Abstract
Metal-rich natural and artificial habitats are extreme environments for the development and evolution of unique microbial communities, which have adapted to the toxic levels of the metals. Diverse bacterial groups have developed abilities to deal with the toxic metals by bioaccumulation of the metal ions inside the cell actively or passively, extracellular precipitation, efflux of heavy metals outside to the microbial cell surface, biotransformation of toxic metals to less toxic forms, and metal adsorption on the cell wall. Metalophilic microbes are found in all bacterial and archaeal groups studied, but mostly appear among aerobic and facultative anaerobic chemoheterotrophic and chemolithoautotrophic microorganisms of the Bacillus, Pseudomonas, Staphylococcus, Actinobacteria, Cuprividus, Acidobacterium, Acidithiobacillus, Thiobacillus, Ferroplasma, and Sulfolobus genera. The phenomenon of microbial heavy metal resistance has fundamental importance and is particularly relevant in microbial ecology, especially in connection with the roles of microbes in biogeochemical cycling of heavy metals and in the bioremediation of metal-contaminated environments. The heavy metal resistance mechanisms and different applications of metal resistant/metalophilic bacteria and archaea have been expounded deeply in this chapter.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
13.1 Heavy Metals and Its Toxicity on Microbes
There is no widely agreed criterion-based definition of a heavy metal. In metallurgy, a heavy metal may be defined on the basis of density, in physics the differentiating criterion might be atomic number, and in chemistry or biology the distinguishing criteria could be atomic mass (Hawkes 1997; Ali and Khan 2018; Meija et al. 2016). Based on density definition, the heavy metals are those elements that have a density above 5 g/cm3 (Nies 1999). Based on atomic number definition, heavy metals are those elements which atomic number greater than 20 (Ca), sometimes this is capped at 92 (U). Definitions based on atomic number have been criticized for including metals with low densities. Atomic mass definitions can range: it reserved those elements with an atomic mass greater than Na (atomic mass 22.98), greater than 50 (Ni (58.69), Cu (63.54), Mo (95.95), etc.) or more than 200 (e.g., Hg (200), TI (204), Pb (207), Bi (209), and the Th series) (Baldwin and Marshall 1999; Ali and Khan 2018; Pourret and Hursthouse 2019).
Correspondingly, the list of heavy metals according to different definitions will include different elements. Of the 90 natural elements, 21 are non-metals, 16 are light metals, and the remaining 53 (including As) are heavy metals (Ali and Khan 2018).
Most heavy metals are transition elements with incompletely filled d orbitals. These d orbitals provide heavy metal cations with the ability to form complex compounds which may or may not be redox-active. Thus, the heavy metal cations which play an important role as micronutrients in the vital processes of microorganisms or other living organisms are essential metals. For example, Mo(II), Fe(II), Cu(II), Mn(II), Zn(II), Ni(II), and Co(II) are involved in the catalytic acceleration of biochemical processes. They can serve as cofactors or be part of enzymes such as nitrogenases, superoxide dismutases, dehydrogenases, cytochrome oxidases, ureases, etc. (Ehrlich 1997a; Nies 1999). Cu(II) and Ni(II) are involved in bacterial cell’s redox processes (Nies 1999). Zn(II) ions stabilize the structure of DNA and proteins of the bacterial cell wall, since they have redox stability at certain pH and Eh values of biological media (Nies 1999). A significant number of bacteria and archaea are able to use ions of certain metals (Fe(III), Mn(II), Cr(VI), etc.) and metalloids as donors or acceptors of electrons in energy metabolism (Ehrlich 1997a). Thus, many archaeal and bacterial species have the ability to derive energy from the reduction of a variety of metals. Archaeal species Archaeoglobus fulgidus, Pyrococcus furiosus, and bacterial species Desulforomonas, Desulfovibrio are capable of reducing Fe(III), and two Pyrobaculum sp. can effectively grow respiring Fe(III) (Vargas et al. 1998; Feinberg et al. 2008; Kashefi et al. 2008). At least one archaeal species, Pyrobaculum arsenaticum, can use arsenate as a terminal electron acceptor for growth (Oremland and Stolz 2005).
Nickel is another important requirement for methanogens: it is required for methanogenesis in Methanobacterium strains (Hartzell et al. 1988) and in the methanogenic archaea Methanobrevibacter smithii and M. barkeri for incorporation into cofactor, a yellow chromophore found in the methylreductase of Methanobacterium (Diekert et al. 1981; Ellefson et al. 1982).
Tungsten and molybdenum have similar chemical properties. Molybdenum is a trace metal required by virtually every species, and tungsten can replace molybdenum in some instances (Kletzin and Adams 1996). Tungsten is an essential trace metal for hyperthermophile archaea P. furiosus, as it involves in aldehyde oxidoreductases activity. Thermococcus litoralis uses another tungsten-containing enzyme, FOR (Dhawan et al. 2000). Several bacterial species, including strains of Pseudomonas, Chloroflexus, Thiobacillus, Alcaligenes, and Thermus genera and archaea Pyrobaculum arsenaticum, P. aerophilum can generate energy either by oxidation or reduction of specific arsenic oxyanions (Ben Fekih et al. 2018).
Some heavy metal ions, for example Cd(II), Pb(II), Sn(II), Hg(II), and Ag(I), do not have vital biological significance for microorganisms, besides form strong toxic complexes, which makes them too dangerous for any physiological function (Bruins et al. 2000). These heavy metals can also show more specific forms of chemical attack through mimicry. In this regard the toxic metals may act as mimics of essential metals, binding to physiological sites that normally are reserved for an essential element. Through mimicry, the toxic metals may gain access to, and potentially disrupt, a variety of important or even critical metal-mediated cellular functions (Cousins et al. 2006; Kasprzak 2002). In the Fig. 13.1 is presented the diagram showing the heavy metal’s classification based on their toxicity.
At high concentrations, all heavy metals (both those that are essential and those that do not have biological significance) are toxic to microbes and other organisms (Nies 1999). Toxicity of heavy metals is manifested in detrimental effects on microorganisms, such as changes in the conformational structures of nucleic acids and proteins, in violation of redox processes and in maintaining the osmotic balance (Ehrlich 1997a; Nies 1999; Igiri et al. 2018). Cd, Hg, Ag ions tend to connect within the cell with sulfhydryl groups, inhibiting the activity of sensitive enzymes. The cations of some metals can replace physiologically significant ions in biomolecules, thereby violating their functions. Ni and Co ions can displace Fe, Zn—Mg ions, Cd and Zn ions—Ca ions (Ehrlich 1997a; Nies 1999). Heavy metal cations can combine with glutathione groups of gram-negative bacteria, forming a bisglutathione complex, which tends to interact with molecular oxygen to form oxidized glutathione (GS-SG) (Kachur et al. 1998). The latter can be reduced in NADPH-dependent reactions, and as a result, the formed metal cations bind other glutathione molecules, thereby causing oxidative stress. Oxygen-containing anions of some heavy metals and metalloids can be involved in the metabolism of structurally similar anions of vital elements, such as S and P. For example, a chromate ion can affect the metabolism of a sulfate ion, arsenate—a metabolism of phosphate (Nies 1999; White and Gadd 2000). Cd and Pb pose deleterious effect on microbes, damage cell membranes, and destroy the structure of DNA. This harmfulness is generated by the displacement of metals from their native binding sites or ligand interactions.
Arsenic is a metalloid that occurs naturally in the environments mainly in tow forms: the trivalent species (As(III)), commonly as the oxyanion arsenite (AsO2−), and the pentavalent species (As(V)), or arsenate (AsO43−). Arsenite is more toxic than arsenate as it is able to bind strongly to sulfhydryl groups in proteins and weakly to thiol groups, such as those in glutathione, lipoic acid, and cysteine. The primary toxic effects of arsenate arise from its transformation to arsenite, besides arsenate has ability to compete with phosphate oxyanions for both transport and energetics functions (Ben Fekih et al. 2018).
The morphology, metabolism, and growth of microbes are affected by changing the nucleic acid structure, causing functional disturbance, disrupting cell membranes, inhibiting enzyme activity, and oxidative phosphorylation (Fig. 13.2) (Ahemad 2012; Igiri et al. 2018).
Cr(VI) is usually present as the oxyanion chromate and based on its high oxidizing potential, considered as the most toxic form of chromium. Toxic effects of chromate for bacteria are associated with its structural similarity to sulfate (SO42−). The CrO42− crosses the cell membrane in some species via the sulfate transport system and cases an oxidative damage to biomolecules. Cr(VI) does not interact directly with DNA, hence its genotoxicity is attributed to its intracellular reduction to Cr(III) via reactive intermediates. The resulting types of DNA damage that are produced can be grouped into two categories: (1) oxidative DNA damage and (2) Cr(III)-DNA interactions (Cervantes and Campos-García 2007; Díaz-Magaña et al. 2009; Luo et al. 2019).
13.2 Microbial Heavy Metal Transporters
To have any physiological or toxic effect, most heavy metal ions should enter the microbial cell. Microorganisms have two main types of transport systems for heavy metal ions. The first type of transport system is fast, nonspecific, which is expressed constitutively and is controlled through the cytoplasmic membrane of bacteria by the proton gradient (pmf—proton motive force) (Silver 1996; Sar et al. 1998; Nies 1999). The second type is a substrate-specific slow transport, often requiring ATP as an energy source in addition to the proton gradient (Table 13.1). This “energetically expensive” type of transport system is inducible and is used by the cell in certain metabolic states, for example, in a state of hunger (Nies 2003, 2007; Nies and Silver 1995).
ATP-binding cassette (ABC) transporters are a major category of membrane-associated bacterial protein structures involved in the transport of a wide range of substrates including heavy metals. For example, Ni can be absorbed by the NikA-E transport system (ABC family transporter), which consists of five components (NikA periplasmic Ni-binding protein, NikB and NikC transmembrane pores for passage of Ni, NikD and NikE ions hydrolyze ATP and use energy to ion transport Ni(II)). The NikA protein can also bind Co, Cu, and Fe ions, but with a tenfold low affinity (Eitinger and Mandrand-Berthelot 2000; Mulrooney and Hausinger 2003). In different microbes, the Znu transport system of the ABC family absorbs Zn ions and has a similar structure to the Nik transporter.
Heavy metal ions like Ni, Co, Zn, and Mn can be accumulated also in gram-negative bacteria and archaea by the fast and nonspecific CorA system (metal inorganic transporter of the MIT family) (Smith and Maguire 1995; Hynninen 2010). In B. subtilis, Mg, Ni, Mn, Co, and Zn ions can be absorbed by the metal citrate transport protein CitM and CitH (Hantke 2001; Krom et al. 2000).
The fast ion transport along the concentration gradient is an important factor contributing to the toxicity of heavy metals. When cells are exposed to high concentrations of heavy metals, which can accumulate through nonspecific transport systems, the “passage” into the cytoplasm can remain open, even at “toxicologically dangerous” concentrations of metals in the cytoplasm, since this process is constitutive (Nies 1999). Despite heavy metal toxicity, microbes possessing different metal resistance strategies, such as detoxification, metal absorption, uptake and accumulation, extracellular precipitation, efflux of heavy metals from the cells.
13.3 Heavy Metal Resistance in Prokaryotes
Heavy metal ions cannot undergo degradation or significant modification like toxic organic compounds in the environment. Microbes, leaving in the heavy metal-polluted environment, develop different mechanisms to tolerate toxic concentrations of the metals (Nies 2007). Microbes can have one or a combination of several different strategies of metal resistance (Bruins et al. 2000; Nies and Silver 1995).
In bacteria, all existing mechanisms that allow surviving in the presence of toxic concentrations of heavy metals in the medium can be attributed to several main types. This is an active release of metal from the cell, restriction of metal intake due to changes in cell permeability, intracellular metal binding and detoxification, extracellular binding, enzymatic metal detoxification into a less toxic form and a decrease in the metal sensitivity of cellular components (Fig. 13.3) (Nies and Silver 1995; Nies 2007; Bruins et al. 2000; Ahemad 2015).
Various bacterial interactions with heavy metals in metal-polluted soil. Biosorption: Precipitation/crystallization of metals occurs due to bacteria-mediated reactions or as a result of the production of specific metabolites. Bioaccumulation: Plasmid-DNA-encoded efflux transporters (e.g., ATPase pumps or chemiosmotic ion/proton pumps) expel the accumulated metals outside the cell. Bioprecipitation: Metals bind to the anionic functional groups (e.g., sulfhydryl, carboxyl, hydroxyl, sulfonate, amine, and amide groups) of extracellular materials present on cell surfaces. Bioleaching: Organic acids secreted by bacteria solubilize the insoluble metal minerals. Biotransformation: Some bacteria utilize methylation as an alternative for metal resistance/detoxification mechanism, which involves the transfer of methyl groups to metals and metalloids
Among the Archaea, thermophiles and hyperthermophiles of the Crenarchaeota and the methanogens and thermophiles of Euryarchaeota utilize P-type ATPases and ABC transporters for metal transport and homeostasis (Coombs and Barkay 2005; Bartolucci et al. 2013).
13.3.1 Active Transport of Heavy Metals
Microbes use active transport mechanisms to efflux toxic metals from the cytoplasm. Metals that do not have physiological significance usually enter into the cell through transport systems designed for the necessary cations, but then quickly get out of the cell by efflux pumps (Ehrlich 1997a). It was found that active ion efflux systems can be either ATP-independent or using ATP energy (see Table 13.1). All of them are highly specific for cations or anions that are exported from the cell (Nies and Silver 1995; Hynninen 2010). A large number of varieties of this mechanism of metal resistance in bacteria and archaea are described (Table 13.1). Three families of transport systems are mainly involved in the export of heavy metal ions from the cell: a three-component transmembrane transporter in Gram-negative bacteria is Capsule biogenesis/assembly (CBA) family transporter, which acts as a chemosmotic antiport; cation diffusion facilitator (CDF), which acts as a chemosmotic ion-proton exchanger and P-type ATPase located in the inner membrane and using ATP energy to export metal ions from the cytoplasm to periplasm (Fig. 13.4) (Hynninen 2010; Nies 2003, 2007; Grass et al. 2001).
The main transporter families that determine bacterial heavy metal resistance. P-type ATPases pump their substrates from cytoplasm to periplasm using energy provided by ATP hydrolysis. CBA transporters are three-component complexes in Gram-negative bacteria that efflux ions from cyto- and periplasm to outside using a chemiosmotic gradient. CDF transporters are driven by a proton motive force and they export ions from cytoplasm to periplasm (Hynninen 2010)
13.3.2 CBA Family Transporters
CBA family transporters are a three-component protein complex that span the whole cell wall of Gram-negative bacteria and expel ions from cyto- and periplasm to outside using a chemiosmotic gradient. The most important component of the transporter is the intramembrane protein RND (resistance, nodulation, and division), which was first described as a bacterial transport protein involved in the resistance processes of heavy metals in R. metallidurans, nodulation of Mesorhizobium loti, and cell division of E. coli (Nies 2003).
An example of the RND family transporter is the Czc system for the active export of Cd(II), Zn(II), Co(II) cations from a bacterial cell. The Czc system is described and studied in detail in the facultative chemolithoautotrophic bacteria Alcaligenes eutrophus CH34. The Czc system is regulated by a proton concentration gradient across the inner membrane and is ATP-independent (Silver 1996; Collard et al. 1994; Diels et al. 1995). The Czc system consists of three main parts (Fig. 13.5) (Rosen 2002; Anton et al. 1999).
Structural models of CBA and CDF families pumps. (a) Czc, functioning as a proton/cation antiport, consisting of intramembrane (CzcA), extramembrane (CzcC) and integral (CzcB) proteins, (b) CzcD, transporting Cd, Zn and Co ions in B. subtilis, (c) CusABC, and (d) CnrABC / NccABC are similar in structure and function to the CzcABC system, (e) ZitB is similar to the CzcD system (modified from Aguilar-Barajas et al. 2010)
13.3.3 CDF Family Transporters
The cation diffusion facilitators (CDFs) are a family of membrane-bound proteins that maintain cellular homeostasis of essential metal ions. Proteins of the secondary cationic CDF transporter catalyzing the efflux of heavy metals and were found in both prokaryotes and eukaryotes. All proteins of the CDF family are substrate-specific. The main substrate for CDF transporters is Zn(II) ions, but Co(II), Ni(II), Cd(II), and Fe(II) can also initiate transporter. The CDF system is regulated by a proton concentration gradient, ΔΨ, ΔpH, or K(I) concentration gradient (Nies 2007; Guffanti et al. 2002; Paulsen and Saier 1997).
CDF coding genes were found in the chromosomes of a number of microorganisms, but protein functionality has been characterized only in few microbes. In B. subtilis, czcD genes are located in the operon along with trikA dehydrogenase gene (Nies 2003). The czcD-trkA operon is complementary to the K(I) transport system in E. coli (Guffanti et al. 2002). CzcD was first described in bacteria Ralstonia metallidurans CH34 as a regulator of czcABC gene expression, but CzcD (Fig. 13.5) can also participate in the transport of Cd(II), Zn(II), Co(II) in the absence of the CzcABC system (Anton et al. 1999; Nies 2003; Scherer and Nies 2009; von Rozycki and Nies 2009).
In B. subtilis, CzcD is regulated by a K(I) concentration gradient and leads to the emission of Cd(II), Zn(II) and Co(II) (Guffanti et al. 2002). In E. coli cells, the CzcD system is regulated by a proton concentration gradient and leads to the emission of Zn(II) and Cd(II) ions, but not Co(II) (Nies 2003; Paulsen and Saier 1997).
In E. coli, the ZitB protein (product of the ybgR gene) of the CDF family has also been described, which determines resistance to Zn ions, reducing ion accumulation (Fig. 13.5) (Grass et al. 2001).
In Staphylococcus aureus, CzcD determines resistance to Zn(II) and Co(II), in Thermus thermophilus determines resistance to Zn(II) and Cd(II). CDF proteins can also export Pb ions (Spada et al. 2002; Xiong and Jayaswal 1998).
13.3.4 P-Type ATPase Family Transporters
P-type ATPase is a family of transport protein that exports ions against a concentration gradient using ATP. It is highly substrate-specific. The substrates are Na, K, Mg, Ca, Cu, Ag, Zn, Cd, Co, and Pb cations. Heavy metal-transporting ATPases have a metal-binding domain (MBD) and are described in both gram-positive and gram-negative bacteria. Prototype of P-type ATPase is ZntA system for active efflux of Zn(II), Cd(II), and Pb(II) from E. coli cell (Fig. 13.6) and CadA for active efflux of Cd(II) from S. aureus cell (Fig. 13.6).
Transport systems of metals—P-type ATPases: (a) CopABCD copper transport system; (b) CopA ATPase P-type transformation of Cu(I) into Cu(II); (c) CadA ATPase of the P-type, removal of Cd, Zn, and Pb ions from the cytoplasm; (d) CopA system of absorption of Cu(II) and CopB of Cu(II) export in E. hirae cells; (e) PbrA ATPase P-type removal of Pb ions from the cytoplasm; ZntA ATPase P-type removal of Zn, Cd and Pb ions from the cytoplasm (modified from Aguilar-Barajas et al. 2010)
CadA consists with six domains located in the membrane, four of which are involved in translocation of cations, and a conservative Cys-Pro-Cys tripeptide. Two intracellular domains common to all P-type ATPases are aspartyl kinase and phosphatase domain. During metal transport, ATP phosphorylates the protein, probably at the location of the invariant aspartic acid (Asp 415). Phosphorylation occurs only in the presence of Cd ions (Tsai et al. 1992). The transport system CadA was also found in the bacteria Bacillus subtilis, Pseudomonas metallidurans, Cupriavidus metallidurans, Synechocystis sp. etc. (Lee et al. 2001; Scherer and Nies 2009).
PbrA system, the member of P-type ATPase, actively removes Pb ions from the cytoplasm of the bacterium Cupriavidus metallidurans (Fig. 13.6). The structure and function of the protein PbrA is similar to the CadA and ZntA. PbrB lipoprotein is located on the outer membrane, which probably transports Pb ions from periplasm to the environment (Aguilar-Barajas et al. 2010).
In Enterobacter hirae have been found the CopA and CopB system of the P-type ATPase family, which are for Cu(II) transport. CopA determines the absorption of Cu ions, and CopB efflux of Cu ions from the cytoplasm. The synthesis of the both proteins is regulated by operon genes (Fig. 13.6) (Argüello et al. 2013). The promoter region of the operon is controlled by the CopY repressor, regulated by Cu ions. CopZ protein, together with Cu ions, activates the promoter. The binding of copper to CopZ leads to the formation of the complex, which attached to CopY, as a result the operon, is activated (Rademacher and Masepohl 2012).
Homologous systems have been described in Pseudomonas syringae, Xanthomonas campestris, and E. coli (Cooksey 1994). In the copper metabolism of P. syringae, two regulatory copRS genes and four structural copABCD genes were found, while in X. campestris and E. coli, the corresponding genes are called pcoRS and pcoABCD. The copR and copS genes are located immediately after the copper tolerance operon (copABCD) on the pPT23D plasmid and transcribed as an operon from two genes of the same constitutive promoter (Mills et al. 1994; Rademacher and Masepohl 2012) (Fig. 13.6).
The product of copS gene is the copper-sensitive CopS protein, which located in the inner membrane. The product of copR gene is the regulatory protein CopR, which located in the cytoplasm. With an increased periplasmic concentration of Cu(II), CopR transphosphorylates the CopS protein and activates transcription of the cop operon (Rademacher and Masepohl 2012; Mills et al. 1994).
The plasmid operon copABCD in the bacterium Pseudomonas syringae is one of the first described copper resistance systems in bacteria. The copABCD operon encodes a system that prevents the penetration of copper into the cell cytoplasm. CopA and CopC are periplasmic proteins that bind copper. The proteins CopA and CopC able to bind 11 and 1 copper atoms, respectively, on the same polypeptide (Aguilar-Barajas et al. 2010). The activation of transporters leads to the accumulation of copper in the periplasmic space, which protects the cell from the toxic effect of the ion. CopA also exhibits oxidase activity, transforming Cu(I) into Cu(II), thereby protecting the periplasmic enzymes from the toxic effect of copper (Argüello et al. 2013).
CopC is probably a chaperone protein that transports Cu ions to the integral CopD protein. CopD consists of eight transmembrane segments and transports copper both into the cytoplasm and from the cytoplasm to the periplasm. CopB is an outer membrane protein that absorbs copper (Aguilar-Barajas et al. 2010).
P-type ATPase has been found in 17 archaea species, by screening the databases from TIGR, NCBI, DOE, and TCDB. In all analyzed archaea species contained 1–3 metal ATPases, which belong to six different phylogenetic TC (Transport Classification) clusters. The proteins belonging to these clusters export (more rarely import), a variety of monovalent or divalent metals (copper, zinc, lead, cadmium, or silver) (De Hertogh et al. 2004). Only three transmembrane motifs for metal-transporting ATPases identified in archaea, which correspond to the group IB-1 (Cu(I)/Ag(I)), group IB-2 (Zn(II)/Cd(II)/Pb(II)), and group IB-3 (Cu(II)/Cu(I)/Ag(I)) motifs (Argüello et al. 2003).
Two metal-transporting ATPase genes CopA and CopB from the thermophilic archae Archaeoglobus fulgidus were cloned in E. coli, purified, and their ATPase activity were biochemically characterized (Mana-Capelli et al. 2003; Mandal and Argüello 2003). The thermophilic ATPase activity of CopA was best activated by the monovalent metals Ag(I) and Cu(I) while CopB was activated by the divalent Cu(II).
ATPases along with the ABC transporters, transcriptional regulators, and certain metallochaperones were found to be involved in metal resistance and homeostasis in the haloarchaeon Halobacterium sp. strain NRC-1 (Kaur et al. 2006). The list of archaea P-type ATPases are shown in the Table 13.2.
13.3.5 Limitation of Metal Intake Due to Changes in Cell Permeability
When a cell is exposed to concentrations of heavy metals in the environment, the microbe may undergo structural changes in the cell wall, membrane, and cytoplasmic membrane. These processes are not always the result of the toxic effects of the metals. They can be a manifestation of induced defense mechanisms that limit the flow of toxic ions into the cell cytoplasm (Bruins et al. 2000; Ehrlich 1997a).
The first sites of cell and heavy metal interaction are at the cell surface. The bacterial cytoplasmic membrane, and to a lesser extent the outer membrane in Gram-negative bacteria, are a major barrier to the entry of hydrophilic substances, including metal ions, into the interior of the cell. In Gram-negative bacteria, like E. coli, the outer membrane contains protein channels called porins, that allow low-molecular-weight substances such as metal ions to diffuse across the membrane into the periplasmic space. In E. coli synthesis of the major porin can be prevented by mutations in a single gene resulting in increased metal resistance. The outer membrane can also act as a limited (i.e., saturable) trap for heavy metals by nonspecifically binding them, therefore contributing to the natural metal tolerance of cells (Rouch et al. 1995).
An unusual mechanism of metal resistance is found in Pseudomonas syringae, which accumulate blue Cu(II) ions in the periplasmic space and outer membrane. At least part of this copper sequestering activity is determined by copper-binding periplasmic CopA protein products of the copper resistance operon (cop). Copper resistance operons related to cop have been described in the related plant pathogen Xanthomonas campestris and in E. coli, but these resistance systems may differ functionally from the P. syringae system (Cooksey 1994).
A significant advantage for survival in environments contaminated with heavy metals is reducing bioavailability or mobility of heavy metal ions by the released exopolysaccharide (EPS). Anionic property of EPS allows the biopolymer to effectively sequester positively charged heavy metal ions and restricts the entry of metal ions into the cell. The anionic property of EPS imparts by abundant active and ionisable functional groups and non-carbohydrate substituents like phosphodiester (techoic acid), phosphate, hydroxyl groups, or acetamido group of chitin, structural polysaccharides of fungi. On contrary to homopolysaccharides, extracellular heteropolysaccharides are often polyanionic due to association of some of such functional groups with polysaccharide backbone. The sorption and immobilization again occurs via different mechanisms like ion exchange, complexation, precipitation, etc. (Gupta and Diwan 2017). As an example can be serve Ochrobactrum anthropi, isolated from activated sludge. This bacteria producing the most EPS for the removal of Cr(VI), Cd(II) and Cu(II) (Ozdemir et al. 2003).
Staphylococcus xylosus and Staphylococcus carnosus strains were characterized by production of surface-exposed chimeric two different polyhistidyl peptides, His3-Glu-His3 and His6 due to the expression of recombinant plasmid genes designed for binding to divalent metal ions. As a result, the entry of Cd ions and other toxic metals into the cell is limited, which suggests that such bacteria could find use in bioremediation of heavy metals (Samuelson et al. 2000).
It has been shown, that EPS synthesized by Arthrobacter viscosus accumulate 2.3 times more Cd(II) than an equivalent weight of intact cells and have 13.7 times the sorptive capacity of Arthrobacter globiformis cells, which do not produce EPS (Hrynkiewicz et al. 2015).
Numerous halophilic bacteria and archaea can also tolerate high concentrations of heavy metals by secrete EPS. Halomonas strains can tolerate high concentrations of Pb(II) and Cd(II) (5 mM) by the EPS-mediated adsorption of the metallic ions (Voica et al. 2016). Dry biomass of the haloarchaeon Halobacterium sp. GUSF was an effective adsorbent for Mn(II) from saline solutions, the process of adsorption involving cell surface carboxyl, amino, phosphate and hydroxyl groups (Naik and Furtado 2014). The high EPS-producing halotolerant cyanobacterium Aphanothece halophytica grown at 6% NaCl (w/v) was capable of accelerated Zn(II) adsorption up to a critical cell density that may result in aggregation, reducing the matrix surface available for metal binding (Incharoensakdi and Kitjaharn 2002).
13.3.6 Intracellular Binding of Toxic Metals and Their Detoxification
The accumulation of metal in the cytoplasm and its detoxification can occur due to the binding of toxic ions to specific proteins, like low-weight cysteine-rich proteins and peptides. A variety of metal-binding peptides like glutathione (GSH) and proteins like metallothioneins and phytochelatins produced by certain microbes like Cyanobacterium synococcus, Synechococcus sp., E. coli, P. putida (Gupta and Diwan 2017; Bruins et al. 2000; Silver 1996).
Citrobacter sp., isolated from metal-polluted soil can resist Cd(II) toxicity by forming insoluble complexes of Cd-phosphate (CdHPO4); this transformation is mediated by a cell-bound phosphatase that precipitates inorganic phosphate with heavy metals. A strain of Pseudomonas putida isolated from sewage can sequester intracellular Cd(II) by producing three low-molecular-weight cysteine-rich proteins related to eukaryotic metallothioneins, while K. aerogenes excretes sulfur into the surrounding environment to immobilize Cd(II) ions as insoluble Cd-sulfide (Hrynkiewicz et al. 2015). The ability to intracellularly accumulate lead phosphate in the form of granules was exhibited by the P. aeruginosa (Naik et al. 2012). For Mycobacterium scrofulaceum, the ability to intracellular accumulation of Cu(II) in the form of sulfide was found (Bruins et al. 2000).
Cyanobacteria at toxic concentrations of free Cu ions in the medium produce extracellular chelating ligands that bind to Cu(II) ions, reducing their bioavailability. In Synechococcus spp., a ubiquitous and important group of phytoplankton, synthesis of chelating ligands is regulated by the concentration of free Cu(II) ions in the medium according to the feedback mechanism (Moffett and Brand 1996).
In some cases, the formation of metal precipitating anions may result from normal cellular metabolism, such as the formation of sulfides under anaerobic conditions by sulfate-reducing bacteria of the genus Desulphovibrio. In other cases, the process is inducible under certain environmental conditions, for example, the formation of sulfides by bacteria of the genus Clostridium (Karnachuk et al. 2003).
Metallothioneins and phytochelatins are not represented in archaeal genomes, however members of the CutA family of metal-binding proteins are found in archaea, bacteria, and eukaryotes. The crystal structure of the Pyrococcus horikoshii CutA has been determined with and without copper, contributing to the clarification of the protein’s function. In fact, binding of heavy metals induced the reversible multimerization of CutA. Thus, a role has been proposed for CutA in the capture and precipitation of metal ions. Interestingly, while the metal-binding site of the E. coli homolog contains Cys and His residues, these amino acids are absent in the Pyrococcus protein (Bini 2010).
13.3.7 Reduction of Heavy Metal Ions and Enzymatic Detoxification
Bacteria and Archaea are reducing a broad spectrum of heavy metal ions: chromate, molybdate, vanadate, iron, etc. (Table 13.3). Some bacteria and archaea can use metals and metalloids as electron donors or acceptors for energy generation. Metals in the oxidized form could serve as terminal acceptors of electrons during anaerobic respiration.
The most studied example of the manifestation of the metal resistance mechanism in bacteria associated with the process of intracellular enzymatic metal detoxification is the Hg ion resistance system (Nies 1999). Stability is due to the functioning of the operon and was revealed both in gram-positive (S. aureus, Bacillus sp.) and gram-negative bacteria (Escherichia coli, Pseudomonas aeruginosa, Serratia marcescens, Thiobacillus ferrooxidans) (Bruins et al. 2000). As a result of the expression of the genes that make up the mer operon, Hg(II) in two stages is reduced to metallic mercury, which then diffuses through the cell membrane and is released into the environment (Fig. 13.7). Due to the volatility of metallic mercury, its content in the medium can rapidly decrease (Silver 1996).
Mechanism of detoxification by the Hg(II) Mer system (Aguilar-Barajas et al. 2010)
In Alcaligenes faecalis bacteria, the mechanisms of enzymatic oxidation of As(III) compounds present in the form of AsO2 to As(V) compounds in the form of AsO4, which are less toxic, have been studied and described (Anderson et al. 2003).
Microorganisms have developed, or acquired, various genetic systems to cope with arsenic toxicity. These systems include the ars operons, groups of genes widely distributed in bacterial and archaeal species. ars operons frequently occur in most prokaryotic genomes, and it has been stressed that they are more common than genes for tryptophan biosynthesis. This operon first has been found in the plasmid pI258 in the clinical bacteria Staphylococcus aureus. The plasmid pI258 was found to encode multiple resistances to antibiotics, arsenate, arsenite and other heavy metal derivatives (Ben Fekih et al. 2018; Novick and Roth 1968). Arsenic resistance genes have identified in R773 plasmid in Escherichia coli strain isolated from a patient with a urinary tract infection (Hedges and Baumberg 1973). The nucleotide sequence of the determinants from the E. coli R773 plasmid identified the arsRDABC operon involved in the arsenic resistance phenotype, and staphylococcal plasmids pI258 and pSX267 both contained similar, but simpler arsRBC operons encoding proteins with homology to those encoded by R773 (Ben Fekih et al. 2018). The distribution of ars operon genes in bacteria and archaea are presented in the Table 13.4.
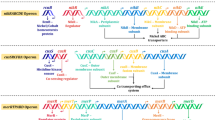
Nearly every organism has resistance pathways for inorganic arsenic. The minimal constituents are usually an As(III)-responsive repressor (ArsR), and an As(III) efflux permease (ArsB or ACR3) that functions to extrude trivalent As(III) from cells. The As(III)-stimulated ATPase (ArsA), and the As(III) metallochaperone (ArsD), which are always associated in ars operons, appears to be later adaptations that enhances the ability of ArsB to extrude As(III) and increase resistance. ArsC and other arsenate reductases are required for resistance to arsenate (Yang and Rosen 2016; Ben Fekih et al. 2018). Recently, a parallel pathway for organic arsenicals has been identified. The ars genes responsible for the organo-arsenical detoxification include arsM, which encodes an As(III) S-adenosylmethionine methyltransferase, arsI, which encodes a CeAs bond lyase, and arsH, which encodes a methylarsenite oxidase (Fig. 13.8).
Pentavalent inorganic arsenate (As(V)) is reduced by the ArsC arsenate reductase to trivalent arsenite (As(III)). Some microbes encode As(III) S-adenosylmethionine methyltransferases ArsM protein, that transform As(III) into the considerably more toxic (for humans, carcinogenic) organo-arsenical MAs(III). Other microbes can produce the ArsI C-As lyase, a dioxygenase that cleaves off the methyl group, forming inorganic As(III). Since As(III) is less toxic than MAs(III), this reaction detoxifies the organo-arsenical product. Other bacteria have the ArsH NADPHFMN oxidoreductase that oxidizes MAs(III) to relatively nontoxic pentavalent MAs(V), also a detoxification process (Yang and Rosen 2016). The protein structure of the ArsC (from S. aureus), ArsM (from Cyanidioschyzon sp.), ArsI (from T. curvata) and ArsH (from S. meliloti), presented in the illustration, were used from Protein Data Bank (https://www.rcsb.org/).
Outer example of the heavy metal detoxification is hexavalent chromate reduction. Bacterial developed the mechanisms for reduction of Cr(VI) to the Cr(III) species and efflux of chromate from cell cytoplasm. Several chromate reductases have been identified in diverse bacterial species (Table 13.5). Most characterized enzymes belong to the NAD(P)H-dependent flavoprotein family of reductases.
Candidatus “Methanoperedens” independently utilizes chromate as electron acceptor to form Cr(III) compounds, or it can oxidizes methane to generate intermediates or electrons, which will be utilized to reduce chromate to Cr(III) compounds by unknown chromate reducers synergistically (Luo et al. 2019).
Efflux of chromate by the ChrA membrane transporter, a plasmid-encoded protein, has been demonstrated in Pseudomonas and Cupriavidus species (Fig. 13.9). Chromate efflux by ChrA consists of an energy-dependent process driven by the membrane potential. The CHR protein family, which includes putative ChrA homologs, currently contains about 135 sequences from all three domains of life. Other mechanisms of bacterial resistance to chromate involve the expression of components of the machinery for repair of DNA damage as well as free-radical scavenging enzymes (Cervantes and Campos-García 2007; Díaz-Magaña et al. 2009).
Schematic diagram of Cr(VI) transport into bacterial cell, its reduction pathways, and efflux (modified from Pradhan et al. 2016)
13.4 Application and Prospects of Heavy Metal Resistant Microbes
Accumulation of high concentrations of heavy metals in environments can cause many human health risks and serious ecological problems. The ability of microorganisms to adsorb heavy metals or change the forms of their presence in the environment attracts wide attention of researchers in connection with the possibility of biotechnological use of heavy metal resistant bacteria or archaea for wastewater treatment, bioremediation of contaminated environments, as well as in biogeotechnology of metals (Volesky 1994; Gadd 2005; White and Gadd 2000).
Bioremediation using microorganisms is receiving much attention due to their good performance and employed in order to transform toxic heavy metals into a less harmful state (Ndeddy Aka and Babalola 2016; Akcil et al. 2015) or using microbial enzymes to clean-up polluted environment (Okoduwa et al. 2017). The technique is environmentally friendly and cost effective in the revitalization of the environment (Turpeinen et al. 2004; Ma et al. 2016). In the Table 13.6 showed a number of microbes which can be used for removing metal ions from solutions. However, bioremediation of heavy metals has limitations. Among these are production of toxic metabolites by microbes and non-biodegradability of heavy metals.
Bioremediation of the environment from toxic metal can be achieved by biosorption ability of the microbes. Biosorption is the group of all processes, during which alive or dead microbial biomass removes heavy metals or other pollutants from solutions (Gavrilescu 2004). Biosorption occurring with the participation of microorganisms may be conducted by surface adsorption concerning the gathering of metals on the cell surface and linking them with extracellular polymers, such as exopolysaccharide (EPS). EPS released out of self-defense against harsh conditions of starvation, pH and temperature, hence it displays exemplary physiological, rheological and physiochemical properties. The ionic nature of metals, its size and charge density in turn regulates its interaction with negatively charged EPS (Gupta and Diwan 2017). In the Table 13.7 is given some microbial EPS involved in heavy metal remediation.
It is often when biosorption occurs as the first phase of the following intracellular accumulation and the process of surface adsorption occurring very fast—during several minutes may have a dominant role in metal linking or may lead to high metal accumulation in the middle of the cell in a longer time (Gavrilescu 2004).
The practical application of biosorption to the removal or the recovery of heavy metals is mainly the result of the reversibility of this process. Desorption allows the recovery of metals (which is profitable in the case of more valuable heavy metals like gold, copper, and zinc) or their removal (Wang and Chen 2009). As the biosorbents can be:
-
Biomass of microorganisms is the secondary product in the sewage or pharmaceutical industry and in sewage treatment processes;
-
Microorganisms from cultured and proliferated on a special base indicating the ability to efficiently metals;
-
Sorbents of vegetable or animal origin (as nutshells, crust-rich tannins, sea plants, humus, moss peat, etc.).
The direct use of microorganisms with distinctive features of catabolic potential and/or their products such as enzymes and bio surfactant is a novel approach to enhance and boost their remediation efficacy (Le et al. 2017; Schenk et al. 2012). Different alternatives have also been anticipated to widen the applications of microbiological techniques toward the remediation of heavy metals. For instance, the use of microbial fuel cell to degrade recalcitrant heavy metals has been explored. Biofilm mediated bioremediation can be applied for cleaning up of heavy metal-contaminated environment.
High bioremediation potential and feasibility of the microbial detoxification of arsenic by reduction, oxidation, and methylation process, make bacteria an impending foundation for green chemistry to exterminate arsenic in the environment (Sher and Rehman 2019).
Many microorganisms are capable of precipitating metal ions. The method of precipitation of metals in the form of sulfides is based on the ability of sulfate-reducing bacteria (Desulfovibrio, Desulfotomaculum, Desulfomonas, Desulfobacter, Desulfobulbus, Desulfococcus, Desulfosarcina, Desulfonema) to form H2S, which precipitates metals from solutions almost completely. Thus, from solutions containing 8.6 g/L Cu, the extraction of Cu was 98.5%. Toxic metals can also precipitate during their recovery. For example, chromium-reducing bacteria under anaerobic conditions reduce Cr(VI) to Cr(III), which is precipitated (Cervantes and Campos-García 2007).
Soil microorganisms, including plant growth promoting bacteria, through toxic metal stress evading mechanisms, can be used as bioinoculant or biofertilizers, which substantially improve the growth of plants implanted in heavy metal-contaminated soils by lowering the metal toxicity (Madhaiyan et al. 2007; Wani and Khan 2010; Khan et al. 2012). In addition, there are other mechanisms of plant growth promotion by bacteria e.g., they protect colonizing plants from the pathogens attack directly by inhibiting/killing pathogens through the production of antibiotics, hydrogen cyanide, and phenazines, etc. (Saravanakumar et al. 2007; Cazorla et al. 2007).
Metalophilic bacteria and archaea play an important role in the process of leaching of metals from ores, concentrates, rocks and solutions, thus they are widely used in biogeometallurgy. In the Table 13.8 showed chemolithotrophic bacteria that oxidize Fe(II), S(II), S, and sulfide minerals important for biohydrometallurgy (Sand et al. 1992; Ehrlich 1997b; Vardanyan and Vardanyan 2018).
Many prokaryotes, including archaea, are capable of transforming the oxidation state of metals in processes leading to either their solubilization or biomineralization. Although these phenomena have been observed in the environment and studied in cultures, there is still much to be learned about the genetic determinants of these metal transformations.
References
Adekanmbi AO, Adelowo OO, Okoh AI, Fagade OE (2019) Metal-resistance encoding gene-fingerprints in some bacteria isolated from wastewaters of selected printeries in Ibadan, South-Western Nigeria. J Taibah Univ Sci 13(1):266–273
Aguilar-Barajas E, Ramírez-Díaz MI, Riveros-Rosas H, Cervantes C (2010) Heavy metal resistance in pseudomonads. In: Ramos JL, Filloux A (eds) Pseudomonas: volume 6: molecular microbiology, infection and biodiversity. Springer, Dordrecht, pp 255–282
Ahemad M (2012) Implications of bacterial resistance against heavy metals in bioremediation: a review. IIOABJ 3(3):39–46
Ahemad M (2015) Phosphate-solubilizing bacteria-assisted phytoremediation of metalliferous soils: a review. 3 Biotech 5(2):111–121
Akcil A, Erust C, Ozdemiroglu S, Fonti V, Beolchini F (2015) A review of approaches and techniques used in aquatic contaminated sediments: metal removal and stabilization by chemical and biotechnological processes. J Clean Prod 86:24–36
Ali H, Khan E (2018) What are heavy metals? Long-standing controversy over the scientific use of the term ‘heavy metals’—proposal of a comprehensive definition. Toxicol Environ Chem 100(1):6–19
Anderson GL, Love M, Zeider BK (2003) Metabolic energy from arsenite oxidation in Alcaligenes faecalis. J Phys IV (Proc) 107:49–52
Anton A, Grosse C, Reissmann J, Pribyl T, Nies DH (1999) CzcD is a heavy metal ion transporter involved in regulation of heavy metal resistance in Ralstonia sp. strain CH34. J Bacteriol 181(22):6876–6881
Argüello JM, Mandal AK, Mana-Capelli S (2003) Heavy metal transport CPx-ATPases from the thermophile Archaeoglobus fulgidus. Ann N Y Acad Sci 986:212–218
Argüello JM, Raimunda D, Padilla-Benavides T (2013) Mechanisms of copper homeostasis in bacteria. Front Cell Infect Microbiol 3:73
Baldwin DR, Marshall WJ (1999) Heavy metal poisoning and its laboratory investigation. Ann Clin Biochem 36(Pt 3):267–300
Bartolucci S, Contursi P, Fiorentino G, Limauro D, Pedone E (2013) Responding to toxic compounds: a genomic and functional overview of archaea. Front Biosci (Landmark Ed) 18:165–189
Ben Fekih I, Zhang C, Li YP, Zhao Y, Alwathnani HA, Saquib Q, Rensing C, Cervantes C (2018) Distribution of arsenic resistance genes in prokaryotes. Front Microbiol 9:2473
Bini E (2010) Archaeal transformation of metals in the environment. FEMS Microbiol Ecol 73(1):1–16
Boyd ES, Barkay T (2012) The mercury resistance operon: from an origin in a geothermal environment to an efficient detoxification machine. Front Microbiol 3:349
Branco R, Chung A-P, Morais PV (2008) Sequencing and expression of two arsenic resistance operons with different functions in the highly arsenic-resistant strain Ochrobactrum tritici SCII24T. BMC Microbiol 8(1):95
Brown NL, Stoyanov JV, Kidd SP, Hobman JL (2003) The MerR family of transcriptional regulators. FEMS Microbiol Rev 27(2–3):145–163
Bruins MR, Kapil S, Oehme FW (2000) Microbial resistance to metals in the environment. Ecotoxicol Environ Saf 45(3):198–207
Butcher BG, Rawlings DE (2002) The divergent chromosomal ars operon of Acidithiobacillus ferrooxidans is regulated by an atypical ArsR protein. Microbiology (Reading) 148(Pt 12):3983–3992
Butcher BG, Deane SM, Rawlings DE (2000) The chromosomal arsenic resistance genes of Thiobacillus ferrooxidans have an unusual arrangement and confer increased arsenic and antimony resistance to Escherichia coli. Appl Environ Microbiol 66(5):1826–1833
Cai J, Salmon K, DuBow MS (1998) A chromosomal ars operon homologue of Pseudomonas aeruginosa confers increased resistance to arsenic and antimony in Escherichia coli. Microbiology (Reading) 144(Pt 10):2705–2713
Carlin A, Shi W, Dey S, Rosen BP (1995) The ars operon of Escherichia coli confers arsenical and antimonial resistance. J Bacteriol 177(4):981–986
Cazorla FM, Romero D, Pérez-García A, Lugtenberg BJJ, Vicente A d, Bloemberg G (2007) Isolation and characterization of antagonistic Bacillus subtilis strains from the avocado rhizoplane displaying biocontrol activity. J Appl Microbiol 103(5):1950–1959
Cervantes C, Campos-García J (2007) Reduction and efflux of chromate by bacteria. In: Nies DH, Silver S (eds) Molecular microbiology of heavy metals. Springer, Berlin, pp 407–419
Chen CM, Misra TK, Silver S, Rosen BP (1986) Nucleotide sequence of the structural genes for an anion pump. The plasmid-encoded arsenical resistance operon. J Biol Chem 261(32):15030–15038
Chen L, Ren Y, Lin J, Liu X, Pang X (2012) Acidithiobacillus caldus sulfur oxidation model based on transcriptome analysis between the wild type and sulfur oxygenase reductase defective mutant. PLoS One 7(9):e39470
Chmurny AB, Quintero EJ, Kneer R (1998) Novel heavy metals sorbents produced from hyphomonas and method of use. Worldwide applications
Collard JM, Corbisier P, Diels L, Dong Q, Jeanthon C, Mergeay M, Taghavi S, van der Lelie D, Wilmotte A, Wuertz S (1994) Plasmids for heavy metal resistance in Alcaligenes eutrophus CH34: mechanisms and applications. FEMS Microbiol Rev 14(4):405–414
Cooksey DA (1994) Molecular mechanisms of copper resistance and accumulation in bacteria. FEMS Microbiol Rev 14(4):381–386
Coombs JM, Barkay T (2005) New findings on evolution of metal homeostasis genes: evidence from comparative genome analysis of Bacteria and archaea. Appl Environ Microbiol 71(11):7083
Cousins RJ, Liuzzi JP, Lichten LA (2006) Mammalian zinc transport, trafficking, and signals. J Biol Chem 281(34):24085–24089
Cuebas M, Villafane A, McBride M, Yee N, Bini E (2011) Arsenate reduction and expression of multiple chromosomal ars operons in Geobacillus kaustophilus A1. Microbiology (Reading) 157(Pt 7):2004–2011
De Hertogh B, Lantin A-C, Baret PV, Goffeau A (2004) The archaeal P-type ATPases. J Bioenerg Biomembr 36(1):135–142
De Philippis R, Paperi R, Sili C (2007) Heavy metal sorption by released polysaccharides and whole cultures of two exopolysaccharide-producing cyanobacteria. Biodegradation 18(2):181–187
Dhawan IK, Roy R, Koehler BP, Mukund S, Adams MW, Johnson MK (2000) Spectroscopic studies of the tungsten-containing formaldehyde ferredoxin oxidoreductase from the hyperthermophilic archaeon Thermococcus litoralis. J Biol Inorg Chem 5(3):313–327
Díaz-Magaña A, Aguilar-Barajas E, Moreno-Sánchez R, Ramírez-Díaz MI, Riveros-Rosas H, Vargas E, Cervantes C (2009) Short-chain chromate ion transporter proteins from Bacillus subtilis confer chromate resistance in Escherichia coli. J Bacteriol 191(17):5441
Díaz-Pérez C, Cervantes C, Campos-García J, Julián-Sánchez A, Riveros-Rosas H (2007) Phylogenetic analysis of the chromate ion transporter (CHR) superfamily. FEBS J 274(23):6215–6227
Diekert G, Konheiser U, Piechulla K, Thauer RK (1981) Nickel requirement and factor F430 content of methanogenic bacteria. J Bacteriol 148(2):459–464
Diels L, Dong Q, van der Lelie D, Baeyens W, Mergeay M (1995) Theczc operon of Alcaligenes eutrophus CH34: from resistance mechanism to the removal of heavy metals. J Ind Microbiol 14(2):142–153
Drewniak L, Dziewit L, Ciezkowska M, Gawor J, Gromadka R, Sklodowska A (2013) Structural and functional genomics of plasmid pSinA of Sinorhizobium sp. M14 encoding genes for the arsenite oxidation and arsenic resistance. J Biotechnol 164(4):479–488
Ehrlich HL (1997a) Microbes and metals. Appl Microbiol Biotechnol 48(6):687–692
Ehrlich HL (1997b) Technical potential for bioleaching and biobeneficiation of ores to recover base metals (other than iron or copper), platinum-group metals and silver. In: Rawlings DE (ed) Biomining: theory, microbes and industrial processes. Springer, Berlin, pp 129–150
Eitinger T, Mandrand-Berthelot MA (2000) Nickel transport systems in microorganisms. Arch Microbiol 173(1):1–9
Ellefson WL, Whitman WB, Wolfe RS (1982) Nickel-containing factor F430: chromophore of the methylreductase of Methanobacterium. Proc Natl Acad Sci U S A 79(12):3707–3710
Feinberg LF, Srikanth R, Vachet RW, Holden JF (2008) Constraints on anaerobic respiration in the hyperthermophilic archaea Pyrobaculum islandicum and Pyrobaculum aerophilum. Appl Environ Microbiol 74(2):396–402
Freedman Z, Zhu C, Barkay T (2012) Mercury resistance and mercuric reductase activities and expression among chemotrophic thermophilic Aquificae. Appl Environ Microbiol 78(18):6568–6575
Freire-Nordi CS, Vieira AAH, Nascimento OR (2005) The metal binding capacity of Anabaena spiroides extracellular polysaccharide: an EPR study. Process Biochem 40(6):2215–2224
Gadd G (2005) Microorganisms in toxic metal-polluted soils. In: Varma A, Buscot F (eds) Microorganisms in soils: roles in genesis and functions soil biology. Springer, Berlin, pp 325–356
Garbinski LD, Rosen BP, Chen J (2019) Pathways of arsenic uptake and efflux. Environ Int 126:585–597
Gavrilescu M (2004) Removal of heavy metals from the environment by biosorption. Eng Life Sci 4(3):219–232
Gihring TM, Bond PL, Peters SC, Banfield JF (2003) Arsenic resistance in the archaeon “Ferroplasma acidarmanus”: new insights into the structure and evolution of the ars genes. Extremophiles 7(2):123–130
Golyshina OV, Pivovarova TA, Karavaiko GI, Kondratéva TF, Moore ER, Abraham WR, Lünsdorf H, Timmis KN, Yakimov MM, Golyshin PN (2000) Ferroplasma acidiphilum gen. Nov., sp. nov., an acidophilic, autotrophic, ferrous-iron-oxidizing, cell-wall-lacking, mesophilic member of the Ferroplasmaceae fam. Nov., comprising a distinct lineage of the archaea. Int J Syst Evol Microbiol 50(Pt 3):997–1006
Grass G, Fan B, Rosen BP, Franke S, Nies DH, Rensing C (2001) ZitB (YbgR), a member of the cation diffusion facilitator family, is an additional zinc transporter in Escherichia coli. J Bacteriol 183(15):4664
Guffanti AA, Wei Y, Rood SV, Krulwich TA (2002) An antiport mechanism for a member of the cation diffusion facilitator family: divalent cations efflux in exchange for K+ and H+. Mol Microbiol 45(1):145–153
Gupta P, Diwan B (2017) Bacterial exopolysaccharide mediated heavy metal removal: a review on biosynthesis, mechanism and remediation strategies. Biotechnol Rep (Amst) 13:58–71
Gutierrez T, Biller DV, Shimmield T, Green DH (2012) Metal binding properties of the EPS produced by Halomonas sp. TG39 and its potential in enhancing trace element bioavailability to eukaryotic phytoplankton. Biometals 25(6):1185–1194
Ha J, Gélabert A, Spormann AM, Brown GE (2010) Role of extracellular polymeric substances in metal ion complexation on Shewanella oneidensis: batch uptake, thermodynamic modeling, ATR-FTIR, and EXAFS study. Geochim Cosmochim Acta 74(1):1–15
Hantke K (2001) Bacterial zinc transporters and regulators. Biometals 14(3):239–249
Hartzell PL, Escalante-Semerena JC, Bobik TA, Wolfe RS (1988) A simplified methylcoenzyme M methylreductase assay with artificial electron donors and different preparations of component C from Methanobacterium thermoautotrophicum delta H. J Bacteriol 170(6):2711–2715
Hawkes SJ (1997) What is a “heavy metal”? J Chem Educ 74(11):1374
Hedges RW, Baumberg S (1973) Resistance to arsenic compounds conferred by a plasmid transmissible between strains of Escherichia coli. J Bacteriol 115(1):459–460
Hohle TH, O'Brian MR (2009) The mntH gene encodes the major Mn(2+) transporter in Bradyrhizobium japonicum and is regulated by manganese via the Fur protein. Mol Microbiol 72(2):399–409
Hrynkiewicz K, Złoch M, Kowalkowski T, Baum C, Niedojadło K, Buszewski B (2015) Strain-specific bioaccumulation and intracellular distribution of Cd2+ in bacteria isolated from the rhizosphere, ectomycorrhizae, and fruitbodies of ectomycorrhizal fungi. Environ Sci Pollut Res Int 22(4):3055–3067
Huber G, Stetter KO (1991) Sulfolobus metallicus, sp. nov., a novel strictly chemolithoautotrophic thermophilic archaeal species of metal-mobilizers. Syst Appl Microbiol 14(4):372–378
Huber G, Spinnler C, Gambacorta A, Stetter KO (1989) Metallosphaera sedula gen, and sp. nov. represents a new genus of aerobic, metal-mobilizing, thermoacidophilic archaebacteria. Syst Appl Microbiol 12(1):38–47
Hynninen A (2010) Zinc, cadmium and lead resistance mechanisms in bacteria and their contribution to biosensing. Academic Dissertation in Microbiology, University of Helsinki. pp 1–52
Ianeva OD (2009) Mechanisms of bacteria resistance to heavy metals. Mikrobiol Zh 71(6):54–65
Igiri BE, Okoduwa SIR, Idoko GO, Akabuogu EP, Adeyi AO, Ejiogu IK (2018) Toxicity and bioremediation of heavy metals contaminated ecosystem from tannery wastewater: a review. Hindawi J Toxicol 2018:2568038
Incharoensakdi A, Kitjaharn P (2002) Zinc biosorption from aqueous solution by a halotolerant cyanobacterium Aphanothece halophytica. Curr Microbiol 45(4):261–264
Ji G, Silver S (1992) Regulation and expression of the arsenic resistance operon from Staphylococcus aureus plasmid pI258. J Bacteriol 174(11):3684–3694
Kachur AV, Koch CJ, Biaglow JE (1998) Mechanism of copper-catalyzed oxidation of glutathione. Free Radic Res 28(3):259–269
Karnachuk OV, Kurochkina SY, Nicomrat D, Frank YA, Ivasenko DA, Phyllipenko EA, Tuovinen OH (2003) Copper resistance in Desulfovibrio strain R2. Antonie Van Leeuwenhoek 83(1):99–106
Kashefi K, Moskowitz BM, Lovley DR (2008) Characterization of extracellular minerals produced during dissimilatory Fe(III) and U(VI) reduction at 100 °C by Pyrobaculum islandicum. Geobiology 6(2):147–154
Kasprzak KS (2002) Oxidative DNA and protein damage in metal-induced toxicity and carcinogenesis1, 3 1 This article is part of a series of reviews on “Oxidative DNA Damage and Repair.” The full list of papers may be found on the homepage of the journal. 3Guest Editor: Miral Dizdaroglu. Free Radic Biol Med 32(10):958–967
Kaur A, Pan M, Meislin M, Facciotti MT, El-Gewely R, Baliga NS (2006) A systems view of haloarchaeal strategies to withstand stress from transition metals. Genome Res 16(7):841–854
Khan MS, Zaidi A, Wani PA, Oves M (2012) Erratum to: role of plant growth promoting rhizobacteria in the remediation of metal contaminated soils. Environ Chem Lett 10(1):105–106
Kim S-Y, Kim J-H, Kim C-J, Oh D-K (1996) Metal adsorption of the polysaccharide produced from Methylobacterium organophilum. Biotechnol Lett 18(10):1161–1164
Kletzin A, Adams MW (1996) Tungsten in biological systems. FEMS Microbiol Rev 18(1):5–63
Krom BP, Warner JB, Konings WN, Lolkema JS (2000) Complementary metal ion specificity of the metal-citrate transporters CitM and CitH of Bacillus subtilis. J Bacteriol 182(22):6374–6381
Lau CK, Krewulak KD, Vogel HJ (2016) Bacterial ferrous iron transport: the Feo system. FEMS Microbiol Rev 40(2):273–298
Le TT, Son M-H, Nam I-H, Yoon H, Kang Y-G, Chang Y-S (2017) Transformation of hexabromocyclododecane in contaminated soil in association with microbial diversity. J Hazard Mater 325:82–89
Lee SW, Glickmann E, Cooksey DA (2001) Chromosomal locus for cadmium resistance in Pseudomonas putida consisting of a cadmium-transporting ATPase and a MerR family response regulator. Appl Environ Microbiol 67(4):1437–1444
Li X, Krumholz LR (2007) Regulation of arsenate resistance in Desulfovibrio desulfuricans G20 by an arsRBCC operon and an arsC gene. J Bacteriol 189(10):3705
Luo J-H, Wu M, Liu J, Qian G, Yuan Z, Guo J (2019) Microbial chromate reduction coupled with anaerobic oxidation of methane in a membrane biofilm reactor. Environ Int 130:104926
Ma Y, Rajkumar M, Zhang C, Freitas H (2016) Beneficial role of bacterial endophytes in heavy metal phytoremediation. J Environ Manage 174:14–25
Madhaiyan M, Poonguzhali S, Sa T (2007) Metal tolerating methylotrophic bacteria reduces nickel and cadmium toxicity and promotes plant growth of tomato (Lycopersicon esculentum L.). Chemosphere 69(2):220–228
Mana-Capelli S, Mandal AK, Argüello JM (2003) Archaeoglobus fulgidus CopB is a thermophilic Cu2+-ATPase: functional role of its histidine-rich-N-terminal metal binding domain. J Biol Chem 278(42):40534–40541
Mandal AK, Argüello JM (2003) Functional roles of metal binding domains of the Archaeoglobus fulgidus cu(+)-ATPase CopA. Biochemistry 42(37):11040–11047
Marchal M, Briandet R, Koechler S, Kammerer B, Bertin PN (2010) Effect of arsenite on swimming motility delays surface colonization in Herminiimonas arsenicoxydans. Microbiology 156(8):2336–2342
Margaryan AA, Panosyan HH, Birkeland N-K, Trchounian AH (2013) Heavy metal accumulation and the expression of the copA and nikA genes in Bacillus subtilis AG4 isolated from the Sotk gold min in Armenia. Yerevan, Armenia: biolog. J Armenia 3(65):51–57
Meija J, Coplen TB, Berglund M, Brand WA, De Bièvre P, Gröning M, Holden NE, Irrgeher J, Loss RD, Walczyk T, Prohaska T (2016) Atomic weights of the elements 2013 (IUPAC technical report). Pure Appl Chem 88(3):265–291
Mills SD, Lim C-K, Cooksey DA (1994) Purification and characterization of CopR, a transcriptional activator protein that binds to a conserved domain (cop box) in copper- inducible promoters of Pseudomonas syringae. Mol Gen Genet 244(4):341–351
Moffett JW, Brand LE (1996) Production of strong, extracellular cu chelators by marine cyanobacteria in response to cu stress. Limnol Oceanogr 41(3):388–395
Moraleda-Muñoz A, Pérez J, Extremera AL, Muñoz-Dorado J (2010) Differential regulation of six heavy metal efflux Systems in the Response of Myxococcus xanthus to copper. Appl Environ Microbiol 76(18):6069
Mourão J, Rebelo A, Ribeiro S, Peixe L, Novais C, Antunes P (2020) Tolerance to arsenic contaminant among multidrug-resistant and copper-tolerant salmonella successful clones is associated with diverse ars operons and genetic contexts. Environ Microbiol 22(7):2829–2842
Muller D, Médigue C, Koechler S, Barbe V, Barakat M, Talla E, Bonnefoy V, Krin E, Arsène-Ploetze F, Carapito C, Chandler M, Cournoyer B, Cruveiller S, Dossat C, Duval S, Heymann M, Leize E, Lieutaud A, Lièvremont D, Makita Y, Mangenot S, Nitschke W, Ortet P, Perdrial N, Schoepp B, Siguier P, Simeonova DD, Rouy Z, Segurens B, Turlin E, Vallenet D, Van Dorsselaer A, Weiss S, Weissenbach J, Lett MC, Danchin A, Bertin PN (2007) A tale of two oxidation states: bacterial colonization of arsenic-rich environments. PLoS Genet 3(4):e53
Mulrooney SB, Hausinger RP (2003) Nickel uptake and utilization by microorganisms. FEMS Microbiol Rev 27(2–3):239–261
Nadar VS, Yoshinaga M, Pawitwar SS, Kandavelu P, Sankaran B, Rosen BP (2016) Structure of the ArsI C-as Lyase: insights into the mechanism of degradation of Organoarsenical herbicides and growth promoters. J Mol Biol 428(11):2462–2473
Naik S, Furtado I (2014) Equilibrium and kinetics of adsorption of Mn2+ by Haloarchaeon Halobacterium sp. GUSF (MTCC3265). Geomicrobiol J 31(8):708–715
Naik MM, Pandey A, Dubey SK (2012) Pseudomonas aeruginosa strain WI-1 from Mandovi estuary possesses metallothionein to alleviate lead toxicity and promotes plant growth. Ecotoxicol Environ Saf 79:129–133
Ndeddy Aka RJ, Babalola OO (2016) Effect of bacterial inoculation of strains of Pseudomonas aeruginosa, Alcaligenes feacalis and Bacillus subtilis on germination, growth and heavy metal (cd, cr, and ni) uptake of brassica juncea. Int J Phytoremediation 18(2):200–209
Nies DH (1999) Microbial heavy-metal resistance. Appl Microbiol Biotechnol 51(6):730–750
Nies DH (2003) Efflux-mediated heavy metal resistance in prokaryotes. FEMS Microbiol Rev 27(2–3):313–339
Nies D (2007) Bacterial transition metal homeostasis. In: Nies D, Silver S (eds) Molecular microbiology of heavy metals microbiology monographs. Springer, Berlin, pp 117–142
Nies DH, Silver S (1995) Ion efflux systems involved in bacterial metal resistances. J Ind Microbiol 14(2):186–199
Nikaido H (2018) RND transporters in the living world. Res Microbiol 169(7–8):363–371
Norris PR, Clark DA, Owen JP, Waterhouse S (1996) Characteristics of Sulfobacillus acidophilus sp. nov. and other moderately thermophilic mineral-sulphide-oxidizing bacteria. Microbiology (Reading) 142(Pt 4):775–783
Novick RP, Roth C (1968) Plasmid-linked resistance to inorganic salts in Staphylococcus aureus. J Bacteriol 95(4):1335–1342
Okoduwa SIR, Igiri B, Udeh CB, Edenta C, Gauje B (2017) Tannery effluent treatment by yeast species isolates from watermelon. Toxics 5(1)
Ordóñez E, Letek M, Valbuena N, Gil JA, Mateos LM (2005) Analysis of genes involved in arsenic resistance in Corynebacterium glutamicum ATCC 13032. Appl Environ Microbiol 71(10):6206–6215
Oremland RS, Stolz JF (2005) Arsenic, microbes and contaminated aquifers. Trends Microbiol 13(2):45–49
Oshima T, Kondo K, Ohto K, Inoue K, Baba Y (2008) Preparation of phosphorylated bacterial cellulose as an adsorbent for metal ions. React Funct Polym 68(1):376–383
Ozdemir G, Ozturk T, Ceyhan N, Isler R, Cosar T (2003) Heavy metal biosorption by biomass of Ochrobactrum anthropi producing exopolysaccharide in activated sludge. Bioresour Technol 90(1):71–74
Páez-Espino AD, Durante-Rodríguez G, de Lorenzo V (2015) Functional coexistence of twin arsenic resistance systems in Pseudomonas putida KT2440. Environ Microbiol 17(1):229–238
Paulsen IT, Saier MH Jr (1997) A novel family of ubiquitous heavy metal ion transport proteins. J Membr Biol 156(2):99–103
Peng ED, Oram DM, Battistel MD, Lyman LR, Freedberg DI, Schmitt MP (2018) Iron and zinc regulate expression of a putative ABC metal transporter in Corynebacterium diphtheriae. J Bacteriol 200(10):e00051–e00018
Porcheron G, Garenaux A, Proulx J, Sabri M, Dozois C (2013) Iron, copper, zinc, and manganese transport and regulation in pathogenic Enterobacteria: correlations between strains, site of infection and the relative importance of the different metal transport systems for virulence. Front Cell Infect Microbiol 3:90
Pourret O, Hursthouse A (2019) It’s time to replace the term “heavy metals” with “potentially toxic elements” when reporting environmental research. Int J Environ Res Public Health 16(22):4446
Pradhan SK, Singh NR, Rath BP, Thatoi H (2016) Bacterial chromate reduction: a review of important genomic, proteomic, and bioinformatic analysis. Crit Rev Environ Sci Technol 46(21–22):1659–1703
Prithivirajsingh S, Mishra SK, Mahadevan A (2001) Detection and analysis of chromosomal arsenic resistance in Pseudomonas fluorescens strain MSP3. Biochem Biophys Res Commun 280(5):1393–1401
Qin J, Rosen BP, Zhang Y, Wang G, Franke S, Rensing C (2006) Arsenic detoxification and evolution of trimethylarsine gas by a microbial arsenite S-adenosylmethionine methyltransferase. Proc Natl Acad Sci U S A 103(7):2075–2080
Quatrini R, Johnson DB (2019) Acidithiobacillus ferrooxidans. Trends Microbiol 27(3):282–283
Rademacher C, Masepohl B (2012) Copper-responsive gene regulation in bacteria. Microbiology 158:2451–2464
Rasulov BA, Yili A, Aisa HA (2013) Biosorption of metal ions by exopolysaccharide produced by Azotobacter chroococcum XU1. J Environ Prot 4:989–993
Raungsomboon S, Chidthaisong A, Bunnag B, Inthorn D, Harvey NW (2006) Production, composition and Pb2+ adsorption characteristics of capsular polysaccharides extracted from a cyanobacterium Gloeocapsa gelatinosa. Water Res 40(20):3759–3766
Rosen BP (2002) Transport and detoxification systems for transition metals, heavy metals and metalloids in eukaryotic and prokaryotic microbes. Comp Biochem Physiol A Mol Integr Physiol 133(3):689–693
Rosenstein R, Peschel A, Wieland B, Götz F (1992) Expression and regulation of the antimonite, arsenite, and arsenate resistance operon of Staphylococcus xylosus plasmid pSX267. J Bacteriol 174(11):3676–3683
Rouch DA, Lee BTO, Morby AP (1995) Understanding cellular responses to toxic agents: a model for mechanism-choice in bacterial metal resistance. J Ind Microbiol 14(2):132–141
Ruangsomboon S, Chidthaisong A, Bunnag B, Inthorn D, Harvey NW (2007) Lead (Pb2+) adsorption characteristics and sugar composition of capsular polysaccharides of cyanobacterium Calothrix marchica. Songklanakarin J Sci Technol 29(2):529–541
Ryan D, Colleran E (2002) Arsenical resistance in the IncHI2 plasmids. Plasmid 47(3):234–240
Salehizadeh H, Shojaosadati SA (2003) Removal of metal ions from aqueous solution by polysaccharide produced from Bacillus firmus. Water Res 37(17):4231–4235
Saltikov CW, Cifuentes A, Venkateswaran K, Newman DK (2003) The ars detoxification system is advantageous but not required for as(V) respiration by the genetically tractable Shewanella species strain ANA-3. Appl Environ Microbiol 69(5):2800–2809
Samuelson P, Wernérus H, Svedberg M, Ståhl S (2000) Staphylococcal surface display of metal-binding polyhistidyl peptides. Appl Environ Microbiol 66(3):1243–1248
Sand W, Rohde K, Sobotke B, Zenneck C (1992) Evaluation of Leptospirillum ferrooxidans for leaching. Appl Environ Microbiol 58(1):85–92
Sar P, Kazy SK, Asthana RK, Singh SP (1998) Nickel uptake by Pseudomonas aeruginosa: role of modifying factors. Curr Microbiol 37(5):306–311
Saravanakumar D, Vijayakumar C, Kumar N, Samiyappan R (2007) PGPR-induced defense responses in the tea plant against blister blight disease. Crop Prot 26(4):556–565
Sato T, Kobayashi Y (1998) The ars operon in the skin element of Bacillus subtilis confers resistance to arsenate and arsenite. J Bacteriol 180(7):1655–1661
Schenk PM, Carvalhais LC, Kazan K (2012) Unraveling plant–microbe interactions: can multi-species transcriptomics help? Trends Biotechnol 30(3):177–184
Scherer J, Nies DH (2009) CzcP is a novel efflux system contributing to transition metal resistance in Cupriavidus metallidurans CH34. Mol Microbiol 73(4):601–621
Segerer A, Neuner A, Kristjansson JK, Stetter KO (1986) Acidianus infernus gen. Nov., sp. nov., and Acidianus brierleyi comb. nov.: facultatively aerobic, extremely acidophilic thermophilic sulfur-metabolizing archaebacteria. Int J Syst Evol Microbiol 36(4):559–564
Sharma M, Kaushik A, Somvir B, K. and Kamra, A. (2008) Sequestration of chromium by exopolysaccharides of Nostoc and Gloeocapsa from dilute aqueous solutions. J Hazard Mater 157(2):315–318
Sher S, Rehman A (2019) Use of heavy metals resistant bacteria-a strategy for arsenic bioremediation. Appl Microbiol Biotechnol 103(15):6007–6021
Silver S (1996) Bacterial resistances to toxic metal ions. Gene 179(1):9–19
Singh R, Dong H, Liu D, Zhao L, Marts AR, Farquhar E, Tierney DL, Almquist CB, Briggs BR (2015) Reduction of hexavalent chromium by the thermophilic methanogen Methanothermobacter thermautotrophicus. Geochim Cosmochim Acta 148:442–456
Smith RL, Maguire ME (1995) Distribution of the CorA Mg2+ transport system in gram-negative bacteria. J Bacteriol 177(6):1638–1640
Spada S, Pembroke JT, Wall JG (2002) Isolation of a novel Thermus thermophilus metal efflux protein that improves Escherichia coli growth under stress conditions. Extremophiles 6(4):301–308
Suzuki K, Wakao N, Kimura T, Sakka K, Ohmiya K (1998) Expression and regulation of the arsenic resistance operon of Acidiphilium multivorum AIU 301 plasmid pKW301 in Escherichia coli. Appl Environ Microbiol 64(2):411–418
Tavares MT, Neves IC (2008) Biosorption system produced from biofilms supported on Faujasite (FAU) zeolite, process for obtaining it and its usage for removal of hexavalent chromium (Cr(VI)). International patent, W0 2007/020588 A1, EP1912905
Tsai KJ, Yoon KP, Lynn AR (1992) ATP-dependent cadmium transport by the cadA cadmium resistance determinant in everted membrane vesicles of Bacillus subtilis. J Bacteriol 174(1):116–121
Tuffin IM, de Groot P, Deane SM, Rawlings DE (2005) An unusual Tn21-like transposon containing an ars operon is present in highly arsenic-resistant strains of the biomining bacterium Acidithiobacillus caldus. Microbiology (Reading) 151(Pt 9):3027–3039
Tuffin IM, Hector SB, Deane SM, Rawlings DE (2006) Resistance determinants of a highly arsenic-resistant strain of Leptospirillum ferriphilum isolated from a commercial biooxidation tank. Appl Environ Microbiol 72(3):2247–2253
Turpeinen R, Kairesalo T, Häggblom MM (2004) Microbial community structure and activity in arsenic-, chromium- and copper-contaminated soils. FEMS Microbiol Ecol 47(1):39–50
Vardanyan NS, Vardanyan AK (2018) Thermophilic chemolithotrophic bacteria in mining sites. In: Egamberdieva D, Birkeland N-K, Panosyan H, Li W-J (eds) Extremophiles in Eurasian ecosystems: ecology, diversity, and applications. Springer, Singapore, pp 187–218
Vargas M, Kashefi K, Blunt-Harris EL, Lovley DR (1998) Microbiological evidence for Fe(III) reduction on early earth. Nature 395(6697):65–67
Voica DM, Bartha L, Banciu HL, Oren A (2016) Heavy metal resistance in halophilic Bacteria and archaea. FEMS Microbiol Lett 363(14):1–9
Volesky B (1994) Advances in biosorption of metals: selection of biomass types. FEMS Microbiol Rev 14(4):291–302
von Rozycki T, Nies DH (2009) Cupriavidus metallidurans: evolution of a metal-resistant bacterium. Antonie Van Leeuwenhoek 96(2):115–139
Wang J, Chen C (2009) Biosorbents for heavy metals removal and their future. Biotechnol Adv 27(2):195–226
Wang G, Kennedy SP, Fasiludeen S, Rensing C, DasSarma S (2004) Arsenic resistance in Halobacterium sp. strain NRC-1 examined by using an improved gene knockout system. J Bacteriol 186(10):3187–3194
Wang L, Chen S, Xiao X, Huang X, You D, Zhou X, Deng Z (2006) arsRBOCT arsenic resistance system encoded by linear plasmid pHZ227 in Streptomyces sp. strain FR-008. Appl Environ Microbiol 72(5):3738–3742
Wani PA, Khan MS (2010) Bacillus species enhance growth parameters of chickpea (Cicer arietinum L.) in chromium stressed soils. Food Chem Toxicol 48(11):3262–3267
Whelan KF, Colleran E (1992) Restriction endonuclease mapping of the HI2 incompatibility group plasmid R478. J Bacteriol 174(4):1197–1204
White C, Gadd GM (2000) Copper accumulation by sulfate-reducing bacterial biofilms. FEMS Microbiol Lett 183(2):313–318
Xiong A, Jayaswal RK (1998) Molecular characterization of a chromosomal determinant conferring resistance to zinc and cobalt ions in Staphylococcus aureus. J Bacteriol 180(16):4024–4029
Yang HC, Rosen BP (2016) New mechanisms of bacterial arsenic resistance. Biom J 39(1):5–13
Yang HC, Cheng J, Finan TM, Rosen BP, Bhattacharjee H (2005) Novel pathway for arsenic detoxification in the legume symbiont Sinorhizobium meliloti. J Bacteriol 187(20):6991–6997
Yang L, Zhao D, Yang J, Wang W, Chen P, Zhang S, Yan L (2019) Acidithiobacillus thiooxidans and its potential application. Appl Microbiol Biotechnol 103(19):7819–7833
Ye J, Yang H-C, Rosen BP, Bhattacharjee H (2007) Crystal structure of the flavoprotein ArsH from Sinorhizobium meliloti. FEBS Lett 581(21):3996–4000
Zeng W, Zhang S, Xia M, Wu X, Qiu G, Shen L (2020) Insights into the production of extracellular polymeric substances of Cupriavidus pauculus 1490 under the stimulation of heavy metal ions. RSC Adv 10(34):20385–20394
Zhang Y, Rodionov DA, Gelfand MS, Gladyshev VN (2009) Comparative genomic analyses of nickel, cobalt and vitamin B12 utilization. BMC Genomics 10(1):78
Acknowledgments
This work was supported by grants from the Eurasia Programme of the Norwegian Center for International Cooperation in Education (CPEA-2011/10081, CPEA-LT-2016/10095) and partially supported by Research Grant from MESCS Science Committee of Armenia, to AM (19YR-1F065), and the Armenian National Science and Education Fund based in New York, USA, to AM (ANSEF-NS-microbio-3869 and 4619).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Margaryan, A., Panosyan, H., Birkeland, NK. (2021). Heavy Metal Resistance in Prokaryotes: Mechanism and Application. In: Egamberdieva, D., Birkeland, NK., Li, WJ., Panosyan, H. (eds) Microbial Communities and their Interactions in the Extreme Environment. Microorganisms for Sustainability, vol 32. Springer, Singapore. https://doi.org/10.1007/978-981-16-3731-5_13
Download citation
DOI: https://doi.org/10.1007/978-981-16-3731-5_13
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-16-3730-8
Online ISBN: 978-981-16-3731-5
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)