Abstract
Understanding of impact dynamics of droplets on oblique plane is relevant for applications like pesticides spraying and internal combustion engines. Experiments were performed using three test fluids to investigate the effect of viscosity and surface tension properties on the elongation factor of droplets impacting on oblique planes. The temporal variation of elongation factor primarily depends upon the surface inclination and surface tension. For a given Weber number, emission of secondary droplet takes place at the earliest time for surfactant solution due to reduction of interfacial energy.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
1 Introduction
Drop impact dynamics on surfaces is a complex phenomenon due to interplay of fluid mechanics, surface physics, and interfacial chemistry. This impact process exhibits a diverse set of intriguing behavior depending upon the wettability of the solid surface with respect to the fluid [1, 2] and the fluid properties [3,4,5,6]. Oblique impact studies may be useful in applications like spraying of pesticides at random angles and impact of supercooled water drops on airplane leading to frost formation [7,8,9]. In IC engines too, fuel injection system sprays fuel obliquely toward the combustion zone [10]. In addition, typical applications such as spray coating, painting, cooling, or inkjet printing are more likely to occur at oblique angles instead of normal impact. Sikalo et al. [11] reported asymmetry in the spreading factor, i.e., the differences in the deformation at the front and back of the droplet after impact. Chiarot et al. [12] and Zheng et al. [13] studied grazing impact of high velocity continuous drop streams on inclined SH surfaces and observed that the shape and structure of the rebounding stream are influenced by the frequency of the drop ejection and velocity. Yeong et al. [14] investigated the dependence of drop dynamics on Weber number. Antonini et al. [15] distinguished six distinct impact regimes at wide range of We for drop impact on tilted hydrophobic and superhydrophobic (SH) substrates. LeClear et al. [16] observed the transition from the superhydrophobic Cassie–Baxter regime to the fully wetted Wenzel regime while studying the impact of water drops on inclined textured SH surfaces.
While a lot of attention has been paid to the spreading dynamics on SH surfaces, the dynamics of lift off of the drops upon inclined surfaces are rarely studied. Therefore, the present study highlights the role of non-dimensional numbers like Reynolds number (Re) and Weber number (We) and surface inclination upon impact of droplets. Experiments have been carried out with different fluids to observe the post-impact elongation characteristics of droplets and the dynamics vis-à-vis physical properties.
2 Methodology
2.1 Experimental Details
The experimental setup consists of a drop dispenser controller (Holmarc Opto-Mechantronics Pvt. Ltd., India) that maintains constant volume of droplet discharged from a syringe pump as shown in Fig. 1. The drop impact images were recorded using a high speed camera (Photron FASTCAM SA4) mounted with a G-type AF-S macrolens of focal length 105 mm (Nikkor, Nikon). The images were taken at 1024 × 1024 pixels resolution at 3600 frame per second. Experiments were performed at ambient conditions (25 °C) on SH surfaces. For impact study, sterile glass slides were thoroughly cleaned with acetone and DI water and then dried in hot air oven. The SH surfaces were created on similar glass substrates using superhydrophobic spray coating (Ultra Tech International Inc., USA). In the present study, three fluids such as DI water, SiO2 water nanocolloids (2.5 and 5% wt.), and different concentrations of sodium dodecyl sulfate (SDS, Merck, India) dissolved in DI water (0.25 and 0.50% of the critical micelle concentration (CMC)) were used as test fluids.
Schematic of the experimental setup [17] (A) drop dispenser controller (B) base (C) substrate inclination apparatus with backlight arrangement (D) syringe pump (E) high-speed camera (F) laptop (G) power source (H) drop (I) target surface (J) syringe
3 Results and Discussions
The present study focuses on experiments with different test fluids, highlighting the role of surface inclination and Weber number (We) and Reynolds number (Re). The Weber number has been defined based on the initial velocity (vi), density (ρl), diameter (di), and fluid surface tension (σl) (Subscripts i, l stand for initial condition before impact and liquid, respectively). The Weber number (We) was varied between 10 and 127 (We = 10–89 for water, We = 12–127 for sodium dodecyl sulfate (SDS) surfactant solutions (0.25 and 0.5 wt % of critical micelle concentration (CMC), and We = 10–89 for aqueous solutions of silica particles (2.5 and 5 wt %)). Similarly, the Reynolds number based on initial velocity is defined as Re = . Re was varied from 28 to 4873, (Re = 1624–4873 for water, Re = 28–1105 for silica colloids and Re = 1411–4587 for surfactant solutions). The initial diameter of droplets of DI water, 0.25 and 0.5 wt. % CMC are considered as 2.9, 2.71, and 2.52 mm, respectively. The initial diameters of 2.5 and 5 wt. % of silica colloidal solutions are almost similar to the initial diameters of water droplets. The measurement uncertainty of initial diameter of droplets for test fluids is within 5% error.
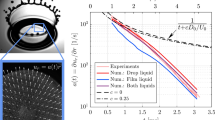
Figure 2 elucidates the elongation dynamics of post-impact drops on different inclination angles on SH surfaces. The elongation factor (β) is expressed as the ratio of height of post-impact drop bouncing off from the target to initial drop diameter. The non-dimensional time (τ) is expressed as the ratio of the product of time (t) at which post-impact image is considered and initial velocity (vi) to the initial droplet diameter (di). It is observed that the elongation factor for droplets of 0.25 and 0.5 wt. % CMC solution increases up to certain time interval followed by a sharp decline. The sharp decline occurs due to emission of secondary droplets during vertical acceleration of the droplets after impact. The vertical momentum and reduction in interfacial energy promotes pinching off event (Fig. 3). At ϕ = 0°, for 0.25 CMC surfactant solution, formation of secondary droplet twice at time t = 13.77 and 18.63 ms corresponds to twice decrease in β (Fig. 2).
Variation of elongation factor with non-dimensional time on SH surface. The test fluids are surfactant solutions considering 0.25 and 0.5 CMC. Considering the surface inclination, the normal Weber number is defined as Wen = \(\rho _{l} v_{i}^{2} d_{i}\) (cos Φ)2/σl , where ϕ is the tilted angle. We represents normal Weber number in the plot
In case of water at initial impact velocity vi = 1 m/s, elongation factor follows the same trend as droplets of surfactant solutions (Fig. 4). The emission of secondary droplets occurs at ϕ = 0°, but this behavior is diminished with increasing surface inclinations. The reduction in normal momentum to the oblique plane inhibits the formation of secondary droplet formation while lifting off from the surface.
Figure 5 investigates the elongation dynamics of three test fluids such as water, silica colloid solutions and surfactant solutions at We = 40 and ϕ = 0° for SH surfaces. It is found that the secondary droplet is formed earlier in case of surfactant solutions compared to water and SiO2 colloidal solution. This is obvious due to the fact that surfactants reduce the interfacial energy, thereby promoting the formation of secondary droplets.
4 Conclusion
The present study investigates the temporal variation of elongation factor using different test fluids on tilted SH surfaces. It is observed that the elongation factor decreases with increase in surface inclination. Again, the suppression of secondary droplet occurs with increase in substrate angle. The time at which secondary droplet departs from the original droplet depends upon the fluid properties. The reduction in normal momentum and interfacial energy are the two key parameters for the above events. This study enables to find the critical parameters for comprehensive study of elongation outcomes on SH surfaces.ables should be pasted within the text column as follows.
Abbreviations
- v :
-
Velocity of droplet (m/s)
- d :
-
Diameter of droplet (m)
- t :
-
Time
- ρ :
-
Density (dimensionless)
- β :
-
Elongation factor (dimensionless)
- ϕ :
-
Tilted angle
- i :
-
Pre-impact
- l :
-
Liquid
References
Clanet, C., Béguin, C., Richard, D., & Quéré, D. (2004). Journal of Fluid Mechanics, 517, 199–208.
Fukai, J., Shiiba, Y., Yamamoto, T., Miyatake, O., Poulikakos, D., Megaridis, C. M., & Zhao, Z. (1995). Physics of Fluids, 7, 236.
Koch, K., & Barthlott, W. (2009). Philosophical Transactions of the Royal Society A, 367, 1487–1509.
Ueda, T., Enomoto, T., & Kanetsuki, M. (1979). Bullet JSME, 22, 724–732.
Park, J. Y., Gardner, A., King, W. P., & Cahill, D. G. (2014). The Journal of Heat Transfer, 136, 092902.
Roisman, I. V. (2009). Physics of Fluids, 21, 052104–1–052104–11.
Yeong, Y. H., Mudafort, R., Steele, A., Bayer, I., & Loth, E. (2012). In 4th AIAA Atmospheric and Space Environments Conference, New Orleans, Louisiana, 2012.
Politovich, M. K. (1989). The Journal of Applied Meteorology, 28, 856–868.
Massinon, M., & Lebeau, F. (2012). Biosystems Engineering, 112, 56–64.
Chen, R., Chiu, S. L., & Lin, T. H. (2007). Experimental Thermal and Fluid Science, 32, 587–595.
Šikalo, Š, Tropea, C., & Ganic, E. N. (2005). Journal of Colloid and Interface Science, 286, 661–669.
Chiarot, P. R., & Jones, T. (2010). Experiments in Fluids, 49, 1109–1119.
Zheng, L., Li, Z., Bourdo, S., Khedir, K. R., Asar, M. P., Ryerson, C. C., & Biris, A. S. (2011). Langm, 27, 9936–9943.
Yeong, Y. H., Burton, J., & Loth, E. (2014). Langm, 30, 12027–12038.
Antonini, C., Villa, F., & Marengo, M. (2014). Experiments in Fluids, 55, 1713–1–9.
LeClear, S. LeClear, J., Abhijeet, Park, K. C., & Choi, W. (2016). Journal of Colloid and Interface Science, 461, 114–121.
Sahoo, N., Khurana, G., Harikrishnan, A. R., Samanta, D., & Dhar, P. (2021). Post impact droplet hydrodynamics on inclined planes of variant wettabilities. European Journal of Mechanics/B Fluids, 79, 27–37.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this paper
Cite this paper
Sahoo, N., Samanta, D., Dhar, P. (2022). Effect of Substrate Inclination on Post-impact Dynamics of Droplets. In: Mahanta, P., Kalita, P., Paul, A., Banerjee, A. (eds) Advances in Thermofluids and Renewable Energy . Lecture Notes in Mechanical Engineering. Springer, Singapore. https://doi.org/10.1007/978-981-16-3497-0_3
Download citation
DOI: https://doi.org/10.1007/978-981-16-3497-0_3
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-16-3496-3
Online ISBN: 978-981-16-3497-0
eBook Packages: EngineeringEngineering (R0)