Abstract
While encountering the Coronavirus disease 2019 (COVID-19) outbreak, yet, there are no specific available drugs against SARS-CoV-2 infection. Hence, the administration of repurposed medications could be one of the most promising strategies to fight the pathogenic disease. This chapter aimed to represent the ongoing clinical trials, and the potent therapeutics and medications used for inhibiting SARS-CoV-2 infection with respect to their targets and mechanism of action. In order to inhibit the virus, enhance or modulate the immune system, manage the deregulated inflammatory responses, and control the symptoms, several antivirals such as remdesivir, favipiravir, lopinavir/ritonavir, umifenovir, and ribavirin alongside interferons, convalescent plasma, interleukin inhibitors, anticitokines and immunosuppressant drugs, corticosteroids, antibiotics, anticoagulants, natural medicine, recombinant proteins, and monoclonal antibodies have been employed either alone or in combination to combat COVID-19.
Hossein Abolhassani, Ghazal Bashiri, and Mahdi Montazeri contributed equally to this work.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
2.1 Introduction
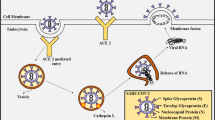
It is demonstrated that Coronavirus disease 2019 (COVID-19) pathogenesis is involved with both the direct harm inflicted by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and on the other hand, excessive inflammatory and immune response from the host [1]. Taking the biology and viral pathogenesis of SARS-CoV-2, and the potential treatment mechanisms of the virus into account thoroughly in previos chapter, many therapeutics and medications have been proposed to be efficacious against the COVID-19 pandemic [1,2,3]. On account of the fact that there are no particular treatment options available for COVID-19, the drug repurposing approach has been taken into consideration as a promising strategy for the treatment of SARS-CoV-2 infection [4, 5]. Among them, antivirals have demonstrated satisfactory inhibitory effects against COVID-19 in vitro, in vivo, and in clinical conditions as well [5, 6]. On the other hand, as the severe patients are generally associated with the acute respiratory distress syndrome (ARDS), acute lung injury (ALI), and cytokine storm, immunomodulators and anti-inflammatory drugs, as well as biological products, have been employed aiming to enhance the innate immune system and alleviate the damage caused by the deregulated inflammatory responses to manage the infection and control the symptoms leading to surviving the severe patients [7, 8]. Many therapeutic strategies and medications acting on targets of the virus or on the targets of the host have been proposed and are being developed in several clinical studies to be evaluated regarding their safety and efficacy against COVID-19 (Table 2.1) [1, 4]. The therapeutic interventions, medications, biological, and natural products alongside combination therapy approach that may have a promising role in suppressing COVID-19 is highlighted based on current evidence in this chapter.
/Para>
2.2 Antivirals/Anti-HIV and Antimalarials
Viruses are obligate, intracellular parasites containing either RNA or DNA that utilize host cells for their reproduction [107]. Viruses such as HIV, herpes simplex, varicella-zoster, respiratory syncytial, cytomegalovirus, HBV, HCV, or influenza virus are known to be associated with the development of a wide range of infections [108]. In the early 1950s, with research on anticancer drugs, advancements in developing antiviral chemotherapy, particularly in compounds preventing viral replication, are commenced [109]. Having expertise in the mechanisms of viral replication has assisted scientists in comprehending the viral life cycle, thereby finding potential antiviral agents for each step of replication [109]. The efficacy of antiviral agents heavily depends on their potency and therapeutic index. That is, besides their damaging effects on viruses, they should remain non-toxic to the host cells. In this regard, target sites special to viruses, without any human homolog, can aid in achieving a high therapeutic index [110]. Since the outbreak of a new infection, COVID-19, health professionals have been trying to find proper drugs for the treatment of infected patients [110].
However, repurposing available antiviral/anti-HIV and antimalarial drugs, with known safety, dosages, and pharmacokinetic properties, is recently gaining attention, given the limited time and high cost required for discovering new drugs [5, 110]. In this regard, many antivirals have been employed to test their efficiency and safety against COVID-19 (Table 2.1). As a result, several therapeutics such as remdesivir, favipiravir, arbidol as well as the combination of lopinavir and ritonavir are identified as potent agents against COVID-19 by WHO [5].
2.2.1 Remdesivir
By the arisen of the COVID-19 pandemic caused by SARS-CoV-2, remdesivir is being considered as one of the highly potential therapeutic agents for the treatment of COVID-19 [111]. The researches on developing remdesivir commenced by the cooperation between the United State Army Medical Research Institute of Infectious Diseases (USAMRIID) and the Gilead—the U.S. Centers for Disease Control and Prevention (CDC) to provide potential anti-viral therapeutic agents against RNA-based viruses, namely, Ebola virus and the Coronaviridae family viruses [9]. This led to the compilation of a library for nucleoside analogs, small molecules with antiviral activity against infections such as HBV, HIV, and herpes viruses [112, 113].
For nucleosides to become their active metabolites, it is requisite to undergo intracellular phosphorylation [14]. It should be noted that the development of the nucleoside monophosphate is the rate-limiting step for their intracellular activation [114]. Accordingly, nucleosides were modified to phosphoramidate, ester, and monophosphate prodrugs, enhancing both their intracellular delivery and activation [14, 112, 115,116,117]. With the outbreak of the Ebola virus in West Africa (2013–2016), the library of nucleoside molecules was appraised to find the most potential ones against the virus, resulting in the identification of remdesivir (formerly GS-5734), a monophosphoramidate prodrug of the 1′-cyano-substituted nucleoside analog (GS-441524) [11, 13, 118]. Even though remdesivir was a potential therapeutic agent for the treatment of the Ebola virus and its safety profile in the human population was established, it was outdone by monoclonal antibodies, namely, Zmapp (triple monoclonal antibody cocktail), MAb114 (single monoclonal antibody), and REGN-EB3 (a cocktail of three monoclonal antibodies), in phase 3 clinical trial. Hence, remdesivir is not being utilized in this regard anymore [10, 119, 120]. However, in addition to Ebola virus, remdesivir has demonstrated wide antiviral activities against MERS-CoV, SARS-CoVs, Marburg virus, respiratory syncytial virus, HCV, and several paramyxoviruses [14, 72, 121, 122].
For remdesivir (GS-5734) to be converted into its active metabolite, it undergoes intracellular metabolic conversion [123]. Once remdesivir (GS-5734) enters cells, it is metabolized into an alanine metabolite (GS-704277), processed into the monophosphate derivative, and then it is converted into its active form of nucleoside triphosphate (NTP) [9, 13]. Owing to the fact that the resultant NTP resembles the natural nucleotide, that is, ATP, it could be misleadingly considered by the RdRp as a nucleotide for incorporation into the nascent RNA strand, thereby bringing the replication of RNA to a halt [124,125,126,127]. It should be noted that CoVs have a proofreading ability enabling the virus to remove wrongly incorporated nucleosides [123, 124]. However, remdesivir seems to be capable of suppressing such activities due to the mechanism of its inhibitory effect and delayed RNA chain termination [128].
As it was shown in a series of recent studies on SARS-CoV-2 RdRp and MERS-CoV RdRp, the inhibition of the RNA replication cannot happen immediately after the addition of remdesivir. Rather, it occurs after three nucleotides were added into the nascent RNA [129]. Thus, remdesivir inhibits further growth of the RNA strand by the delayed RNA chain termination phenomenon; meanwhile, the three added nucleotides might account for the protection of inhibitor (remdesivir) from excision by the viral 3’–5’ exonuclease activity, which is responsible for the proofreading ability of the CoVs [12, 125].
Many clinical studies are aiming to assess the efficiency of remdesivir for SARS-CoV-2-infected patients. Remdesivir is known to be well tolerated in clinical studies and compassionate use [130,131,132]. However, its main adverse effects may include multiple organ-dysfunction syndromes, septic shock, acute kidney injury (AKI), and hypotension [133]. In a compassionate use of remdesivir for patients with COVID-19 infection, patients received a 10-day course of treatment with remdesivir (200 mg on day1 and 100 mg daily for 9 days). The results showed 68% (36 of 53 patients) clinical improvement in patients. However, increased hepatic enzymes, diarrhea, rash, renal impairment, and hypotension were the most common adverse events experienced by patients under treatment, particularly those patients receiving invasive ventilation. Serious adverse events were observed for 12 patients (23%), among which multiple organ-dysfunction syndrome, septic shock, acute kidney injury, and hypotension were most common in patients receiving invasive ventilation at the baseline [132].
In a randomized, double-blind, placebo-controlled, multicenter trial on patients with severe COVID-19 at 10 hospitals in Hubei, China, 237 patients randomly received either placebo or remdesivir (158 remdesivir and 79 placebo). The results showed that patients under treatment with remdesivir had faster clinical improvements compared with those receiving placebo; however, differences were not significant. 66% of patients receiving remdesivir and 64% of those receiving placebo experienced some adverse events [134].
Based on the preliminary results of the first stage of the Adaptive COVID-19 Treatment Trial (ACTT-1), in which patients with COVID-19 were randomly given either remdesivir or placebo as a control group, from among 1059 patients, 538 were assigned to remdesivir and 521 to placebo while the outcome of the study was the time to recovery. The results showed that the patients receiving remdesivir recovered in 11 days; however, recovery time for those receiving placebo was 15 days. Moreover, the Kaplan–Meier estimates of the death rate by 14 days in patients treated with remdesivir and placebo were 7.1% and 11.9%, respectively [131].
In a phase 3 SIMPLE trial, the effect of receiving remdesivir for either 5 or 10 days plus standard of care versus standard of care alone was assessed for patients with moderate COVID-19 pneumonia. The results showed that patients receiving remdesivir for 5 days were 65% more likely to have clinical improvement at day 11 compared with those receiving standard of care alone; however, no significant differences were observed between patients treated with remdesivir for 10 days and those receiving SOC alone.
In the SIMPLE-severe study on patients with SARS-CoV-2 receiving remdesivir for either 5 days or 10 days (200 mg on day 1 and 100 mg daily), from among 397 patients, 200 patients were under treatment for 5 days while 197 patients were under treatment for 10 days. For both groups, the percentage of patients with adverse events was similar (70% in the 5-day group and 74% in the 10-day group). Among all patients, 21% of patients treated for 5 days and 35% of patients treated for 10 days experienced serious adverse events. Also, serious adverse events of Grade 3 or higher for patients receiving remdesivir for 5 days and 10 days were 30% and 43%, respectively. The most common adverse events experienced were nausea (10% in the 5-day group vs. 9% in the 10-day group), increased alanine aminotransferase (6% vs. 8%), acute respiratory failure (6% vs. 11%), and constipation (7% in both groups) while 4% of the 5-day group and 10% of the 10-day group discontinued treatment due to adverse events. The most common serious adverse events in patients receiving remdesivir for 10 days were the acute respiratory failure (9%, vs. 5%) and respiratory failure (5%, vs. 2%). It should be mentioned that for patients with severe COVID-19 independent of mechanical ventilation, no noticeable differences were observed for patients treated with remdesivir for 5 days and 10 days [130]. According to the newest information released from mortality trials, recommended by the WHO expert groups, in hospitalized patients infected with COVID-19, remdesivir had little or no effect on inpatient overall mortality, initiation of ventilation, and duration of hospital stay [135]. Nevertheless, remdesivir, as the first treatment for COVID-19 patients requiring hospitalization, was approved by the FDA after a phase 3 clinical study sponsored by Gilead Sciences (NCT04292899).
2.2.2 Chloroquine and Hydoroxychloroquine
Chloroquine, an amine acidotropic form of quinine, was first synthesized as an antimalaria drug in 1934 and has been utilized for the treatment and prophylaxis of malaria for many years [15]. In 1946, hydroxychloroquine sulfate, hydroxyl analog of the chloroquine, was synthesized by introducing a hydroxyl group into CQ. Both CQ and HCQ have been utilized for the treatment of malaria, lupus, and rheumatoid arthritis for many years [16, 136]. They share similarities such as pharmacokinetics, mode of action, indications, and type of drug toxicity; however, they slightly differ in the clinical indications and toxic doses [15, 137]. Even though the utilization of both CQ and HCQ for the treatment of malaria is being limited owing to the arisen of chloroquine-resistant P. falciparum strains, they have shown broad-spectrum activities against bacterial, fungal, and viral infections such as autoimmune diseases [15, 138].
Inhibitory effects of CQ and HCQ against CoVs could be fulfilled in various ways [139, 140]. A complete review of their mechanisms of action can be found elsewhere [141]. Despite observed controversy regarding the exact mechanism of action, it was proved that the very mechanism of action, for CoVs entry, is mainly dependent on not only the type of the virus but also the type of the host cells [139, 142, 143]. Since the interaction of COVID-19 S protein with the receptor ACE2 on the host cells is a critical step for initiating the infection process, one of the feasible inhibitory effects of CQ on viral attachment could be through impairing terminal glycosylation of the ACE2 receptor, thereby impeding viral binding and its subsequent entry [18, 144]. On the other hand, another possible way of inhibition could happen through the interaction of CQ and HCQ with viral S proteins, thus preventing the binding of S proteins on the host cell membrane receptors according to some in silico studies [145]. Moreover, as it is known, for CoVs, the endocytic pathway is one of the chief mechanisms of viral entry into host cells [139]. In this regard, on account of the weak diprotic base nature of CQ and HCQ, their accumulation in acidic organelles such as lysosomes and endosomes increases the pH of their surrounding ambient [5]. Hence, CQ and HCQ are able to prevent the attachment and subsequent entry of the virus mainly dependent on the acidic endo-lysosomal pH, by inhibiting the acidification of the lysosome in that enzymatic protease activities responsible for the cleavage of S protein and subsequent viral entry [146]. In addition, the elevation of pH caused by CQ and HCQ could impair not only the correct maturation and recognition of viral antigens by dendritic cells but also the maturation process of viral proteins completed in the ERGIC and trans-Golgi network (TGN) vesicles both of which require acidic pH [15, 141]. Furthermore, the inhibition of the autophagic process by CQ and HCQ could be involved with the effects of COVID-19 prevention. The viral assembly process occurs in the ERGIC, related directly to autophagosome biogenesis. After the use of CQ/HCQ, the autophagic process could be inhibited by the subsequent pH elevation in lysosomes leading to the SARS-COV-2 halt. Besides, the inhibition of the autophagic process might also associate with the activity suppression of the recycled materials accompanying the autophagic process accounting for the nucleation and replication process of COVID-19 [16].
Moreover, apart from its anti-viral activity, HCQ could act as an anti-inflammatory agent capable of decreasing the production of some cytokines [17, 147]. The secretion of cytokines, such as IL-1β, IL-1RA, IL-6, IL-7, IL-8, IL-2R, TNF-α, known as the cytokine storm, is associated with the disease severity [148, 149]. The possible mechanisms of CQ and HCQ are their involvement in anti-thrombotic activities, and suppressing the release of IL-6, IL-1β, and TNF-α, which are key modulators of inflammation [141, 148].
There are several clinical studies conducted to assess the efficacy of HCQ and CQ on patients infected with COVID-19 [16]. In a study, it was observed that the patients treated with CQ experienced a faster and higher rate of viral suppression compared with those patients in the control group [150]. In another study, the effects of high dosage and low dosage of the CQ on patients infected with severe COVID-19 was assessed, and the results indicated that the higher dosage of CQ should not be used for severe COVID-19 patients, since it might cause a safety hazard, particularly when used with azithromycin and oseltamivir [151]. According to the results of a study, HCQ brought a decreased mortality in critical patients infected with COVID-19 [152]. However, contradictory results were also obtained. For instance, Mahevas et al. found that HCQ could not significantly decrease the admission to ICU, death, or ARDS in COVID-19 patients with hypoxemic pneumonia [153]. According to the findings of another study, it was also demonstrated that CQ cannot prevent the SARS-CoV-2 entry into the lung cells in vitro, in that CQ targets a pathway for viral activation that is not active in the lung cells [154]. Similarly, Mallat et al. indicated that HCQ resulted in a slower viral clearance and mild to moderate disease compared to the control group in patients infected with COVID-19 [155]. On June 15, 2020, the FDA revoked the emergency use authorization for both CQ and HCQ [16] and according to the newest information released from mortality trials, recommended by the WHO expert groups, in hospitalized patients infected with COVID-19, HCQ had little or no effect on inpatient overall mortality, initiation of ventilation, and duration of the hospital stay [135]. Nonetheless, the efficiency of CQ/HCQ as an antiviral treatment for COVID-19 is still assessing in phase 4 clinical studies in the USA (NCT04331600, NCT04382625). HCQ was proved to have 40% less toxicity in animals [156]. However, the most common side effects of both CQ and HCQ at therapeutic doses include myopathy, electrocardiographic changes, bleaching of hair, retinopathy, pruritus, headaches, and gastrointestinal symptoms [5].
2.2.3 Favipiravir (Avigan)
Favipiravir (6-fluoro-3-hydroxy-2-pyrazinecarboxamide, T-705, Avigan), was first discovered by Toyama Chemical Co., Ltd for antiviral activity against the influenza virus and has been approved for the treatment of Influenza in Japan since 2014 because of its proven safety and effectiveness on humans in clinical trials [5, 26, 27]. Concerning COVID-19, favipiravir was approved to be utilized on 15 February 2020, in China against SARS-CoV-2 [157]. For favipiravir to be converted to its active form, that is, favipiravir-RTP (T-705 RTP) undergoes intra-cellularly phosphoribosylation, consequently exerting its antiviral activity as a pro-drug [27]. Also, it was shown that favipiravir-RTP could be efficiently recognized as a guanosine and an adenosine analog by influenza A virus polymerase [158]. Favipiravir triphosphate, a purine nucleoside analog, is believed to directly inhibit the RdRp activity of influenza A virus polymerase [25, 158]. However, the exact mode of action and accurate molecular interaction between the nucleotide and the viral polymerase has yet to be explicated [158]. In a study conducted on the influenza A (H1N1) virus, it was demonstrated that a high rate of mutation is induced with favipiravir generating a non-viable viral phenotype, a lethal mutagenesis which is a key antiviral mechanism of T-705 [159].
Favipiravir has antiviral activity against a great variety of influenza viruses such as A (H1N1) pdm09, A (H5N1), and recently emerged A (H7N9) avian virus. Moreover, favipiravir is capable of inhibiting the influenza strains resisting current antiviral drugs and showing a synergic effect in combination with oseltamivir, thus expanding influenza treatment options [160]. It was shown that its antiviral activity performs in a dose-dependent manner while it has a short half-life of 2–5.5 h [161, 162]. In addition, the metabolism of favipiravir occurs in the liver mainly by aldehyde oxidase (AO), and partially by xanthine oxidase, thereby producing an inactive oxidative metabolite, T-705M1 that is excreted by the kidneys [162]. In a small clinical study conducted on 168 critically ill patients infected with influenza, patients received either a combination of favipiravir and oseltamivir or oseltamivir alone. The results showed that the combination therapy of favipiravir and oseltamivir results in accelerating clinical recovery [163].
Favipiravir, chloroquine, arbidol, and remdesivir are under clinical studies in china to assess their efficacy and safety against SARS-CoV-2 [157]. According to preliminary clinical results obtained from an open-label comparative controlled study of patients infected with COVID-19, patients receiving favipiravir compared with those receiving lopinavir/ritonavir experienced not only faster viral clearance but also better chest computed tomography changes [164]. Furthermore, in an in vitro study conducted on Vero E6 cells, favipiravir inhibited SARS-CoV-2 replication with EC50 values of 61.88 μM (9.4 μg/mL). Nevertheless, another study reported EC50 values > 100 μM (15. 7 μg/mL) for favipiravir [24, 165]. The need for metabolic activation in the host cells for favipiravir could explain the differences between these two studies [24]. In a randomized, controlled, open-label multicenter trial performed on 240 patients infected with COVID-19, patients randomly received arbidol or favipiravir in a 1:1 ratio. According to the results, favipiravir could not considerably improve the clinical recovery rate on day 7 in comparison to arbidol. However, favipiravir appreciably improved the latency to relief for pyrexia and cough and showed mild and manageable adverse effects, including raised serum uric acid, psychiatric symptom reactions, digestive tract reaction, and abnormal LFT [166]. In a double-blinded, placebo-controlled, randomized, phase 3 trial, favipiravir is being administered as a potential therapy for mild to moderate COVID-19 outpatients (NCT04600895).
2.2.4 Lopinavir/Ritonavir (Kaletra)
Lopinavir/ritonavir combination, available under the brand name Kaletra, and developed by Abbott Laboratories, USA, is known as an anti-retroviral drug and was approved by FDA for the treatment of patients infected with HIV in 2000 [5]. Ritonavir, a potent inhibitor of cytochrome P450 3A4, inhibits the metabolism of lopinavir and increases its bioavailability, it was shown that the co-administration of these drugs in healthy volunteers increases the area under the lopinavir plasma concentration–time curve > 100-fold [19, 167]. PLpro, a crucial factor in the protease activity and proper replication of the SARS-CoVs genome has been a target of interest in the treatment of COVID-19 patients [168]. It was demonstrated that lopinavir is a non-covalent, competitive, and potential inhibitor for inhibiting the PLpro of CoVs and subsequently blocking the virus replication [169]. The administration of lopinavir/ritonavir during the early peak viral replication phase (initial 7–10 days) has been reported to be crucial for the efficiency of drugs [170].
In a study, it was demonstrated that after the administration of lopinavir/ritonavir, the viral load and clinical symptoms dramatically decreased [171]. In another study conducted on 36 pediatric patients (aged 0–16 years) infected with COVID-19, all patients received IFN-α by aerosolization twice a day, 14 (39%) patients received lopinavir/ritonavir syrup (twice a day), and 6 patients needed oxygen inhalation. The results indicated that all patients were cured and the hospital stay meantime was 14 days [172]. On the contrary, in a randomized controlled, open-label clinical trial conducted on 199 patients infected with severe COVID-19, no specific difference was observed in patients treated with lopinavir/ritonavir compared to those who received standard care, and gastrointestinal disturbances were more prevalent adverse events between patients treated with lopinavir/ritonavir than patients in the control group [173].
In an open-label, randomized, phase 2 trial in adults infected with COVID-19, patients were assigned to either a 14-day triple combination of IFN-β-1b, lopinavir/ritonavir and ribavirin or a control group (lopinavir/ritonavir); results showed that triple combination therapy was superior to control group regarding decreasing the time of hospital stay and alleviating symptoms in patients with mild to moderate COVID-19 [174]. In another study, four patients infected with COVID-19 underwent treatment with lopinavir/ritonavir (lopinavir 400 mg/ritonavir 100 mg, q12 h through oral route), arbidol (0.2 g, three times in a day through oral route), and Chinese traditional medicine Shufeng Jiedu capsule (SFJDC) (2.08 g, three times in a day through oral route) while the duration of treatment was 6–15 days. According to the obtained results, from among four patients, three patients showed considerable improvement in pneumonia-associated symptoms, and for the other patients suffering from severe pneumonia, signs of improvement were observed [175]. The most common adverse effects of lopinavir/ritonavir have been reported to be diarrhea, nausea, and, vomiting (gastrointestinal adverse effects from mild to moderate). However, less common adverse effects observed in patients treated with lopinavir/ritonavir consist of an allergic reaction, asthenia, malaise, headache, myalgias, arthralgias, myocardial infarction, seizures, and lactic acidosis [20, 167, 176]. Lopinavir/ritonavir is still in phase 4 of a clinical study in China to be evaluated for COVID-19 patients (NCT04252885).
2.2.5 Umifenovir (Arbidol)
Arbidol, or umifenovir, an indole-derivative with broad-spectrum activity against both enveloped and non-enveloped viruses, was initially approved in China and Russia for the treatment of influenza A and B [177, 178]. Arbidol is believed to block the entry of influenza virus (A and B) into the host cells by increasing the stability of the hemagglutinin (HA) and hampering low pH reorganizations necessary for fusion machinery of hemagglutinin with the membrane [5, 21, 23]. Arbidol could interfere with advanced stages of the viral life cycle, in that it is capable of interacting with both viral proteins and lipids [4, 179]. Regarding its structure, the presence of amine in position 4 and the hydroxyl moiety in position 5 is crucial for its antiviral activity [39]. It is reported that 40% of the drug could be excreted unchanged after the administration while its half-life is between 17 and 21 h [22].
In a study conducted on 69 patients infected with SARS-CoV-2 in Wuhan, arbidol therapy led to not only a decrease in the mortality rate but also an improvement in the discharge rate [180]. In another study, the therapeutic efficacy of co-administration of arbidol and lopinavir/ritonavir compared to only lopinavir/ritonavir on COVID-19 patients was evaluated, and the results showed that the combination of arbidol and lopinavir/ritonavir culminates in slowing down the development of lung lesions, decreasing the feasibility of respiratory and gastrointestinal transmission toward decreasing the viral load of COVID-19 [181]. In a clinical trial, 27 COVID-19 patients were recruited, among them, 10 of the patients received chloroquine phosphate, 11 received arbidol, and 6 received lopinavir/ritonavir; the results indicated that both CQ and arbidol decreased the hospitalization time as well as hospitalization expenses and shortened the viral shedding interval [182].
Furthermore, in a study, 200 inpatients infected with common-type COVID-19 received either arbidol hydrochloride capsules (control groups) or a combination of arbidol hydrochloride capsules and Shufeng Jiedu Capsule (SFJDC) (experiment group) for 14 days. The results demonstrated that combining traditional Chinese and western allopathic medicine not only improves recovery time but also has better clinical efficiency and safety [183]. On the contrary, in a clinical trial performed on 141 patients infected with COVID-19, 70 patients received IFN-α-2b, while 71 of them received a combination of arbidol and IFN-α-2b. The outcomes demonstrated that patients receiving co-administration of arbidol and IFN-α-2b experienced neither a decrease in their hospitalization time nor an acceleration in COVID-19 RNA clearance [184]. Likewise, an inefficiency is reported for umifenovir in non-ICU patients [185]. Despite the inconsistent results, arbidol is currently in phase 4 of a clinical trial, which has been conducted on 380 patients with pneumonia caused by SARS-CoV-2 in Ruijin Hospital, Shanghai, China (NCT04260594) [186].
2.2.6 Darunavir
Darunavir, a non-peptidic protease inhibitor (PI) approved by the FDA, is particularly used for the treatment of HIV-1 infection and is majorly utilized in combination with a low boosting dose of ritonavir [38]. Darunavir is more potent compared with other protease inhibitors due to its distinct chemical structure increasing binding affinity and reducing dissociation rate [5]. It has been proved that it is able to prevent viral maturation by inhibiting the cleavage of HIV gag and gag-pol polyproteins alongside inhibiting proteolytic activity and subsequent HIV-1 replication by suppressing dimerization of HIV-1 protease [33]. Therefore, darunavir is recognized as a protease inhibitor while cobicistat could be a supplement for enhancing both pharmacodynamics and pharmacokinetics of darunavir through inhibiting cytochrome P450 (CYP3A) [4, 187]. Darunavir is thoroughly metabolized by hepatic cytochrome P450 (CYP) 3A4 enzymes and is rapidly absorbed after oral intake; moreover, its terminal elimination half-life is 15 h [34]. In a study, it was demonstrated that administration of darunavir is accompanied by an increase in the risk of myocardial infarction in patients infected with HIV. Hence, employing darunavir as a potential therapeutic may be associated with enhancing the risk of cardiovascular diseases [188].
In an open-label trial conducted on 30 patients infected with COVID-19, patients randomly received either darunavir/cobicistat for 5 days on top of IFN-α-2b inhaling or IFN-α-2b inhaling alone. The results showed that darunavir/cobicistat therapy did not change the viral clearance rate at day 7 in comparison to the control group; furthermore, for patients receiving darunavir/cobicistat the median duration of viral shedding from randomization was 8 days, while 7 days in the control group. However, no statistical significance was observed, and the recurrence of adverse events in both groups was similar. On the other hand, one of the patients receiving darunavir/cobicistat developed anemia (a decrease in the level of hemoglobin from 11.3 to 9.9 g/dL). Other observed adverse events were elevated transaminase levels and renal dysfunction. It should be noted that all of the adverse events were mild [189]. In a phase 3 clinical study, the efficacy and safety of darunavir and cobicistat are evaluating on COVID-19 patients in China (NCT04252274).
2.2.7 Ribavirin
Ribavirin, a guanosine analog, is an antiviral drug used for the treatment of patients infected with HCV and respiratory syncytial virus [5]. Its mechanisms of action could be divided into indirect and direct mechanisms. The direct mechanisms consist of interfering with RNA capping, polymerase inhibition, as well as lethal mutagenesis, and indirect mechanisms are comprised of inosine monophosphate dehydrogenase inhibition and immunomodulatory effects [190]. Ribavirin has established a good reputation for being utilized in emergency clinical plans against CoVs infection due to its availability and low cost. The most convincing outcomes generally have been obtained with early administration upon presentation with pneumonia and before sepsis or organ system failure [30]. Its half-life time is estimated to be 3.7 h, with an oral bioavailability of 52%, which could be because of the first-pass metabolism in the liver [31, 191]. Even though ribavirin is known as a potential therapeutic for the treatment of HCV, it is highly toxic. Hence, it is recommended to be used in combination therapy with IFNs or lopinavir/ritonavir in the Diagnosis and Treatment Guidelines of COVID-19 in China [39].
A combination of ribavirin and IFN-α-2b was utilized for the treatment of MERS-CoV infected rhesus macaques and demonstrated a decrease in viral replication, moderating the host response, and improving clinical results [192]. In addition, according to an open-label, randomized, phase 2 trial conducted on patients infected with COVID-19, triple combination therapy of patients with interferon-β-1b, lopinavir/ritonavir, as well as ribavirin was much safer and superior to the administration of only lopinavir/ritonavir regarding the decreasing symptoms, reducing the time of hospital stay, and viral shedding in patients infected with mild to moderate COVID-19 [174]. In order to compare the efficacy and safety of three antivirals, namely, ribavirin, lopinavir/ritonavir, and IFN-α-1b for the treatment of patients infected with COVID-19, three different therapeutic regimes were applied in a clinical trial, that is, ribavirin plus IFN-α1b or lopinavir/ritonavir plus IFN-α1b and or ribavirin plus lopinavir/ritonavir plus IFN-α1b. According to the obtained results, the combination of ribavirin plus lopinavir/ritonavir caused a considerable increase in gastrointestinal adverse effects [193]. A combination of ribavirin, nitazoxanide, and ivermectin for a duration of 7 days is assessed for COVID-19 treatment at Mansoura University in Egypt (NCT04392427).
2.2.8 Oseltamivir (Tamiflu)
Oseltamivir (Tamiflu), a neuraminidase inhibitor (NAIs) licensed for the treatment of both influenzas A and B, was synthesized through employing two natural products from plants, namely, quinic acid, and shikimic acid [29, 194]. Oseltamivir prodrug is known as oseltamivir phosphate [28]. In the liver, oseltamivir is metabolized and converted to its active metabolite, that is, oseltamivir carboxylate [28]. It is able to prevent the release of viral particles from the host cells by binding to influenza viral neuraminidase, thereby decreasing the spread of the virus in the respiratory tract [4, 28]. Nevertheless, according to the result of a study performed on patients infected with COVID-19 in china, no positive results were obtained for patients receiving tamiflu [195]. However, the administration of oseltamivir and its combination with other drugs such as CQ, arbidol, lopinavir/ritonavir, and favipiravir are under clinical studies to evaluate their potential in the treatment of SARS-CoV-2 infection [85, 196]. In an open, prospective/retrospective, randomized controlled cohort study, the efficiency of three antiviral drugs including oseltamivir, arbidol hydrochloride, and lopinavir/ritonavir is compared for COVID-19 treatment in China (NCT04255017).
2.2.9 Ivermectin
Ivermectin, approved as both an anti-parasitic and anthelmintic agent, is a macrolide endectocide macrocyclic lactone that was originally derived from an actinomycete (streptomyces avermitilis) [5, 38]. Its antiviral activity was initially found by its capability in inhibiting the interaction between the nuclear transport receptor importin α/β (IMP) and integrase molecule of HIV [4, 48]. In fact, its antiviral mechanism of action involves the dissociation of the preformed IMPα/β1 heterodimer, responsible for the transport of viral proteins to the nuclear [77, 197].
According to the result of a study conducted in Australia, ivermectin demonstrated antiviral activity against SARS-CoV-2 in clinical isolate in vitro Vero-hSLAM cells with the addition of a single dose 2 h post-infection, and it was able to reduce viral RNA around 5,000 times. Moreover, the hypothesized mechanism of action for ivermectin was observed to be likely through inhibiting IMPα/β1-mediated nuclear import of viral proteins as anticipated [198]. This drug is currently under a phase 3 clinical trial against COVID-19 in the USA, Pennsylvania, Temple University (NCT04530474).
2.2.10 Tenofovir
Tenofovir, a nucleotide analog (NA) of adenosine 5’-monophosphate, is a reverse transcriptase inhibitor with two different formulations, namely, tenofovir disoproxil fumarate (TDF) and tenofovir alafenamide (TAF) [35]. They are commercially available prodrugs of tenofovir capable of improving their oral bioavailability and membrane permeability [35, 36]. Tenofovir alafenamide is able to selectively activate presenting preferential distribution in lymphatic tissues, and it is formulated to reduce adverse events associated with the administration of tenofovir disoproxil fumarate [199]. Both of them are vital components for the treatment of HIV and HBV [35]. Tenofovir is one of the potential nucleotide analogs under investigation for the treatment of SARS-CoV-2 [200]. A combinational administration of tenofovir/emtricitabine in addition to the use of personal protective equipment (PPE) is currently under phase 2/3 of a clinical trial for COVID-19 patients by Hospital Universitario San Ignacio, Colombia (NCT04519125).
2.2.11 Camostat Mesylate
Camostat mesylate, a serine protease inhibitor first used for the treatment of dystrophic epidermolysis, chronic pancreatitis, and oral squamous cell carcinoma, was initially manufactured by the Nichi-Iko Pharmaceutical Co., Ltd. in contribution with Ono Pharmaceutical, Japan [38, 201]. It should be noted that the S protein of human CoVs is primed by TMPRSS2, which is a serine protease [37]. In this regard, camostat mesylate may be able to inhibit the SARS-CoV-2 entry into the host cell, since it is a serine protease inhibitor blocking TMPRSS2 activity [39, 202]. In a study conducted on a pathogenic animal model of SARS-CoV-1 infection, it was observed that camostat has the potential to prevent viral spread and pathogenesis of SARS-CoV-1 [203]. Camostat mesylate is currently under phase 3 of a clinical trial for the treatment of COVID-19 patients in French (NCT04608266).
2.2.12 Nafamostat Mesylate
Nafamostat, a synthetic serine protease inhibitor that is known as an anticoagulant in nature, was first brought to the Japanese market in 1986 for the treatment of acute symptoms of pancreatitis and for applying to certain bleeding complications. This drug is capable of inhibiting different enzymatic systems such as complement, kallikrein-kinin, fibrinolytic systems, and coagulation. It has been also utilized for the prevention of liver transplantation and post-transplant syndrome [5, 38, 204]. Nafamostat is able to prevent viral entry through the host cell surface membrane. Hence, it is considered as one of the potential repurposing drugs against COVID-19 [205]. Its mechanism of action is anticipated to be through inhibiting the human protein TMPRSS2, the S protein-dependent enzyme that cleaves and thereby activates the S protein for binding to ACE2 [206, 207]. In an in vitro study, nafamostat prevented the entry of MERS-CoV, and it was demonstrated as the most potential protease inhibitor among all 1000 drugs screened [40]. This drug is currently under phase 3 of the clinical trial on patients infected with COVID-19 by the University of Edinburgh, the UK (NCT04473053).
2.2.13 Molnupiravir
Molnupiravir, or MK-4482/EIDD-2801, pro-drug of the nucleoside analog N4-hydroxycytidine (NHC), is an RNA polymerase inhibitor that is orally available and was originally developed for the treatment of influenza [43,44,45]. It has shown appreciable anti-influenza activity in ferrets, mice, guinea pigs, and human airway epithelium organoids [44, 208, 209]. As a result of the collaboration between Ridgeback Biotherapeutics and Merck, molnupiravir is developing for the treatment of COVID-19 patients [43]. In a study, the effect of molnupiravir in a Syrian hamster SARS-CoV-2 infection model was investigated, and the results showed that molnupiravir considerably decreased not only infectious virus titers but also viral RNA loads in the lungs, thereby improving lung histopathology [210]. Moreover, in another in vivo study conducted on animals infected with SARS-CoV-2, molnupiravir was proved to be a potential oral drug capable of considerably decreasing the viral load in the upper respiratory tract and preventing the spread to untreated contact animals [45]. Molnupiravir administration is currently in phase 2/3 of a multicenter clinical trial by Merck Sharp & Dohme Corp (NCT04575584).
2.2.14 Sofosbuvir
Sofosbuvir, a direct-acting antiviral drug that was initially approved as an anti-HCV, could be utilized as a repurposed antiviral drug for the treatment of COVID-19 [46, 47]. Among the studies, it was predicted that sofosbuvir might be capable of binding to the SARS-CoV-2 RdRp enzyme, thereby inhibiting its activity [64, 211, 212]. In a single-center, randomized controlled trial in patients infected with moderate COVID-19, patients received either a combination therapy of sofosbuvir/daclatasvir/ribavirin or standard care. The results demonstrated that the combinational administration of these three drugs engendered recovery and lower mortality rates for patients. Nevertheless, an imbalance was observed in the baseline characteristics between the arms. Thus, larger randomized trials are needed to prove these results [213]. Additionally, according to a molecular docking study, ribavirin, remdesivir, sofosbuvir, galidesivir, and tenofovir are potent drugs against COVID-19 that tightly bind to the RdRp of the SARS-CoV-2 strain, thereby preventing its function [214]. Sofosbuvir is currently under phase 2/3 of a clinical trial in Egypt by Tanta University (NCT04497649).
2.2.15 Famotidine
Famotidine, a histamine-2 receptor antagonist (H2RA), reduces the production of gastric acid [50, 51]. An in vitro study of this drug demonstrated that H2RA has anti-viral activity against HIV replication [215]. Regarding the treatment of COVID-19, according to the results obtained from in silico molecular docking, Famotidine could inhibit PLpro enzyme activity in the viral replication cycle [216]. Hence, this drug is capable of inhibiting vital enzymes in the life cycle of SARS-CoV-2 and consequently mediating the maturation of non-structural proteins [51]. In a multi-site, randomized, double-blind phase 3 clinical study, the efficiency of famotidine is evaluating for COVID-19 patients in the USA (NCT04370262).
2.2.16 Nitazoxanide
Nitazoxanide, a synthetic nitrothiazolyl-salicylamide derivative, is a broad-spectrum antiviral agent used for the treatment of a wide range of viruses, including influenza A, B, and Ebola viruses [3, 6, 54]. Nitazoxanide demonstrated in vitro antiviral activity against MERS-CoV and other CoVs; also, this drug suppresses the production of pro-inflammatory cytokines in peripheral blood mononuclear cells and IL-6 in mice [217]. Moreover, the antiviral activity of this drug could be attributed not to the virus-specific pathways, but rather to its interference with host-regulated pathways involved in viral replication [5, 217, 218]. Nitazoxanide is currently in phase 4 of clinical trials for COVID-19 treatment in Mexico by Laboratorios Liomont (NCT04406246).
2.2.17 Nelfinavir
Nelfinavir, a non-peptidic, competitive HIV protease inhibitor, is considered as one of the potential drugs against COVID-19 [55]. This drug was approved by the FDA for the treatment of HIV infection in 1997 [219]. According to the results of a study in which HIV protease inhibitors were screened to find potential drugs, CoVs, it was indicated that nelfinavir is capable of inhibiting the replication cycle of SARS-CoV-1 [56]. Similarly, in another in vitro study, it was shown that nelfinavir could inhibit 3CLpro of the virus and consequently suppressing the replication cycle of SARS-CoV-2 with EC50 and EC90 of 1.13 µM and 1.76 µM, respectively [57, 220]. According to an in silico study, based on the combinational administration of nelfinavir and cepharanthine, nelfinavir could bind the SARS-COV-2 main protease, thereby inhibiting the viral replication cycle, while cepharanthine is able to prevent viral attachment and entry into cells. Hence, their combination could be a potent multidrug for the treatment of COVID-19 [221].
2.2.18 Auranofin
Auranofin is a gold-containing triethyl phosphine that has been explored for therapeutic applications against a wide range of diseases, including cancer, neurodegenerative disorders, HIV, as well as parasitic and bacterial infections [58, 59]. According to the results of one in vitro study, auranofin inhibits the replication of SARS-COV-2 in human cells at a low micromolar concentration by reducing the viral RNA up to 95% at 48 h after infection [58]. Its mechanism of action consists of inhibiting the redox enzymes as well as induction of ER stress, thereby interfering with the protein synthesis of SARS-CoV-2 [58,59,60,61, 222].
2.2.19 Carmofur
Carmofur, an approved antineoplastic drug that was derived from 5-fluorouracil (5-FU) and was explored for the treatment of breast, gastric, bladder, and colorectal cancers, is shown to have inhibitory effects against SARS-CoV-2 [222,223,224]. It was demonstrated that carmofur inhibits the main protease (3CLpro) activity of the SARS-CoV-2 with an IC50 value of 1.82 μM and prevents viral replication in cells with an EC50 value of 24.30 μM [63, 64].
2.2.20 Galidesivir
Galidesivir (BCX4430, Immucillin-A), an adenosine analog as well as RdRp inhibitor that was first developed for the treatment of HCV, has shown antiviral activity against a wide variety of viruses, including togaviruses, filoviruses, arenaviruses, paramyxoviruses, orthomyxovirus bunyaviruses, CoVs, picornavirus, and flaviviruses [38, 66, 67]. This drug is currently under phase 1 of clinical trials for COVID-19 treatment in Brazil (NCT03891420).
2.2.21 Azvudine
Azvudine or 2′-deoxy-2′-β-fluoro-4′-azidocytidine (FNC) was first developed for the treatment of HIV and has antiviral activity against HBV and HCV [68, 225]. Azvudine might be able to inhibit the reverse enzyme transcriptase vital in viral transcription, thereby interfering with the replication of the CoVs [38]. This drug is currently under phase 3 of a randomized clinical trial for patients infected with COVID-19 in Brazil (NCT04668235).
2.3 Immunomodulators and Anti-inflammatory Drugs
The immune (innate and adaptive) system includes cells, molecules, and processes working together to provide the body protection against aggressive viruses, bacteria, toxins, parasites, fungi, and cancer cells [7]. However, the immune system may be weakened in individuals owing to high age or immunodeficiency disorders [1]. On the other hand, the inflammatory phase, the third phase after the mild infection and pulmonary phases, is initiated and accompanied by cytokine storm due to the excessive immune response of the host upon infection and may create complications like ARDS leading to death in many cases [148]. Considering the biology of SARS-CoV-2 and by exploring the molecular mechanisms employed by the virus regarding its interactions with host cells, the development of host immune response could be illuminated, which may lead to proposing efficient drugs for inhibiting COVID-19 [7]. The medications that have the potential to interact with the host immune system generally can fall into two main categories, the remedies with the aim of boosting the immune system and the therapeutics that intervene in the host immune response and play their role in immunomodulation or alleviating damages caused by the dysregulated inflammatory responses [1]. In this respect, many immunomodulatory therapies and anti-inflammatory drugs such as NKs, IFNs, MSCs, convalescent plasma, interleukin inhibitors, anticitokines, anticoagulants, corticosteroids, and monoclonal antibodies have been administrated to control the symptoms, modulate the immune system leading to COVID-19 treatment (Table 2.1) [1, 8, 85].
2.3.1 Natural Killer Cells
The higher mortality rate of elderly patients compared to other generation individuals infected with COVID-19 could be explained by the weakening of the immune system with age and considered somehow as aging-associated diseases in chronic disease states [77, 101, 226]. NK cells as practical components of the innate immune system are against viral infections. NK cells are able to rapidly release granzymes and perforins inducing cell lysis. In addition, they are crucial sources of interferon-gamma (IFN-γ) capable of mobilizing antigen-presenting cells (APCs) and activating antiviral immunity [7]. Hence, the injection of NK cells into elder and fragile patients’ bodies could be efficacious in SARS-CoV-2 clearance with no severe side effects [1, 77]. Chen et al. indicated that macrophages and NK cells have a crucial function in the clearance of SARS-CoV-1 [227]. The safety and efficacy of CYNK-001, an immunotherapy containing NK cells derived from human placental CD34 + cells on moderate COVID-19 patients, are currently assessing in a phase 2/3 clinical study at multicenters in the USA (NCT04365101).
2.3.2 Mesenchymal Stem Cells
In severe patients, SARS-CoV-2 infection may generate a threatening cytokine storm in the lungs. MSCs, as a safe and well-tolerated therapeutic option, can be used to benefit through their immunomodulatory, antimicrobial, antiapoptotic, and regenerative effects [70]. The anti-inflammatory activity of MSCs has been associated with decreasing pro-inflammatory cytokines and producing paracrine factors leading to regenerative medicine in pulmonary epithelial cells [1]. MSCs are able to regulate the function of both innate and adaptive immune systems through either passive or active cell–cell interaction, secretion of trophic factors, or activation of regulatory T cells [2, 70]. Clinically, the employing of intravenous transplantation of MSCs for severe SARS-CoV-2 patients has been reported to be safe and effective [71, 228, 229]. It has been reported that MSC therapy for COVID-19 patients exerts no adverse effects on the patients [230, 231]. The efficacy and safety of the MSC remestemcel-L administration are evaluating on COVID-19 patients with ARDS in phase 3 multicenter clinical study in the USA (NCT04371393).
2.3.3 Interferons
IFNs are soluble endogenous signaling proteins with high antiviral activity secreted by cells including cells with hematopoietic origin upon viral or bacterial infection. The interferon-stimulating genes (ISG) generally associated with immunomodulation, signaling, and inflammation are activated by the INF fixation on interferon α/β receptor (IFNAR), the receptors found at most cells plasma membrane [4, 232]. IFNs have been utilized as a therapeutic option against autoimmune disorders, different cancers, and viral infections including hepatitis B and C [1, 232]. IFNs play significant roles in enhancing the immune system by restricting the spread of infectious viral, adjusting innate immunity responses, and activation of adaptive immune responses [7, 233, 234]. Hence, employing recombinant human interferons (rhIFNs) has been considered as a potential treatment method against COVID-19 while SARS-CoV-2 has shown sensitivity to some human type I IFNs like IFN-α and IFN-β [94, 235, 236]. However, the adverse reactions of fever, myalgias, headaches, leukopenia, lymphopenia, and autoimmune hepatitis may be associated with the administration of IFNs [72].
2.3.3.1 Interferon-α
IFN-α is a cytokine secreted by the immune cells in the body capable of eliciting a practical host-mediated immune cell response for various cancer treatments and inhibition of replication of viruses like SARS-CoVs, HIV, and HCV while SARS-CoV-2 has shown significantly high sensitivity to IFN-α [8, 72, 237]. IFN-α has been exploited as a favorable antiviral activity due to low toxicity and its crucial roles in the inhibition of the virus replication at the early stage of infection, moderating the symptoms of the acute phase of the disease, shortening the disease duration leading to the survival of severe patient [8]. To enhance the stability of IFN-α and prolong its half-life from 4.6 to 22–60 h, Pegylated IFN-α-2b has been utilized providing a situation in which the lower dosing could be injected frequently [72]. In a retrospective multicenter cohort clinical trial, 242 of 446 COVID-19 patients received IFN-α-2b as a treatment in Hubei, China. It was observed that early administration (≤5 days after admission) of IFN-α-2b was responsible for the reduced in-hospital mortality while peculiarly, late administration of IFN-α-2b was involved with increased mortality [238]. In a phase 3 clinical study, the efficiency of rhIFN-α-1b in preventing COVID-19 is currently assessing on a large number of patients at Taihe Hospital Shiyan, Hubei, China (NCT04320238).
2.3.3.2 Interferon-β
IFN-β is a signaling cytokine that has a broad range of applications against viral infections like HCV and HBV. It is able to activate cytoplasmic enzymes stimulating them to prevent viral replication [38, 74]. IFN-β with the ability of maintaining endothelial barrier activity, pro-inflammatory response, and defensive function in the lungs has demonstrated the highest potency among the IFNs in prophylactic protection and antiviral potential post-infection effects [74, 234, 239]. IFN-β-1 has demonstrated efficacy against SARS-CoV-1 [240]. Consequently, on account of the high similarity of SARS-CoV-1 with SARS-CoV-2, IFN-β-1 has been introduced as a potential therapeutic in COVID-19 treatment [241]. Despite inhibiting the production of IFN-β and obstructing the innate immune system response by SARS-CoV-2, the virus has shown sensitivity to the antiviral activity of externally administrated type I IFNs [236].
In a phase 2 clinical study (NCT04385095), safety and efficacy of inhaled nebulized IFN-β-1-a (SNG001) were assessed for the treatment of patients admitted to hospital with COVID-19, and it was demonstrated that in comparison with patients who received placebo, treated patients with SNG001 had a greater chance of treatment with more rapid recovery [73]. Likewise, IFN-β-1-b has indicated potency in inhibiting SARS-CoV-1, MERS-CoV, and SARS-CoV-2 infections [241]. Also, a combination therapy of IFN-β-1-b, lopinavir/ritonavir, and ribavirin was evaluated on COVID-19 patients admitted to hospital in a phase 2 clinical study (NCT04276688) and demonstrated a high potential in alleviating symptoms and reducing the disease duration and hospital stay in patients with mild to moderate SARS-CoV-2 infection [174].
2.3.4 Convalescent Plasma or Intravenous Immunoglobulin
The pooled plasma or hyperimmune immunoglobulins derived from recovered patients of a disease, termed convalescent plasma (CP) has been widely employed to treat many infectious diseases such as MERS-CoV, SARS-CoV-1, Ebola, and SARS-CoV-2 with favorable outcomes through passive immunity delivery and replacement therapy for antibody deficiencies [38, 242, 243]. On account of the fact that CP or intravenous immunoglobulin (IVIG) possesses neutralizing antibodies to SARS-CoV-2, which could be exploited as a potential therapy to directly neutralize the virus, modulate the inflammatory response, and control the overactive immune system (i.e., cytokine storm) [75, 76, 243]. In the hope of minimizing morbidity and mortality, all these benefits of CP are expected to be attained if used in early administration and non-critically hospitalized patients [8, 76].
Improvement in the clinical status of small sample sizes of critically ill patients was reported in some studies by the administration of CP with low serious adverse reactions [243,244,245,246,247]. Despite the risks involved with CP and IVIG administration for COVID-19 patients, including transfusion-associated lung injury and circulatory overload, allergic/anaphylactic reactions and less common risks like transmission of infections and red blood cell alloimmunization [41], very low adverse events have been reported in a safety study of CP for 20,000 hospitalized patients implying that CP is safe in hospitalized patients with COVID-19 [246]. In a phase 3 clinical trial, CP has been employed for treating hospitalized COVID-19 patients in New York, the USA (NCT04418518).
2.3.5 Anticitokines, Immunosuppressants, and JAK Inhibitors
Since COVID-19 is associated with a significant increase in the level of serum cytokines, that is, the cytokine storm, repurposing of available anticytokines with proven safety has come to the fore [248, 249]. In this regard, considering the role of the Janus kinase signal transducer and activator of transcription (JAK-STAT) pathway in Angiotensin II type 1 receptor, the receptor that is expressed on peripheral tissues and immune cells as cytokine receptors with the role of the renin-angiotensin system (RAS) signal transduction, targeting of this pathway in hospitalized patients by employing JAK and Adaptor-associated kinase (AAK) inhibitors like sirolimus, baricitinib, ruxolitinib, imatinib, cyclosporine, and tofacitinib capable of inhibiting type I/II cytokine receptors could not only reduce the clinical symptoms in organs like lung, kidney, and heart but also inhibit the cytokine storm in ARDS condition associated with severe SARS-CoV-2 infection [3, 250, 251].
2.3.5.1 Sirolimus
Sirolimus, known as rapamycin (trademark name: Rapamune), is a natural product isolated from the bacterium Streptomyces hygroscopicus in Easter Island. Sirolimus was initially isolated as an antifungal agent with potential anticandida activity [92, 252]. Nevertheless, its antitumor/antiproliferative and immunosuppressive properties were proved by further studies [252]. Also, sirolimus is capable of weakening the immune system, surprisingly strengthening T cells activity in the course of pathogenic invasions, delaying age-related illnesses in humans, and having an inhibitory effect on the mammalian target of rapamycin complex 1 (mTORC1) receptor [91].
On account of the fact that mTORC1 has a pivotal role in the viral replication of different viruses, including orthohantavirus and CoVs, sirolimus could be a potential therapeutic agent for repurposing against COVID-19 [85, 91, 253]. According to the results obtained from an in vitro study, sirolimus affected PI3K/AKT/mTOR pathway, thereby inhibiting MERS-CoV activity [254]. Moreover, an in silico study showed that sirolimus could be a potential drug for the treatment of patients infected with COVID-19 using a network-based drug repurposing model [253]. This drug is currently in phase 2 of a clinical trial for patients infected with COVID-19 performing in the USA by the University of Cincinnati (NCT04341675).
2.3.5.2 Baricitinib
Baricitinib under the brand name olumiant, an inhibitor of cytokine-release approved in 2018 for the treatment of rheumatoid arthritis, is a potential JAK inhibitor that selectively inhibits the JAK1 and 2, consequently reducing inflammation in patients infected with COVID-19 [3, 255,256,257]. Moreover, baricitinib is capable of inhibiting AAK1 and Cyclin G-associated kinase (GAK) [3]. Both AAK1 and GAK are important regulators of endocytosis. Hence, targeting AAK1 and GAK makes baricitinib also a potential candidate for not only inhibiting the viral entry but also interfering with the virus assembly [258]. The effectiveness and safety of baricitinib have been shown through conclusive results such as lower fatality rate and higher discharge rate obtained from clinical studies conducted on COVID-19 patients receiving baricitinib [256, 259]. This drug is undergoing phase 3 of a clinical study performing on 1400 COVID-19 patients in the USA (NCT04421027).
2.3.5.3 Ruxolitinib
Ruxolitinib, also known as INC424 or INCB18424, is a potent inhibitor of JAK1 and 2 that was approved by the FDA against myelofibrosis, polycythemia vera, and acute graft-versus-host disease [38, 93, 260, 261]. Its main mechanism of action includes interfering with the JAK-STAT, one of the chief regulator cell signaling pathways, through interacting with JAK and preventing the activation of STAT, thereby reducing the elevated levels of cytokines [93, 257]. Given these points, ruxolitinib could be considered as one of the potent drugs against a wide range of diseases, including COVID-19. According to studies conducted on COVID-19 patients receiving ruxolitinib, obtained results demonstrated that this drug successfully reduced both the inflammatory blood cytokine levels such as IL-6 and the acute phase protein ferritin; moreover, the administration of ruxolitinib brought about rapid respiratory and cardiac improvement, significant chest computed tomography (CT) improvement, faster recovery from lymphopenia, clinical stabilization, as well as favorable side-effect profile [262, 263]. Ruxolitinib is under phase 3, randomized, double-blind, placebo-controlled, multicenter clinical study on patients infected with COVID-19 in the USA (NCT04377620).
2.3.5.4 Fingolimod
Fingolimod, known as FTY720, is an orally administered compound acting as the modulator of sphingosine-1-phosphate (S1P) receptors and was chemically derived from an immunosuppressive metabolite (myriocin) isolated from a fungus (Isaria sinclairii) [38, 264, 265]. In fact, fingolimod could play a role as a potent functional antagonist of S1P1 receptors on T cells subsequently sequestering lymphocytes in lymph nodes [1]. This drug has shown conclusive results in the treatment of multiple sclerosis (MS) on account of its capability to reduce the inflammatory damages and effect on the central nervous system (CNS) [95, 266]. Based on pathological findings, besides ventilator support, immune modulators such as fingolimod should be taken into consideration, in that, their combination might prevent the progression of ARDS [196].
2.3.5.5 Thalidomide
Thalidomide, which is an antiangiogenic, anti-inflammatory, as well as anti-fibrotic agent, was initially synthesized by the CIBA pharmaceutical company in 1954 and used as a sedative, antiemetic, and tranquilizer for morning sickness [267]. Thalidomide has inhibitory effects on TNF-α synthesis and is used for the treatment of multiple inflammatory diseases, including Behçets’ disease and Crohn’s disease [77, 96]. Even though the exact mechanism of action for the anti-inflammatory effects of thalidomide has yet to be found, researchers have attributed its anti-inflammatory effects to its ability for accelerating the degradation of messenger RNA in blood cells, thereby reducing the blood serum level of TNF-α, which is a cytokine involved in systemic inflammation and cytokine storm [268, 269]. Due to its properties, clinical studies are conducting to assess the immunomodulatory effects of thalidomide on reducing lung damage caused by SARS-CoV-2 [77]. Thalidomide is under phase 2 of a randomized, multicenter, placebo-controlled, double-blind clinical trial on 100 patients infected with COVID-19 performing by Wenzhou Medical University in China (NCT04273529).
2.3.6 Non-steroidal Anti-inflammatory Drugs (NSAIDs)
NSAIDs have been widely utilized for controlling acute and chronic inflammatory circumstances [270]. Generally, NSAIDs act by curbing prostaglandin synthases 1 and 2, known as cyclooxygenase enzymes (COX-1 and COX-2) that are responsible for producing prostaglandins (PGs) and provoking pain and fever [271,272,273]. NSAIDs have been employed to reduce fever and muscle pain caused by COVID-19. However, there is a heated controversy as to whether these drugs are safe [270]. On account of the fact that most studies postulating not protective effects of NSAIDs have been majorly in vitro or on animals rather than on humans [272], further studies are needed to determine the role of NSAIDs in the context of SARS-CoV-2 infection.
2.3.6.1 Naproxen
Naproxen a member of NSAIDs is a propionic acid derivative, which is administered orally and rectally, has been widely used against rheumatic diseases and non-rheumatic circumstances [97, 98]. It was demonstrated by Zheng et al. that naproxen is capable of exerting antiviral activity against influenza A and B viruses, in that this drug obstructs the nuclear export of the viral nucleoproteins, hampering influenza replication [274]. Additionally, in another study conducted on COVID-19 patients receiving ibuprofen and naproxen, conclusive results such as diminishing the probability of hospitalization and requiring mechanical ventilation were obtained [275]. Currently, the efficacy of naproxen on hospitalized COVID-19 patients is assessing through phase 3 of a randomized clinical study performing by Hôpitaux de Paris (NCT04325633).
2.3.6.2 Ibuprofen
Ibuprofen is NSAID with analgesic, antipyretic, and anti-inflammatory properties, which was first introduced in the UK in 1969 and has been used against symptoms of acute pain, inflammation, fever, osteoarthritis, rheumatoid arthritis, ankylosing spondylitis, gout, and Bartter’s syndromes [38, 276, 277]. Its mechanisms of action consist of reducing the activity of COX enzyme, thereby inhibiting the production of prostaglandins [278]. Regarding the effectiveness of ibuprofen against COVID-19 infection, there are quite a few contradictory suggestions made by studies. For example, whether ibuprofen could facilitate the cleavage of the ACE2 receptor on host cells, thereby interfering with the viral entry, or whether ibuprofen may decrease the excess inflammation or cytokine release in COVID-19 infection has been discussing. However, due to a lack of substantiated evidence, these claims are just possible protective effects of ibuprofen against CoV infection [279, 280]. Moreover, even though it is hypothesized that ibuprofen might increase the severity of COVID-19 infection, according to one study performed on 430 patients infected with COVID-19, ibuprofen was not associated with worse clinical outcomes, compared with paracetamol or no antipyretic. Nevertheless, further clinical studies are required to confirm their results [281]. This drug is currently under phase 4 of a clinical study conducting on 230 participants infected with COVID-19 by King’s College London in the UK (NCT04334629).
2.3.6.3 Paracetamol
Paracetamol (acetaminophen), which was initially synthesized through its precursor, that is, phenacetin in 1878, has been used to relieve acute and chronic pain worldwide [282, 283]. This drug is currently the most prevalent analgesic in the world and possesses weak inhibitory effects on the synthesis of prostaglandins [283, 284]. According to suggested warnings regarding the administration of ibuprofen for COVID-19 patients, paracetamol was recommended as a safer option. Although paracetamol has been reported to have no or insignificant anti-inflammatory and antiplatelet activity, this drug has been constantly used for the control of COVID-19 [285, 286]. Nonetheless, paracetamol might cause glutathione (GSH) depletion, which might result in developing severe COVID-19, particularly in more vulnerable groups. Therefore, clinical studies are needed to investigate the efficacy and adverse effects of this drug on patients infected with COVID-19 [286].
2.3.7 Corticosteroids
Corticosteroids have been proposed to be utilized for the suppression of lung inflammation in SARS-CoV-1 and MERS-CoV owing to their anti-inflammatory effects and the potential in reducing mortality [72, 287]. Prognosis improvement and clinical recovery promotion have been reported in a systematic review of corticosteroid therapy for severe COVID-19 patients [288]. Nevertheless, the efficacy and safety of the administration of corticosteroids like methylprednisolone, dexamethasone, and budesonide for the management of SARS-CoV-2 infection are still controversial [200, 287, 289]. The administration of corticosteroids in the management of COVID-19 may bring the risk of damages like prolonged mechanical ventilation, delayed viral clearance, and avascular necrosis. Thus, it demonstrates the need for a high consideration in corticosteroid administration for COVID-19 patients while also requiring more clinical data [289].
2.3.7.1 Methylprednisolone
Whether methylprednisolone could be a potential drug for the suppression of unwanted immune reactions is questionable [1]. However, it is believed by many medical researchers that methylprednisolone could improve the deregulation of the host immune response and increase the blood pressure where is low due to the cytokine storm [85]. Wu et al. reported that the risk of mortality was decreased by the administration of methylprednisolone for severe patients with ARDS. In fact, 23 of 50 (46%) patients who received methylprednisolone died while the mortality rate in patients with no methylprednisolone treatment was higher (61.8% (21 of 34 patients)) [101]. The routine use of corticosteroids including methylprednisolone is opposed by the Infectious Diseases Society of American. On the other hand, they do recommend the administration of corticosteroids for the patients with developed ARDS in order to set the cytokine storm in the context of a clinical trial [290]. The efficacy of different hormone doses of methylprednisolone is evaluating on severe COVID-19 patients in a phase 4 clinical study in Hubei, China (NCT04263402).
2.3.7.2 Dexamethasone
Dexamethasone is on the list of essential medicine of the WHO, which is available worldwide at low cost [291]. It is published that early treatment of ARDS with dexamethasone could reduce the ventilator days and mortality in patients generally with established moderate-to-severe ARDS [102]. A lower 28-day mortality was reported by employing dexamethasone treatment at a dose of 6 mg once daily for up to 10 days in hospitalized patients with COVID-19 who were receiving respiratory support (NCT04381936) [291]. In a phase 3 clinical study (NCT04327401), administration of intravenous dexamethasone plus standard care, compared with standard care alone for the COVID-19 patients with moderate or severe ARDS, showed promising results and caused a significant increase in the number of days alive and free of mechanical ventilation over 28 days [103]. In a phase 4 clinical trial in Brno, Czechia, the effect of two different doses of dexamethasone is assessing on COVID-19 patients with ARDS (NCT04663555).
2.3.8 Antibiotics
2.3.8.1 Azithromycin
Azithromycin, a broad-spectrum antibiotic, is an orally administered acid-stable antibacterial drug that is structurally related to erythromycin with analogous antimicrobial activity [38, 292, 293]. This drug is known for its antimicrobial activity against some gram-negative organisms, especially Haemophilus influenza that is associated with respiratory tract infections [293]. Besides its antibacterial activity, azithromycin has shown a wide variety of antiviral and immunomodulatory activities. Hence, this drug could be a potent candidate in suppressing viral infections, particularly COVID-19 [294]. Further, regarding SARS-CoV-2, it was shown that azithromycin inhibits the viral entry into the host cells by interacting with SARS-CoV-2 S protein and ACE2 [4, 100]. Also, it should be noted that, in the context of COVID-19, the combination of HCQ and azithromycin has been associated with serious adverse events, including a higher risk of cardiac toxicity and arrhythmias [294]. However, in one randomized-controlled clinical trial, results showed that treatment with a combination of azithromycin and HCQ was associated with a reduction in mortality of COVID-19 patients, and while administered alone, azithromycin did not show a higher risk of adverse events compared with the administration of the combination of HCQ and azithromycin or HCQ alone [295]. Azithromycin versus usual care is under phase 3 of a multicenter open-label clinical trial in ambulatory care of COVID19 by the collaboration of Pfizer in the UK (NCT04381962).
2.3.8.2 Teicoplanin
Teicoplanin is a glycopeptide antibiotic that besides its antibacterial activities against gram-positive bacteria, including staphylococci, streptococci, and enterococci, has shown conclusive results against the Ebola virus, influenza virus, flavivirus, HCV, HIV, and CoVs such as MERS-CoV and SARS-CoV-1 [62, 296]. As for CoVs, including SARS CoV-2, teicoplanin is capable of preventing the replication of the virus-cell cycle by inhibiting the viral RNA release. This could happen simply because teicoplanin inhibits the low pH cleavage of viral S protein by cathepsin L in the endosome [4]. Moreover, the concentration of teicoplanin needed in vitro for inhibiting 50% of SARS-CoV-2 viruses (IC50) was 1.66 µM, which was far lower than the IC50 reached in human blood (8.78 µM for a daily dose of 400 mg) [297, 298]. Nonetheless, these results are required to be confirmed by further clinical studies.
2.3.8.3 Tetracyclines
Tetracyclines (doxycycline, tetracyclines, minocycline) are polyketide antibiotics that have a broadspectrum antimicrobial activity [4, 299]. Tetracycline’s mechanism of action, that is, blocking protein synthesis in staphylococcus aureus cells and inhibiting cell growth in a bacteriostatic manner was first delineated in 1953; further studies showed that these drugs act through binding to bacterial ribosomes [300]. Studies on the skin showed that tetracyclines are also capable of reducing the levels of inflammatory cytokines [301]. Thus, due to their anti-inflammatory effects, tetracyclines could be considered for the treatment of COVID-19 patients. Another possible mechanism of action of tetracyclines against COVID-19 is related to its chelating activity. In better words, tetracyclines are capable of chelating zinc from host matrix metalloproteinases (MMPs), thereby limiting viral replication, and this happens because CoVs bind to the host MMPs for viral survival [302,303,304]. Doxycycline is under phase 3 of a multicenter, randomized, clinical study performed on 330 COVID-19 patients receiving either doxycycline or placebo conducting by Nantes University Hospital in France (NCT04371952).
2.3.9 Low Molecular Weight Heparins as Anticoagulants
Owing to the fact that coagulopathy has been one of the major causes of morbidity and mortality in COVID-19 patients, anticoagulation therapy has been considered as one of the potential ways of combating COVID-19 [305]. In this regard, heparins are clinically approved anti-coagulants, and low molecular weight heparins (LMWHs), derived from unfractionated heparins either by chemical or by enzymatic depolymerization, are glycosaminoglycans that have been utilized in the prophylaxis of post-surgical venous thromboembolism as well as non-surgical patients with acute pathology and reduced mobility [77, 99, 306]. Due to the fact that unfractionated heparins and LMWHs have inhibitory effects on proteases, including factor Xa, thrombin, furin, and cathepsin-L, it has been hypothesized that LMWH and unfractionated heparin could be considered as potential drugs for not only targeting protease cleavage but also the viral entry of SARS-CoV-2 [307]. Nevertheless, these suggestions need to be confirmed by in vitro and clinical studies. According to a retrospective clinical study, from among 42 patients with COVID-19, 21 underwent LMWH treatment, and 21 were assigned to the control group during hospitalization. Results showed that LMWH treatment not only caused improvement in the coagulation dysfunction of patients but also exerts anti-inflammatory effects through reducing IL-6 and increasing lymphocyte percent [308]. Another retrospective study was performed to assess the safety of intermediate-dose regimens of one LWMH, that is, enoxaparin in COVID-19 patients with pneumonia, especially in older patients. Their results proposed that the use of an intermediate dose of LWMH seems to be safe and possible for COVID-19 patients, but further clinical studies are needed to substantiate these suggestions [305]. LMWH is currently under phase 4 of a randomized clinical trial performing by Ain Shams University of Egypt (NCT04584580). Moreover, another phase 3 ongoing clinical trial, in the USA, is performing to compare the effects of full dose administration of enoxaparin vs. prophylactic or intermediate dose of enoxaparin in high-risk COVID-19 patients (NCT04401293).
2.3.10 Adjunctive Supplements and Vitamins
2.3.10.1 Vitamin D
Vitamin D, a crucial group of fat-soluble secosteroids, is generally known for its functions in the maintenance of bone health and calcium-phosphorus metabolism [309]. A wide range of antioxidant, immunomodulatory, anti-inflammatory, and antifibrotic functions have been recently attributed to vitamin D. It has been considered to be able to inhibit cytokine storm in SARS-CoV-2 infection and decrease the expression levels of pro-inflammatory type 1 cytokines like IL-12, IL-16, IL-8, TNF-α, IFN-γ while increasing regulatory T cells and type 2 cytokines including IL-4, IL-5, IL-10 [69, 104, 309]. The elderly patients and individuals with common variable immunodeficiency and bronchiectasis who are recognized with vitamin D deficiency are reported to be at a high risk of viral respiratory tract infections, acute lung injury, and particularly COVID-19 infection [104, 310]. On the contrary, no relationship between vitamin D concentration and the severity of COVID-19 in hospitalized patients was reported by Hernández et al. [311]. However, considering the several beneficial functions of vitamin D and its effects on immune cell proliferation and activity, pulmonary ACE2 expression, and priming effects against the viral replication, it has been proposed to employ high-dose vitamin D as a safe adjuvant therapeutic intervention to reduce the risk of COVID-19 severity and mortality [38, 104, 309]. Nevertheless, further studies are still needed to validate this association between vitamin D and COVID-19. Two clinical trials are currently in phase 4 to evaluate the efficiency of high doses of cholecalciferol (vitamin D3) on morbidity and mortality of COVID-19 patients in Spain and Argentina (NCT04552951, NCT04411446).
2.3.10.2 Vitamin C
Vitamin C (ascorbic acid) as an essential micronutrient and a potent antioxidant agent could play significant roles in neutralizing free radicals, preventing cellular damage, and associating with immune health [105]. Vitamin C has been reported to be effective against viruses like influenza viruses and reducing the duration and severity of upper respiratory infections [69, 85]. Many studies have demonstrated that vitamin C could involve with the development and maturation of T lymphocytes and NKs in the immune defense. Generally, it accumulates in phagocytic cells like neutrophils and can contribute to enhancing chemotaxis, phagocytosis, inhibition of reactive oxygen species (ROS) generation, preventing the neutrophils accumulation in the lung, and modulation of cytokine production network in the host inflammation response. Hence, employing vitamin C appears to be effective in preventing and treating respiratory and systemic infections [85, 105, 312]. The high dosage administration of vitamin C, a safe supportive treatment with no major side effects, has been considered as a potential treatment for reducing the cytokine storm and recovering COVID-19 patients [38, 85, 313, 314]. Currently, in a phase 3 clinical trial, the effect of high-dose intravenous vitamin C is evaluated on the mortality or persistent organ dysfunction of COVID-19 patients in Canada (NCT04401150).
2.3.10.3 Zinc
Zinc, as a micronutrient food supplement, has anti-inflammatory and antioxidant activities with an evident function in immunity on account of its roles as a cofactor, signaling molecule, and a structural element [69, 315]. Zinc deficiency has been reported to be responsible for the up-regulation of TNF-α, IFN-γ, JAK signaling in the lungs, cytokine production, and induction of apoptosis in lung epithelial cells [316]. Zinc could play its role in preventing viral pathogenesis through inhibiting viral entry, fusion, and replication alongside attenuating the risk of hyper-inflammation, preserving natural tissue barriers while protecting cells and tissues from oxidative damage and dysfunction [69, 106, 315]. The administration of zinc as a potential well-tolerated supplementary therapeutic against COVID-19 has been considered in several studies owing to its possible anti-inflammatory, antioxidant, immunomodulatory, and direct antiviral effects [69]. The efficacy of zinc in higher risk COVID-19 outpatients is currently assessing in a phase 4 clinical placebo-controlled trial in the USA (NCT04621461).
2.3.11 Miscellaneous Therapies
2.3.11.1 Nitric Oxide
Nitric oxide (NO) is an important cellular signaling molecule that is produced by nitric oxide synthase (NOS) by converting arginine and oxygen into citrulline and nitric oxide [317,318,319]. NOS exists in a wide range of cells such as neurons, macrophages, airway epithelial cells, and vascular endothelial cells, and mediate neurotransmission, smooth muscle contraction, and mucin secretions [2]. It was demonstrated that NO possesses broadspectrum antiviral activity against ectromelia virus, vaccinia virus, herpes simplex type 1 viruses, CoVs, and influenza A and B viruses [319,320,321]. Besides, different inflammatory stimuli like cytokines can bring about high and sustained production of NO by inducible nitric oxide synthase (iNOS). Furthermore, iNOS causes anti-inflammatory or pro-inflammatory responses, cytotoxicity, or cytoprotection [319]. Concerning SARS-CoV-2, inhalation of nitric oxide is being evaluated for use against COVID-19, because inhaled NO has a chief role in pulmonary and cardiovascular physiology [322]. Inhaled nitric oxide gas is under phase 2 of a randomized clinical trial performing on 470 COVID-19 patients by Massachusetts General Hospital (NCT04312243).
2.3.11.2 Statins
Statins are inhibitors of 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) with anti-inflammatory and immunomodulatory, anti-thrombotic, and antioxidant activities. Therefore, they could be considered as repurposing drugs for restoring viral infection-induced endothelial dysfunction, decreasing the severity of the lung injury and mortality rate caused by SARS-CoV-2, and maintaining the homeostasis of the patients [5, 323]. Moreover, it was postulated that stains might decrease the fatality rate caused by MERS-CoV [324]. Nevertheless, studies on animal models infected with SARS and MERS infections proposed that the administration of statins that suppress the myeloid differentiation primary response protein (MYD88) signaling might worsen the disease condition [5, 325]. It should be noted that since statins have a potency for drug–drug interaction with some protease inhibitor drugs, their co-administration is contraindicated [326]. Additionally, myopathy and severe rhabdomyolysis are two major side effects of statins, and less prevalent adverse effects include peripheral neuropathy, hepatotoxicity, impaired myocardial contractility, and autoimmune diseases [327]. Phase 2 of a randomized clinical study is currently conducting on COVID-19 patients to assess the efficacy of atorvastatin as an adjunctive treatment by Mount Auburn hospital in the USA (NCT04380402).
2.3.11.3 Losartan
Losartan, an angiotensin-receptor antagonist without agonist properties, is a selectively and orally available ACE2 inhibitor that plays a role by blocking the vasoconstrictor and aldosterone-secreting effects of angiotensin 2 through inhibiting the binding of it to the angiotensin II type 1 receptor [38, 328]. Thus, it is considered as a repurposing drug against COVID-19 infection. While orally administered, roughly 14% of the losartan is converted to its metabolite, E 3174, which is 10-to-40-fold more active compared with its original compound, with an estimated terminal half-life from 6 to 9 h [328]. Losartan is currently under phase 3 of a multicenter clinical trial for assessing its protective effect against COVID-19 (NCT04606563).
2.4 Recombinant Proteins and Monoclonal Antibodies
Three phases of infection have been proposed for COVID-19, the first one is mild infection requiring only symptomatic treatment. The pulmonary phase is the second phase necessitating mostly antiviral treatment where recombinant proteins like APN01, meplazumab and novaferon could play a vital role by inhibiting the viral entry and replication. The third phase of the SARS-CoV-2 infection is the inflammatory response phase mainly experienced by severe COVID-19 patients. The third phase is generally associated with complications and immune-inflammatory response accompanied by abundant macrophages, neutrophils, lymphocytes, immune mediators, and pro-inflammatory cytokines. IL-1, IL-6, and TNF-α are the most prominent pro-inflammatory cytokines in the body [62, 148].
IL-1 binds to the IL-1 receptor to modulate its function leading to the production of other pro-inflammatory cytokines. IL-6, another key pro-inflammatory cytokine, binds to the IL-6 receptor expressed on monocytes, neutrophils, macrophages, and other leukocytes and interact with membrane-bound gp130 to activate its downstream JAK signal. The excessive IL-6 signaling may cause a faster decline of lung elasticity, more severe bronchoalveolar inflammation, and organ damages. The need for mechanical ventilation has been recently reported to be strongly connected to the elevated IL-6 [1, 148, 329]. In this regard, the administration of monoclonal antibodies (mAbs) and recombinant proteins could be highly effective. Several mAbs and proteins such as tocilizumab, sarilumab, bevacizumab, and anakinra have been utilized to reduce hyper inflammation and the risk of ARDS and organ dysfunction (Table 2.1) [38, 330].
2.4.1 APN01
APN01, a recombinant human ACE2 (rhACE2) that was originally developed by Apeiron Biologics, is currently utilized for the treatment of patients infected with COVID-19 [38, 41]. APN01, which is a soluble recombinant ACE2, may prevent SARS-CoV-2 entry, as it imitates the innate human enzyme ACE2 on the surface of the host cells employed by the virus for entering the cells [3, 42]. By so doing, the S protein of the virus binds not to the ACE2 on the host cell, but rather to the soluble ACE2, (APN01), thereby preventing viral infection and increasing the viral load [77]. In the meantime, APN01 decreases damaging inflammatory reactions in the lungs and reduces lung injuries [3, 331]. It was demonstrated that the administration of APN01 as a therapeutic could decrease the level of Angiotensin II, thus preventing the ACE enzyme from accessing its substrate. This mechanism has the potential to inhibit further activations of the ACE2 angiotensin receptor [77, 332]. In a study, it was demonstrated that APN01 can reduce SARS-CoV-2 recovery from Vero cells by a factor of 1,000–5,000 in a dose-dependent manner [333]. APN01 is currently under phase 2 in a randomized, double-blind clinical trial for COVID-19 therapy, which is performing in a multicenter in Austria, Denmark, Germany, Russian Federation, and the UK by Apeiron Biologics (NCT04335136).
2.4.2 Novaferon
Novaferon (Nova) is a novel recombinant IFN-α like protein with 176 amino acids. Novaferon has exhibited anticancer and antiviral activities. In China, this drug has been approved for the treatment of chronic HBV. Li et al. in 2014, by studying the antitumor effects of novaferon and comparing it with recombinant humanized IFN-α-2b (rhIFN-α-2b), demonstrated that novaferon has stronger antitumor effects than rhIFN-α-2b [334, 335]. Novafen is able to block the virus replication in COVID-19-infected cells and also prevent the virus from entering healthy cells.
Zhang et al. reported a significantly higher clearance rate of SARS-CoV-2 employing novafron alone or by its combination with lopinavir/ritonavir compared with lopinavir/ritonavir alone [52, 53, 336]. Novaferon is currently under Phase 3 of randomized, double-blind clinical trials for hospitalized COVID-19 patients’ treatment (NCT04669015).
2.4.3 Tocilizumab
Tocilizumab or atlizumab is a recombinant humanized monoclonal antibody (rhmAb) against IL-6 under the trade name Actemra [337]. It has FDA approval for the treatment of rheumatoid and polyarticular juvenile idiopathic arthritis and systematic juvenile idiopathic. Tocilizumab plays its role through the membrane and soluble IL-6 receptor blockade and inhibiting the cytokine release syndrome process (CRS) [38]. Actemra is able to restrain the cytokine storm induced by SARS-CoV-2 [330]. Several studies have shown that a single dose of 400 ml administration of tocilizumab could render benefits through better breathing, faster fever reduction while it is also capable of reducing the inflammation response, vasopressor support, and mortality rate in COVID-19 [8, 77, 338]. Nevertheless, some side effects regarding the administration of tocilizumab have been reported including increased upper respiratory infections, hypertension, hematological effects, gastrointestinal perforation and hepatotoxicity [77]. For the management of the severe SARS-CoV-2 infection, tocilizumab has been employed in phase 4 and 3 clinical trials (NCT04377750, NCT04320615).
2.4.4 Sarilumab
Sarilumab, developed by Regeneron Pharmaceuticals and Sanofi, is another immunomodulatory drug. In May 2017, the FDA approved Sarilumab for the treatment of rheumatoid arthritis under the Kevzara brand name. Sarilumab has the potential to suppress the growth of some xenograft prostate and lung tumors either as a single drug or in combination with other therapeutics [339, 340]. Sarilumab, like tocilizumab, is an IL-6 rhmAb that could suppress COVID-19-associated overactive inflammatory immune responses and cytokine storm. The most common side effects of this drug include cough or sore throat, thrombocytopenia, blocked or runny nose, urinary and respiratory tract infections, neutropenia, hypercholesterolemia, mild hepatotoxicity, and cold sores [78, 341]. In a randomized, embedded, multifactorial, adaptive platform trial for community-acquired pneumonia (REMAP-CAP), a clinical phase 4 study, the efficiency of a range of interventions including sarilumab has been evaluating on ICU admitted COVID-19 patients (NCT02735707).
2.4.5 Eculizumab
Eculizumab has the FDA approval for the treatment of atypical hemolytic uremic syndrome (aHUS), paroxysmal nocturnal hemoglobinuria (PNH) diseases [342]. One of the most effective methods for preventing tissue damage is to suppress the production of excessive inflammation responses and cytokines caused by SARS CoV-2. Eculizumab with the brand name of soliris as a rhmAb against the complement protein C5 could inhibit the cleavage of C5 into C5a and C5b [343]. Consequently, it is able to prevent the terminal complement complex C5b-9 and subsequently the membrane attack complex formation, production of reactive oxygen species, and initiation of releasing cytokine storm. In addition, it could inhibit the formation of the C5a, responsible for the development of acute lung injury [80]. Despite the benefits of eculizumab, it may exhibit some side effects such as bradycardia, atrioventricular block, and hypertension in some patients [79]. Eculizumab is currently under phase 2 clinical trial against COVID-19 in France (NCT04346797).
2.4.6 Bevacizumab
Bevacizumab (under brand name avastin) is a humanized monoclonal antivascular endothelial growth factor (anti-VEGF) antibody that has been approved for the treatment of several cancers such as breast, brain, and renal cancers by the FDA. Studies have shown that upon ARDS the amount of VEGF produced by epithelial and inflammatory cells increases in patients. The increase in VEGF causes vascular permeability and pulmonary edema [1, 344, 345]. Bevacizumab with a specific ability in binding to VEGF and subsequent inhibition of its linking to VEGF receptor on the surface of endothelial cells could be a potential therapeutic for the treatment of ARDS and ALI caused by COVID-19 [81, 346]. However, the administration of bevacizumab is associated with an increased risk of cardiovascular events in some cases [347]. In multiple cohort randomized controlled trials, bevacizumab is under phase 2 for COVID-19 treatment at Hôpitaux de Paris in France (NCT04344782).
2.4.7 Infliximab
Infliximab is a chimeric monoclonal anti-tumor necrosis factor (anti-TNF) alpha antibody (IgG1), which could inhibit TNF from binding to its receptors by targeting it [82, 84]. TNF is a cytokine that releases in the acute phase of inflammation and binds to its receptors (TNFRI and TNFRII) in all cells except erythrocytes. Several signaling pathways including transcription factor activation (nuclear factor-κB), proteases (caspases), and protein kinases (MAP kinase, c-Jun N-terminal kinase) are activated by connecting TNF to its receptor leading to the activation of the target cells for immune and inflammatory responses by the release of apoptotic pathway initiation and several cytokines. TNF also plays role in the activation of lymphocytes (B, T) and macrophages, production of proinflammatory cytokines such as IL-1, IL-6, and expression of adhesion molecules (ICAM-1, E-selectin) [83]. Studies have shown that TNF could induce cytokine cascade in rheumatoid arthritis and promote pathogenesis in SARS-CoV-2 [348]. Infliximab has been approved in the USA since 1988 as a drug for the treatment of some autoimmune inflammatory diseases. Infliximab could be a proper option to reduce inflammation, cytokine storm, and subsequent organ failure due to COVID-19 [82, 84, 349]. Some reactions may occur upon infliximab Infusion, which could be prevented by employing antihistamines, acetaminophen, and corticosteroids as pre-medications [83]. Infliximab is currently under phase 3 of clinical trials for severe COVID-19 patients in multi centers of the USA (NCT04593940).
2.4.8 Anakinra
Anakinra under the brand name of kinert is a modified recombinant human IL-1 receptor antagonist with a short half-life of about 3–4 h and a proper safety profile that is approved for use in rheumatoid arthritis and neonatal-onset multisystem inflammatory treatment [85, 86]. IL-1 receptors induce the innate immune response and are associated with excessive inflammation response of the host [350]. It has been hypothesized that anakinra could assist in neutralizing the SARS-CoV-2-related hyperinflammatory state, one of the main causes of ARDS in COVID-19 patients [86, 351]. Anakinra was administered for severe COVID-19 patients in a cohort study and it was observed that it can reduce both the need for invasive mechanical ventilation and mortality without serious side effects [86]. As an anti-proinflammatory cytokine drug, it has been employed in several clinical studies against COVID-19 with encouraging results and it is currently under phase 3 in multi centers of Greece (NCT04680949).
2.4.9 Emapalumab
Emapalumab is a humanized monoclonal anti-IFN-γ antibody, which is known as gamifant brand name. FDA approved Emapalumab for the treatment of hemophagocytic lymphohistiocytosis (HLH) (an illness caused by an overactive immune system) [87, 148, 352, 353]. Emapalumab could prevent the binding of IFN-γ to its cell surface receptors subsequently inhibiting the activation of inflammatory signals and cytokine release syndrome caused by SARS-COV-2 [88, 148]. In order to minimize the rate of inflammation, and the needing mechanical ventilation emapalumab was utilized in combination with anakinra in a phase 2/3 multicenter randomized clinical trial against COVID-19 (NCT04324021). Immunosuppression is reported as one of the side effects of utilizing emapalumab in some patients. Thus, patients with weak immune systems should take this drug with caution [87].
2.4.10 Meplazumab
The main entry route of the virus is to bind host cells through ACE2 receptors at the surface of the host cells. SARS-CoV-2 can also enter the host cells through the cluster of differentiation 147 (CD147). In fact, CD147 can act as a receptor for the SARS-CoV-2 spike protein [89, 354]. Moreover, CD147 could function as a mediator in the inflammatory response as a receptor for cyclophilin A (CyPA), the activator of the intracellular antiviral response, and a potent chemotactic factor for inflammatory leukocytes [90, 355]. Meplazumab is a humanized monoclonal anti-CD147 antibody (IgG2) that inhibits SARS-CoV-2 from entering the cell by blocking the expression of CD147 and reducing the infection caused by the virus. Meplazumab also plays a critical role in reducing the cytokine storm caused by COVID-19 by suppressing cyclophilin A from linking to CD147 [356, 357]. In a phase 2/3 multicenter clinical study, the safety and efficacy of meplazumab are assessing for hospitalized COVID-19 patients (NCT04586153).
2.5 Bioactive Natural Compounds and Herbal Medicines
Natural compounds as highly safe and available products have exhibited promising biological and pharmacological activities including anticancer, antiviral, antimicrobial, anti-inflammatory, and antioxidant properties. Medicinal plant-based natural compounds and traditional herbal medicines have demonstrated antiviral properties against several viruses like the influenza virus, HBV, HCV, SARS-CoV-1, and MERS-CoV. The intervention in both the viral life cycle and host response is attributed to the antiviral functions of natural compounds [358, 359]. Due to the high similarity between SARS-CoV-2 and SARS-CoV-1 in respect to genomics, epidemiologic, and pathogenesis, some herbal and natural medicines are used for the treatment of SARS-CoV-1 could be employed for inhibiting SARS-CoV-2 as well [85]. In this regard, natural compounds and herbal medicines such as theaflavin, cepharanthine, lectins, silvestrol, tryptanthrin, hirsutenoone, tanshinones I-VII, celastrol, pristimererin, iguesterin, tingenone have indicated the potential to prevent SARS-CoV-2 infection through inhibiting RdRp, ACE2, PLpro, or 3CLpro [94, 358, 359].
On the other hand, curcumin and piperine, quercetin, emodin, and scutellarein have been reported to be able to associate with the inhibition of COVID-19 while rendering their anti-inflammatory activities [358]. Particularly, extracted food supplements from plants like curcumin, piperine, and quercetin have the potential to interfere in cellular entry and replication of SARS-CoV-2 and play their roles by immune-boosting, antioxidant and anti-inflammatory functions and by repairing the tissue damages induced by COVID-19. The immunomodulatory and anticytokine effects are also proposed for these agents. Furthermore, these drugs are highly potent to be employed as adjuvants to enhance the bioavailability of other drugs by rendering their multidrug resistance (MDR) effect [69, 360,361,362]. The nanoencapsulation of quercetin, curcumin/piperine has been practiced and developed due to their hydrophobicity and utilized for the treatment of cancer cells with the capability of suppressing MDR [363, 364]. Likewise, despite the uncertainty of the precise mechanism of many natural compounds, Traditional Chinese Medicines (TCMs) like Glycyrrhiza uralensis, Saposhnikoviae divaricata, Astragalus membranaceus, Rhizoma Atractylodis Macrocephalae have been reported to be effective in inhibiting COVID-19 and its subsequent lung inflammation or acute lung injury [85, 94].
2.6 Combination Therapy Approach for COVID-19
Numerous drugs have been reported to be effective against SARS-CoV-2 infection employing the drug repurposing approach that targets viral entry, fusion, replication, and translation alongside regulating immunity and inflammatory response attenuating [234, 365]. In this respect, antivirals, immunomodulators, and anti-inflammatory drugs possessing different mechanisms of action could be exploited in combination to simultaneously inhibit viral functions while providing support and symptomatic treatment for SARS-CoV-2 patients. Besides, the treatment of the severe COVID-19 patients, most at the risk of dying due to the cytokine storm should be practiced with utmost importance by the combination therapy while considering drug–drug interactions and side effects [234, 366]. Some promising combinational administrations of drugs for COVID-19 therapy are presented in Table 2.2.
2.7 Perspectives and Conclusion
SARS-CoV-2 infection is associated with both direct damages induced by the virus and host inflammatory and immune response. In this regard, many antivirals have been administered to inhibit the virus while immunomodulators and anti-inflammatory drugs, as well as biological and natural compounds, have been utilized to either enhance the innate immune system or manage the deregulated inflammatory responses and control the symptoms leading to quick recovery of patients and reducing mortality. Accordingly, combination therapy could be more effective against SARS-CoV-2 infection in the case being utilized timely by taking drug-drug interactions into account. In many clinical trials, combinational administration of antivirals, immunomodulators, and anti-inflammatory drugs has been proposed considering different targets to inhibit the infection.
Alternatively, nanotechnology as a promising strategy could be applied to the COVID-19 treatment principle. Highly biocompatible natural-based vehicles such as proteins and polysaccharides are highly potent to be employed to encapsulate the potential COVID-19 therapeutics and deliver them in an efficient way by enhancing the stability and bioavailability of drugs like favipiravir alongside reducing their side effects. Besides, by utilizing the targeted delivery, the nanoparticle-based therapeutics could be triggered toward susceptible alveolar cells prone to be infected by SARS-CoV-2 to either protect them against COVID-19 or provide them with inhibitory drugs. It is worth noting that the nanoparticle-based vaccines have already been taken into consideration for the control of the COVID-19 pandemic and prevent its higher outbreak.
As we obtain more information about the potency of the drug formulations against COVID-19 with respect to their mechanism of action particularly in severe patients, we will be better equipped to optimize therapeutic strategies.
References
Tu Y-F, Chien C-S, Yarmishyn AA, Lin Y-Y, Luo Y-H, Lin Y-T, Lai W-Y, Yang D-M, Chou S-J, Yang Y-P (2020) A review of SARS-CoV-2 and the ongoing clinical trials. Int J Mol Sci 21(7):2657
Oroojalian F, Haghbin A, Baradaran B, Hemat N, Shahbazi M-A, Baghi HB, Mokhtarzadeh A, Hamblin MR (2020) Novel insights into the treatment of SARS-CoV-2 infection: an overview of current clinical trials. Int J Biol Macromol
Gil C, Ginex T, Maestro I, Nozal V, Barrado-Gil L, Cuesta-Geijo MA, Urquiza J, Ramírez D, Alonso C, Campillo NE (2020) COVID-19: drug targets and potential treatments. J Med Chem
Pandey A, Nikam AN, Shreya AB, Mutalik SP, Gopalan D, Kulkarni S, Padya BS, Fernandes G, Mutalik S, Prassl R (2020) Potential therapeutic targets for combating SARS-CoV-2: drug repurposing, clinical trials and recent advancements. Life Sci 117883
Singh TU, Parida S, Lingaraju MC, Kesavan M, Kumar D, Singh RK (2020) Drug repurposing approach to fight COVID-19. Pharmacol Rep 1–30
Santos IdA, Grosche VR, Bergamini FRG, Sabino-Silva R, Jardim ACG (2020) Antivirals against coronaviruses: candidate drugs for SARS-coV-2 treatment? Front Microbiol 11:1818
Alnefaie A, Albogami S (2020) Current approaches used in treating COVID-19 from a molecular mechanisms and immune response perspective. Saudi Pharmaceut J
Yang X, Liu Y, Liu Y, Yang Q, Wu X, Huang X, Liu H, Cai W, Ma G (2020) Medication therapy strategies for the coronavirus disease 2019 (COVID-19): recent progress and challenges. Exp Rev Clin Pharmacol 13(9):957–975
Eastman RT, Roth JS, Brimacombe KR, Simeonov A, Shen M, Patnaik S, Hall MD (2020) Remdesivir: a review of its discovery and development leading to emergency use authorization for treatment of COVID-19. ACS Central Sci
Nili A, Farbod A, Neishabouri A, Mozafarihashjin M, Tavakolpour S, Mahmoudi H (2020) Remdesivir: a beacon of hope from Ebola virus disease to COVID‐19. Rev Med Virol e2133
Santoro MG, Carafoli E (2020) Remdesivir: from ebola to COVID-19. Biochem Biophys Res Commun
Gordon CJ, Tchesnokov EP, Woolner E, Perry JK, Feng JY, Porter DP, Götte M (2020) Remdesivir is a direct-acting antiviral that inhibits RNA-dependent RNA polymerase from severe acute respiratory syndrome coronavirus 2 with high potency. J Biol Chem 295(20):6785–6797
Jorgensen SC, Kebriaei R, Dresser LD (2020) Remdesivir: review of pharmacology, pre‐clinical data and emerging clinical experience for COVID‐19. Pharmacother J Human Pharmacol Drug Ther
Malin JJ, Suárez I, Priesner V, Fätkenheuer G, Rybniker J (2020) Remdesivir against COVID-19 and other viral diseases. Clin Microbiol Rev 34(1)
Devaux CA, Rolain J-M, Colson P, Raoult D (2020) New insights on the antiviral effects of chloroquine against coronavirus: what to expect for COVID-19? Int J Antimicrob Agents 105938
Hoffmann M, Schroeder S, Kleine-Weber H, Müller MA, Drosten C, Pöhlmann S (2020) Nafamostat mesylate blocks activation of SARS-CoV-2: new treatment option for COVID-19. Antimicrob Agents Chemother
Naghipour S, Ghodousi M, Rahsepar S, Elyasi S (2020) Repurposing of well-known medications as antivirals: hydroxychloroquine and chloroquine–from HIV-1 infection to COVID-19. Exp Rev Anti-Infect Ther 18(11):1119–1133
Vincent MJ, Bergeron E, Benjannet S, Erickson BR, Rollin PE, Ksiazek TG, Seidah NG, Nichol ST (2005) Chloroquine is a potent inhibitor of SARS coronavirus infection and spread. Virology J 2(1):1–10
Hurst M, Faulds D (2000) Lopinavir. Drugs 60(6):1371–1379
Cvetkovic RS, Goa KL (2003) Lopinavir/ritonavir. Drugs 63(8):769–802
Blaising J, Polyak SJ, Pécheur E-I (2014) Arbidol as a broad-spectrum antiviral: an update. Antiviral Res 107:84–94
Deng P, Zhong D, Yu K, Zhang Y, Wang T, Chen X (2013) Pharmacokinetics, metabolism, and excretion of the antiviral drug arbidol in humans. Antimicrob Agents Chemother 57(4):1743–1755
Villalaín J (2010) Membranotropic effects of arbidol, a broad anti-viral molecule, on phospholipid model membranes. J Phys Chem B 114(25):8544–8554
Boretti A (2020) Favipiravir use for SARS CoV-2 infection. Pharmacol Rep 72(6):1542–1552
Coomes EA, Haghbayan H (2020) Favipiravir, an antiviral for COVID-19? J Antimicrob Chemother
Shiraki K, Daikoku T (2020) Favipiravir, an anti-influenza drug against life-threatening RNA virus infections. Pharmacol Therapeut 107512
Furuta Y, Komeno T, Nakamura T (2017) Favipiravir (T-705), a broad spectrum inhibitor of viral RNA polymerase. Proc Jpn Acad Series B 93(7):449–463
Uyeki TM (2018) Oseltamivir treatment of influenza in children. Oxford University Press, US
Yousefi B, Valizadeh S, Ghaffari H, Vahedi A, Karbalaei M, Eslami M (2020) A global treatments for coronaviruses including COVID‐19. J Cell Physiol
Khalili JS, Zhu H, Mak NSA, Yan Y, Zhu Y (2020) Novel coronavirus treatment with ribavirin: groundwork for an evaluation concerning COVID‐19. J Med Virol
Preston SL, Drusano GL, Glue P, Nash J, Gupta S, McNamara P (1999) Pharmacokinetics and absolute bioavailability of ribavirin in healthy volunteers as determined by stable-isotope methodology. Antimicrob Agents Chemother 43(10):2451–2456
Back D, Sekar V, Hoetelmans R (2008) Darunavir: pharmacokinetics and drug interactions. Antiviral Ther 13(1):1
Deeks ED (2014) Darunavir: a review of its use in the management of HIV-1 infection. Drugs 74(1):99–125
Rittweger M, Arasteh K (2007) Clinical pharmacokinetics of darunavir. Clin Pharmacokinet 46(9):739–756
Wassner C, Bradley N, Lee Y (2020) A review and clinical understanding of tenofovir: tenofovir disoproxil fumarate versus tenofovir alafenamide. J Int Associat Provid AIDS Care (JIAPAC) 19:2325958220919231
Clososki GC, Soldi RA, Silva RMd, Guaratini T, Lopes JN, Pereira PR, Lopes JL, Santos Td, Martins RB, Costa CS (2020) Tenofovir disoproxil fumarate: new chemical developments and encouraging in vitro biological results for SARS-CoV-2. J Brazil Chem Soc 31(8):1552–1556
Uno Y (2020) Camostat mesilate therapy for COVID-19. Int Emerg Med 1–2
Sarkar C, Mondal M, Torequl Islam M, Martorell M, Docea AO, Maroyi A, Sharifi-Rad J, Calina D (2020) Potential therapeutic options for COVID-19: current status, challenges, and future perspectives. Front Pharmacol 11:1428
Zheng L, Zhang L, Huang J, Nandakumar KS, Liu S, Cheng K (2020) Potential treatment methods targeting 2019-nCoV infection. Eur J Med Chem 112687
Yamamoto M, Matsuyama S, Li X, Takeda M, Kawaguchi Y, Inoue J-I, Matsuda Z (2016) Identification of nafamostat as a potent inhibitor of Middle East respiratory syndrome coronavirus S protein-mediated membrane fusion using the split-protein-based cell-cell fusion assay. Antimicrob Agents Chemother 60(11):6532–6539
Chary MA, Barbuto AF, Izadmehr S, Hayes BD, Burns MM (2020) COVID-19: therapeutics and their toxicities. J Med Toxicol 16(3):10.1007
Yang P, Gu H, Zhao Z, Wang W, Cao B, Lai C, Yang X, Zhang L, Duan Y, Zhang S (2014) Angiotensin-converting enzyme 2 (ACE2) mediates influenza H7N9 virus-induced acute lung injury. Sci Rep 4:7027
Corum J, Wu KJ, Zimmer C (2020) Coronavirus drug and treatment tracker. The New York Times
Shang Z, Chan SY, Liu WJ, Li P, Huang W (2020) Recent Insights into emerging coronavirus: SARS-CoV-2. ACS Infect Dis
Cox RM, Wolf JD, Plemper RK (2020) Therapeutically administered ribonucleoside analogue MK-4482/EIDD-2801 blocks SARS-CoV-2 transmission in ferrets. Nat Microbiol 1–8
Nourian A, Khalili H (2020) Sofosbuvir as a potential option for the treatment of COVID-19. Acta Bio Medica: Atenei Parmensis 91(2):239
Sayad B, Sobhani M, Khodarahmi R (2020) Sofosbuvir as repurposed antiviral drug against COVID-19: why were we convinced to evaluate the drug in a registered/approved clinical trial? Arch Med Res 51(6):577–581
Wagstaff KM, Sivakumaran H, Heaton SM, Harrich D, Jans DA (2012) Ivermectin is a specific inhibitor of importin α/β-mediated nuclear import able to inhibit replication of HIV-1 and dengue virus. Biochem J 443(3):851–856
Altay O, Mohammadi E, Lam S, Turkez H, Boren J, Nielsen J, Mardinoglu A (2020) Current status of COVID-19 therapies and drug repositioning applications. iScience 23(7):101303
Sethia R, Prasad M, Jagannath S, Nischal N, Soneja M, Garg P (2020) Efficacy of famotidine for COVID-19: a systematic review and meta-analysis, medRxiv
Aguila EJT, Cua IHY (2020) Repurposed GI drugs in the treatment of COVID-19. Dig Dis Sci 65(8):2452–2453
Kumari P, Singh A, Ngasainao MR, Shakeel I, Kumar S, Lal S, Singhal A, Sohal SS, Singh IK, Hassan MI (2020) Potential diagnostics and therapeutic approaches in COVID-19. Clinica Chimica Acta; Int J clinical Chem 510:488–497
Zheng F, Zhou Y, Zhou Z, Ye F, Huang B, Huang Y, Ma J, Zuo Q, Tan X, Xie J (2020) Novel protein drug, novaferon, as the potential antiviral drug for COVID-19, medRxiv
Rossignol J-F (2014) Nitazoxanide: a first-in-class broad-spectrum antiviral agent. Antiviral Res 110:94–103
Bardsley-Elliot A, Plosker GL (2000) Nelfinavir. Drugs 59(3):581–620
Yamamoto N, Yang R, Yoshinaka Y, Amari S, Nakano T, Cinatl J, Rabenau H, Doerr HW, Hunsmann G, Otaka A (2004) HIV protease inhibitor nelfinavir inhibits replication of SARS-associated coronavirus. Biochem Biophys Res Commun 318(3):719–725
Bolcato G, Bissaro M, Pavan M, Sturlese M, Moro S (2020) Targeting the coronavirus SARS-CoV-2: computational insights into the mechanism of action of the protease inhibitors lopinavir, ritonavir and nelfinavir. Sci Rep 10(1):20927
Rothan HA, Stone S, Natekar J, Kumari P, Arora K, Kumar M (2020) The FDA-approved gold drug Auranofin inhibits novel coronavirus (SARS-COV-2) replication and attenuates inflammation in human cells. Virology
Harbut MB, Vilchèze C, Luo X, Hensler ME, Guo H, Yang B, Chatterjee AK, Nizet V, Jacobs WR, Schultz PG (2015) Auranofin exerts broad-spectrum bactericidal activities by targeting thiol-redox homeostasis. Proc Natl Acad Sci 112(14):4453–4458
Thangamani S, Mohammad H, Abushahba MF, Sobreira TJ, Seleem MN (2016) Repurposing auranofin for the treatment of cutaneous staphylococcal infections. Int J Antimicrob Agents 47(3):195–201
May HC, Yu J-J, Guentzel MN, Chambers JP, Cap AP, Arulanandam BP (2018) Repurposing auranofin, ebselen, and PX-12 as antimicrobial agents targeting the thioredoxin system. Front Microbiol 9:336
Ahamad S, Branch S, Harrelson S, Hussain MK, Saquib M, Khan S (2020) Primed for global coronavirus pandemic: emerging research and clinical outcome. Eur J Med Chem 112862
Jin Z, Zhao Y, Sun Y, Zhang B, Wang H, Wu Y, Zhu Y, Zhu C, Hu T, Du X (2020) Structural basis for the inhibition of SARS-CoV-2 main protease by antineoplastic drug carmofur. Nat Struct Mol Biol 27(6):529–532
Chien M, Anderson TK, Jockusch S, Tao C, Li X, Kumar S, Russo JJ, Kirchdoerfer RN, Ju J (2020) Nucleotide analogues as inhibitors of SARS-CoV-2 polymerase, a key drug target for COVID-19. J Proteome Res 19(11):4690–4697
Asselah T, Durantel D, Pasmant E, Lau G, Schinazi RF (2020) COVID-19: discovery, diagnostics and drug development. J Hepatol
Ataei M, Hosseinjani H (2020) Molecular mechanisms of galidesivir as a potential antiviral treatment for COVID-19. J Pharmaceut Care 8(3):150–151
Westover JB, Mathis A, Taylor R, Wandersee L, Bailey KW, Sefing EJ, Hickerson BT, Jung K-H, Sheridan WP, Gowen BB (2018) Galidesivir limits Rift Valley fever virus infection and disease in Syrian golden hamsters. Antiviral Res 156:38–45
Yu B, Chang J (2020) Azvudine (FNC): a promising clinical candidate for COVID-19 treatment. Signal Transduct Target Ther 5(1):1–2
Mrityunjaya M, Pavithra V, Neelam R, Janhavi P, Halami P, Ravindra P (2020) Immune-boosting, antioxidant and anti-inflammatory food supplements targeting pathogenesis of COVID-19. Front Immunol 11
Durand N, Mallea J, Zubair AC (2020) Insights into the use of mesenchymal stem cells in COVID-19 mediated acute respiratory failure. npj Regenerat Med 5(1):1–9
Sadeghi S, Soudi S, Shafiee A, Hashemi SM (2020) Mesenchymal stem cell therapies for COVID-19: current status and mechanism of action. Life Sci 262:118493
Barlow A, Landolf KM, Barlow B, Yeung SYA, Heavner JJ, Claassen CW, Heavner MS (2020) Review of emerging pharmacotherapy for the treatment of coronavirus disease 2019. Pharmacother J Human Pharmacol Drug Ther 40(5):416–437
Monk PD, Marsden RJ, Tear VJ, Brookes J, Batten TN, Mankowski M, Gabbay FJ, Davies DE, Holgate ST, Ho L-P (2020) Safety and efficacy of inhaled nebulised interferon beta-1a (SNG001) for treatment of SARS-CoV-2 infection: a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Respirat Med
Hensley LE, Fritz EA, Jahrling PB, Karp C, Huggins JW, Geisbert TW (2004) Interferon-β 1a and SARS coronavirus replication. Emerg Infect Dis 10(2):317
Chen L, Xiong J, Bao L, Shi Y (2020) Convalescent plasma as a potential therapy for COVID-19. Lancet Infect Dis 20(4):398–400
Rojas M, Rodríguez Y, Monsalve DM, Acosta-Ampudia Y, Camacho B, Gallo JE, Rojas-Villarraga A, Ramírez-Santana C, Díaz-Coronado JC, Manrique R (2020) Convalescent plasma in Covid-19: possible mechanisms of action. Autoimmun Rev 102554
Nittari G, Pallotta G, Amenta F, Tayebati SK (2020) Current pharmacological treatments for SARS-COV-2: a narrative review. Eur J Pharmacol 173328
Montesarchio V, Parella R, Iommelli C, Bianco A, Manzillo E, Fraganza F, Palumbo C, Rea G, Murino P, De Rosa R (2020) Outcomes and biomarker analyses among patients with COVID-19 treated with interleukin 6 (IL-6) receptor antagonist sarilumab at a single institution in Italy. J Immunother Cancer 8(2)
Liu J, Virani SS, Alam M, Denktas AE, Hamzeh I, Khalid U (2020) Coronavirus disease‐19 and cardiovascular disease: a risk factor or a risk marker? Rev Med Virol e2172
Annane D, Heming N, Grimaldi-Bensouda L, Frémeaux-Bacchi V, Vigan M, Roux A-L, Marchal A, Michelon H, Rottman M, Moine P (2020) Eculizumab as an emergency treatment for adult patients with severe COVID-19 in the intensive care unit: a proof-of-concept study. EClinicalMedicine 28:100590
Zhang J, Xie B, Hashimoto K (2020) Current status of potential therapeutic candidates for the COVID-19 crisis. Brain Behav Immun
Stallmach A, Kortgen A, Gonnert F, Coldewey SM, Reuken P, Bauer M (2020) Infliximab against severe COVID-19-induced cytokine storm syndrome with organ failure—a cautionary case series. Crit Care 24(1):1–3
Gerriets V, Bansal P, Khaddour K (2019) Tumor necrosis factor (TNF) inhibitors. StatPearls [Internet], StatPearls Publishing
Feldmann M, Maini RN, Woody JN, Holgate ST, Winter G, Rowland M, Richards D, Hussell T (2020) Trials of anti-tumour necrosis factor therapy for COVID-19 are urgently needed. Lancet 395(10234):1407–1409
Wu R, Wang L, Kuo H-CD, Shannar A, Peter R, Chou PJ, Li S, Hudlikar R, Liu X, Liu Z (2020) An update on current therapeutic drugs treating COVID-19. Curr Pharmacol Rep 1
Huet T, Beaussier H, Voisin O, Jouveshomme S, Dauriat G, Lazareth I, Sacco E, Naccache J-M, Bézie Y, Laplanche S (2020) Anakinra for severe forms of COVID-19: a cohort study. Lancet Rheumatol
Henderson LA, Canna SW, Schulert GS, Volpi S, Lee PY, Kernan KF, Caricchio R, Mahmud S, Hazen MM, Halyabar O (2020) On the alert for cytokine storm: immunopathology in COVID‐19. Arthrit Rheumatol
Boettler T, Newsome PN, Mondelli MU, Maticic M, Cordero E, Cornberg M, Berg T (2020) Care of patients with liver disease during the COVID-19 pandemic: EASL-ESCMID position paper. JHEP Rep 100113
Ulrich H, Pillat MM (2020) CD147 as a target for COVID-19 treatment: suggested effects of azithromycin and stem cell engagement. Stem Cell Rev Rep 1–7
Xia P, Dubrovska A (2020) Tumor markers as an entry for SARS-CoV-2 infection? FEBS J 287(17):3677–3680
Naserifar M, Hosseinjani H (2020) Novel immunological aspects of sirolimus as a new targeted therapy for COVID-19. J Pharmaceut Care 8(3):152–153
Seto B (2012) Rapamycin and mTOR: a serendipitous discovery and implications for breast cancer. Clin Translat Med 1(1):1–7
Bagca BG, Avci CB (2020) Overview of the COVID-19 and JAK/STAT pathway inhibition: ruxolitinib perspective. Cytokine Growth Factor Rev (2020)
Maurya VK, Kumar S, Bhatt ML, Saxena SK (2020) Therapeutic development and drugs for the treatment of COVID-19. In: Coronavirus disease 2019 (COVID-19). Springer, pp 109–126
Chun J, Hartung H-P (2010) Mechanism of action of oral fingolimod (FTY720) in multiple sclerosis. Clin Neuropharmacol 33(2):91
Vargesson N (2015) Thalidomide-induced teratogenesis: history and mechanisms. Birth Def Res Part C Embryo Today Rev 105(2):140–156
Brogden R, Heel R, Speight T, Avery G (1979) Naproxen up to date: a review of its pharmacological properties and therapeutic efficacy and use in rheumatic diseases and pain states. Drugs 18(4):241–277
Todd PA, Clissold SP (1990) Naproxen. Drugs 40(1):91–137
Jamwal S, Gautam A, Elsworth J, Kumar M, Chawla R, Kumar P (2020) An updated insight into the molecular pathogenesis, secondary complications and potential therapeutics of COVID-19 pandemic. Life Sci 118105
Damle B, Vourvahis M, Wang E, Leaney J, Corrigan B (2020) Clinical pharmacology perspectives on the antiviral activity of azithromycin and use in COVID‐19. Clin Pharmacol Therapeut
Wu C, Chen X, Cai Y, Zhou X, Xu S, Huang H, Zhang L, Zhou X, Du C, Zhang Y (2020) Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Int Med
Villar J, Ferrando C, Martínez D, Ambrós A, Muñoz T, Soler JA, Aguilar G, Alba F, González-Higueras E, Conesa LA (2020) Dexamethasone treatment for the acute respiratory distress syndrome: a multicentre, randomised controlled trial. Lancet Respirat Med 8(3):267–276
Tomazini BM, Maia IS, Cavalcanti AB, Berwanger O, Rosa RG, Veiga VC, Avezum A, Lopes RD, Bueno FR, Silva MVA (2020) Effect of dexamethasone on days alive and ventilator-free in patients with moderate or severe acute respiratory distress syndrome and COVID-19: the CoDEX randomized clinical trial. JAMA 324(13):1307–1316
Ebadi M, Montano-Loza AJ (2020) Perspective: improving vitamin D status in the management of COVID-19. Eur J Clin Nutrit 1–4
Carr AC, Maggini S (2017) Vitamin C and immune function. Nutrients 9(11):1211
Wessels I, Rolles B, Rink L (2020) The potential impact of zinc supplementation on COVID-19 pathogenesis. Front Immunol 11:1712
Jassim SAA, Naji MA (2003) Novel antiviral agents: a medicinal plant perspective. J Appl Microbiol 95(3):412–427
Antonelli G, Turriziani O (2012) Antiviral therapy: old and current issues. Int J Antimicrob Agents 40(2):95–102
Safrin S (2001) Antiviral agents. Basic Clin Pharmacol 11:845–875
Chan S-W (2020) Current and future direct-acting antivirals against COVID-19. Front Microbiol 11:2880
Ita K (2020) Coronavirus disease (COVID-19): current status and prospects for drug and vaccine development. Arch Med Res
Jordan PC, Stevens SK, Deval J (2018) Nucleosides for the treatment of respiratory RNA virus infections. Antiviral Chem Chemother 26:2040206618764483
De Clercq E (2011) A 40-year journey in search of selective antiviral chemotherapy. Annu Rev Pharmacol Toxicol 51:1–24
Siegel D, Hui HC, Doerffler E, Clarke MO, Chun K, Zhang L, Neville S, Carra E, Lew W, Ross B (2017) Discovery and synthesis of a phosphoramidate prodrug of a pyrrolo [2, 1-f][triazin-4-amino] adenine C-nucleoside (GS-5734) for the treatment of ebola and emerging viruses. ACS Publications
De Clercq E, Herdewijn P (2010) Strategies in the design of antiviral drugs. Pharmaceut Sci Encycl Drug Discov Develop Manufact 1–56
Seley-Radtke KL, Yates MK (2018) The evolution of nucleoside analogue antivirals: a review for chemists and non-chemists. Part 1: Early structural modifications to the nucleoside scaffold. Antiviral Res 154:66–86
Lo MK, Jordan R, Arvey A, Sudhamsu J, Shrivastava-Ranjan P, Hotard AL, Flint M, McMullan LK, Siegel D, Clarke MO (2017) GS-5734 and its parent nucleoside analog inhibit Filo-, Pneumo-, and Paramyxoviruses. Sci Rep 7:43395
Warren TK, Jordan R, Lo MK, Ray AS, Mackman RL, Soloveva V, Siegel D, Perron M, Bannister R, Hui HC (2016) Therapeutic efficacy of the small molecule GS-5734 against Ebola virus in rhesus monkeys. Nature 531(7594):381–385
Mulangu S, Dodd LE, Davey Jr RT, Tshiani Mbaya O, Proschan M, Mukadi D, Lusakibanza Manzo M, Nzolo D, Tshomba Oloma A, Ibanda A (2019) A randomized, controlled trial of Ebola virus disease therapeutics. N Engl J Med 381(24):2293–2303
Lamb YN (2020) Remdesivir: first approval. Drugs 1–9
Sheahan TP, Sims AC, Graham RL, Menachery VD, Gralinski LE, Case JB, Leist SR, Pyrc K, Feng JY, Trantcheva I (2017) Broad-spectrum antiviral GS-5734 inhibits both epidemic and zoonotic coronaviruses. Sci Translat Med 9(396)
Kaddoura M, AlIbrahim M, Hijazi G, Soudani N, Audi A, Alkalamouni H, Haddad S, Eid A, Zaraket H (2020) COVID-19 therapeutic options under investigation. Front Pharmacol 11
Zhu W, Chen CZ, Gorshkov K, Xu M, Lo DC, Zheng W (2020) RNA-dependent RNA polymerase as a target for COVID-19 drug discovery. SLAS DISCOVERY Adv Sci Drug Discov 2472555220942123
Amirian ES, Levy JK (2020) Current knowledge about the antivirals remdesivir (GS-5734) and GS-441524 as therapeutic options for coronaviruses. One Health 100128
Gordon CJ, Tchesnokov EP, Feng JY, Porter DP, Götte M (2020) The antiviral compound remdesivir potently inhibits RNA-dependent RNA polymerase from Middle East respiratory syndrome coronavirus. J Biol Chem 295(15):4773–4779
Cao Y-c, Deng Q-x, Dai S-x (2020) Remdesivir for severe acute respiratory syndrome coronavirus 2 causing COVID-19: an evaluation of the evidence. Travel Med Infect Dis 101647
Ramezankhani R, Solhi R, Memarnejadian A, Nami F, Hashemian SM, Tricot T, Vosough M, Verfaillie C (2020) Therapeutic modalities and novel approaches in regenerative medicine for COVID-19. Int J Antimicrob Agents 106208
Liu W, Morse JS, Lalonde T, Xu S (2020) Learning from the past: possible urgent prevention and treatment options for severe acute respiratory infections caused by 2019‐nCoV. Chembiochem
Kirchdoerfer RN (2020) Halting coronavirus polymerase. J Biol Chem 295(15):4780–4781
Goldman JD, Lye DC, Hui DS, Marks KM, Bruno R, Montejano R, Spinner CD, Galli M, Ahn M-Y, Nahass RG (2020) Remdesivir for 5 or 10 days in patients with severe Covid-19. N Engl J Med
Beigel JH, Tomashek KM, Dodd LE, Mehta AK, Zingman BS, Kalil AC, Hohmann E, Chu HY, Luetkemeyer A, Kline S (2020) Remdesivir for the treatment of Covid-19—preliminary report. N Engl J Med
Grein J, Ohmagari N, Shin D, Diaz G, Asperges E, Castagna A, Feldt T, Green G, Green ML, Lescure F-X (2020) Compassionate use of remdesivir for patients with severe Covid-19. N Engl J Med 382(24):2327–2336
Khan Z, Karataş Y, Ceylan AF, Rahman H (2020) COVID-19 and therapeutic drugs repurposing in hand: the need for collaborative efforts. Le Pharmacien Hospitalier et Clinicien
Wang Y, Zhang D, Du G, Du R, Zhao J, Jin Y, Fu S, Gao L, Cheng Z, Lu Q (2020) Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial. Lancet
WST Consortium (2020) Repurposed antiviral drugs for COVID-19—interim WHO SOLIDARITY trial results. N Engl J Med
Schrezenmeier E, Dörner T (2020) Mechanisms of action of hydroxychloroquine and chloroquine: implications for rheumatology. Nat Rev Rheumatol 1–12
Khuroo MS, Sofi AA, Khuroo M (2020) Chloroquine and Hydroxychloroquine in Coronavirus Disease 2019 (COVID-19). Facts, fiction & the hype. A critical appraisal. Int J Antimicrob Agents 106101
Rolain JM, Colson P, Raoult D (2007) Recycling of chloroquine and its hydroxyl analogue to face bacterial, fungal and viral infections in the 21st century. Int J Antimicrob Agents 30(4):297–308
Yang N, Shen H-M (2020) Targeting the endocytic pathway and autophagy process as a novel therapeutic strategy in COVID-19. Int J Biolog Sci 16(10):1724
Iyer M, Jayaramayya K, Subramaniam MD, Lee SB, Dayem AA, Cho S-G, Vellingiri B (2020) COVID-19: an update on diagnostic and therapeutic approaches. BMB Rep 53(4):191
Roldan EQ, Biasiotto G, Magro P, Zanella I (2020) The possible mechanisms of action of 4-aminoquinolines (chloroquine/hydroxychloroquine) against Sars-Cov-2 infection (COVID-19): a role for iron homeostasis? Pharmacol Res 104904
Wang H, Yang P, Liu K, Guo F, Zhang Y, Zhang G, Jiang C (2008) SARS coronavirus entry into host cells through a novel clathrin-and caveolae-independent endocytic pathway. Cell Res 18(2):290–301
Inoue Y, Tanaka N, Tanaka Y, Inoue S, Morita K, Zhuang M, Hattori T, Sugamura K (2007) Clathrin-dependent entry of severe acute respiratory syndrome coronavirus into target cells expressing ACE2 with the cytoplasmic tail deleted. J Virol 81(16):8722–8729
Yan R, Zhang Y, Li Y, Xia L, Guo Y, Zhou Q (2020) Structural basis for the recognition of SARS-CoV-2 by full-length human ACE2. Science 367(6485):1444–1448
Fantini J, Di Scala C, Chahinian H, Yahi N (2020) Structural and molecular modeling studies reveal a new mechanism of action of chloroquine and hydroxychloroquine against SARS-CoV-2 infection. Int J Antimicrob Agents 105960
Simmons G, Reeves JD, Rennekamp AJ, Amberg SM, Piefer AJ, Bates P (2004) Characterization of severe acute respiratory syndrome-associated coronavirus (SARS-CoV) spike glycoprotein-mediated viral entry. Proc Natl Acad Sci 101(12):4240–4245
Liu J, Cao R, Xu M, Wang X, Zhang H, Hu H, Li Y, Hu Z, Zhong W, Wang M (2020) Hydroxychloroquine, a less toxic derivative of chloroquine, is effective in inhibiting SARS-CoV-2 infection in vitro. Cell Discov 6(1):1–4
Magro G (2020) COVID-19: Review on latest available drugs and therapies against SARS-CoV-2. Coagulation and inflammation cross-talking. Vir Res 198070
Qin C, Zhou L, Hu Z, Zhang S, Yang S, Tao Y, Xie C, Ma K, Shang K, Wang W (2020) Dysregulation of immune response in patients with COVID-19 in Wuhan, China. Clin Infect Dis
Huang M, Li M, Xiao F, Pang P, Liang J, Tang T, Liu S, Chen B, Shu J, You Y (2020) Preliminary evidence from a multicenter prospective observational study of the safety and efficacy of chloroquine for the treatment of COVID-19. Natl Sci Rev 7(9):1428–1436
Borba MGS, Val FFA, Sampaio VS, Alexandre MAA, Melo GC, Brito M, Mourão MPG, Brito-Sousa JD, Baía-da-Silva D, Guerra MVF (2020) Effect of high vs low doses of chloroquine diphosphate as adjunctive therapy for patients hospitalized with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection: a randomized clinical trial. JAMA Netw Open 3(4):e208857–e208857
Yu B, Wang DW, Li C (2020) Hydroxychloroquine application is associated with a decreased mortality in critically ill patients with COVID-19, medRxiv
Mahevas M, Tran V-T, Roumier M, Chabrol A, Paule R, Guillaud C, Gallien S, Lepeule R, Szwebel T-A, Lescure X (2020) No evidence of clinical efficacy of hydroxychloroquine in patients hospitalized for COVID-19 infection with oxygen requirement: results of a study using routinely collected data to emulate a target trial. MedRxiv
Hoffmann M, Mösbauer K, Hofmann-Winkler H, Kaul A, Kleine-Weber H, Krüger N, Gassen NC, Müller MA, Drosten C, Pöhlmann S (2020) Chloroquine does not inhibit infection of human lung cells with SARS-CoV-2. Nature 585(7826):588–590
Mallat J, Hamed F, Balkis M, Mohamed MA, Mooty M, MalikA, Nusair A, Bonilla F (2020) Hydroxychloroquine is associated with slower viral clearance in clinical COVID-19 patients with mild to moderate disease: a retrospective study, medRxiv
McChesney EW (1983) Animal toxicity and pharmacokinetics of hydroxychloroquine sulfate. Am J Med 75(1):11–18
Dong L, Hu S, Gao J (2020) Discovering drugs to treat coronavirus disease 2019 (COVID-19). Drug Discov Therapeut 14(1):58–60
Jin Z, Smith LK, Rajwanshi VK, Kim B, Deval J (2013) The ambiguous base-pairing and high substrate efficiency of T-705 (favipiravir) ribofuranosyl 5′-triphosphate towards influenza A virus polymerase. PLoS ONE 8(7):e68347
Baranovich T, Wong S-S, Armstrong J, Marjuki H, Webby RJ, Webster RG, Govorkova EA (2013) T-705 (favipiravir) induces lethal mutagenesis in influenza A H1N1 viruses in vitro. J Virol 87(7):3741–3751
Furuta Y, Gowen BB, Takahashi K, Shiraki K, Smee DF, Barnard DL (2013) Favipiravir (T-705), a novel viral RNA polymerase inhibitor. Antiviral Res 100(2):446–454
de Mello CPP, Tao X, Kim TH, Vicchiarelli M, Bulitta JB, Kaushik A, Brown AN (2018) Clinical regimens of favipiravir inhibit Zika virus replication in the hollow-fiber infection model. Antimicrob Agents Chemother 62(9)
Du YX, Chen XP (2020) Favipiravir: pharmacokinetics and concerns about clinical trials for 2019‐nCoV infection. Clin Pharmacol Therapeut
Wang Y, Fan G, Salam A, Horby P, Hayden FG, Chen C, Pan J, Zheng J, Lu B, Guo L (2020) Comparative effectiveness of combined favipiravir and oseltamivir therapy versus oseltamivir monotherapy in critically ill patients with influenza virus infection. J Infect Dis 221(10):1688–1698
Cai Q, Yang M, Liu D, Chen J, Shu D, Xia J, Liao X, Gu Y, Cai Q, Yang Y (2020) Experimental treatment with favipiravir for COVID-19: an open-label control study. Engineering
Choy K-T, Wong AY-L, Kaewpreedee P, Sia S-F, Chen D, Hui KPY, Chu DKW, Chan MCW, Cheung PP-H, Huang X (2020) Remdesivir, lopinavir, emetine, and homoharringtonine inhibit SARS-CoV-2 replication in vitro. Antiviral Res 104786
Chen C, Huang J, Cheng Z, Wu J, Chen S, Zhang Y, Chen B, Lu M, Luo Y, Zhang J (2020) Favipiravir versus arbidol for COVID-19: a randomized clinical trial. MedRxiv
Chandwani A, Shuter J (2008) Lopinavir/ritonavir in the treatment of HIV-1 infection: a review. Ther Clin Risk Manag 4(5):1023
Maciorowski D, Idrissi SZE, Gupta Y, Medernach BJ, Burns MB, Becker DP, Durvasula R, Kempaiah P (2020) A review of the preclinical and clinical efficacy of remdesivir, hydroxychloroquine, and lopinavir-ritonavir treatments against COVID-19. SLAS DISCOVERY Advan Sci Drug Discov 2472555220958385
Ratia K, Pegan S, Takayama J, Sleeman K, Coughlin M, Baliji S, Chaudhuri R, Fu W, Prabhakar BS, Johnson ME (2008) A noncovalent class of papain-like protease/deubiquitinase inhibitors blocks SARS virus replication. Proc Natl Acad Sci 105(42):16119–16124
Sanders JM, Monogue ML, Jodlowski TZ, Cutrell JB (2020) Pharmacologic treatments for coronavirus disease 2019 (COVID-19): a review. JAMA 323(18):1824–1836
Lim J, Jeon S, Shin H-Y, Kim MJ, Seong YM, Lee WJ, Choe K-W, Kang YM, Lee B, Park S-J (2020) Case of the index patient who caused tertiary transmission of COVID-19 infection in Korea: the application of lopinavir/ritonavir for the treatment of COVID-19 infected pneumonia monitored by quantitative RT-PCR. J Kor Med Sci 35(6)
Qiu H, Wu J, Hong L, Luo Y, Song Q, Chen D (2020) Clinical and epidemiological features of 36 children with coronavirus disease 2019 (COVID-19) in Zhejiang, China: an observational cohort study. Lancet Infect Dis
Cao B, Wang Y, Wen D, Liu W, Wang J, Fan G, Ruan L, Song B, Cai Y, Wei M (2020) A trial of lopinavir–ritonavir in adults hospitalized with severe Covid-19. N Engl J Med
Hung IF-N, Lung K-C, Tso EY-K, Liu R, Chung TW-H, Chu M-Y, Ng Y-Y, Lo J, Chan J, Tam AR (2020) Triple combination of interferon beta-1b, lopinavir–ritonavir, and ribavirin in the treatment of patients admitted to hospital with COVID-19: an open-label, randomised, phase 2 trial. Lancet 395(10238):1695–1704
Wang Z, Chen X, Lu Y, Chen F, Zhang W (2020) Clinical characteristics and therapeutic procedure for four cases with 2019 novel coronavirus pneumonia receiving combined Chinese and Western medicine treatment. Biosci Trends
Bongiovanni M, Cicconi P, Landonio S, Meraviglia P, Testa L, Di Biagio A, Chiesa E, Tordato F, Bini T, Monforte AdA (2005) Predictive factors of lopinavir/ritonavir discontinuation for drug-related toxicity: results from a cohort of 416 multi-experienced HIV-infected individuals. Int J Antimicrob Agents 26(1):88–91
Wang X, Cao R, Zhang H, Liu J, Xu M, Hu H, Li Y, Zhao L, Li W, Sun X (2020) The anti-influenza virus drug, arbidol is an efficient inhibitor of SARS-CoV-2 in vitro. Cell Discov 6(1):1–5
Jomah S, Asdaq SMB, Al-Yamani MJ (2020) Clinical efficacy of antivirals against novel coronavirus (COVID-19): a review. J Infect Public Health
Zeng L-Y, Yang J, Liu S (2017) Investigational hemagglutinin-targeted influenza virus inhibitors. Expert Opin Investig Drugs 26(1):63–73
Wang Z, Yang B, Li Q, Wen L, Zhang R (2020) Clinical features of 69 cases with coronavirus disease 2019 in Wuhan, China. Clin Infect Dis
Deng L, Li C, Zeng Q, Liu X, Li X, Zhang H, Hong Z, Xia J (2020) Arbidol combined with LPV/r versus LPV/r alone against corona virus disease 2019: a retrospective cohort study. J Infect
Huang H, Guan L, Yang Y, Le Grange JM, Tang G, Xu Y, Yuan J, Lin C, Xue M, Zhang X (2020) Chloroquine, arbidol (umifenovir) or lopinavir/ritonavir as the antiviral monotherapy for COVID-19 patients: a retrospective cohort study
Chen J, Lin S, Niu C, Xiao Q (2020) Clinical evaluation of Shufeng Jiedu Capsules combined with umifenovir (Arbidol) in the treatment of common-type COVID-19: a retrospective study. Exp Rev Respirat Med 1–9
Xu P, Huang J, Fan Z, Huang W, Qi M, Lin X, Song W, Yi L (2020) Arbidol/IFN-α2b therapy for patients with corona virus disease 2019: a retrospective multicenter cohort study. Microbes Infect 22(4–5):200–205
Lian N, Xie H, Lin S, Huang J, Zhao J, Lin Q (2020) Umifenovir treatment is not associated with improved outcomes in patients with coronavirus disease 2019: a retrospective study. Clin Microbiol Infect
Jieming Q (2020) Clinical study of arbidol hydrochloride tablets in the treatment of pneumonia caused by novel coronavirus. NCT04260594
Santos JR, Curran A, Navarro-Mercade J, Ampuero MF, Pelaez P, Perez-Alvarez N, Clotet B, Paredes R, Molto J (2019) Simplification of antiretroviral treatment from darunavir/ritonavir monotherapy to darunavir/cobicistat monotherapy: effectiveness and safety in routine clinical practice. AIDS Res Hum Retroviruses 35(6):513–518
Triant VA, Siedner MJ (2020) Darunavir and cardiovascular risk: evaluating the data to inform clinical care. J Infect Dis 221(4):498–500
Chen J, Xia L, Liu L, Xu Q, Ling Y, Huang D, Huang W, Song S, Xu S, Shen Y (2020) Antiviral activity and safety of darunavir/cobicistat for the treatment of COVID-19, Open forum infectious diseases. Oxford University Press, US, p ofaa241
Graci JD, Cameron CE (2006) Mechanisms of action of ribavirin against distinct viruses. Rev Med Virol 16(1):37–48
Loustaud-Ratti V, Stanke-Labesque F, Marquet P, Gagnieu M-C, Maynard M, Babany G, Trépo C (2009) Optimizing ribavirin dosage: a new challenge to improve treatment efficacy in genotype 1 hepatitis C patients. Gastroentérologie clinique et biologique 33(6–7):580–583
Falzarano D, De Wit E, Rasmussen AL, Feldmann F, Okumura A, Scott DP, Brining D, Bushmaker T, Martellaro C, Baseler L (2013) Treatment with interferon-α2b and ribavirin improves outcome in MERS-CoV–infected rhesus macaques. Nat Med 19(10):1313–1317
Zeng Y-M, Xu X-L, He X-Q, Tang S-Q, Li Y, Huang Y-Q, Harypursat V, Chen Y-K (2020) Comparative effectiveness and safety of ribavirin plus interferon-alpha, lopinavir/ritonavir plus interferon-alpha, and ribavirin plus lopinavir/ritonavir plus interferon-alpha in patients with mild to moderate novel coronavirus disease 2019: study protocol. Chin Med J 133(9):1132–1134
Zhang ZJ, Morris-Natschke SL, Cheng YY, Lee KH, Li RT (2020) Development of anti-influenza agents from natural products. Med Res Rev 40(6):2290–2338
Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, Wang B, Xiang H, Cheng Z, Xiong Y (2020) Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus–infected pneumonia in Wuhan, China. Jama 323(11):1061–1069
Rosa SGV, Santos WC (2020) Clinical trials on drug repositioning for COVID-19 treatment. Revista Panamericana de Salud Pública 44:e40
Caly L, Wagstaff KM, Jans DA (2012) Nuclear trafficking of proteins from RNA viruses: potential target for antivirals? Antiviral Res 95(3):202–206
Caly L, Druce JD, Catton MG, Jans DA, Wagstaff KM (2020) The FDA-approved drug ivermectin inhibits the replication of SARS-CoV-2 in vitro. Antiviral Res 104787
De Salazar PM, Ramos J, Cruz VL, Polo R, Del Amo J, Martínez-Salazar J (2020) Tenofovir and remdesivir ensemble docking with the SARS-CoV-2 polymerase and template-nascent RNA. Authorea Preprints
Drożdżal S, Rosik J, Lechowicz K, Machaj F, Kotfis K, Ghavami S, Łos MJ (2020) FDA approved drugs with pharmacotherapeutic potential for SARS-CoV-2 (COVID-19) therapy. Drug Resist Updates 100719
Ohkoshi M, Oka T (1984) Clinical experience with a protease inhibitor [N, N-dimethylcarbamoylmethyl 4-(4-guanidinobenzoyloxy)-phenylacetate] methanesulfate for prevention of recurrence of carcinoma of the mouth and in treatment of terminal carcinoma. J Maxillofacial Surg 12:148–152
Hoffmann M, Kleine-Weber H, Schroeder S, Krüger N, Herrler T, Erichsen S, Schiergens TS, Herrler G, Wu N-H, Nitsche A (2020) SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell
Zhou Y, Vedantham P, Lu K, Agudelo J, Carrion R Jr, Nunneley JW, Barnard D, Pöhlmann S, McKerrow JH, Renslo AR (2015) Protease inhibitors targeting coronavirus and filovirus entry. Antiviral Res 116:76–84
Bittmann S, Luchter E, Weissenstein A, Villalon G, Moschuring-Alieva E (2020) TMPRSS2-inhibitors play a role in cell entry mechanism of COVID-19: an insight into camostat and nefamostat. J Regen Biol Med 2(2):1–3
Jang S, Rhee J-Y (2020) Three cases of treatment with Nafamostat in elderly patients with COVID-19 pneumonia who need oxygen therapy. Int J Inf Dis
Ragia G, Manolopoulos VG (2020) Inhibition of SARS-CoV-2 entry through the ACE2/TMPRSS2 pathway: a promising approach for uncovering early COVID-19 drug therapies. Eur J Clin Pharmacol 1–8
Hempel T, Raich L, Olsson S, Azouz NP, Klingler AM, Rothenberg ME, Noé F (2020) Molecular mechanism of SARS-CoV-2 cell entry inhibition via TMPRSS2 by Camostat and Nafamostat mesylate. BioRxiv
Toots M, Yoon J-J, Cox RM, Hart M, Sticher ZM, Makhsous N, Plesker R, Barrena AH, Reddy PG, Mitchell DG (2019) Characterization of orally efficacious influenza drug with high resistance barrier in ferrets and human airway epithelia. Sci Translat Med 11(515)
Toots M, Yoon J-J, Hart M, Natchus MG, Painter GR, Plemper RK (2020) Quantitative efficacy paradigms of the influenza clinical drug candidate EIDD-2801 in the ferret model. Translat Res 218:16–28
Abdelnabi R, Foo CS, Kaptein SJ, Zhang X, Langendries L, Vangeel L, Vergote V, Heylen E, Dallmeier K, Chatterjee A (2020) Molnupiravir (EIDD-2801) inhibits SARS-CoV2 replication in Syrian hamsters model
Eslami G, Mousaviasl S, Radmanesh E, Jelvay S, Bitaraf S, Simmons B, Wentzel H, Hill A, Sadeghi A, Freeman J (2020) The impact of sofosbuvir/daclatasvir or ribavirin in patients with severe COVID-19. J Antimicrob Chemother 75(11):3366–3372
Ju J, Li X, Kumar S, Jockusch S, Chien M, Tao C, Morozova I, Kalachikov S, Kirchdoerfer R, Russo JJ (2020) Nucleotide analogues as inhibitors of SARS-CoV polymerase. BioRxiv
Abbaspour Kasgari H, Moradi S, Shabani AM, Babamahmoodi F, Davoudi Badabi AR, Davoudi L, Alikhani A, Hedayatizadeh Omran A, Saeedi M, Merat S (2020) Evaluation of the efficacy of sofosbuvir plus daclatasvir in combination with ribavirin for hospitalized COVID-19 patients with moderate disease compared with standard care: a single-centre, randomized controlled trial. J Antimicrob Chemother 75(11):3373–3378
Elfiky AA (2020) Ribavirin, remdesivir, sofosbuvir, galidesivir, and tenofovir against SARS-CoV-2 RNA dependent RNA polymerase (RdRp): a molecular docking study, Life Sci 117592
Bourinbaiar AS, Fruhstorfer EC (1996) The effect of histamine type 2 receptor antagonists on human immunodeficiency virus (HIV) replication: identification of a new class of antiviral agents. Life Sci 59(23):PL365–PL370
Wu C, Liu Y, Yang Y, Zhang P, Zhong W, Wang Y, Wang Q, Xu Y, Li M, Li X (2020) Analysis of therapeutic targets for SARS-CoV-2 and discovery of potential drugs by computational methods. Acta Pharmaceutica Sinica B
Rossignol J-F (2016) Nitazoxanide, a new drug candidate for the treatment of Middle East respiratory syndrome coronavirus. J Infect Public Health 9(3):227–230
Yavuz S, Ünal S (2020) Antiviral treatment of COVID-19. Turk J Med Sci 50(SI-1):611–619
Xu Z, Yao H, Shen J, Wu N, Xu Y, Lu X, Li L-J (2020) Nelfinavir is active against SARS-CoV-2 in Vero E6 cells
Yamamoto N, Matsuyama S, Hoshino T, Yamamoto N (2020) Nelfinavir inhibits replication of severe acute respiratory syndrome coronavirus 2 in vitro. BioRxiv
Ohashi H, Watashi K, Saso W, Shionoya K, Iwanami S, Hirokawa T, Shirai T, Kanaya S, Ito Y, Kim KS (2020) Multidrug treatment with nelfinavir and cepharanthine against COVID-19, bioRxiv
Cui W, Yang K, Yang H (2020) Recent progress in the drug development targeting SARS-CoV-2 main protease as treatment for COVID-19. Front Mole Biosci 7
Sakamoto J, Hamada C, Rahman M, Kodaira S, Ito K, Nakazato H, Ohashi Y, Yasutomi M (2005) An individual patient data meta-analysis of adjuvant therapy with carmofur in patients with curatively resected colon cancer. Jpn J Clin Oncol 35(9):536–544
Morimoto K, Koh M (2003) Postoperative adjuvant use of carmofur for early breast cancer. Osaka City Med J 49(2):77–84
Wang R-R, Yang Q-H, Luo R-H, Peng Y-M, Dai S-X, Zhang X-J, Chen H, Cui X-Q, Liu Y-J, Huang J-F (2014) Azvudine, a novel nucleoside reverse transcriptase inhibitor showed good drug combination features and better inhibition on drug-resistant strains than lamivudine in vitro. PLoS ONE 9(8):e105617
Phillip JM, Wu P-H, Gilkes DM, Williams W, McGovern S, Daya J, Chen J, Aifuwa I, Lee JS, Fan R (2017) Biophysical and biomolecular determination of cellular age in humans. Nat Biomed Eng 1(7):0093
Chen J, Lau YF, Lamirande EW, Paddock CD, Bartlett JH, Zaki SR, Subbarao K (2010) Cellular immune responses to severe acute respiratory syndrome coronavirus (SARS-CoV) infection in senescent BALB/c mice: CD4 + T cells are important in control of SARS-CoV infection. J Virol 84(3):1289–1301
Leng Z, Zhu R, Hou W, Feng Y, Yang Y, Han Q, Shan G, Meng F, Du D, Wang S (2020) Transplantation of ACE2-mesenchymal stem cells improves the outcome of patients with COVID-19 pneumonia. Aging Dis 11(2):216
Canham MA, Campbell JD, Mountford JC (2020) The use of mesenchymal stromal cells in the treatment of coronavirus disease 2019. J Translat Med 18(1):1–15
Cao Y, Wu H, Zhai W, Wang Y, Li M, Li M, Yang L, Tian Y, Song Y, Li J (2020) A safety consideration of mesenchymal stem cell therapy on COVID-19. Stem Cell Res 49:102066
Rajarshi K, Chatterjee A, Ray S (2020) Combating COVID-19 with Mesenchymal stem cell therapy. Biotechnol Rep e00467
Lin F-C, Young HA (2014) Interferons: success in anti-viral immunotherapy. Cytokine Growth Factor Rev 25(4):369–376
Ivashkiv LB, Donlin LT (2014) Regulation of type I interferon responses. Nat Rev Immunol 14(1):36–49
Nile SH, Nile A, Qiu J, Li L, Jia X, Kai G (2020) COVID-19: pathogenesis, cytokine storm and therapeutic potential of interferons. Cytokine Growth Factor Rev
Sallard E, Lescure F-X, Yazdanpanah Y, Mentre F, Peiffer-Smadja N, Florence A, Yazdanpanah Y, Mentre F, Lescure F-X, Peiffer-Smadja N (2020) Type 1 interferons as a potential treatment against COVID-19. Antiviral Res 104791
Schreiber G (2020) The role of type I interferons in the pathogenesis and treatment of COVID-19. Front Immunol 11
Lokugamage KG, Hage A, Schindewolf C, Rajsbaum R, Menachery VD (2020) SARS-CoV-2 is sensitive to type I interferon pretreatment. BioRxiv
Wang N, Zhan Y, Zhu L, Hou Z, Liu F, Song P, Qiu F, Wang X, Zou X, Wan D (2020) Retrospective multicenter cohort study shows early interferon therapy is associated with favorable clinical responses in COVID-19 patients. Cell Host Microbe 28(3):455–464, e2
Cinatl J, Morgenstern B, Bauer G, Chandra P, Rabenau H, Doerr H (2003) Treatment of SARS with human interferons. Lancet 362(9380):293–294
Kindler E, Thiel V, Weber F (2016) Interaction of SARS and MERS coronaviruses with the antiviral interferon response. Adv Virus Res 96:219–243
Abdolvahab MH, Moradi-Kalbolandi S, Zarei M, Bose D, Majidzadeh-A K, Farahmand L (2020) Potential role of interferons in treating COVID-19 patients. Int Immunopharmacol 107171
Bloch EM, Shoham S, Casadevall A, Sachais BS, Shaz B, Winters JL, van Buskirk C, Grossman BJ, Joyner M, Henderson JP (2020) Deployment of convalescent plasma for the prevention and treatment of COVID-19. J Clin Investig 130(6):2757–2765
Shen C, Wang Z, Zhao F, Yang Y, Li J, Yuan J, Wang F, Li D, Yang M, Xing L (2020) Treatment of 5 critically ill patients with COVID-19 with convalescent plasma. JAMA 323(16):1582–1589
Duan K, Liu B, Li C, Zhang H, Yu T, Qu J, Zhou M, Chen L, Meng S, Hu Y (2020) Effectiveness of convalescent plasma therapy in severe COVID-19 patients. Proc Natl Acad Sci 117(17):9490–9496
Zhang B, Liu S, Tan T, Huang W, Dong Y, Chen L, Chen Q, Zhang L, Zhong Q, Zhang X (2020) Treatment with convalescent plasma for critically ill patients with SARS-CoV-2 infection. Chest
Joyner MJ, Bruno KA, Klassen SA, Kunze KL, Johnson PW, Lesser ER, Wiggins CC, Senefeld JW, Klompas AM, Hodge DO (2020) Safety update: COVID-19 convalescent plasma in 20,000 hospitalized patients. In: Mayo Clinic Proceedings, Elsevier, pp 1888–1897
Ye M, Fu D, Ren Y, Wang F, Wang D, Zhang F, Xia X, Lv T (2020) Treatment with convalescent plasma for COVID‐19 patients in Wuhan, China. J Med Virol
Mangalmurti N, Hunter CA (2020) Cytokine storms: understanding COVID-19. Immunity
Channappanavar R, Perlman S (2017) Pathogenic human coronavirus infections: causes and consequences of cytokine storm and immunopathology, Seminars in immunopathology, Springer, pp 529–539
Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, Manson JJ, HAS Collaboration (2020) COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet (London, England) 395(10229):1033
Seif F, Aazami H, Khoshmirsafa M, Kamali M, Mohsenzadegan M, Pornour M, Mansouri D (2020) JAK inhibition as a new treatment strategy for patients with COVID-19. Int Arch Allergy Immunol 181(6):467–475
Sehgal S (2003) Sirolimus: its discovery, biological properties, and mechanism of action. In: Transplantation proceedings. Elsevier, pp S7–S14
Zhou Y, Hou Y, Shen J, Huang Y, Martin W, Cheng F (2020) Network-based drug repurposing for novel coronavirus 2019-nCoV/SARS-CoV-2. Cell Discov 6(1):1–18
Kindrachuk J, Ork B, Hart BJ, Mazur S, Holbrook MR, Frieman MB, Traynor D, Johnson RF, Dyall J, Kuhn JH (2015) Antiviral potential of ERK/MAPK and PI3K/AKT/mTOR signaling modulation for Middle East respiratory syndrome coronavirus infection as identified by temporal kinome analysis. Antimicrob Agents Chemother 59(2):1088–1099
Mogul A, Corsi K, McAuliffe L (2019) Baricitinib: the second FDA-approved JAK inhibitor for the treatment of rheumatoid arthritis. Ann Pharmacother 53(9):947–953
Cantini F, Niccoli L, Matarrese D, Nicastri E, Stobbione P, Goletti D (2020) Baricitinib therapy in COVID-19: a pilot study on safety and clinical impact. J Infect
Stebbing J, Phelan A, Griffin I, Tucker C, Oechsle O, Smith D, Richardson P (2020) COVID-19: combining antiviral and anti-inflammatory treatments. Lancet Infect Dis 20(4):400–402
Richardson P, Griffin I, Tucker C, Smith D, Oechsle O, Phelan A, Stebbing J (2020) Baricitinib as potential treatment for 2019-nCoV acute respiratory disease. Lancet, Lancet Publishing Group
Tang JW, Young S, May S, Bird P, Bron J, Mohamedanif T, Bradley C, Patel D, Holmes CW, Kwok KO (2020) Comparing hospitalised, community and staff COVID-19 infection rates during the early phase of the evolving COVID-19 epidemic. J Infect
Mesa RA, Yasothan U, Kirkpatrick P, Ruxolitinib, Nature Publishing Group (2012)
Harrison C, Kiladjian J-J, Al-Ali HK, Gisslinger H, Waltzman R, Stalbovskaya V, McQuitty M, Hunter DS, Levy R, Knoops L (2012) JAK inhibition with ruxolitinib versus best available therapy for myelofibrosis. N Engl J Med 366(9):787–798
Neubauer A, Wiesmann T, Vogelmeier CF, Mack E, Skevaki C, Gaik C, Keller C, Figiel J, Sohlbach K, Rolfes C (2020) Ruxolitinib for the treatment of SARS-CoV-2 induced acute respiratory distress syndrome (ARDS). Leukemia 34(8):2276–2278
Li H, Liu H (2020) Whether the timing of patient randomization interferes with the assessment of the efficacy of Ruxolitinib for severe COVID-19. J Allergy Clin Immunol 146(6):1453
Fujita T, Inoue K, Yamamoto S, Ikumoto T, Sasaki S, Toyama R, Chiba K, Hoshino Y, Okumoto T (1994) Fungal metabolites. Part 11. A potent immunosuppressive activity found in Isaria sinclairii metabolite. J Antibiot 47(2):208–215
Brinkmann V (2009) FTY720 (fingolimod) in multiple sclerosis: therapeutic effects in the immune and the central nervous system. Br J Pharmacol 158(5):1173–1182
Ingwersen J, Aktas O, Kuery P, Kieseier B, Boyko A, Hartung H-P (2012) Fingolimod in multiple sclerosis: mechanisms of action and clinical efficacy. Clin Immunol 142(1):15–24
Franks ME, Macpherson GR, Figg WD (2004) Thalidomide. The Lancet 363(9423):1802–1811
Newfield C (2018) New medical indications for thalidomide and its derivatives. Sci J Lander Coll Arts Sci 12(1):3
Paravar T, Lee DJ (2008) Thalidomide: mechanisms of action. Int Rev Immunol 27(3):111–135
Robb CT, Goepp M, Rossi AG, Yao C (2020) Non-steroidal anti-inflammatory drugs, prostaglandins, and COVID-19. Br J Pharmacol 177(21):4899–4920
FitzGerald GA (2020) Misguided drug advice for COVID-19. Science 367(6485):1434
Capuano A, Scavone C, Racagni G, Scaglione F (2020) NSAIDs in patients with viral infections, including Covid-19: victims or perpetrators? Pharmacol Res 104849
Crosby JC, Heimann MA, Burleson SL, Anzalone BC, Swanson JF, Wallace DW, Greene CJ (2020) COVID‐19: a review of therapeutics under investigation. J Am Coll Emer Phys Open
Zheng W, Fan W, Zhang S, Jiao P, Shang Y, Cui L, Mahesutihan M, Li J, Wang D, Gao GF (2019) Naproxen exhibits broad anti-influenza virus activity in mice by impeding viral nucleoprotein nuclear export. Cell Rep 27(6):1875–1885, e5
Castro VM, Ross RA, McBride SM, Perlis RH (2020) Identifying common pharmacotherapies associated with reduced COVID-19 morbidity using electronic health records, medRxiv
Rainsford K (2009) Ibuprofen: pharmacology, efficacy and safety. Inflammopharmacology 17(6):275–342
Mititelu RR, Pădureanu R, Băcănoiu M, Pădureanu V, Docea AO, Calina D, Barbulescu AL, Buga AM (2020) Inflammatory and oxidative stress markers—mirror tools in rheumatoid arthritis. Biomedicines 8(5):125
Cole GM, Frautschy SA (2010) Mechanisms of action of non-steroidal anti-inflammatory drugs for the prevention of Alzheimer’s disease. CNS Neurol Disorders-Drug Targets (Formerly Current Drug Targets-CNS & Neurological Disorders) 9(2):140–148
Smart L, Fawkes N, Goggin P, Pennick G, Rainsford K, Charlesworth B, Shah N A narrative review of the potential pharmacological influence and safety of ibuprofen on coronavirus disease 19 (COVID-19), ACE2, and the immune system: a dichotomy of expectation and reality. Inflammopharmacology 1–12
Batlle D, Wysocki J, Satchell K (2020) Soluble angiotensin-converting enzyme 2: a potential approach for coronavirus infection therapy? Clin Sci 134(5):543–545
Rinott E, Kozer E, Shapira Y, Bar-Haim A, Youngster I (2020) Ibuprofen use and clinical outcomes in COVID-19 patients. Clin Microbiol Inf
McCrae J, Morrison E, MacIntyre I, Dear J, Webb D (2018) Long-term adverse effects of paracetamol–a review. Br J Clin Pharmacol 84(10):2218–2230
Graham GG, Scott KF (2005) Mechanism of action of paracetamol. Am J Ther 12(1):46–55
Roberts E, Nunes VD, Buckner S, Latchem S, Constanti M, Miller P, Doherty M, Zhang W, Birrell F, Porcheret M (2016) Paracetamol: not as safe as we thought? A systematic literature review of observational studies. Ann Rheumat Dis 75(3):552–559
Leont’ev D, Babaev B, Shishkov M, Ostreĭkov I (2005) Effect of nonsteroidal anti-inflammatory drugs and paracetamol on hemodynamic changes during postoperative analgesia in children. Anesteziologiia i reanimatologiia (1):22
Sestili P, Fimognari C (2020) Paracetamol use in COVID-19: friend or enemy?
Ye Z, Wang Y, Colunga-Lozano LE, Prasad M, Tangamornsuksan W, Rochwerg B, Yao L, Motaghi S, Couban RJ, Ghadimi M (2020) Efficacy and safety of corticosteroids in COVID-19 based on evidence for COVID-19, other coronavirus infections, influenza, community-acquired pneumonia and acute respiratory distress syndrome: a systematic review and meta-analysis. CMAJ
Cheng W, Li Y, Cui L, Chen Y, Shan S, Xiao D, Chen X, Chen Z, Xu A (2020) Efficacy and safety of corticosteroid treatment in patients with COVID-19: a systematic review and meta-analysis. Front Pharmacol 11:1378
Russell CD, Millar JE, Baillie JK (2020) Clinical evidence does not support corticosteroid treatment for 2019-nCoV lung injury. Lancet 395(10223):473–475
Bhimraj A, Morgan RL, Shumaker AH, Lavergne V, Baden L, Cheng VC-C, Edwards KM, Gandhi R, Muller WJ, O’Horo JC (2020) Infectious diseases Society of America guidelines on the treatment and management of patients with COVID-19. Clin Inf Dis
Dexamethasone in hospitalized patients with Covid-19—preliminary report. N Engl J Med (2020)
Peters DH, Friedel HA, McTavish D (1992) Azithromycin. Drugs 44(5):750–799
Dunn CJ, Barradell LB (1996) Azithromycin. Drugs 51(3):483–505
Echeverría-Esnal D, Martin-Ontiyuelo C, Navarrete-Rouco ME, De-Antonio Cuscó M, Ferrández O, Horcajada JP, Grau S (2020) Azithromycin in the treatment of COVID-19: a review. Exp Rev Anti-inf Ther 1–17
Arshad S, Kilgore P, Chaudhry ZS, Jacobsen G, Wang DD, Huitsing K, Brar I, Alangaden GJ, Ramesh MS, McKinnon JE (2020) Treatment with hydroxychloroquine, azithromycin, and combination in patients hospitalized with COVID-19. Int J Infect Dis 97:396–403
Campoli-Richards DM, Brogden RN, Faulds D (1990) Teicoplanin. Drugs 40(3):449–486
Baron SA, Devaux C, Colson P, Raoult D, Rolain J-M (2020) Teicoplanin: an alternative drug for the treatment of coronavirus COVID-19. Int J Antimicrob Agents 105944(10.1016)
Zhang J, Ma X, Yu F, Liu J, Zou F, Pan T, Zhang H (2020) Teicoplanin potently blocks the cell entry of 2019-nCoV. BioRxiv
Klein NC, Cunha BA (1995) Tetracyclines. Med Clin North Am 79(4):789–801
Nelson ML, Levy SB (2011) The history of the tetracyclines. Ann N Y Acad Sci 1241(1):17–32
Henehan M, Montuno M, De Benedetto A (2017) Doxycycline as an anti-inflammatory agent: updates in dermatology. J Eur Acad Dermatol Venereol 31(11):1800–1808
Aggarwal HK, Jain D, Talapatra P, Yadav RK, Gupta T, Kathuria KL (2010) Evaluation of role of doxycycline (a matrix metalloproteinase inhibitor) on renal functions in patients of diabetic nephropathy. Ren Fail 32(8):941–946
Phillips JM, Gallagher T, Weiss SR (2017) Neurovirulent murine coronavirus JHM. SD uses cellular zinc metalloproteases for virus entry and cell-cell fusion. J Virol 91(8)
Poinas A, Boutoille D, Vrignaud F, Nguyen J-M, Bonnet F, Rat C, Garcia G, Dompmartin A, Leccia M-T, Piroth L (2020) Impact of doxycycline on Covid-19 patients with risk factors of disease degradation: dynamic, a randomised controlled double-blind trial
Mattioli M, Benfaremo D, Mancini M, Mucci L, Mainquà P, Polenta A, Baldini PM, Fulgenzi F, Dennetta D, Bedetta S (2020) Safety of intermediate dose of low molecular weight heparin in COVID-19 patients. J Thromb Thrombol 1–7
Cosmi B, Hirsh J (1994) Low molecular weight heparins. Curr Opin Cardiol 9(5):612–618
Belen-Apak FB, Sarialioglu F (2020) The old but new: Can unfractioned heparin and low molecular weight heparins inhibit proteolytic activation and cellular internalization of SARS-CoV2 by inhibition of host cell proteases? Med Hypotheses 109743
Shi C, Wang C, Wang H, Yang C, Cai F, Zeng F, Cheng F, Liu Y, Zhou T, Deng B (2020) The potential of low molecular weight heparin to mitigate cytokine storm in severe COVID-19 patients: a retrospective clinical study. Medrxiv
Panfili FM, Roversi M, D’Argenio P, Rossi P, Cappa M, Fintini D (2020) Possible role of vitamin D in Covid-19 infection in pediatric population. J Endocrinol Invest
Hansdottir S, Monick MM (2011) Vitamin D effects on lung immunity and respiratory diseases. Vit Hormon, Elsevier 217–237
Hernández JL, Nan D, Fernandez-Ayala M, García-Unzueta M, Hernández-Hernández MA, López-Hoyos M, Muñoz-Cacho P, Olmos JM, Gutiérrez-Cuadra M, Ruiz-Cubillán JJ (2020) Vitamin D status in hospitalized patients with SARS-CoV-2 infection. J Clin Endocrinol Metabol
Van Gorkom GN, Klein Wolterink RG, Van Elssen CH, Wieten L, Germeraad WT, Bos GM (2018) Influence of vitamin C on lymphocytes: an overview. Antioxidants 7(3):41
Feyaerts AF, Luyten W (2020) Vitamin C as prophylaxis and adjunctive medical treatment for COVID-19? Nutrition 79:110948
Cheng RZ (2020) Can early and high intravenous dose of vitamin C prevent and treat coronavirus disease 2019 (COVID-19)? Med Drug Discov 5:100028
Pal A, Squitti R, Picozza M, Pawar A, Rongioletti M, Dutta AK, Sahoo S, Goswami K, Sharma P, Prasad R (2020) Zinc and COVID-19: basis of current clinical trials. Biol Trace Element Res 1–11
Bao S, Knoell DL (2006) Zinc modulates cytokine-induced lung epithelial cell barrier permeability. Am J Physiol-Lung Cell Molecul Physiol 291(6):L1132–L1141
Lowenstein CJ, Dinerman JL, Snyder SH (1994) Nitric oxide: a physiologic messenger. Ann Intern Med 120(3):227–237
Bruckdorfer R (2005) The basics about nitric oxide. Mol Aspects Med 26(1–2):3–31
Darwish I, Miller C, Kain KC, Liles WC (2012) Inhaled nitric oxide therapy fails to improve outcome in experimental severe influenza. Int J Med Sci 9(2):157
De Groote MA, Fang FC (1995) NO inhibitions: antimicrobial properties of nitric oxide. Clin Inf Dis 21(Supplement_2):S162–S165
Croen KD (1993) Evidence for antiviral effect of nitric oxide. Inhibition of herpes simplex virus type 1 replication. J Clin Investigat 91(6):2446–2452
Martel J, Ko Y-F, Young JD, Ojcius DM (2020) Could nasal nitric oxide help to mitigate the severity of COVID-19?
Subir R (2020) Pros and cons for use of statins in people with coronavirus disease-19 (COVID-19). Diab Metabol Syn Clin Res Rev 14(5):1225–1229
Yuan S (2015) Statins may decrease the fatality rate of Middle East respiratory syndrome infection. MBio 6(4)
Totura AL, Baric RS (2015) Reply to “statins may decrease the fatality rate of MERS infection. Mbio 6(5)
Chauvin B, Drouot S, Barrail-Tran A, Taburet A-M (2013) Drug–drug interactions between HMG-CoA reductase inhibitors (statins) and antiviral protease inhibitors. Clin Pharmacokinet 52(10):815–831
Bełtowski J, Wójcicka G, Jamroz-Wiśniewska A (2009) Adverse effects of statins—mechanisms and consequences. Curr Drug Saf 4(3):209–228
Sica DA, Gehr TW, Ghosh S (2005) Clinical pharmacokinetics of losartan. Clin Pharmacokinet 44(8):797–814
Salvi R, Patankar P (2020) Emerging pharmacotherapies for COVID-19. Biomed Pharmacother 110267
Alzghari SK, Acuña VS (2020) Supportive treatment with tocilizumab for COVID-19: a systematic review. J Clin Virol 127:104380
Wösten‐van Asperen RM, Lutter R, Specht PA, Moll GN, van Woensel JB, van der Loos CM, van Goor H, Kamilic J, Florquin S, Bos AP (2011) Acute respiratory distress syndrome leads to reduced ratio of ACE/ACE2 activities and is prevented by angiotensin‐(1–7) or an angiotensin II receptor antagonist. J Pathol 225(4):618–627
Khan A, Benthin C, Zeno B, Albertson TE, Boyd J, Christie JD, Hall R, Poirier G, Ronco JJ, Tidswell M (2017) A pilot clinical trial of recombinant human angiotensin-converting enzyme 2 in acute respiratory distress syndrome. Crit Care 21(1):1–9
Monteil V, Kwon H, Prado P, Hagelkrüys A, Wimmer RA, Stahl M, Leopoldi A, Garreta E, Del Pozo CH, Prosper F (2020) Inhibition of SARS-CoV-2 infections in engineered human tissues using clinical-grade soluble human ACE2. Cell
Li M, Rao C, Pei D, Wang L, Li Y, Gao K, Wang M, Wang J (2014) Novaferon, a novel recombinant protein produced by DNA-shuffling of IFN-α, shows antitumor effect in vitro and in vivo. Cancer Cell Int 14(1):8
Mousavi SM, Hashemi SA, Parvin N, Gholami A, Ramakrishna S, Omidifar N, Moghadami M, Chiang W-H, Mazraedoost S (2020) Recent biotechnological approaches for treatment of novel COVID-19: from bench to clinical trial. Drug Metabol Rev 1–30
Zheng F, Zhou Y, Zhou Z, Ye F, Huang B, Huang Y, Ma J, Zuo Q, Tan X, Xie J (2020) SARS-CoV-2 clearance in COVID-19 patients with Novaferon treatment: a randomized, open-label, parallel-group trial. Int J Infect Dis 99:84–91
Luo P, Liu Y, Qiu L, Liu X, Liu D, Li J (2020) Tocilizumab treatment in COVID-19: a single center experience. J Med Virol 92(7):814–818
Fu B, Xu X, Wei H (2020) Why tocilizumab could be an effective treatment for severe COVID-19? J Translat Med 18(1):1–5
Yao X, Huang J, Zhong H, Shen N, Faggioni R, Fung M, Yao Y (2014) Targeting interleukin-6 in inflammatory autoimmune diseases and cancers. Pharmacol Ther 141(2):125–139
Yousefi H, Mashouri L, Okpechi SC, Alahari N, Alahari SK (2020) Repurposing existing drugs for the treatment of COVID-19/SARS-CoV-2 infection: a review describing drug mechanisms of action. Biochem Pharmacol 114296
López RL, Fernández SC, Pérez LL, Palacios AR, Fernández-Roldán MC, Alonso EA, Camacho IP, Rodriguez-Baño J, Merchante N, Olalla J (2020) Efficacy and safety of early treatment with sarilumab in hospitalised adults with COVID-19 presenting cytokine release syndrome (SARICOR STUDY): protocol of a phase II, open-label, randomised, multicentre, controlled clinical trial. BMJ Open 10(11):e039951
Mahajan R, Lipton M, Broglie L, Jain NG, Uy NS (2020) Eculizumab treatment for renal failure in a pediatric patient with COVID-19. J Nephrol 33(6):1373–1376
Dixit SB, Zirpe KG, Kulkarni AP, Chaudhry D, Govil D, Mehta Y, Jog SA, Khatib KI, Pandit RA, Samavedam S (2020) Current approaches to COVID-19: therapy and prevention. Ind J Crit Care Med: Peer-Reviewed, Official Publication of Indian Society of Critical Care Medicine 24(9):838
Scavone C, Brusco S, Bertini M, Sportiello L, Rafaniello C, Zoccoli A, Berrino L, Racagni G, Rossi F, Capuano A (2020) Current pharmacological treatments for COVID‐19: what’s next? Br J Pharmacol
Rudrapal M, Khairnar SJ, Borse LB, Jadhav AG (2020) Coronavirus disease-2019 (COVID-19): an updated review. Drug Res 70(9):389
Pang J, Xu F, Aondio G, Li Y, Fumagalli A, Lu M, Valmadre G, Wei J, Bian Y, Canesi M (2020) Efficacy and tolerability of bevacizumab in patients with severe Covid-19, medRxiv
Kumar A, Dey AD, Behl T, Chadha S, Aggarwal V (2020) Exploring the multifocal therapeutic approaches in COVID-19: a ray of hope. Int Immunopharmacol
Abdin SM, Elgendy SM, Alyammahi SK, Alhamad DW, Omar HA (2020) Tackling the cytokine storm in COVID-19, challenges, and hopes. Life Sci 118054
Nicolela Susanna F, Pavesio C (2020) A review of ocular adverse events of biological anti-TNF drugs. J Ophthal Inflamm Infect 10:1–9
Dinarello CA (2018) Overview of the IL-1 family in innate inflammation and acquired immunity. Immunol Rev 281(1):8–27
Monteagudo LA, Boothby A, Gertner E (2020) Continuous intravenous anakinra infusion to calm the cytokine storm in macrophage activation syndrome. ACR Open Rheumatol 2(5):276–282
Al-Salama ZT (2019) Emapalumab: first global approval. Drugs 79(1):99–103
Liu B, Li M, Zhou Z, Guan X, Xiang Y (2020) Can we use interleukin-6 (IL-6) blockade for coronavirus disease 2019 (COVID-19)-induced cytokine release syndrome (CRS)? J Autoimmun 102452
Jin Y, Yang H, Ji W, Wu W, Chen S, Zhang W, Duan G (2020) Virology, epidemiology, pathogenesis, and control of COVID-19. Viruses 12(4):372
Landras A, Reger de Moura C, Jouenne F, Lebbe C, Menashi S, Mourah S (2019) CD147 is a promising target of tumor progression and a prognostic biomarker. Cancers 11(11):1803
Trivedi N, Verma A, Kumar D (2020) Possible treatment and strategies for COVID-19: review and assessment. Eur Rev Med Pharmacol Sci 24:12593–12608
Bian H, Zheng Z-H, Wei D, Zhang Z, Kang W-Z, Hao C-Q, Dong K, Kang W, Xia J-L, Miao J-L (2020) Meplazumab treats COVID-19 pneumonia: an open-labelled, concurrent controlled add-on clinical trial. MedRxiv
Xian Y, Zhang J, Bian Z, Zhou H, Zhang Z, Lin Z, Xu H (2020) Bioactive natural compounds against human coronaviruses: a review and perspective. Acta Pharmaceutica Sinica B
Mani JS, Johnson JB, Steel JC, Broszczak DA, Neilsen PM, Walsh KB, Naiker M (2020) Natural product-derived phytochemicals as potential agents against coronaviruses: a review. Virus Res 197989
Soni VK, Mehta A, Ratre YK, Tiwari AK, Amit A, Singh RP, Sonkar SC, Chaturvedi N, Shukla D, Vishvakarma NK (2020) Curcumin, a traditional spice component, can hold the promise against COVID-19? Eur J Pharmacol 173551
Abolhassani H, Shojaosadati SA (2019) A comparative and systematic approach to desolvation and self-assembly methods for synthesis of piperine-loaded human serum albumin nanoparticles. Colloids Surf, B 184:110534
Srinivasan K (2007) Black pepper and its pungent principle-piperine: a review of diverse physiological effects. Crit Rev Food Sci Nutr 47(8):735–748
Abolhassani H, Safavi MS, Handali S, Nosrati M, Shojaosadati SA (2020) Synergistic effect of self-assembled curcumin and piperine co-loaded human serum albumin nanoparticles on suppressing cancer cells. Drug Dev Ind Pharm 46(10):1647–1655
Kumari A, Yadav SK, Pakade YB, Singh B, Yadav SC (2010) Development of biodegradable nanoparticles for delivery of quercetin. Colloids Surf, B 80(2):184–192
Siddiqui AJ, Jahan S, Ashraf SA, Alreshidi M, Ashraf MS, Patel M, Snoussi M, Singh R, Adnan M (2020) Current status and strategic possibilities on potential use of combinational drug therapy against COVID-19 caused by SARS-CoV-2. J Biomole Struct Dyn 1–14
Shyr ZA, Gorshkov K, Chen CZ, Zheng W (2020) Drug discovery strategies for SARS-CoV-2. J Pharmacol Exp Ther 375(1):127–138
Kalil AC, Patterson TF, Mehta AK, Tomashek KM, Wolfe CR, Ghazaryan V, Marconi VC, Ruiz-Palacios GM, Hsieh L, Kline S (2020) Baricitinib plus remdesivir for hospitalized adults with Covid-19. N Engl J Med
Zhao H, Zhu Q, Zhang C, Li J, Wei M, Qin Y, Chen G, Wang K, Yu J, Wu Z (2020) Tocilizumab combined with favipiravir in the treatment of COVID-19: a multicenter trial in a small sample size. Biomed Pharmacother 133:110825
Gautret P, Lagier J-C, Parola P, Meddeb L, Mailhe M, Doudier B, Courjon J, Giordanengo V, Vieira VE, Dupont HT (2020) Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Int J Antimicrob Agents 105949
Chen H, Zhang Z, Wang L, Huang Z, Gong F, Li X, Chen Y (2020) First clinical study using HCV protease inhibitor danoprevir to treat naive and experienced COVID-19 patients. MedRxiv
Peng H, Gong T, Huang X, Sun X, Luo H, Wang W, Luo J, Luo B, Chen Y, Wang X (2020) A synergistic role of convalescent plasma and mesenchymal stem cells in the treatment of severely ill COVID-19 patients: a clinical case report. Stem Cell Res Therapy 11(1):1–6
Colunga Biancatelli RML, Berrill M, Catravas JD, Marik PE (2020) Quercetin and vitamin C: an experimental, synergistic therapy for the prevention and treatment of SARS-CoV-2 related disease (COVID-19). Front Immunol 11:1451
Acknowledgements
The authors would like to thank Maria Tajbakhsh Rigi (MD) for sharing her experience in the treatment of COVID-19 patients.
Author information
Authors and Affiliations
Corresponding authors
Editor information
Editors and Affiliations
Appendices
Financial support
This research did not receive any specific fundings.
Conflict of interest
The authors declare no conflict of interests.
Rights and permissions
Copyright information
© 2021 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Abolhassani, H., Bashiri, G., Montazeri, M., Kouchakzadeh, H., Shojaosadati, S.A., Siadat, S.E.R. (2021). Ongoing Clinical Trials and the Potential Therapeutics for COVID-19 Treatment. In: Rahmandoust, M., Ranaei-Siadat, SO. (eds) COVID-19. Springer, Singapore. https://doi.org/10.1007/978-981-16-3108-5_2
Download citation
DOI: https://doi.org/10.1007/978-981-16-3108-5_2
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-16-3107-8
Online ISBN: 978-981-16-3108-5
eBook Packages: Physics and AstronomyPhysics and Astronomy (R0)