Abstract
The treatment of ovarian cancer has changed significantly over the past few years, particularly in the case of hereditary breast and ovarian cancer (HBOC) syndrome. Genetic testing for BRCA1 and BRCA2 is used not only for a diagnosis for HBOC but also a biomarker for PARP inhibitors, which is of great importance in the treatment of ovarian cancer. The characteristics of ovarian cancer in HBOC have been reported of the highest prevalence in high-grade serous carcinoma subtype, high sensitivity to platinum salt chemotherapies and PARP inhibitors, and a better prognosis compared to BRCA-negative ovarian cancer. It is important to note that ovarian cancer with a family history is also associated with Lynch syndrome, although less frequently than HBOC. In addition, recent multi-panel genetic analysis has led to the identification of genes other than HBOC that are involved in the development of ovarian cancer, which may require further clinical practice.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
7.1 Overview of Ovarian Cancer
Ovarian cancer is the most lethal gynecologic malignancy with more than 200,000 cases every year [1]. Due to the lack of effective screening methods, most patients are diagnosed at advanced stages. Less than 40% of women with ovarian cancer can be cured.
7.1.1 Symptoms
Symptoms associated with ovarian cancer were reported as pelvic/abdominal pain, urinary urgency/frequency, increased abdominal size/bloating, and difficulty eating/feeling full when they were frequently present for <1 year [2]. However, the screening by these symptoms, especially in patients with early-stage ovarian cancer, did not show enough sensitivity or specificity [3, 4]. Thus, ovarian cancer is still called as “a silent killer.”
7.1.2 Histologic Subtypes
Epithelial ovarian cancer has four main histologic subtypes, including serous, endometrioid, mucinous, and clear cell. High-grade serous carcinoma (HGSC) characterized by TP53 mutations is the most common and aggressive subtype. This subtype is related to hereditary breast and ovarian cancer (HBOC) syndrome, and its origin is said to be the fallopian tube or ovarian epithelium. Low-grade serous, mucinous, clear cell, and endometrioid tumors are believed to have developed from inclusion cysts or implants in the ovarian surface epithelium. They also have KRAS, BRAF, or PTEN mutations [5, 6]. Clear cell carcinoma has characteristics of being resistant to anticancer drugs, contrary to its slow growth, and is more common in Japan [7].
7.1.3 Risk Factors
The risk factors of developing ovarian cancer are age, nulliparity, and age (>35 years) at first pregnancy or first birth. Thirty percent to sixty percent decreased risk for cancer, in contrast, is associated with younger age at first pregnancy or first birth (≤25 years), the use of oral contraceptives, and history of breastfeeding [5]. As we will discuss later, having a family cancer syndrome is the most relevant risk of developing ovarian cancer.
7.1.4 Screening
Screening for ovarian cancer did not reduce mortality in two large screening trials. The Prostate, Lung, Colorectal and Ovarian (PLCO) Cancer Screening Trial by annual screening with serum CA125 and ultrasound showed no reduction in mortality [8]. The other result from the UK Collaborative Trial of Ovarian Cancer Screening (UKCTOCS) based on serum CA125-based screening seemed encouraging, but the mortality reduction was not significant [9].
7.1.5 Treatment
For epithelial ovarian cancer, primary treatment consists of appropriate surgical staging and debulking surgery, followed by systemic chemotherapy in most, but not all, patients. For most patients, initial surgery includes total abdominal hysterectomy and bilateral salpingo-oophorectomy with comprehensive staging with omentectomy, pelvic and para-aortic lymphadenectomy, and peritoneal biopsy [5, 10]. Debulking surgery is recommended for patients at stage II, III, or IV, because the maximal cytoreduction improves survival [11, 12]. Neoadjuvant chemotherapy followed by interval debulking surgery is recommended for patients diagnosed as advanced disease and the optimal surgery is difficult [10]. Regarding systematic lymphadenectomy, one recent RCT has shown that systematic pelvic and paraaortic lymphadenectomy after maximal cytoreduction did not improve survival and might cause postoperative complications when lymph nodes have no suspicious findings [13].
Most patients with epithelial ovarian cancer receive postoperative systemic chemotherapy. The combination of platinum and taxane agents is typically administered as a first-line chemotherapy for ovarian cancer. The effect of bevacizumab, anti-angiogenesis agent, was assessed by two RCTs, ICON7 and GOG218. These trials showed that the addition of bevacizumab to upfront chemotherapy with carboplatin/paclitaxel followed by bevacizumab as maintenance therapy improved PFS (hazard ratio[HR] 0.72, 0.81) and is recommended for patients at stage III or IV [14, 15].
The effect of poly(ADP-ribose) polymerase (PARP) inhibitors as maintenance therapy was assessed by several RCTs. The SOLO1 study demonstrated that PFS was prolonged substantially by using olaparib as a maintenance therapy in patients with a germline or somatic BRCA1/BRCA2 variant (HR 0.30) [16]. In Japan, olaparib is currently available for advanced ovarian cancer patients with BRCA1/BRCA2 variants as maintenance therapy. Furthermore, the three RCTs of PARP inhibitors—veliparib, niraparib, and olaparib plus bevacizumab—have recently been shown to improve PFS [HR 0.68, 0.62, 0.59] when used after primary treatment regardless of BRCA1/BRCA2 variant, but the better outcome was seen in homologous recombination deficiency (HRD)-positive patients [17,18,19].
The recurrent disease is categorized by platinum-sensitive disease (if the patients have the disease ≥6 months after completion prior platinum-based therapy) or platinum-resistant disease (if the patients have the disease <6 months after completion prior platinum-based therapy). For platinum-sensitive disease, six cycles of platinum-based chemotherapy are preferred. The addition of bevacizumab to standard chemotherapy and maintenance therapy until progression improved PFS for platinum-sensitive recurrent ovarian cancer. OS was also improved in GOG213 [20, 21]. PARP inhibitors as maintenance therapy improved PFS of platinum-sensitive disease with germline BRCA1/BRCA2 variant, platinum-sensitive recurrent HGSC, and HRD positive [22,23,24,25]. For platinum-resistant disease, non-platinum-based agents or regimens are preferred. The prognosis is poor, but adding bevacizumab to chemotherapy improved PFS [26].
7.2 Hereditary Ovarian Cancer
It is now known that at least 10% of epithelial ovarian cancer have germline pathogenic variant in ovarian cancer-susceptibility genes, commonly BRCA1/BRCA2 and DNA mismatch repair (MMR) genes. Of 1915 ovarian cancer patients, 347 (18%) carried a germline mutation in a gene associated with ovarian cancer risk [27]. In Japan, of 230 unselected women with ovarian cancer, 17.8% women had pathogenic germline variant. The variants include genes associated with BRCA1 (prevalence; 8.3%), BRCA2 (3.5%), and mismatch repair genes (2.6%) [28]. Patients with HGSC may have germline variants in other genes involved in HR, including BRIP1, BARD1, RAD51C, and RAD51D, but the frequency is less compared to BRCA1 and BRCA2 [29]. In addition, some large studies using multiple-gene, next-generation sequencing panels and whole-exome sequencing were conducted, and gene-phenotype associations were examined. Table 7.1 shows the list of genes related to hereditary ovarian cancer and their risks of ovarian cancer reported in NCCN guidelines, and these studies [30, 31, 37]. Detailed personal and family history of cancer is important for cancer risk assessment and choice of gene testing. Comprehensive testing with multigene panel for BRCA1-/BRCA2-negative patients and individuals without a known familial pathogenic variant should be considered. In gynecological clinics, a self-administered questionnaire would be a useful tool for screening patient’s medical history and familial cancer history [38].
7.2.1 Ovarian Cancer in HBOC
7.2.1.1 Frequency of HBOC in Ovarian Cancer
The frequency of germline BRCA1/BRCA2 variants in ovarian cancer patients was reported to be 13–15% in some large studies [39, 40]. In Japan, one multicenter analysis reported that the overall prevalence of germline BRCA1/BRCA2 variants was 14.7% (93/634), where germline BRCA1 mutations (9.9%) were more common than germline BRCA2 mutations (4.7%) [41]. In another report, of 230 unselected Japanese women with ovarian cancer, 11.7% women had pathogenic germline variants of BRCA1/BRCA2 [28].
7.2.1.2 Germline Testing for Ovarian Cancer Patients
Based on personal and familial cancer history, germline BRCA1/BRCA2 testing should be considered for individuals from a family without a known BRCA1/BRCA2 variant. NCCN guidelines recommend testing should be considered for patients at any age with a personal history of ovarian cancer (including fallopian tube cancer or peritoneal cancer) and those with a first- or second-degree blood relative of ovarian cancer [42]. Since 2020, Japanese public health insurance has covered 70% of germline BRCA1/BRCA2 testing costs for cancer patients suspected of HBOC, and this includes ovarian cancer patients, too.
Detailed personal and family history of cancer is important for cancer risk assessment and choice of gene testing. For those without a known familial pathogenic variant or BRCA1-/BRCA2-negative patients, comprehensive testing with multigene panel should be considered. BRCA-related ovarian cancers are associated with epithelial, non-mucinous histology as discussed below; however, bear in mind that Lynch syndrome or other syndromes could be associated with both non-mucinous and mucinous histology.
7.2.1.3 Penetrance of BRCA1/BRCA2 Variant Carriers in Ovarian Cancer
Women with a BRCA1/BRCA2 pathogenic variant are at increased risk of ovarian cancers (including fallopian tube cancer and primary peritoneal cancer). The reliable prediction of developing ovarian cancer (the penetrance) is critical in genetic counseling and a gynecological practice for BRCA1/BRCA2 variant carriers. A meta-analysis showed the mean cumulative ovarian cancer risks for BRCA1/BRCA2 variant carriers at age 70 years were 40% (95% CI, 35% to 46%) for BRCA1 and 18% (95% CI, 13% to 23%) for BRCA2 variant carriers [43]. A large prospective cohort study of 6036 BRCA1 and 3820 BRCA2 female variant carriers showed that the cumulative ovarian cancer risks to age 80 years were 44% (95% CI, 36%–53%) for BRCA1 and 17% (95% CI, 11%–25%) for BRCA2 carriers [44]. The risk of ovarian cancer is not the same for all BRCA1/BRCA2 mutations. A large observational study from data collected by the Consortium of Investigators of Modifiers of BRCA (CIMBA) initiative revealed that women with a variant in the central part of BRCA1/BRCA2, especially in exon11 where ovarian cancer cluster regions (OCCRs) were identified, will have a higher lifetime risk of ovarian cancer [45]. The estimated penetrance of ovarian cancer can be influenced by allelic heterogeneity, modifier genes, and environmental and hormonal cofactors, such as oral-contraceptive use or parity and nationality [46].
7.2.1.4 Histology of Ovarian Cancer in HBOC
There are four main histologic subtypes of epithelial ovarian cancer: serous carcinoma (low-grade and high-grade), mucinous carcinoma, endometrioid carcinoma, and clear cell carcinoma. Germline BRCA1/BRCA2 variants were reported in all histologic subtypes except mucinous carcinoma (Table 7.2). In several large studies, high-grade serous carcinoma had the highest prevalence of BRCA1/BRCA2 variant [27, 39]. In Japan, a multicenter analysis reported that 28.5% of high-grade serous carcinoma has germline BRCA variants [41]. Another multivariate analysis showed that the high-grade serous carcinoma subtype is an independent predictive factor for pathogenic germline BRCA1/BRCA2 variants [28]. It should be noted that the prevalence of germline BRCA1/BRCA2 variants differs between studies, especially in clear cell carcinoma. This is because the frequency of germline BRCA1/BRCA2 variants has not been clarified, due to the low incidence of clear cell carcinoma in Western countries. Large-scale studies will be necessary in the future.
7.2.1.5 Ovarian Cancer Initiation in HBOC
After risk-reducing salpingo-oophorectomy (RRSO), a precursor lesion called tubal intraepithelial carcinoma (TIC) was detected in 5–10% of cases in women with BRCA variants [47,48,49,50]. The distal fallopian tube is suspected to be the dominant origin of early malignancies found in RRSO samples [47, 50, 51]. TICs and their associated ovarian carcinomas share identical mutations of TP53 [52]. Although the idea of fallopian tube to be the origin of many serous carcinomas of ovary for BRCA1/BRCA2 variant carriers is now generally accepted, there is a subset of HGSC with no apparent precursor lesion in the fallopian tube, so further study is needed to understand how these cancers develop [29]. It is not clear whether surgical staging and/or adjuvant chemotherapy is beneficial for women with STIC.
7.2.1.6 Prognosis of BRCA1/BRCA2 Variant Carriers with Ovarian Cancer
Recently, meta-analysis of women’s survival with ovarian cancer was done. This study was based on 26 reports including data from 1213 epithelial ovarian cancer patients with germline BRCA1/BRCA2 variants and 2666 noncarriers. Germline variants in BRCA1 or BRCA2 are associated with higher 5-year overall survival among patients with ovarian cancer. After adjusting the methods of studies and years of diagnosis, BRCA1/BRCA2 variant carriers showed better survival than noncarriers (for BRCA1, hazard ratio [HR], 0.78; 95% CI, 0.68–0.89, and for BRCA2, HR, 0.61; 95% CI, 0.50–0.76) [53]. However, other reports suggested a positive effect of germline BRCA1/BRCA2 variant, where mortality in patients with ovarian cancer decreased to 10 years [54].
7.2.1.7 Chemosensitivity and HRD of Ovarian Cancer in HBOC
Both BRCA1 and BRCA2 take part in DNA repair such as homologous recombination (HR) and the maintenance of genomic integrity. Cells with defective BRCA1 or BRCA2 are hypersensitive to agents that crosslink DNA strands. These are also sensitive to agents that produce breaks in double-stranded DNA, such as platinum salt chemotherapies [46]. Multiple case-control studies compared the effect of primary therapy between ovarian cancer patients with and without BRCA1/BRCA2 variants. These studies revealed that BRCA-related ovarian cancer showed better survival outcomes and platinum sensitivity [39, 55, 56]. However, one study showed that, among women with high-grade serous ovarian cancer, BRCA2 mutation, but not BRCA1 deficiency, was associated with improved survival and chemotherapy response [57]. Not only germline BRCA1/BRCA2 variant but also germline variants of other cancer-associated genes such as BRIP1, RAD51C, RAD51D, PALB2, and BARD1 were more frequent in patients with ovarian cancer than in the general population. There wasn’t a significant difference in survival rate between women with mutations in BRCA1 and other ovarian cancer-associated genes [27]. In addition to germline variant, ovarian cancer with somatic BRCA1/BRCA2 variants or somatic variants in other homologous recombination DNA repair genes, such as ATM, BARD1, BRIP1, CHEK1, CHEK2, FAM175A, MRE11A, NBN, PALB2, RAD51C, and RAD51D, had higher primary platinum sensitivity and improved overall survival than those without variants [39, 58].
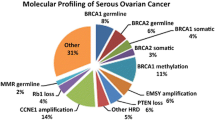
Almost 50% of epithelial ovarian cancers exhibit defects within the homologous recombination DNA repair (HRR) pathway. As cells with double-strand break repair deficiency have synthetic lethality to PARP inhibitors (PARPi), ovarian cancer with homologous recombination repair deficiency (HRD) exhibits a sensitivity to PARPi and platinum salt chemotherapies [58]. HRD is often caused by loss of function mutations in HRR genes, such as BRCA1, BRCA2, RAD51C, RAD51D, or PALB2, promoter hypermethylation of the BRCA1 and RAD51C gene promoter (leading to reduced expression), or unknown mechanisms. HRD testing is hoped to be a predictive biomarker for PARPi sensitivity. A wide range of assays, referred as “HRD tests,” have been developed to define which cancers have HRD. These HRD tests fall into three main categories: (1) HRR pathway, which is related to genes that identify specific causes of HRD, (2) genomic “scars” or mutational signatures which identify the patterns of somatic mutations that accumulate in HRD cancers irrespective of the underlying defect, and (3) functional assays that have the potential to provide a real-time readout of HRD or homologous recombination proficiency (HRP) [59]. A commercially available assay, Myriad MyChoice®, can now be used as a biomarker for PARPi in Japan. This test is the combination of BRCA1/BRCA2 variant and genomic instability scores (GIS). GIS includes loss of heterozygosity (LOH), telomeric allelic imbalance (TAI), and large-scale state transitions (LST), which is categorized by the genomic scar assay. Although there are several clinical benefits of HRD testing on PARPi response in ovarian cancer, HRD testing is not completely overlapped to PARPi sensitivity, where HRP ovarian cancer has sensitivity to PARPi. Better biomarkers are needed for HGSC management [59] (Fig. 7.1). When analyzing cancer genome by next-generation sequencing like HRD testing, we should bear in mind that mutations in DNA of a tumor may reveal germline variants with clinical significance [60]. Further detail about significance of PARPi for ovarian cancer is described in another section.
7.2.1.8 Surveillance for Ovarian Cancer
Women with a BRCA1/BRCA2 pathogenic variant are at increased risk of having ovarian cancers. Several studies on significance of ovarian cancer screening had been conducted. Phase II study of the UK Familial Ovarian Cancer Screening Study (UK FOCSS) included 4348 women with an estimated lifetime ovarian cancer risk of ≥10% and did not choose risk-reducing salpingo-oophorectomy (RRSO). They were assessed by serum CA-125 tests (every 3 months, with using the risk of ovarian cancer algorithm [ROCA]) and TVUS (annually or within 2 months of an abnormal ROCA result). Thirteen ovarian cancer patients were screen-detected, and 5 (38.5%) of the 13 patients were diagnosed at an early stage (stages I to II). Sensitivity, positive predictive value, and negative predictive value for detecting ovarian cancer within 1 year were 94.7%, 10.8%, and 100%, respectively [61].
In another study, 3692 women with a strong family history of breast/ovarian cancer or BRCA1/BRCA2 variant were assessed by serum CA125 (every 3 months, with using the risk of ovarian cancer algorithm [ROCA]) and transvaginal ultrasound (TVUS) (if ROCA increased above a baseline). Three (50%) of six incidental ovarian cancers were at early stage. ROCA flagged 50% of incidental cases. This method had better early-stage sensitivity at high specificity, but low PPV compared with CA125 every 6 months or annually [62].
Given its high sensitivity and significance in stage shift, these surveillance methods could be an option for BRCA1/BRCA2 variant carriers who did not choose RRSO. However, significance of these strategies to improve survival rate in screened BRCA1/BRCA2 variant carriers remains unknown. In NCCN guidelines, RRSO is the standard method of ovarian cancer risk management in BRCA1/BRCA2 carriers. For those patients who did not select RRSO, regular checkup by transvaginal ultrasound and serum CA-125 for ovarian cancer screening may be considered from the age of 30 to 35, although its benefit is not certain [42]. Further details about RRSO and chemoprevention for ovarian cancer are described in other sections.
7.2.2 Ovarian Cancer in Lynch Syndrome
Lynch syndrome is a hereditary syndrome associated with familial cancers, including colorectal cancer and Lynch syndrome-related cancers, such as endometrial cancer. The cause of the disease is the germline variant of DNA mismatch repair (MMR) genes, such as MLH1, MSH2, MSH6, and PMS2, characterized by autosomal dominant inheritance. Women with Lynch syndrome are also at increased risk of ovarian cancer.
The histological types of ovarian cancer were mixed type (mucinous/endometrioid/clear cell carcinomas) 33%, endometrioid carcinoma 25%, serous carcinoma 22%, clear cell carcinoma 12%, and mucinous carcinoma 4%. Most tumors (65%) were diagnosed at an early stage [63].
Microsatellites are short DNA repeat sequences that increase or decrease in number when MMR is dysfunctional. An MSI test is recommended before examining germline mutation when a patient is suspected of suspected Lynch syndrome. Screening of ovarian cancer specimens by MSI may be an efficient way to diagnose Lynch syndrome [64].
There is no definite evidence to support routine screening for ovarian cancers in Lynch syndrome. Total abdominal hysterectomy and bilateral salpingo-oophorectomy are options that may be considered for risk reduction in women with Lynch syndrome who have completed childbearing [5].
7.2.3 Other Germline Variants Associated with Ovarian Cancer
7.2.3.1 RAD51C, RAD51D, BRIP1
DNA recombinase RAD51 protein is a central player in homologous recombination and DNA repair. BRIP1 encodes the BRCA1-interacting protein C-terminal helicase 1 protein, which is required for the normal double-strand break repair function of BRCA1. RAD51C and RAD51D, genes in the RAD51 protein family, and BRIP1 have been shown to be associated with increased risk for ovarian cancer [32,33,34,35,36].
The frequency of germline RAD51C/RAD51D variants and BRIP1 variants in ovarian cancer patients was reported to be about 1% [30, 31].
In carriers of a RAD51D variant or BRIP1 variant, the cumulative risk of ovarian cancer approaches 2.6% around 50 to 54 years of age, which is the expected lifetime risk for a woman with a BRCA-negative family history of ovarian cancer [65]. The NCCN guidelines recommend that RRSO in carriers of RAD51C, RAD51D, or BRIP1 pathogenic or likely pathogenic variants be considered beginning at 45 to 50 years of age. In women with variants in these genes who also have a family history of ovarian cancer in a first-degree relative, the risk threshold might cross earlier and the timing for RRSO should be considered [42].
7.2.3.2 NBN, ATM, PALB2
Some studies suggest that there may be a moderately increased risk for ovarian cancer in carriers of an NBN, ATM, or PALB2 variant, but there is currently insufficient evidence to recommend RRSO in these carriers [27, 30, 31].
References
Bray F, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. https://doi.org/10.3322/caac.21492.
Goff BA, et al. Development of an ovarian cancer symptom index: possibilities for earlier detection. Cancer. 2007;109(2):221–7. https://doi.org/10.1002/cncr.22371.
Gilbert L, et al. Assessment of symptomatic women for early diagnosis of ovarian cancer: results from the prospective DOvE pilot project. Lancet Oncol. 2012;13(3):285–91. https://doi.org/10.1016/S1470-2045(11)70333-3.
Lim AWW, et al. Predictive value of symptoms for ovarian cancer: comparison of symptoms reported by questionnaire, interview, and general practitioner notes. J Natl Cancer Inst. 2012;104:114–24. https://doi.org/10.1093/jnci/djr486.
NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®). Ovarian cancer including fallopian tube cancer and primary peritoneal cancer version 1. 2020.
Wentzensen N, et al. Ovarian cancer risk factors by histologic subtype: an analysis from the ovarian cancer cohort consortium. J Clin Oncol. 2016;34(24):2888–98. https://doi.org/10.1200/JCO.2016.66.8178.
Takano M, Tsuda H, Sugiyama T. Clear cell carcinoma of the ovary: is there a role of histology-specific treatment? J Exp Clin Cancer Res. 2012; https://doi.org/10.1186/1756-9966-31-53.
Buys SS, et al. Effect of screening on ovarian cancer mortality: the prostate, lung, colorectal and ovarian (PLCO) cancer screening randomized controlled trial. JAMA. 2011;305(22):2295–302. https://doi.org/10.1001/jama.2011.766.
Jacobs IJ, et al. Ovarian cancer screening and mortality in the UK collaborative trial of ovarian Cancer screening (UKCTOCS): a randomised controlled trial. Lancet. 2016;387(10022):945–56. https://doi.org/10.1016/S0140-6736(15)01224-6.
JSGO Guidelines. Guidelines for treatment of ovarian cancer, fallopian tube cancer and primary peritoneal cancer. 2020 edition. Japan Society of Gynecologic Oncology (JSGO); 2020.
Bristow RE, et al. Survival effect of maximal cytoreductive surgery for advanced ovarian carcinoma during the platinum era: a meta-analysis. J Clin Oncol. 2002;20(5):1248–59. https://doi.org/10.1200/jco.2002.20.5.1248.
Panici PB, et al. Systematic aortic and pelvic lymphadenectomy versus resection of bulky nodes only in optimally debulked advanced ovarian cancer: a randomized clinical trial. J Natl Cancer Inst. 2005;97(8):560–6. https://doi.org/10.1093/jnci/dji102.
Harter P, et al. A randomized trial of lymphadenectomy in patients with advanced ovarian neoplasms. N Engl J Med. 2019;380(9):822–32. https://doi.org/10.1056/nejmoa1808424.
Burger RA, et al. Incorporation of bevacizumab in the primary treatment of ovarian cancer. N Engl J Med. 2011;365(26):2473–83. https://doi.org/10.1056/nejmoa1104390.
Perren TJ, et al. A phase 3 trial of bevacizumab in ovarian cancer. N Engl J Med. 2011;365(26):2484–96. https://doi.org/10.1056/nejmoa1103799.
Moore K, et al. Maintenance olaparib in patients with newly diagnosed advanced ovarian cancer. N Engl J Med. 2018;379(26):2495–505. https://doi.org/10.1056/nejmoa1810858.
Coleman RL, et al. Veliparib with first-line chemotherapy and as maintenance therapy in ovarian cancer. N Engl J Med. 2019;381(25):2403–15. https://doi.org/10.1056/nejmoa1909707.
González-Martín A, et al. Niraparib in patients with newly diagnosed advanced ovarian cancer. N Engl J Med. 2019;381(25):2391–402. https://doi.org/10.1056/nejmoa1910962.
Ray-Coquard I, et al. Olaparib plus bevacizumab as first-line maintenance in ovarian cancer. N Engl J Med. 2019;381(25):2416–28. https://doi.org/10.1056/nejmoa1911361.
Aghajanian C, et al. OCEANS: a randomized, double-blind, placebo-controlled phase III trial of chemotherapy with or without bevacizumab in patients with platinum-sensitive recurrent epithelial ovarian, primary peritoneal, or fallopian tube cancer. J Clin Oncol. 2012;30(17):2039–45. https://doi.org/10.1200/JCO.2012.42.0505.
Coleman RL, Brady MF, et al. Bevacizumab and paclitaxel–carboplatin chemotherapy and secondary cytoreduction in recurrent, platinum-sensitive ovarian cancer (NRG oncology/gynecologic oncology group study GOG-0213): a multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2017;18(6):779–91. https://doi.org/10.1016/S1470-2045(17)30279-6.
Coleman RL, Oza AM, et al. Rucaparib maintenance treatment for recurrent ovarian carcinoma after response to platinum therapy (ARIEL3): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;390(10106):1949–61. https://doi.org/10.1016/S0140-6736(17)32440-6.
Ledermann J, et al. Olaparib maintenance therapy in platinum-sensitive relapsed ovarian cancer. N Engl J Med. 2012;366(15):1382–92. https://doi.org/10.1056/nejmoa1105535.
Ledermann J, et al. Olaparib maintenance therapy in patients with platinum-sensitive relapsed serous ovarian cancer: a preplanned retrospective analysis of outcomes by BRCA status in a randomised phase 2 trial. Lancet Oncol. 2014;15(8):852–61. https://doi.org/10.1016/S1470-2045(14)70228-1.
Mirza MR, et al. Niraparib maintenance therapy in platinum-sensitive, recurrent ovarian cancer. N Engl J Med. 2016;375(22):2154–64. https://doi.org/10.1056/nejmoa1611310.
Pujade-Lauraine E, et al. Bevacizumab combined with chemotherapy for platinum-resistant recurrent ovarian cancer: the AURELIA open-label randomized phase III trial. J Clin Oncol. 2014;32(13):1302–8. https://doi.org/10.1200/JCO.2013.51.4489.
Norquist BM, et al. Inherited mutations in women with ovarian carcinoma. JAMA Oncol. 2016;2(4):482. https://doi.org/10.1001/jamaoncol.2015.5495.
Hirasawa A, et al. Prevalence of pathogenic germline variants detected by multigene sequencing in unselected Japanese patients with ovarian cancer. Oncotarget. 2017;8(68):112258–67. https://doi.org/10.18632/oncotarget.22733.
Bowtell DD, et al. Rethinking ovarian cancer II: reducing mortality from high-grade serous ovarian cancer. Nat Rev Cancer. 2015;15(11):668–79. https://doi.org/10.1038/nrc4019.
Kurian AW, et al. Breast and ovarian cancer penetrance estimates derived from germline multiple-gene sequencing results in women. JCO Precis Oncol. 2017;1:1–12. https://doi.org/10.1200/po.16.00066.
Lilyquist J, et al. Frequency of mutations in a large series of clinically ascertained ovarian cancer cases tested on multi-gene panels compared to reference controls. Gynecol Oncol. 2017;147(2):375–80. https://doi.org/10.1016/j.ygyno.2017.08.030.
Loveday C, et al. Germline mutations in RAD51D confer susceptibility to ovarian cancer. Nat Genet. 2011;43(9):879–82. https://doi.org/10.1038/ng.893.
Loveday C, et al. Germline RAD51C mutations confer susceptibility to ovarian cancer. Nat Genet. 2012;44(5):475–6. https://doi.org/10.1038/ng.2224.
Rafnar T, et al. Mutations in BRIP1 confer high risk of ovarian cancer. Nat Genet. 2011;43(11):1104–7. https://doi.org/10.1038/ng.955.
Ramus SJ, et al. Germline mutations in the BRIP1, BARD1, PALB2, and NBN genes in women with ovarian cancer. J Natl Cancer Inst. 2015;107(11) https://doi.org/10.1093/jnci/djv214.
Song H, et al. Contribution of germline mutations in the RAD51B, RAD51C, and RAD51D genes to ovarian cancer in the population. J Clin Oncol. 2015;33(26):2901–7. https://doi.org/10.1200/JCO.2015.61.2408.
Lu HM, et al. Association of Breast and Ovarian Cancers with predisposition genes identified by large-scale sequencing. JAMA Oncol. 2019;5(1):51–7. https://doi.org/10.1001/jamaoncol.2018.2956.
Masuda K, et al. Clinical utility of a self-administered questionnaire for assessment of hereditary gynecologic cancer. Jpn J Clin Oncol. 2017;47(5):401–6. https://doi.org/10.1093/jjco/hyx019.
Alsop K, et al. BRCA mutation frequency and patterns of treatment response in BRCA mutation-positive women with ovarian cancer: a report from the Australian ovarian cancer study group. J Clin Oncol. 2012;30(21):2654–63. https://doi.org/10.1200/JCO.2011.39.8545.
Risch HA, et al. Population BRCA1 and BRCA2 mutation frequencies and cancer penetrances: a kin-cohort study in Ontario, Canada. J Natl Cancer Inst. 2006;98(23):1694–706. https://doi.org/10.1093/jnci/djj465.
Enomoto T, et al. The first Japanese nationwide multicenter study of BRCA mutation testing in ovarian cancer: CHARacterizing the cross-sectionaL approach to ovarian cancer geneTic TEsting of BRCA (CHARLOTTE). Int J Gynecol Cancer. 2019;29(6):1043–9. https://doi.org/10.1136/ijgc-2019-000384.
NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®). Genetic/familial high-risk assessment: breast, ovarian, and pancreatic. 2020.
Chen S, Parmigiani G. Meta-analysis of BRCA1 and BRCA2 penetrance. J Clin Oncol. 2007;25(11):1329–33. https://doi.org/10.1200/JCO.2006.09.1066.
Kuchenbaecker KB, et al. Risks of breast, ovarian, and contralateral breast cancer for BRCA1 and BRCA2 mutation carriers. JAMA. 2017;317(23):2402–16. https://doi.org/10.1001/jama.2017.7112.
Rebbeck TR, et al. Association of type and location of BRCA1 and BRCA2 mutations with risk of breast and ovarian cancer. JAMA. 2015;313(13):1347–61. https://doi.org/10.1001/jama.2014.5985.
Narod SA, Foulkes WD. BRCA1 and BRCA2: 1994 and beyond. Nat Rev Cancer. 2004;4(9):665–76. https://doi.org/10.1038/nrc1431.
Callahan MJ, et al. Primary fallopian tube malignancies in BRCA-positive women undergoing surgery for ovarian cancer risk reduction. J Clin Oncol. 2007;25(25):3985–90. https://doi.org/10.1200/JCO.2007.12.2622.
Powell BC, et al. Risk-reducing salpingo-oophorectomy in BRCA mutation carriers: role of serial sectioning in the detection of occult malignancy. J Clin Oncol. 2005;23(1):127–32. https://doi.org/10.1200/JCO.2005.04.109.
Powell CB, et al. Risk-reducing salpingo-oophorectomy (RRSO) in BRCA mutation carriers experience with a consecutive series of 111 patients using a standardized surgical-pathological protocol. Int J Gynecol Cancer. 2011;21(5):846–51. https://doi.org/10.1097/IGC.0b013e31821bc7e3.
Shaw PA, et al. Candidate serous cancer precursors in fallopian tube epithelium of BRCA1/2 mutation carriers. Mod Pathol. 2009;22(9):1133–8. https://doi.org/10.1038/modpathol.2009.89.
Medeiros F, et al. The tubal fimbria is a preferred site for early adenocarcinoma in women with familial ovarian cancer syndrome. Am J Surg Pathol. 2006;30(2):230–6. https://doi.org/10.1097/01.pas.0000180854.28831.77.
Lee Y, et al. A candidate precursor to serous carcinoma that originates in the distal fallopian tube. J Pathol. 2007;211(1):26–35. https://doi.org/10.1002/path.2091.
Bolton KL, et al. Association between BRCA1 and BRCA2 mutations and survival in women with invasive epithelial ovarian cancer. JAMA. 2012;307(4):382–90. https://doi.org/10.1001/jama.2012.20.
Candido-dos-Reis FJ, et al. Germline mutation in BRCA1 or BRCA2 and ten-year survival for women diagnosed with epithelial ovarian cancer. Clin Cancer Res. 2015;21(3):652–7. https://doi.org/10.1158/1078-0432.CCR-14-2497.
Gallagher DJ, et al. Survival in epithelial ovarian cancer: a multivariate analysis incorporating BRCA mutation status and platinum sensitivity. Ann Oncol. 2011;22(5):1127–32. https://doi.org/10.1093/annonc/mdq577.
Vencken PMLH, et al. Chemosensitivity and outcome of BRCA1- and BRCA2-associated ovarian cancer patients after first-line chemotherapy compared with sporadic ovarian cancer patients. Ann Oncol. 2011;22(6):1346–52. https://doi.org/10.1093/annonc/mdq628.
Yang D, et al. Association of BRCA1 and BRCA2 mutations with survival, chemotherapy sensitivity, and gene mutator phenotype in patients with ovarian cancer. JAMA. 2011;306(14):1557–65. https://doi.org/10.1001/jama.2011.1456.
Pennington KP, et al. Germline and somatic mutations in homologous recombination genes predict platinum response and survival in ovarian, fallopian tube, and peritoneal carcinomas. Clin Cancer Res. 2014;20(3):764–75. https://doi.org/10.1158/1078-0432.CCR-13-2287.
Miller RE, et al. ESMO recommendations on predictive biomarker testing for homologous recombination deficiency and PARP inhibitor benefit in ovarian cancer. Ann Oncol. 2020; https://doi.org/10.1016/j.annonc.2020.08.2102.
Meric-Bernstam F, et al. Incidental germline variants in 1000 advanced cancers on a prospective somatic genomic profiling protocol. Ann Oncol. 2016;27(5):795–800. https://doi.org/10.1093/annonc/mdw018.
Rosenthal AN, et al. Evidence of stage shift in women diagnosed with ovarian cancer during phase II of the United Kingdom familial ovarian cancer screening study. J Clin Oncol. 2017;35(13):1411–20. https://doi.org/10.1200/JCO.2016.69.9330.
Skates SJ, et al. Early detection of ovarian cancer using the risk of ovarian cancer algorithm with frequent CA125 testing in women at increased familial risk – combined results from two screening trials. Clin Cancer Res. 2017;23(14):3628–37. https://doi.org/10.1158/1078-0432.CCR-15-2750.
Helder-Woolderink JM, et al. Ovarian cancer in lynch syndrome; a systematic review. Eur J Cancer. 2016;55:65–73. https://doi.org/10.1016/j.ejca.2015.12.005.
Akbari MR, et al. Correlation between germline mutations in MMR genes and microsatellite instability in ovarian cancer specimens. Familial Cancer. 2017;16(3):351–5. https://doi.org/10.1007/s10689-017-9973-1.
Tung N, et al. Counselling framework for moderate-penetrance cancer-susceptibility mutations. Nat Rev Clin Oncol. 2016;13:581–8. https://doi.org/10.1038/nrclinonc.2016.90.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Masuda, K., Satake, M., Aoki, D. (2021). Hereditary Ovarian Cancer. In: Nakamura, S., Aoki, D., Miki, Y. (eds) Hereditary Breast and Ovarian Cancer . Springer, Singapore. https://doi.org/10.1007/978-981-16-4521-1_7
Download citation
DOI: https://doi.org/10.1007/978-981-16-4521-1_7
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-16-4520-4
Online ISBN: 978-981-16-4521-1
eBook Packages: MedicineMedicine (R0)