Abstract
Energy security, environmental concerns, and the increasing demand of a growing population present opportunities for adopting alternative pathways for energy and chemicals. Microbial biotechnologies have been making progress in the context of renewable energy production towards creating more sustainable societies. While the state-of-the-art production of bio-based energy, chemicals, and materials promises competitive functionality and quality, evaluation of their sustainability is crucial, particularly for emerging biotechnologies. Analytical methods such as techno-economic analysis (TEA) and life cycle assessment (LCA) are standardized techniques that are used to quantify economic viability and environmental sustainability of processes and products and offer decision-making information on their research, development, and deployment. However, challenges still exist for TEA and LCA studies to support biotechnology transition to a more sustainable future. Examples of such challenges include data availability and accessibility considering technology readiness levels in TEA studies, broadening the impact assessment to categories other than a single impact indicator (e.g., global warming potential), and estimating full life cycle performance in LCA studies. To address these challenges and to promote a sustainable bio-based economy, this chapter provides a systematic overview of the status of renewable bioenergy and biochemicals commercialization, markets, and policies. Additionally, the chapter discusses possible knowledge-based process design approaches, identifying the interrelations between the challenges and development regarding resource efficiency and waste minimization, and bridging the gap between research and commercialization. Case studies of biobutanol production pathways are also discussed for learning and optimization potential for sustainability gains. Finally, the chapter emphasizes the engagement of multiplayers for interdisciplinary work to bring renewable energy into reality.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
14.1 Introduction
Techno-economic analysis (TEA) and life cycle assessment (LCA) are prospective methods in the assessment of green/sustainable technologies. Green products or technologies, by definition, are to be environment friendly. Research, development, and deployment (RD&D) of green products or technologies are actions taken to achieve different sustainable development goals (SDGs), such as the ones adopted by United Nations (UN) in 2015, including affordable and clean energy, climate action, economic growth, and clean water, for creating a more sustainable future. While technical feasibility and functionality of biotechnologies is a knockout criterion for researchers and interested industry players to identify opportunities, further assessments such as economic viability, ecologic sustainability, and social acceptance are also essential. For example, “yields,” “energy efficiency,” and “reaction rate” are typical technical indicators; however, high scores in these indicators may be accompanied by downsides such as expensive equipment, high global warming potentials, or high eutrophication risks. Extreme cases are green technologies that are neither “green” nor affordable but run against the SDGs. Environmental and economic impacts are two essential criteria for these green chemical technologies, not only to their qualification for lower environmental impact than their conventional counterparts but also their ability to replace conventional technologies commercially.
The fundamental idea of renewable energy production through microbial processes is the processing of biobased resources into energy/chemicals/materials. The challenge for such process development is the scarcity of resources in terms of natural capital and money (Buchner et al. 2018). Thus, the process development needs to achieve three goals: (1) maximizing utilization of all biomass components and minimize waste, (2) evaluating the tradeoffs resulting from the interactions between technical advances and sustainability parameters, and (3) building the decision-making platform of resource allocation for raw material suppliers, producers and stakeholders (Wu et al. 2019). Based on these objectives, this chapter presents the structure and content of TEA and LCA methodologies to understand the framework of sustainability analysis; reviews and discusses challenges and opportunities for microbial processes in renewable energy production to address the importance of biotechnology for a biobased economy; and finally illustrates TEA and LCA applications through case studies. In the end, key factors and concepts are discussed for the roadmap to bring the microbial process in renewable energy production into reality.
14.2 TEA and LCA Methodologies
Developing appropriate tools and methods for measuring sustainability is necessary for inducing new technologies, especially those in a position of making a difference in developing our sustainable future. This chapter focuses on the specific context of two popular ones: TEA and LCA.
LCA is a systematic technique to assess the environmental impacts associated with all the stages (production, distribution, use, and end-of-life phases) of a product’s or service’s life. During an LCA, the upstream and downstream processes throughout the entire life cycle of a product, process, or service are included. For example, in the LCA of bioenergy, the environmental impacts cover biomass cultivation with all relevant inputs and outputs from the environment (e.g., carbon dioxide emission or sequestration and water consumption) as well as emissions from incineration into the air, water, and soil.
Techno-economic analysis (TEA) measures the technical and economic performance of a process, product, and service. To evaluate a specific technology (e.g., compare different options, analyze commercialization feasibility), TEA is an integral tool that usually combines process design and simulation/model with establishing capital and operating cost profiles. For profit-oriented stakeholders, TEA is the most important basis for decisions about research, development, and deployment (RD&D). Specifically, TEA connects research, engineering, and business. Having the capability of being conducted at different technological stages and production scales, TEA can be used as a basis for making a variety of decisions. For example, researchers can use TEA to identify process hotspots of production cost at bench scale, engineers can compare process conditions and configurations for financial impact during process design and development, and investors can determine the potential economic viability of a project by averting unnecessary expenditures.
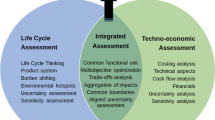
TEA and LCA share similar logic for contents. They are assessments of a product or process that provide essential decision-making information. As defined by ISO standards, LCA consists of four phases: goal and scope definition, life cycle inventory (LCI) analysis, life cycle impact assessment (LCIA), and interpretation of results (Fig. 14.1). Cost estimation and market investment are important components of TEA, where cost and revenue are calculated for profitability analysis. Similar to TEA, life cycle costing (LCC) is a cost assessment tool over the life of a project. Therefore, LCC and LCA have analogous procedures with a consistent definition of the product system and measures the financial impacts. Considering the scope and boundaries, TEA can be applied as the basis for life cycle costs inside the plant gate. In a broader concept, LCC and LCA together with social life cycle assessment are three pillars of life cycle sustainability assessment (LCSA). LCSA is an integrated framework for the application of life cycle thinking. For example, in modern business practice, life cycle management is a comprehensive decision process, which addresses the three pillars and assesses the cost and performance tradeoffs.
When carried out in parallel, TEA and LCA have usually the same goal and scope, as well as they overlap in inventories such as mass and energy balances in terms of physical, chemical, and biological flows (Fig. 14.2). The assessment outcomes can be reflected in different category indicators/indices/metrics. For example, the sustainability metrics cover a wide range of aspects including economic, environmental, and social factors. Currently, no universal metrics are recognized for evaluating the sustainability of a product or process; however, many studies (Bare et al. 2006; Horváth et al. 2017; Tabone et al. 2010) employ widely used approaches such as green chemistry metrics and life cycle assessment. A positive correlation has been found between adherence to green design principles and a reduction of the environmental impacts of a process (Tabone et al. 2010). The principles for green chemistry and green engineering (green metrics) are well known for the design of chemical products and processes that utilize resources (e.g., raw materials, energy) efficiently and reduce waste and toxic/hazardous chemicals use. Mass-based metrics such as atom economy and E-factor (environmental factor), which are core parts of green process design, need to be augmented by metrics of measuring the environmental impact and assessing economic viability (Sheldon 2018). With limited information and resources, identifying the essential metrics could be important for decision-making in chemical manufacturing processes.
Data access and quality are common issues faced by both TEA and LCA methods, which can complicate conducting such analyses. One crucial concept, here, is the technology readiness level (TRL). TRL rates the technological maturity of R&D projects, which indicates the data availability and the corresponding accuracy of the results. For LCA practitioners, the method of building life cycle inventory is also closely related to the database, which could be challenging considering both time requirement and model accuracy. Building and running TEA and LCA models require extensive data collection and analysis for inventories. For example, cost inventory requires operational and capital expenditure and life cycle inventory requires material and energy flow data sets, not only for the studied process but also for upstream chemicals/materials processes. To address the missing data issue, process simulation is a valuable method for inventory data estimation, especially either for chemicals that are not currently commercially produced or for which the primary industrial data are not accessible. An example of such is that the process to produce some chemicals at commercial scales is kept confidential. Figure 14.3 shows the logic of process modeling as a simplified approach for TEA and LCA. The process simulation, however, still requires sufficient data such as concept proof/validation in the laboratory, knowledge of detailed process design parameters and operating conditions, which could have an impact on the quality of inventory data obtained from the simulation. Therefore, the more details achieved in the process model, the more accurate results are obtained from those assessments. In this chapter, we will also introduce a computer simulation platform integrating sustainability assessment tools along with technical performance to compare biotechnology alternatives.
14.3 Microbial Process in Renewable Energy Production: Challenges and Opportunities
Biotechnology uses microorganisms and enzymes for renewable energy production. Microorganisms are “unseen majority”—abundant and diversified, which have the potential to help solve the global energy and climate change challenge (Cavicchioli et al. 2019). Specifically, microbial technologies provide mitigation solutions such as biofuels and CO2 fixation from the contribution of marine and territorial biome. Biofuels, as renewable energies, could be a large-scale approach. Microorganisms and their environment interact and affect each other. On the one hand, microorganisms convert nutrients to various potentially useful by-products (e.g., biofuel) with evolved metabolic strategies under changing environments; on the other hand, the environment is influenced by the products (e.g., methane) generated by the microorganisms. In addition to a systematic understanding of the biological mechanism of energy and carbon transformation, in many cases, the development of microbial processes requires economically viable options, optimized process design, scale-up concepts, and ecological insights. With this in mind, this section presents the challenges for R&D, commercialization aspects, and success/failure cases and closely relates TEA and LCA in the following aspects: (1) early stages for directing research efforts, (2) commercial-scale production for developing a framework, and (3) promote biotechnology contributions to solving environmental sustainability problems.
14.3.1 Status of Renewable Bioenergy/Biochemicals Commercialization, Markets, and Policies
Until now, various biofuel types (alcohols, biogas, hydrogen, biodiesel, hydrocarbons) using a variety of feedstocks (e.g., lignocellulosic, algal biomass, industrial waste) and strains of microorganisms have been researched and developed at different levels from laboratory scales to industrial scales. However, substantial commercial production of biofuels such as cellulosic biofuels is still limited if any available. An example is cellulosic ethanol, an important biofuel whose production has been scaled up for commercialization. Among the three major commercial startup projects in 2014, namely DowDuPont, POET-DSM, and Abengoa SA, none produces cellulosic ethanol commercially at present. Abengoa sold its U. S. ethanol plants in 2016, DowDuPont sold its plant to the company Verbio in 2018, which produces renewable natural gas instead of ethanol, and POET- DSM’s plant is converted to an R&D facility while their ethanol production ceased in 2019. The commercialization of cellulosic ethanol has been tried without success. The reason for this could be three-fold: operational, feedstock-related, and socio-economic aspects. Technically, operation difficulties such as temperature control, microbes contamination, solids handling, and equipment functioning have made processing conditions suboptimal, which prevent the laboratory results (yields and conversion efficiencies) from being realized and economically viable at industrial scales. Likewise, the supply of biomass, including the quantities, collection, transportation, and storage feedstock is still not reliable. Moreover, the socio-economic aspects such as market competition with traditional corn ethanol, the overall “blend-wall” for ethanol, social acceptance of vehicles with high ethanol blends (e.g., E85), and regulatory uncertainties (e.g., investment deterrence) have hampered the development of cellulosic ethanol.
Biodiesel has been commercialized, especially in Europe, as a key biofuel. Currently, the main feedstock for biodiesel conversion is still plant oil, which may be a controversial topic for the potential impact on food markets. Waste and microbial oil (e.g., microalgal lipids) show good future potential for biodiesel development. Renewable natural gas, which is from the biogas product of anaerobic digestion, has been increasingly addressed, for its flexibility in utilizing renewable waste materials (e.g., agricultural residue, municipal solids waste, urban wastewater, livestock manure). Biohydrogen produced through biological means promises merits as a clean fuel with high energy content, however, its commercialization needs to be further validated by improving yield, storage, and transportation logistics, and overcoming the difficulties in strains, fermentation (e.g., substrate), engineering aspects (e.g., bioreactors design). Algal biofuel has been a very active research field since 2005, for its promising features (e.g., high photosynthetic efficiency, using low non-arable land and low-quality water) over terrestrial feedstocks. Although algal biomass depicts a bright future of sustainable energy, additional effort including strain selection, cultivation conditions, and the downstream process is required to advance the practical utilization of algal biomass. Table 14.1 shows the commercialization status of different types of renewable energy through a variety of microbial processes, industrial plants, and future deployment considerations. Policies play important roles in the development of biofuels, both in the R&D and market stages. In general, policies related to bioenergy in the US include feed-in tariffs, carbon tax, biofuel standards for transportation, sustainability standards, and certification, and electricity and heat policies. Due to the complex interaction of various factors, policies have been a controversial topic for promoting bioenergy use and lowering emissions. For example, a carbon tax may affect some economic sectors such as the coal industry and interfere the social equity, while the policy itself may to some extent be limited in impacting climate change. However, good practices and considerations could be designed to adapt to target-specific policies (Smolinksi and Cox 2016). For instance, flexible rates and differentiating payments according to different scenarios (e.g., fuel type, project size, upstream producers/downstream customers), and integrating other policies such as water/land/agriculture could be more resilient and effective in facing the implementation challenges. Policy innovation could also be a powerful tool in reducing risks, thereby encouraging investments in promising bioenergy technologies. One example is the suggestion of a reverse auction instead of government subsidies for corn stover biofuels to reduce the long-term risk for investors as presented in TEA research by Petter and Tyner (2014). Overall, for bioenergy markets and bioeconomies, proper policies could play a key role, especially in a phenomenon of fragile crude oil price and market fluctuations, to secure a structural transition to a downward trend in non-renewables.
14.3.2 TEA and LCA in Research, Development, and Technology Deployment
The scientific literature explicitly using TEA and LCA or an economic and life-cycle approach, to estimate the economic and environmental impacts of bioenergy production and use, as well as other sustainability dimensions has been increasing. These publications can be classified into three main categories: (1) technological system and its direct impacts (e.g., financial performance, emissions), (2) development of evaluation tools (e.g., TEA and LCA methods, sustainability metrics), and (3) sustainability trade-offs and indirect impacts (e.g., social benefits, land use, food security, biodiversity). Intensive research addresses the first category, due to the relatively low technology readiness level of the entire bioenergy industry. Specifically, for the microbial process, the fermentation step is emphasized (Crater et al. 2018). For example, effective microbial communities by systems biotechnology and enzyme/biocatalyst engineering in fermentation have improved capabilities in bioenergy conversion (e.g., higher yields, less inhibition) (Srivastava 2019). This is critical because the fermentation step has a direct impact not only on the economics but also on the technical performance of downstream processing. The importance of “begin with the end” should be also noted for understanding scale-up effects. In many studies, feedstock, pretreatment, and geographical information are starting points within the context of a conceptual design and early guidance for reliable scale-up results of end production. Cherubini and Strømman (2011) have reviewed evolving bioenergy LCA studies and found most research results show more favorable environmental impacts of bioenergy than that of fossil fuels in terms of GHG emission reductions and fossil energy consumption. However, the economic outcomes are more diversified depending on the assumptions (e.g., government incentives, feedstock compositions, geographic and seasonal factors) (Vasco-Correa et al. 2018). Nevertheless, these discussions about bioenergy encourage moving the research and technology deployment towards the direction of development in a more sustainable manner. The areas can be the following technical aspects.
14.3.2.1 Biorefinery Concept
The biorefinery concept has been increasingly focused by researchers, especially TEA and LCA practitioners. Strategies such as byproducts valorization and diversifying product portfolio could potentially reduce the economic risk of investing in a single product by maximizing resource utilization and minimizing “waste”. Accordingly, methodological progress is needed such as allocation of LCA, which should be selected to represent the system with less uncertainty or avoided by using the proper functional unit and defining different system boundaries.
14.3.2.2 “Waste” Materials/Non-food Crops as Feedstock
Biomass as a feedstock for bioenergy production could be an expensive choice, which may also entail environmental burdens to some extent. For example, food-based feedstock cultivation could require substantial inputs such as fertilizer and water and has an indirect influence on the food price, which could result in an increase in both monetary values and mass inflows in the system. As discussed previously, using “waste” materials does not necessarily mean automatic cost-effectiveness or an eco-efficient process. For instance, the process of using lignocellulosic biomass to produce bioenergy or other bio-based products is still limited to the R&D stage, due to the technical difficulties and trade-offs across various sustainability sectors.
14.3.2.3 Process Enhancement
Despite the endeavors by researchers and engineers to use the biomass more efficiently in process hotspots such as pretreatments, microbial culturing and processing, and integrated downstream processes, there is no clear breakthrough technology that significantly makes changes to the energy conversion and the developed system that delivers gains in both bio-based production/processing and waste treatment. At least, fundamental issues such as microorganisms’ potentials, plant phenotyping, reaction mechanism, and inter-and transdisciplinary research need to be more thoroughly understood.
Complexity and diversity of the bioenergy systems (e.g., system boundaries in LCA, production capacity in TEA) have made different studies non-comparable, which means there is still space to improve the methodology for knowledge-based decisions. For example, considering end-of-life scenarios and environmental portfolios including indirect effects (not just GHG emission), addressing data scarcity, and building the analysis framework. Moreover, the 2020 trade-offs should be addressed such as industry bearing and competing for scenery (e.g., regions revenue, market growth trends, manufacturers) (Escobar and Laibach 2020). The example of the first generation of bioethanol and cellulosic ethanol could illustrate the concept. The existence of first-generation bioethanol (such as sugar-based) with its market and suppliers, although criticized by many researchers for its long-term impacts on the environment and food security, could be the result of trade-offs in economic drivers, energy security, resource re-allocation, and the farmers’ benefits. The rare success stories of cellulosic ethanol could be partially attributed to the competition with traditional ethanol, either first-generation or fossil-based, where the underlying approaches are the interactions of different groups of interest. For the chemical industry using biomass as the raw materials, sustainability metrics such as green chemistry metrics need to be taken into account when designing the process and evaluating its economic, environmental, and societal impacts. To bring bioeconomy into the reality, as harnessed by bioenergy, it requires the efforts of players from a wide range such as chemists, engineers, microbiologists, economists, governments, stakeholders, and the communities.
14.4 Case Studies: Lignocellulosic Butanol as an Advanced Biofuel
To illustrate TEA and LCA in evaluating the learning and optimization potential of bioenergy technologies, the biobutanol production processes were investigated and compared as a case study, which covered novel approaches, traditional fermentation methods, and the fossil-based benchmark. This section presents the background of biobutanol production, TEA and LCA modeling details, and key aspects to reinvigorate butanol for bioenergy applications.
14.4.1 TEA of Biobutanol Production Alternatives
The traditional fermentation method for butanol production is called Acetone–butanol–ethanol (ABE) fermentation. Although ABE fermentation has been industrially exploited in the US since the beginning of the last century, it was replaced by the petrochemical industry around the 1960s (Ezeji et al. 2007). The main problems included high feedstock cost, product inhibition, low ABE yield, low productivities, and inefficient recovery processes. However, butanol has increasingly attracted researchers’ attention for its various advantages (high energy content, low water solubility, high blending ratio in gasoline, etc.). Specifically, utilizing cost-effective cellulosic feedstock has motivated the biosynthesis of butanol in the recent era (Kumar et al. 2012). Table 14.2 shows the status of leading biofuel companies producing bio-butanol.
Economic analysis of ABE fermentation has been performed by several researchers (Pfromm et al. 2010; Kumar et al. 2012; Tao et al. 2014; Qureshi et al. 2013) with regard to different feedstocks and process parameters (fermenter size, plant capacity, microbial strains, production yield, etc.). In these studies, the ABE fermentation butanol yields are 0.11–0.3 g/g biomass. Many of these studies were performed on the lab scale and multiple additional assumptions. The low yields were due to the low concentration of butanol in the fermentation broth (12–18 g/L) and the presence of a variety of inhibitory chemicals (furfural, hydroxymethylfurfural (HMF), etc.) generated before and during fermentation. The industrially confirmed yield of 0.11 g butanol/g of corn corresponds to 34 wt% conversions of solvents (Pfromm et al. 2010). Debates exist in energy yield comparison between ethanol fermentation and acetone–butanol–ethanol (ABE) fermentation (Wu et al. 2007; Swana et al. 2011; Tao et al. 2014). To improve the yields of bio-butanol production as an advanced biofuel, a new and promising scheme for the “hybrid conversion” process employs anaerobic bacteria to produce an alternative intermediate—butyric acid, which has a higher titer (more than 60 g/L) and then converting butyric acid to butanol through a catalytic process (more than 98% conversion rate) (Lee et al. 2014).
There is very limited research on the comparisons of traditional ABE fermentation and the butyric acid to butanol catalytic process from domestic lignocellulosic biomass such as corn stover and wheat straw, which are representative of their high cellulose content and biomass yield per unit area (Swana et al. 2011). Thus, this research will focus on bio-butanol production with lignocellulosic feedstock and concentrate on one of the major bottlenecks in the overall process—the difficulty in product purification from the fermentation broth. To address the challenge, different biorefinery scenarios (conversion and product recovery) are discussed to separate butyric acid/butanol from other byproducts, mainly acetic acid/ethanol in both perspectives of energy and economic analysis.
14.4.1.1 TEA Method
This research is focused on the catalytic process for converting butyric acid to butanol of the “hybrid” conversion process. Here, butyric acid is used as direct input in the fermentation broth. Information such as the butyric acid concentration and yields fermentation process is based on literature (Sjöblom et al. 2015). Since the fermentation broth contains butyric acid and other coproducts (mainly acetic acid), two scenarios were investigated:
Scenario 1: First catalytically convert the acids (butyric acid and acetic acid) in the mixture from fermentation broth to alcohols and then separate the alcohols to around 95% mass purity.
Scenario 2: First separate the two acids in the mixture, catalytically convert each of them to their corresponding alcohol, and finally purify the alcohol to 95% mass purity.
The thermodynamic properties of butyric acid and acetic acid are shown in Table 14.3. Considering a plant capacity of 30 million gallons/year of butanol, assumptions made in this study are as the following:
-
Acetic acid and butyric acid could be catalyzed by the same catalyst (ZnO-supported Ru-Sn bimetallic catalyst).
-
The catalysts have the same selectivity (99.9%) and conversion rates (98.6%) on both acetic acids and butyric acid.
-
The concentration of acetic acids and butyric acid does not affect the catalyst’s selectivity and conversion rates.
-
The catalytic process was operated on the same condition: 265 °C and 25 atm.
-
The concentration of butyric acid and acetic acid in the fermentation is 58.8 g/L and 11.46 g/L, respectively.
-
The capital cost is borrowed at an interesting rate of 10% for 20 years.
The catalytic process is through the conversion of hydrogenation of acids in the vapor phase by a stable and selective catalyst. Metal catalysts such as Cu/ZnO/Al2O3 and ZnO-supported Ru-Sn bimetallic catalysts could have more than 98% yield of butanol from biomass-derived butyric acid. The selectivity (ratio of substrate converted to desired product to total substrate converted, addressing unwanted reactions) and conversion rates are important criteria in selecting the catalysts. Here, the main reactions are:
Then Aspen plus V8.8 was used to simulate the processes of the two scenarios and the economic performance is evaluated.
Scenario 1: The process flow diagram (PFD) of scenario 1 is shown in Fig. 14.4. The feed broth and hydrogen are introduced into the catalytic reactor R1, where acetic acid and butyric acid are converted to ethanol and butanol through hydrogenation reaction, respectively. The effluent from the reactor goes into a distillation column (BEERCOL). Here, two azeotropes are formed ethanol and water, butanol, and water (as analyzed by ASPEN, shown in Fig. 14.5). The distillate (S2) contains most ethanol and butanol as well as a portion of water. The S2 is sent for further distillation SEPDIST, where ethanol and butanol are separated for individual distillation for a 95% mass purity. The distillation column ETOHD produces the target ethanol and column BTOHD produces the target butanol. For butanol purification, a decanter is used for two liquid phase separation for removing water. The n-butanol/water azeotrope is heterogeneous, which is different from the ethanol/water system (homogeneous), and therefore the constituents of the mixture are not completely miscible in the decanter (two liquid phases). This process refers to the double effect distillation to obtain ABE as final products (Naleli 2016). Here, the property method chosen is UNIQUAC (universal quasichemical). Vapor-liquid equilibrium for ethanol and butanol is shown in Figs. 14.6 and 14.7. The ternary diagram for butanol ethanol and water is shown in Fig. 14.8.
Scenario 2: The flowsheet of the process of scenario 1 is shown in Fig. 14.9. Different from scenario 1, in this scenario, the mixture of butyric acid and acetic acid is sent to the distillation column DIST01 for separation. Here, the acetic acid solution AA is obtained at the bottom of the distillation column, and the azeotrope of butyric acid and water is obtained as distillate, as analyzed by the azeotrope search report (Fig. 14.10) in ASPEN. Then, acetic acid and butyric acid are sent to the catalytic process separately. In reactors RAA and RBB, each acid is converted to its alcohol. The ethanol and butanol solutions obtained are sent for purification by distillation. Then, over 95% mass purity alcohols are obtained. The butanol purification process is similar to that of scenario 1.
14.4.1.2 Results and Discussion
The capital cost and operation cost were obtained by ASPEN Process Economic Analyzer with its built-in evaluation method of sizing based on the mass and energy balance. The economic analysis summary is shown in Table 14.4. The cost of the main equipment is shown in Table 14.5. The utilities include electricity, steam, refrigerant, and cooling water. The overall economic performance of scenario 1 is better than that of scenario 2 due to the significant savings in operating costs. The high capital and operating costs of Scenario 2 are mainly caused by the distillation difficulties in separating butyric acid and acetic acid and huge utility requirements. Here, without considering the butyric acid fermentation cost, the unit cost for scenario 1 is 0.21 $/L butanol, while scenario 2 has a unit cost of 0.84 $/L butanol. Thus, the process in which the butyric acid fermentation broth was catalyzed before products recovery has better economic performance.
The butyric acid fermentation process is similar to the bioethanol fermentation process. The major difference is the microbes involved in the fermentation. Considering the butyric acid concentration of 58.8 g/L (Sjöblom et al. 2015), ethanol fermentation has a similar titer. The lignocellulosic ethanol fermentation process (Wu 2018) was used as a reference for the economic analysis of butyric acid production cost. The butyric acid production cost is estimated to be 0.71 US$/L. To produce 1 kg of butanol, 1.19 kg of butyric acid is required. The butanol production cost is estimated to be 0.87 U$/L in Scenario 1. Due to limited studies available in the literature about the production cost of butyric acid, future work of evaluating the production cost of butyric acid for the specific fermentation methods is necessary.
Baral and Shah (2016) estimated the butanol production cost from traditional ABE fermentation to be 1.8 $/L. Qureshi et al. (2013) also presented a techno-economic analysis of ABE fermentation with a production cost of 1US$/L. However, different assumptions were made regarding the plant capacity, biorefinery concepts, and recovery methods. Therefore, it is difficult to make comparisons in many aspects.
The butanol purification process could be further optimized as the following: Butanol-water system will form two liquid phases once condensed. This is a steady-state simulation of an azeotrope mixture of system butanol and water in which case two columns were used with a decanter located in between (Luyben 2008). Decanter separated two liquid phases and returned on the aqueous phase and organic (butanol rich) phase to a column as a reflux stream. Recycling and recovering steps for the remaining product in the waste stream are needed but not discussed in this study, which could be further investigated in future work.
The TEA work studied different scenarios about the butyric acid to butanol catalytic process to obtain the final product—butanol. Catalytically converting the acids (butyric acid and acetic acid) in the fermentation broth to alcohols before separating the alcohols shows promising economic advantages. With the advantage of a higher titer than ABE fermentation, butyric acid fermentation still needs a more detailed techno-economic analysis to investigate whether it achieves a competitive cost or not. Besides, the waste stream from the whole process is another area for future research with the purpose of recovering energy and improving economic performance.
14.4.2 Environmental Impacts Considerations: LCA of Butanol Production Alternatives
An LCA study was carried out to evaluate environmental impacts along with process design for the implementation of bio-butanol technologies, support the strategic decision-making process, and analyze and compare different production alternatives of butanol. A wide variety of processes for butanol production have been studied through LCA such as the effect of different pretreatment methods (Baral et al. 2018), conversion methods such as oxo synthesis (Brito and Martins 2017), and ABE fermentation (Pereira et al. 2015), different feedstocks (e.g., corn and wheat straw) (Wu et al. 2007), different microbial strains (e.g., clostridia, cyanobacteria) (Nilsson et al. 2020), and different separation processes (Mahmud and Rosentrater 2020). The assessment applied in this case study used the TEA results from the previous section, which compare different alternatives of the butyric acid catalytic process to show the economic potential.
14.4.2.1 LCA Method
We used the LCA method as a tool to environmentally assess, identify hotspots and recommend strategies to improve the butanol production process. The LCA model was built according to the international standards ISO 14040 and ISO 14044. To conduct the LCA, the SimaPro 9.0 and Traci 2.1 V1.05/US 2008 methods were used.
14.4.2.2 Goal and Scope Definition, Functional Units, and System Boundary
The main purpose of the assessment was to compare different proposed process configurations for butanol production as a fuel. Here, three cases were investigated (Fig. 14.11): (1) butanol production through the catalytic process of butyric acid (two scenarios) from lignocellulosic (wheat straw), (2) butanol production through direct fermentation: ABE by clostridia (wheat straw) and fermentation by E.coli of lignocellulosic (corn stover) biomass, and (3) Butanol production through the petrochemical pathway. The functional unit is defined as 1 MJ of butanol product. A “well to wheel (WTW)” system boundary is considered in this case study, which uses butanol as the final product of the industrial production facility.
14.4.2.3 Life Cycle Inventory
The materials and energy inputs of the inventories for the production of butanol using different processes are obtained from a combination of sources: process simulation results were mainly used and literature and Ecoinvent database v.3 were used for data gaps when needed. The inventory for the petrochemical pathway was directly provided by the Ecoinvent database v.3. Specifically, the petrochemical process includes propylene hydroformylation (oxo synthesis) with subsequent hydrogenation of the aldehydes formed. The hybrid conversion process includes two main processes: butyric acid fermentation, and butyric acid to butanol catalytic process. The butyric acid fermentation process data were based on the process model developed by Baroi et al. (2017) (Table 14.6), where the yield and concentration of butyric acid are in the same range as the TEA model presented in the previous section. Considering the substitutive catalytic process, minor modifications for the inventory data were made to exclude extraction and purification steps. Mass allocation was considered for the two main products: butyric acid and acetic acid. The energy consumption for the following butanol catalytic process was estimated by the previous process simulation section for the industrial scenarios. As a benchmark for the hybrid conversion process, two butanol production through direct fermentation processes were also evaluated: ABE process data from Brito and Martins (2017) and butanol conversion process using corn stover hydrolyzed sugars from the GREET model by Argonne National Laboratory (Dunn et al. 2015). The summary of data sources for processes of different cases considered in the LCA is shown in Table 14.7.
14.4.2.4 Results and Discussion
This case study of LCA covers a wide range of mostly hypothetical processes, which also means the process is still in the R&D stage and currently does not exist at a commercial scale. The overall objective of this study was to explore the potential of butanol production through a novel catalytic process (the modeled hybrid conversion system) by comparing it with the traditional configurations. Our results could be helpful for researchers to focus on areas for sustainability in the future.
14.4.2.5 Environmental Impact Assessment
The main difference observed in the environmental impacts between the process pathways is related to energy consumption in terms of electricity, heat, and cooling energy. Figure 14.12 presents the comparison of impacts for 1 MJ of butanol through all the cases investigated. The hybrid conversion processes include two scenarios of process design, where scenario 1 (butanol 01) performs better in all environmental categories than scenario 2 (butanol 02). It can be inferred that the separation of final products after the catalytic reaction shows benefits in the environmental impacts, which is also in agreement with the economic results (lower operational cost due to lower utility usage). Thus, the results highlight the importance of catalytic process development in the aqueous phase for both economic and environmental advantages. The comparison also points out the environmental desirability of the hybrid conversion process for butanol, compared to the traditional ABE fermentation, since both scenarios of the hybrid conversion process show less environmental burden in most TRACI 2.1 categories, especially acidification, non-carcinogenic, and ecotoxicity. It should be noted that although butanol production from corn stover through fermentation of E.coli shows promising results in all the categories, it was modeled on additional assumptions such as fermentation temperature and retention time, as described in the GREET model, where further process refinement is required. Surprisingly, most of the bio-based routes for butanol production, except butanol from corn stover, have more environmental burden than the fossil-based route. This may partially be due to a lack of optimization of energy networks for the bio-based systems, whereas the fossil-based route is a mature industrial technology. Thus, we can conclude that at the current stage of biobutanol production, it may be difficult to compete with fossil-based butanol, both economically and environmentally.
For the process improvement, Fig. 14.13 shows the impact analysis of butanol hybrid conversion for both scenarios 1 and 2. Heat energy consumption and butyric acid are the main contributors to the environmental burdens, regardless, scenario 2 is more energy-intensive in terms of product purification steps. Figure 14.14 shows the environmental impacts in ten categories for 1 kg of butyric acid production. Electricity is a key factor for the technology, where it was mainly used for removing and recovering organic acids (butyric acid and acetic acid) using membranes. The separation of organic acids is essential for the downstream processes, either catalytic process or direct distillation. The connections between the steps determine the success of the biotechnologies’ development to some extent. Similar to butanol hybrid conversion scenario 2, the ABE fermentation process requires significant amounts of steam for butanol purification (Fig. 14.15). Nevertheless, the bottleneck for butanol production could be the energy-intensive downstream product purification process. The high-energy demand will also be reflected in a higher production cost. For the fermentation step in both the butyric acid and ABE process, enzymes inputs are the second major contributors to the environmental impacts, especially in categories such as eutrophication, non-carcinogenic, and ecotoxicity. It should also be noted that the waste stream treatment process is not considered for all the cases, which could add credits (energy and nutrients recovery) to these emerging biotechnologies.
14.4.3 Uncertainty
Variability in system parameters including inputs and design leads to the inherent uncertainty in the outcomes. Similar to other technology assessment methods, LCA and TEA could be associated with uncertainty risks when used for informing decisions. In the presented case studies, variability in feedstock composition, production and processing, allocation decision, geographic factors, and data estimation methods could cause variation in the environmental impacts and therefore uncertainty in the results. The sustainability impact scores may change substantially if variations in the input parameters are taken into account. Identifying the sources of uncertainty in the model and addressing them through a comprehensive uncertainty analysis will help increase the robustness of the results and reliability of recommendations for a wide range of potential process and market conditions. This highlighted data-driven research as a powerful tool in minimizing risks and maximizing benefits.
14.5 Conclusions, Guidelines, and Roadmap for the Future
This chapter provides state-of-the-art information and presents knowledge of analytical methods such as techno-economic analysis (TEA) and life cycle assessment (LCA) as standardized techniques that are used to quantify economic viability and environmental sustainability of microbial processes and products and assist with decision-making. R&D challenges while incorporating sustainability analysis to support biotechnology transition to a more sustainable future were discussed, especially in the area of data availability and accessibility considering technology readiness levels. Both challenges and opportunities for microbial process in renewable energy production were presented by a systematic review of commercialization status of renewable bioenergy/biochemicals, where success/failure stories were discussed and the main TEA and LCA findings in R&D and Technology Deployment were summarized.
The application of TEA and LCA tools and their main features in assessing the economic viability and environmental performance of microbial processes were further demonstrated through a case study of biobutanol production. The study evaluated the economic and environmental implications of different biobutanol production pathways representing the development of technologies, as well as improved configurations in the future. In general, an integrated or combined butanol production pathway (microbial and chemical) can be beneficial in terms of sustainability performance such as exhibiting lower environmental impacts as well as promising outcomes in the financial assessment. Although compared to a fossil-based route, the results of the case study may depict an unfavorable situation for the biobased route under current technological conditions, key sustainability improvements can be obtained by considering technological advances, waste treatment, and optimized energy networks. Compared to fossil-based energy, the microbial process in energy application still needs to overcome many shortcomings such as the input enzyme production, product recovery, and the interactions between the upstream and downstream steps. To bridge the gap between research and commercialization, this chapter emphasizes the role of interdisciplinary work, namely, analytics, science, engineering, politics, business, and society in building a harmonized and realistic roadmap for future sustainable biotechnology.
References
Baral NR, Shah A (2016) Techno-economic analysis of cellulosic butanol production from corn stover through acetone–butanol–ethanol fermentation. Energy Fuel 30(7):5779–5790
Baral NR, Quiroz-Arita C, Bradley TH (2018) Probabilistic lifecycle assessment of butanol production from corn stover using different pretreatment methods. Environ Sci Technol 52(24):14528–14537. https://doi.org/10.1021/acs.est.8b05176
Bare J, Gloria T, Norris G (2006) Development of the method and US normalization database for life cycle impact assessment and sustainability metrics. Environ Sci Technol 40(16):5108–5115. https://doi.org/10.1021/es052494b
Baroi GN, Gavala HN, Westermann P, Skiadas IV (2017) Fermentative production of butyric acid from wheat straw: economic evaluation. Ind Crop Prod 104:68–80
Brito M, Martins F (2017) Life cycle assessment of butanol production. Fuel 208:476–482. https://doi.org/10.1016/j.fuel.2017.07.050
Buchner GA, Zimmermann AW, Hohgräve AE, Schomäcker R (2018) Techno-economic assessment framework for the chemical industry—based on technology readiness levels. Ind Eng Chem Res 57(25):8502–8517. https://doi.org/10.1021/acs.iecr.8b01248
Cavicchioli R, Ripple WJ, Timmis KN, Azam F, Bakken LR, Baylis M et al (2019) Scientists’ warning to humanity: microorganisms and climate change. Nat Rev Microbiol 17(9):569–586. https://doi.org/10.1038/s41579-019-0222-5
Cherubini F, Strømman AH (2011) Life cycle assessment of bioenergy systems: state of the art and future challenges. Bioresour Technol 102(2):437–451. https://doi.org/10.1016/j.biortech.2010.08.010
Crater JS, Lievense JC (2018) Scale-up of industrial microbial processes. FEMS Microbiol Lett 365(13):fny138
Dunn JB, Adom F, Sather N, Han J, Snyder S, He C, … & You F (2015) Life-cycle analysis of bioproducts and their conventional counterparts in GREET (No. ANL/ESD-14/9 Rev.). Argonne National Lab.(ANL), Argonne
Escobar N, Laibach N (2020) Sustainability check for bio-based technologies: a review of process-based and life cycle approaches. Renew Sustain Energy Rev 135:110213. https://doi.org/10.1016/j.rser.2020.110213
Ezeji T, Qureshi N, Blaschek HP (2007) Butanol production from agricultural residues: impact of degradation products on Clostridium beijerinckii growth and butanol fermentation. Biotechnol Bioeng 97:1460–1469. https://doi.org/10.1002/bit.21373
Horváth IT, Cséfalvay E, Mika LT, Debreczeni M (2017) Sustainability metrics for biomass-based carbon chemicals. ACS Sustain Chem Eng 5(3):2734–2740. https://doi.org/10.1021/acssuschemeng.6b03074
Kumar M, Goyal Y, Sarkar A, Gayen K (2012) Comparative economic assessment of ABE fermentation based on cellulosic and non-cellulosic feedstocks. Appl Energy 93:193–204. https://doi.org/10.1016/j.apenergy.2011.12.079
Lee JM, Upare PP, Chang JS, Hwang YK, Lee JH, Hwang DW et al (2014) Direct hydrogenation of biomass-derived butyric acid to n-butanol over a ruthenium–tin bimetallic catalyst. ChemSusChem 7(11):2998–3001. https://doi.org/10.1002/cssc.201402311
Luyben WL (2008) Control of the heterogeneous azeotropic n-butanol/water distillation system. Energy Fuel 22:4249–4258. https://doi.org/10.1021/ef8004064
Mahmud N, Rosentrater KA (2020) Life-cycle assessment (LCA) of different pretreatment and product separation technologies for butanol bioprocessing from oil palm frond. Energies 13(1):155. https://doi.org/10.3390/en13010155
Naleli K (2016) Process modelling in production of biobutanol from lignocellulosic biomass via ABE fermentation. (Master thesis). Stellenbosch University. http://hdl.handle.net/10019.1/98620
Nilsson A, Shabestary K, Brandão M, Hudson EP (2020) Environmental impacts and limitations of third-generation biobutanol: life cycle assessment of n-butanol produced by genetically engineered cyanobacteria. J Ind Ecol 24(1):205–216. https://doi.org/10.1111/jiec.12843
Pereira LG, Chagas MF, Dias MO, Cavalett O, Bonomi A (2015) Life cycle assessment of butanol production in sugarcane biorefineries in Brazil. J Clean Prod 96:557–568. https://doi.org/10.1016/j.jclepro.2014.01.059
Petter R, Tyner WE (2014) Technoeconomic and policy analysis for corn stover biofuels. International Scholarly Research Notices, 2014
Pfromm PH, Amanor-Boadu V, Nelson R, Vadlani P, Madl R (2010) Bio-butanol vs. bio-ethanol: a technical and economic assessment for corn and switchgrass fermented by yeast or Clostridium acetobutylicum. Biomass Bioenergy 34(4):515–524. https://doi.org/10.1016/j.biombioe.2009.12.017
Qureshi N, Saha BC, Cotta MA, Singh V (2013) An economic evaluation of biological conversion of wheat straw to butanol: a biofuel. Energy Convers Manag 65:456–462. https://doi.org/10.1016/j.enconman.2012.09.015
Sheldon RA (2018) Metrics of green chemistry and sustainability: past, present, and future. ACS Sustain Chem Eng 6(1):32–48. https://doi.org/10.1021/acssuschemeng.7b03505
Sjöblom M, Matsakas L, Christakopoulos P, Rova U (2015) Production of butyric acid by Clostridium tyrobutyricum (ATCC25755) using sweet sorghum stalks and beet molasses. Ind Crop Prod 74:535–544. https://doi.org/10.1016/j.indcrop.2015.05.041
Smolinksi S, Cox S (2016) Policies to enable bioenergy deployment: key considerations and good practices (No. NREL/TP-7A40-66322). National Renewable Energy Lab.(NREL), Golden, CO (United States). https://doi.org/10.2172/1253707
Srivastava RK (2019) Bio-energy production by contribution of effective and suitable microbial system. Mater Sci Energy Technol 2(2):308–318. https://doi.org/10.1016/j.mset.2018.12.007
Swana J, Yang Y, Behnam M, Thompson R (2011) An analysis of net energy production and feedstock availability for biobutanol and bioethanol. Bioresour Technol 102(2):2112–2117. https://doi.org/10.1016/j.biortech.2010.08.051
Tabone MD, Cregg JJ, Beckman EJ, Landis AE (2010) Sustainability metrics: life cycle assessment and green design in polymers. Environ Sci Technol 44(21):8264–8269. https://doi.org/10.1021/es101640n
Tao L, Tan EC, McCormick R, Zhang M, Aden A, He X, Zigler BT (2014) Techno-economic analysis and life-cycle assessment of cellulosic isobutanol and comparison with cellulosic ethanol and n-butanol. Biofuels Bioprod Biorefin 8(1):30–48. https://doi.org/10.1002/bbb.1431
United Nations (2015) Transforming our world: the 2030 agenda for sustainable development. http://www.un.org/ga/search/view_doc.asp?symbol=A/RES/70/1&Lang=E
Vasco-Correa J, Khanal S, Manandhar A, Shah A (2018) Anaerobic digestion for bioenergy production: global status, environmental and techno-economic implications, and government policies. Bioresour Technol 247:1015–1026. https://doi.org/10.1016/j.biortech.2017.09.004
Wu N (2018) Cultivation, techno-economic analysis of biofuels production. Doctoral dissertation, University of Florida
Wu M, Wang M, Liu J, Huo H (2007) Life-cycle assessment of corn-based butanol as a potential transportation fuel (No. ANL/ESD/07-10). Argonne National Lab.(ANL), Argonne, IL (United States). https://doi.org/10.2172/925379
Wu N, Moreira C, Zhang Y, Doan N, Yang S, Phlips E, ..., Pullammanappallil P (2019) Techno-economic analysis of biogas production from microalgae through anaerobic digestion. In: Biogas. IntechOpen. https://doi.org/10.5772/intechopen.86090
Acknowledgments
This work was supported by funding from (1) North Dakota State University, Department of Coatings and Polymeric Materials and Center for Sustainable Materials Science (CSMS), and (2) the U.S. Department of Energy’s Office of Energy Efficiency and Renewable Energy, Bioenergy Technologies Office and sponsored by the U.S. Department of Energy’s International Affairs under award number, DEPI0000031.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Wu, N., Yang, S., Pullammanappallil, P., Pourhashem, G. (2022). Techno-economic and Life Cycle Assessments of Microbial Process in Renewable Energy Production. In: Saini, J.K., Sani, R.K. (eds) Microbial Biotechnology for Renewable and Sustainable Energy. Clean Energy Production Technologies. Springer, Singapore. https://doi.org/10.1007/978-981-16-3852-7_14
Download citation
DOI: https://doi.org/10.1007/978-981-16-3852-7_14
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-16-3851-0
Online ISBN: 978-981-16-3852-7
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)