice (Luo et al. 2017).
15.6.6 mRNA Vaccines for SARS-CoV-2
For the development of the mRNA coronavirus vaccine, the standard vaccine development approach was used, enabling new age of vaccine development. A lipid nanoparticle-encapsulated mRNA-base mrNA-1273, expressing the full-length SARS-CoV-2 stabilized spike protein(S), has been developed by the Moderna (Biotech company) with the National Institutes of Health (NIH) which induces robust neutralizing antibodies and increases T-cell response in nonhuman primates (Corbett et al. 2020). Clinical studies have shown that m-1273 induces high neutralization antibodies and Th1-biased CD+4 T human cell response, with 94.1% protective efficacy against severe COVID-19 cases and 100% protective efficacy against moderate COVID-19 cases (Jackson et al. 2020). Moderna’s vaccine mRNA-1273 against COVID-19 has been approved for emergency use by the US Food and Drug Administration (FDA) and the WHO. This mRNA-1273 vaccine has been shown to be effective against two new SARS-CoV-2 variants, B.1.1.7 and 501Y.V2.
BioNTech and Pfizer have developed another mRNA-based vaccine candidate, BNT162 [BNT162b1: encodes an optimized SARS-CoV-2 receptor-binding domain (RBD) antigen and BNT162b2: encodes an optimized SARS-CoV-2 full-length spike protein antigen], having robust functional antibody titers with a 95% protective efficacy rate (ClinicalTrials.gov. 20202/NCT04380701 2020). Moderna BNT162 has received Emergency Use Authorization (EUA) from the US Food and Drug Administration. The LUNAR-COV19 (ARCT-021) vaccine with lipid-mediated delivery system developed by self-transcribing and replicating mRNA (STARRTM) technology induces strong IgG antibody response, with balanced Th1/Th2 CD4+ T-cell response, and increases protective efficacy, according to Arcturus Therapeutics Vaccines Company. Sanofi and Translate Bio are designing multiple mRNA constructs as COVID-19 vaccine candidates and have begun a phase I/II clinical trial (Sanofi and Translate Bio. Press release on 12 March 2021). Imperial College London is conducting a phase I/II clinical trial for a thermostable and immunogenic vaccine based on self-amplifying RNA technology (saRNA) and DNA/RNA stabilization technologies (Imperial College London). On June 15, 2020, a press statement was issued. The CureVac Company obtained approval from the German Paul-Ehrlich-Institute (PEI) and the Belgian Federal Agency for Medicines and Health Products (FAMHP) for clinical trials of an mRNA vaccine (CVnCoV), which encodes the SARS-CoV-2 spike protein (CureVac. Press release on 18 June 2020).
15.7 Conclusion
mRNA-based vaccines are a promising new platform that is highly versatile, scalable, and low cost and does not require a cold chain. They also bridge the gap between an emerging pandemic infectious disease and an abundant supply of successful vaccines. The preclinical and clinical findings indicate that mRNA vaccines are safe and well tolerated in animal models and humans, and that mRNA prophylaxis and therapy may be useful for preventing infectious disease and treating tumors. Future changes can also boost antigen-specific immune responses as well as the size of memory immune cell responses, such as memory B- and T-cell responses. We conclude that mRNA vaccine platform may be ideally suited for vaccine development, provided that production costs continue to decrease.
References
Ablasser A, Poeck H, Anz D, Berger M, Schlee M, Kim S, Bourquin C, Goutagny N, Jiang Z, Fitzgerald KA, Rothenfusser S, Endres S, Hartmann G, Hornung V (2009) Selection of molecular structure and delivery of RNA oligonucleotides to activate TLR7 versus TLR8 and to induce high amounts of IL-12p70 in primary human monocytes. J Immunol 182:6824–6833
Agarwal P, Raghavan A, Nandiwada SL, Curtsinger JM, Bohjanen PR, Mueller DL, Mescher MF (2009) Gene regulation and chromatin remodeling by IL-12 and type I IFN in programming for CD8 T cell effector function and memory. J Immunol 183:1695–1704
Alnylam Pharmaceuticals (2018). Alnylam announces first-ever FDA approval of an RNAi therapeutic, ONPATTRO™ (patisiran) for the treatment of the polyneuropathy of hereditary transthyretin-mediated amyloidosis in adults. http://investors.alnylam.com/news-releases/news-release-details/alnylam-announces-first-everfda-approval-rnai-therapeutic
Baeza Garcia A, Siu E, Sun T, Exler V, Brito L, Hekele A, Otten G, Augustijn K, Janse CJ, Ulmer JB (2018) Neutralization of the Plasmodium-encoded MIF ortholog confers protective immunity against malaria infection. Nat Commun 9:2714
Bahl K, Senn JJ, Yuzhakov O, Bulychev A, Brito LA, Hassett KJ, Laska ME, Smith M, Almarsson Ö, Thompson J (2017) Preclinical and clinical demonstration of immunogenicity by mRNA vaccines against H10N8 and H7N9 influenza viruses. Mol Ther 25:1316–1327
Bogers WM, Oostermeijer H, Mooij P, Koopman G, Verschoor EJ, Davis D, Ulmer JB, Brito LA, Cu Y, Banerjee K (2015) Potent immune responses in rhesus macaques induced by nonviral delivery of a self-amplifying RNA vaccine expressing HIV type 1 envelope with a cationic nanoemulsion. J Infect Dis 211:947–955
Chahal JS, Khan OF, Cooper CL, McPartlan JS, Tsosie JK, Tilley LD, Sidik SM, Lourido S, Langer R, Bavari S (2016) Dendrimer-RNA nanoparticles generate protective immunity against lethal Ebola, H1N1 influenza, and Toxoplasma gondii challenges with a single dose. Proc Natl Acad Sci U S A 113:E4133–E4142
Chahal JS, Fang T, Woodham AW, Khan OF, Ling J, Anderson DG, Ploegh HL (2017) An RNA nanoparticle vaccine against Zika virus elicits antibody and CD8+ T cell responses in a mouse model. Sci Rep 7:252
Charles A, Janeway J, Travers P, Walport M, Shlomchik MJ (2001) B-cell activation by armed helper T cells. In: Janeway C, Travers P, Walport M, Shlomchik MJ (eds) Immunobiology: the immune system in health and disease, 5th edn. Garland Science, New York
ClinicalTrials.gov (2020). A trial investigating the safety and effects of four BNT162 vaccines against COVID-2019 in healthy adults. NCT04380701. https://www.clinicaltrials.gov/ct2/show/ NCT04380701.
Corbett KS, Flynn B, Foulds KE et al (2020) Evaluation of the mRNA-1273 vaccine against SARS-CoV-2 in nonhuman primates. N Engl J Med. https://doi.org/10.1056/NEJMoa2024671
Cullis PR, Hope MJ (2017) Lipid nanoparticle systems for enabling gene therapies. Mol Ther 25:1467–1475
CureVac (2018). CureVac announces first study participant enrolled in phase I clinical trial testing prophylactic mRNA. https://www.curevac.com/newsroom/news/curevac-announces-first-study-participant-enrolled-in-phase-i-clinical-trial-testingprophylactic-mr/.)
CureVac (2020). CureVac to trial Covid-19 vaccine in Germany and Belgium. https://www.clinicaltrialsarena.com/news/curevac-covid-19-vaccine-trial/).
De Beuckelaer A, Grooten J, De Koker S (2017) Type I interferons modulate CD8+ T cell immunity to mRNA vaccines. Trends Mol Med 23:216–226
Démoulins T, Milona P, Englezou PC, Ebensen T, Schulze K, Suter R, Pichon C, Midoux P, Guzmán CA, Ruggli N, McCullough KC (2016) Polyethylenimine-based polyplex delivery of self-replicating RNA vaccines. Nanomedicine (Lond) 12:711–722
Edwards DK, Jasny E, Yoon H, Horscroft N, Schanen B, Geter T, Fotin-Mleczek M, Petsch B, Wittman V (2017) Adjuvant effects of a sequence-engineered mRNA vaccine: translational profiling demonstrates similar human and murine innate response. J Transl Med 15:1
Englezou PC, Sapet C, Démoulins T, Milona P, Ebensen T, Schulze K, Guzman CA, Poulhes F, Zelphati O, Ruggli N, McCullough KC (2018) Self-amplifying replicon RNA delivery to dendritic cells by cationic lipids. Mol Ther Nucleic Acids 12:118–134
Erasmus JH, Khandhar AP, Guderian J, Granger B, Archer J, Archer M, Gage E, Fuerte-Stone J, Larson E, Lin S et al (2018) A nanostructured lipid carrier for delivery of a replicating viral RNA provides single, low-dose protection against Zika. Mol Ther 26:2507–2522
Houseley J, Tollervey D (2009) The many pathways of RNA degradation. Cell 136:763–776
Iavarone C, O’Hagan TD, Yu D, Delahaye NF, Ulmer JB (2017) Mechanism of action of mRNA-based vaccines. Expert Rev Vaccines 16:871–881. https://doi.org/10.1080/14760584.2017.1355245
Jackson LA, Anderson EJ, Rouphael NG et al (2020) An mRNA vaccine against SARS-CoV-2—preliminary report. N Engl J Med. NEJMoa2022483.
Jansen RP (2001) mRNA localization: message on the move. Nat Rev Mol Cell Biol 2:247–256
Jirikowski GF, Sanna PP, Maciejewski-Lenoir D, Bloom FE (1992) Reversal of diabetes insipidus in Brattleboro rats: intrahypothalamic injection of vasopressin mRNA. Science 255:996–998
Johanning FW, Conry RM, LoBuglio AF, Wright M, Sumerel LA, Pike MJ, Curiel DT (1995) A Sindbis virus mRNA polynucleotide vector achieves prolonged and high level heterologous gene expression in vivo. Nucleic Acids Res 23:1495–1501
Karikó K, Muramatsu H, Welsh FA, Ludwig J, Kato H, Akira S, Weissman D (2008) Incorporation of pseudouridine into mRNA yields superior nonimmunogenic vector with increased translational capacity and biological stability. Mol Ther 16:1833–1840
Krammer F, Palese P (2015) Advances in the production of influenza virus vaccines. Nat Rev Drug Discov 14:167–182
Lee BL, Barton GM (2014) Tracking of endosomal Toll-like receptors. Trends Cell Biol 24:360–369
Lindgren G, Ols S, Liang F, Thompson EA, Lin A, Hellgren F, Bahl K, John S, Yuzhakov O, Hassett KJ et al (2017) Induction of robust B cell responses after influenza mRNA vaccination is accompanied by circulating hemagglutinin-specific ICOS+ PD-1+ CXCR3+ T follicular helper cells. Front Immunol 8:1539
Ljungberg K, Liljeström P (2015) Self-replicating alphavirus RNA vaccines. Expert Rev Vaccines 14:177–194
Luo F, Zheng L, Hu Y, Liu S, Wang Y, Xiong Z, Hu X, Tan F (2017) Induction of protective immunity against Toxoplasma gondii in mice by nucleoside triphosphate hydrolase-II (NTPase-II) self-amplifying RNA vaccine encapsulated in lipid nanoparticle (LNP). Front Microbiol 8:605
Lutz J, Lazzaro S, Habbeddine M, Schmidt KE, Baumhof P, Mui BL, Tam YK, Madden TD, Hope MJ, Heidenreich R, Fotin-Mleczek M (2017) Unmodified mRNA in LNPs constitutes a competitive technology for prophylactic vaccines. NPJ Vaccines 2:29
Magini D, Giovani C, Mangiavacchi S, Maccari S, Cecchi R, Ulmer JB, De Gregorio E, Geall AJ, Brazzoli M, Bertholet S (2016) Self-amplifying mRNA vaccines expressing multiple conserved influenza antigens confer protection against homologous and heterosubtypic viral challenge. PLoS One 11:e0161193
Maruggi G, Chiarot E, Giovani C, Buccato S, Bonacci S, Frigimelica E, Margarit I, Geall A, Bensi G, Maione D (2017) Immunogenicity and protective efficacy induced by self-amplifying mRNA vaccines encoding bacterial antigens. Vaccine 35:361–368
Maruggi G, Zhang C, Li J, Ulmer JB, Yu D (2019) mRNA as a transformative technology for vaccine development to control infectious diseases. Mol Ther 27(4):757–772
Mauro VP, Chappell SA (2014) A critical analysis of codon optimization in human therapeutics. Trends Mol Med 20:604–613
Pardi N, Tuyishime S, Muramatsu H, Kariko K, Mui BL, Tam YK, Madden TD, Hope MJ, Weissman D (2015) Expression kinetics of nucleoside-modified mRNA delivered in lipid nanoparticles to mice by various routes. J Control Release 217:345–351
Pardi N, Hogan MJ, Pelc RS, Muramatsu H, Andersen H, DeMaso CR, Dowd KA, Sutherland LL, Scearce RM, Parks R et al (2017) Zika virus protection by a single low-dose nucleoside-modified mRNA vaccination. Nature 543:248–251
Pardi N, Hogan MJ, Naradikian MS, Parkhouse K, Cain DW, Jones L, Moody MA, Verkerke HP, Myles A, Willis E et al (2018) Nucleoside-modified mRNA vaccines induce potent T follicular helper and germinal center B cell responses. J Exp Med 215:1571–1588
Pepini T, Pulichino AM, Carsillo T, Carlson AL, Sari-Sarraf F, Ramsauer K, Debasitis JC, Maruggi G, Otten GR, Geall AJ et al (2017) Induction of an IFN-mediated antiviral response by a self-amplifying RNA vaccine: implications for vaccine design. J Immunol 198:4012–4024
Petsch B, Schnee M, Vogel AB, Lange E, Hoffmann B, Voss D, Schlake T, Thess A, Kallen KJ, Stitz L, Kramps T (2012) Protective efficacy of in vitro synthesized, specific mRNA vaccines against influenza A virus infection. Nat Biotechnol 30:1210–1216
Pilkinton MA, Nicholas KJ, Warren CM, Smith RM, Yoder SM, Talbot HK, Kalams SA (2017) Greater activation of peripheral T follicular helper cells following high dose influenza vaccine in older adults forecasts seroconversion. Vaccine 35:329–336
Rauch S, Lutz J, Kowalczyk A, Schlake T, Heidenreich R (2017) RNActive technology: generation and testing of stable and immunogenic RNA vaccines. Methods Mol Biol 1499:89–107
Richner JM, Jagger BW, Shan C, Fontes CR, Dowd KA, Cao B, Himansu S, Caine EA, Nunes BTD, Medeiros DBA et al (2017) Vaccine mediated protection against Zika virus-induced congenital disease. Cell 170:273–283.e12
Ross J (1995) mRNA stability in mammalian cells. Microbiol Rev 59:423–450
Sabbah A, Chang TH, Harnack R, Frohlich V, Tominaga K, Dube PH, Xiang Y, Bose S (2009) Activation of innate immune antiviral responses by Nod2. Nat Immunol 10:1073–1080
Sanofi and Translate Bio. (2021) Sanofi and translate bio initiate phase 1/2 clinical trial of mRNA COVID-19 vaccine candidate. https://investors.translate.bio/news-releases/news-release-details/sanofi-and-translate-bio-initiate-phase-12-clinical-trial-mrna.
Schnee M, Vogel AB, Voss D, Petsch B, Baumhof P, Kramps T, Stitz L (2016) An mRNA vaccine encoding rabies virus glycoprotein induces protection against lethal infection in mice and correlates of protection in adult and newborn pigs. PLoS Negl Trop Dis 10:e0004746
Stanton MG (2018) Current status of messenger RNA delivery systems. Nucleic Acid Ther 28:158–165
Suan D, Sundling C, Brink R (2017) Plasma cell and memory B cell differentiation from the germinal center. Curr Opin Immunol 45:97–102
Takeuchi O, Akira S (2010) Pattern recognition receptors and inflammation. Cell 140:805–820
Tanabe Y, Nishibori T, Su L, Arduini RM, Baker DP, David M (2005) Cutting edge: role of STAT1, STAT3, and STAT5 in IFN—responses in T lymphocytes. J Immunol 174:609–613
Thess A et al (2015) Sequence-engineered mRNA without chemical nucleoside modifications enables an effective protein therapy in large animals. Mol Ther 23:1456–1464
Vogel AB, Lambert L, Kinnear E, Busse D, Erbar S, Reuter KC, Wicke L, Perkovic M, Beissert T, Haas H et al (2018) Self-amplifying RNA vaccines give equivalent protection against influenza to mRNA vaccines but at much lower doses. Mol Ther 26:446–455
Weissman D (2015) mRNA transcript therapy. Expert Rev Vaccines 14:265–281
Wen Y, Monroe J, Linton C, Archer J, Beard CW, Barnett SW, Palladino G, Mason PW, Carfi A, Lilja AE (2014) Human cytomegalovirus gH/gL/UL128/UL130/UL131A complex elicits potently neutralizing antibodies in mice. Vaccine 32:3796–3804
Wolff JA et al (1990) Direct gene transfer into mouse muscle in vivo. Science 247:1465–1468
Zhang C, Maruggi G, Shan H, Li J (2019) Advances in mRNA vaccines for infectious diseases. Front Immunol 10:594
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 The Author(s), under exclusive licence to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Kushwaha, V., Anuprabha, Sobti, R.C. (2021). mRNA Vaccine: An Advanced and Transformative Technology for Vaccine Development. In: Sobti, R.C., Dhalla, N.S., Watanabe, M., Sobti, A. (eds) Delineating Health and Health System: Mechanistic Insights into Covid 19 Complications. Springer, Singapore. https://doi.org/10.1007/978-981-16-5105-2_15
Download citation
DOI: https://doi.org/10.1007/978-981-16-5105-2_15
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-16-5104-5
Online ISBN: 978-981-16-5105-2
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)