Abstract
Sediment microbial fuel cells (SMFCs) have been considered as a promising source for green energy in the field of microbial fuel cell (MFC) technologies. By replicating a natural pond system in laboratory scale, this study tries to introduce a novel concept of utilizing duckweed for maintaining self-sustainability and optimum operating conditions required for a SMFC. Installation of fuel cell setups in natural water bodies is a challenging task; the dissolved oxygen being one of the major hurdles, as it interferes with the functioning of the anodic chamber. In this context, the use of duckweed in the SMFC setup is anticipated to reduce the dissolved oxygen (DO) content in the water and create an anaerobic anodic chamber naturally at the bottom. Another advantage of introduction of duckweed into the system is that being a green plant they perform photosynthesis and a portion of the synthesized carbohydrates and minerals gets excreted through the roots into the water body, which helps to naturally maintain the nutrient and mineral availability to the microbial community in the sediment layer. In this study, SMFC systems have been constructed in 250 ml glass containers, which were filled with sediment and water in a height ratio of 1:2. Spirodela species of the Lemnacae family was selected as the model duckweed for the SMFC systems. The sediment and the water were collected from natural pond to construct the SMFC systems. It was observed that introduction of duckweed into the SMFC configuration reduced the DO level in the water gradually from top to bottom of the water column and created an anaerobic condition near the sediment layer. Duckweed inoculation enhanced the cathode potential and also reduced the startup time for SMFC operation. After 31 days of operation, it was observed that the SMFC configuration could produce a stable maximum potential of 796 mV, which could be enhanced to power small scale devices by connecting the SMFC systems in series or parallel as per requirement.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
1 Introduction
Energy generation in the form of electricity has been a major concern for the society since several decades. Meanwhile there have been many techniques or procedures for the production of electricity. However, there is the need of finding alternatives for energy generation since the traditional resources like fossil fuels are depleting due to the ever increasing need [1]. The renewable energy sources possess the potential to replace the conventional sources for production of energy. In this regard, the fuel cells are the best example for the production of electricity of which, the microbial fuel cells (MFCs) are one of the types.
A fuel cell is an electrochemical cell that converts the chemical energy of fuel into electricity by virtue of an electrochemical reaction in the presence of an oxidation and a reduction catalyst [2]. In an MFC, the microorganisms convert the chemical energy associated with organic substances to electricity and if the organic source is a water body then during the electrochemical reactions the source is simultaneously bio-remediated by the microorganisms [3].
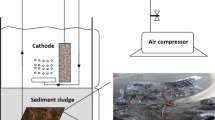
Sediment microbial fuel cells (SMFCs) are such type of MFCs where along with electricity generation, bio-remediation also takes place [4] (Fig. 1). The SMFCs differ from the basic MFC technology in that they are completely anoxic and lack a membrane to separate the two electrode chambers [1]. They possess an extensive range of operational parameters and have the ability to utilize a wide variety of organic substances as fuel, such as glucose, glutamic acid, river water, cysteine, acetate and starch to name a few [1].
The SMFCs over the years are gaining a substantial scientific interest due to the long-term advantages associated with energy production. They can produce electrical energy resiliently without high-maintenance need and without production of any environment impairing components. Additionally, their ability to remediate the sediments of heavy metals has rendered them prominence. However, the low potential and current production of the SMFCs are limiting their prevalent use for practical applications. In most scenarios, the SMFCs are installed in mainstream rivers, ponds and seas [4,5,6,7].
Donovan et. al. (2008, 2011) installed SMFC setups in mainstream river [4, 5]. Meehan et. al. (2009) deployed a SMFC setup inside sea to power hydrophones with the help of power management systems [6]. Installing setups in such locations is also a challenging task and there are limitations in power production even with large sized anodes [4,5,6,7,8]. To increase the power production, connections such as electrical series or parallel was also not possible since the SMFCs were deployed in same water body [6, 7]. There are reports of SMFCs being constructed in laboratory scale also, however, they reported about limitations in power production and the systems were not self-sustainable [9].
This work proposes for a SMFC system with introduction of duckweed (Spirodela sp.) into the system. Duckweed generally grows in lakes and ponds. Scientifically, these group of aquatic plants belong to the family of monocotyledon floating plants called Lemnaceae [10]. Duckweed possesses some additional exceptional properties like phenomenal growth rate, high-protein content, waste water treatment capability and their potential to be used for bio-fuel production. Moreover, duckweed directly effects the DO, pH and conductivity of the water body [11]. It is anticipated that introduction of duckweed into the system would help create an anaerobic environment in the water level below and thereby facilitate the conditions for the anodic chamber. The dissolved oxygen (DO) is an important parameter in SMFCs as it effects the cathode performance [12]. The duckweed assisted SMFC system is expected to help enhance the cathode potential and impart self-sustainability. This SMFC configuration would be very useful to power low-scale devices in rural areas due to its low cost and simple mode of construction.
2 Methodology
2.1 Construction of the SMFC
The duckweed assisted SMFC was constructed in a 250 ml glass beaker. The beaker was filled with 175 g of sediment to cover a height of 2.54 cm inside the glass beaker. This was, followed by addition of water into the system which, covered a height of 5 cm. The height of the water layer was maintained by replenishing water into the SMFC from time to time. Two isomolded graphite plate (purchased from GraphiteStore.com, USA) having dimensions 6 cm × 2 cm × 0.3 cm and 3 cm × 4 cm × 0.3 cm were used as cathode and anode, respectively. The anode was placed horizontally inside the sediment layer and the cathode was placed vertically at the top of the beaker with a support to enable 50% of the cathode to be immersed in water and the rest of the portion to be in direct contact with air. Eventually, duckweed was added to complete the arrangement of the system. A natural pond nearby the Indian Institute of Technology, Guwahati campus (26.1903° N, 91.6920° E) was the source for the sediment, water and duckweed. Figure 2 shows a schematic of the laboratory scale SMFC.
2.2 Operational Conditions
A 10–14 h day-night condition was maintained inside the laboratory for the entire period of SMFC operation, which facilitated the duckweed in the SMFC system to grow naturally. The duckweed assisted SMFC was operated at ambient temperature, which was recorded to vary between 25 and 31 °C during the 31 days of operation.
2.3 Characterization of the SMFC
The open circuit potential of the duckweed assisted SMFC was monitored under the aforementioned laboratory conditions for a duration of 31 days. When the open circuit potential of the SMFC system achieved a stationary potential level, a 10 KΩ resistive load was connected and the stable potential across the load was recorded. The internal resistance of the SMFC system was calculated by applying Eq. 1.
The introduction of duckweed was anticipated to influence the content of dissolved oxygen (DO) in the SMFC system and was monitored using a DO700 (Eutech Instruments).
3 Results and Discussions
3.1 Effect of Duckweed on DO Content of the SMFC System
Figure 3 shows the dissolved oxygen content of the SMFC system at various levels from the surface to a depth of 5 cm before and after addition of duckweed. The DO content at the depth of 5 cm was reduced by 11.19% after introduction of duckweed to the SMFC system. Thus, an anaerobic condition with DO content of 3.7 mg/L could be achieved just at the interface of sediment and water layer. This is very encouraging as the dissolved oxygen content is reported to be one of the parameters hindering the SMFC operation and such arrangement enables the replication of natural water systems for construction of SMFC systems [12]. Another advantage of having duckweed in the SMFC system is that being a green plant it performs photosynthesis and a part of the produced food is released by the roots, which gets deposited at the bottom and helps maintain the nutrient level as well as the microbial diversity in the sediment layer [13]. So, the introduction of duckweed empowers the SMFC system self-sustainability.
3.2 Characterization of the SMFC
Figure 4 shows the open circuit potentials of the duckweed assisted SMFC system monitored for a period of 31 days. The potential achieved maximum value of 796 mV on the12th day of operation and from day 22 onwards, the output potential was relatively stable. On addition of duckweed the cathode potential increased to 264 mV where it was 259 mV prior to addition of duckweed. The reduction in dissolve oxygen helped the anodic reactions to occur and a maximum of −519 mV anode potential was recorded during the experimental period (Fig. 4). Furthermore, it has been observed that the OCP could be enhanced by connecting additional SMFC systems in series or parallel depending upon our requirement. Since internal resistance is the factor which governs the output characteristics of the fuel cells [16, 17]; after 31 days of open circuit operation the SMFC was connected to a 10 KΩ load. The potential across the load was documented over a period of 30 min (until the potential was steady). Finally, against the steady voltage across the load the internal resistance of the cell was calculated as 9.8 KΩ (average of triplicate data).
4 Conclusion
A self-sustainable duckweed assisted sediment microbial fuel cell system was constructed in this work. Introduction of duckweed into the system helped to create an anaerobic condition at a depth of 5 cm water level and helped to maintain a stable OCP of maximum 796 mV. The proposed SMFC configuration can be connected in series or parallel as per need to power low-power consuming electronic devices.
Abbreviations
- DO:
-
Dissolve oxygen (mg/L)
- Rint:
-
Internal Resistance (Ohm)
- RLoad:
-
Load Resistance (Ohm
- SMFC:
-
Sediment Microbial Fuel Cell
- V:
-
Voltage (Volts)
- Vocv:
-
Open Circuit Voltage (mV)
- VLoad:
-
Voltage across Load (mV)
- Ω:
-
Resistance (Ohm)
- °C:
-
Temperature (Degree Celsius)
References
Syed, Z. A., Rafatullah, M., Norli, I., & Muhammad, I. S. (2017). A review on sediment microbial fuel cells as a new source of sustainable energy and heavy metal remediation: Mechanisms and future prospective. International Journal of Energy Research, 41, 1242–1264.
Khurmi, R. S (2014). Material Science. edn.
Bond, D. R., Holmes, D. E., & Tender, L. M., Lovley, D. R., (2002). Electrode-reducing microorganisms that harvest energy from marine sediments. Science 295. https://doi.org/10.1126/science.1066771
Donovan, C., Dewan, A., Heo, D., & Beyenal, A. (2008). Batteryless, wireless sensor powered by a sediment microbial fuel cell. Environmental Science and Technology, 42, 8591–8596.
Donovan, C., Dewan, A., Peng, H., Heo, D., & Beyenal, H. (2011). Power management system for a 2.5W remote sensor powered by a sediment microbial fuel cell. Journal of Power Sources, 196, 1171–1177.
Meehan, A., Gao, H., & Lewandowski, Z. (2009). Energy harvest with microbial fuel cell and power management system. IEEE 978-1-4244-2893-9/09/$25.00. https://doi.org/10.1109/ECCE.2009.5316034
Gao, H., Meehan, A., & Lewandowski, Z. (2011). New microbial fuel cell power system for efficiency improvement. International Conference on Electrical Machines and Systems, Beijing, pp. 1–5.
Wanga, G., Yua, M., Xieb, K., Zhaoa, R., Fua, Y., & Chen, T. (2019). Graphene modified polyacrylonitrile fiber as high-performance cathode for marine sediment microbial fuel cells. Journal of Power Sources 438, 227002.
Song, T.-S., Yan, Z.-S., Zhao, Z.-W., & Jiang, H.-L. (2011). Construction and operation of freshwater sediment microbial fuel cell for electricity generation. Bioprocess and Biosystems Engineering, 34, 621–627.
Renslow, R., Donovan, C., Shim, M., Babauta, J., Nannapaneni, S., Schenk, J., & Beyenal, H. (2011). Oxygen reduction kinetics on graphite cathodes in sediment microbial fuel cells. Physical Chemistry Chemical Physics: PCCP, 13, 21573–21584.
Hubenova, Y., & Mitov, M. (2012). Conversion of solar energy into electricity by using duckweed in Direct Photosynthetic Plant Fuel Cell. Bioelectrochemistry, 87, 185–191.
Landolt, E. (1986). The family Lemnaceae, a monographic study. Vol 1 Morphology, karyology, ecology, geographic distribution, systematic position, nomenclature, descriptions. Veroff. Geobot. Inst. ETH, Stiftung Rubel, 71: 566.
Sajana, T. K., Ghangrekar, M. M., Mitra, A. (2013). Effect of pH and distance between electrodes on the performance of a sediment microbial fuel cell. Water Science & Technology 68(3). https://doi.org/10.2166/wst.2013.271
Arif, M., Cheung, S. C. P., & Andrews, J. (2020). A systematic approach for matching simulated and experimental polarization curves for a PEM fuel cell. International Journal of Hydrogen Energy, 45, 3-2206-2223.
Huang, X., Zhang, Z., Jiang, J. (2006) Fuel cell technology for distributed generation: An overview. Industrial Electronics, IEEE International Symposium on, vol. 2. https://doi.org/10.1109/ISIE.2006.295713
Miller, A., Singh, L., Wang, L., & Liu, H. (2019). Linking internal resistance with design and operation decisions in microbial electrolysis cells. Environment International, 126, 611–618.
Vazquez-Larios, A. L., Solorza-Feria, O., Vazquez-Huerta, G., & Ríos-Leal, E. (2011). Internal resistance and performance of microbial fuel cells: influence of cell configuration and temperature. Journal of New Materials for Electrochemical Systems, 14(2), 67–139.
Acknowledgements
The authors gratefully acknowledge the financial support of the Department of Science and Technology, Government of India, for the above project (DST/INT/RMES/P-10/2016).
Author information
Authors and Affiliations
Corresponding authors
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this paper
Cite this paper
Dutta, A., Barbora, L., Thakuria, A., Goswami, P., Stom, D. (2022). Duckweed Assisted Sediment Microbial Fuel Cell for Powering Small Scale Devices. In: Mahanta, P., Kalita, P., Paul, A., Banerjee, A. (eds) Advances in Thermofluids and Renewable Energy . Lecture Notes in Mechanical Engineering. Springer, Singapore. https://doi.org/10.1007/978-981-16-3497-0_40
Download citation
DOI: https://doi.org/10.1007/978-981-16-3497-0_40
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-16-3496-3
Online ISBN: 978-981-16-3497-0
eBook Packages: EngineeringEngineering (R0)