Abstract
Shrimp meat is consumed globally on a large scale, and their processing releases a large amount of shell waste. The major constituents of shrimp shells are chitin, proteins, calcium carbonate, and lipids. To extract chitin from the shrimp shell, it has to undergo deproteination (DP) to remove the proteins and demineralization (DM) to separate the minerals. Traditionally shrimp shell wastes were dried and directly added as a fertilizer to soil or added in animal feed or dumped in landfills. In recent years, shrimp shell wastes are valorized for producing chitin, chitosan, and other beneficial products like protein hydrolysates, carotenoids, lactic acid, etc. Industries producing chitin are employing chemicals like hydrochloric acid and sodium hydroxide for demineralization and deproteination, respectively, and the residual water is dumped into the water bodies. Considering environmentally friendly approaches, the usage of microorganisms has been tried out for chitin extraction from the shrimp shell. The recent review highlights the production of chitin using microorganisms and mentions other recent greener approaches in chitin production.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
15.1 Introduction
The seafood industry supports the livelihood of 10–12% of the world population (FAO 2020). The proliferation of the different seafood industries across the world has enhanced the problem of waste handling and disposal. The global volume of shellfish food such as prawn, shrimp, crab, lobster, etc. reached 9.3 billion tons according to FAO (2020) reports. Since the shells or exoskeletons of the crustaceans are inedible, a significant portion of the shellfish ends up as waste and finds its way to landfills or water bodies polluting the environment and causing health hazards. Shrimp wastes are alkaline with a pH range of 7.5–8 that supports the growth of putrefying microbes that are hazardous to the environment (Bhaskar et al. 2007).
Due to the massive scale of shellfish landing and its processing, the waste generation is also huge, and the amount is increasing annually. Currently, there is no satisfactory technology for the valorization of these entire shellfish wastes to value-added products. In some Southeast Asian countries like Indonesia, Thailand, and the Philippines, the monetary value of dry shellfish wastes is very low, with prices ranging from 100 to 120 USD per ton. Considering their lack of profitability, the shellfish wastes are not utilized and eventually get disposed in water bodies or land filled causing environmental pollution. In developed countries like Australia and Canada, the shellfish waste disposal is costly, with a processing cost of up to 150 USD per ton. There are several active programs in the developed seafaring nations for valorizing this resource which includes eco-friendly waste management strategies in Canada; production of lime for construction removal of heavy metals and usage as pre-formed baits in fishery, etc. in the UK; conversion to aquaculture feed in Japan; and chitin and chitosan production in the USA and most Scandinavian countries (www.seafish.org). Interestingly, Norway has developed a technology to utilize seafood-processing waste involving enzyme treatment followed by membrane filtration at nano-level to target value-added products (The Marine Products Export Development Authority [MPEDA] 2013). However, a fully integrated process/technology for an effective total shrimp shell waste management is yet to emerge globally.
The shrimp shell composition varies from species, seasonal variation, and geographic locations. The constituents of the shrimp shell wastes include 10–25% chitin, 13–50% protein, 15–70% mineral matter (Babu et al. 2008), and low-fat content (Cira et al. 2002). The major mineral found in the shrimp shell cuticle is calcium carbonate, which helps in strengthening the exoskeleton. Depending on the tons of renewable shrimp shell waste generated annually, the potential value of these wastes is left unexplored. It is necessary to consider a greener prawn shell waste management methodology benefitting the environment and produce value-added products for economic development. The value-added products like proteins generated from the prawn shell waste are used in animal feed for livestock and aquaculture (Evers and Carroll 1998; Sumardiono and Siqhny 2018). Calcium carbonate derived from the prawn shell wastes are in greater demand due to their biological components and superior origin than limestone and marble. Chitin is the most significant component derived from the shellfish wastes with applications in different fields varying from water purification to biomedical applications. The current commercial method for shellfish waste management uses harmful chemicals, creating environmental and economic issues. Utilization of crustacean shell wastes for the extraction of chitin and other bioactive compounds has been studied using different methods including enzymatic approaches (Hayes et al. 2008), microwave irradiation (El Knidri et al. 2016), and ultrasonication (Kjartansson et al. 2006). Strategy for chitin extraction from shrimp wastes includes demineralization (DM), deproteination (DP), and bleaching/depigmentation; and deacetylation can yield chitosan (CHS) which is an even more valuable product finding applications as surgical sutures and wound dressings (Değim et al. 2002). All these processes use acidic and basic solutions under elevated temperature and longer incubation times.
Addition of strong acids and bases for the chitin extraction affects the physiochemical properties of chitin and releases effluent wastewater containing chemicals, requiring further purification. The use of proteolytic bacteria for DP and lactic acid bacteria for DM could curtail the application of concentrated bases and acids. Therefore, biological methods using microbes or microbial enzymes are in demand due to their better reproducibility, lower processing times, easier handling, less solvent and chemical requirements, and lower energy input for producing value-added products (Hayes et al. 2008). Bio-based chitin has distinct properties like biodegradability, non-toxicity, and biocompatibility and is applied in agriculture, medicine, pharmaceutics, environmental waste management, biotechnology, and food processing (Kaur and Dhillon 2015). The protein-rich liquid fractions find applications in human and animal feed (Mizani et al. 2005). Bioprocessing of shrimp wastes for chitin production is reported using lactic acid bacteria and proteolytic bacteria/enzyme for DM and DP as single-stage fermentation (Rao and Stevens 2006), two-stage fermentation (Xu et al. 2008) and cofermentation (Francisco et al. 2015).
15.2 Economic Aspects of Chitin
The main source of raw material for synthesizing chitin is from the waste materials obtained from seafood pre-processing centers deshelling crab, shrimp, prawn, lobster, etc. (Hamdi 2017; Maruthiah and Palavesam 2017). The shrimp wastes are rich in pigments like astaxanthin, β-carotene, and other carotenoids. For several years, chitin is considered as a promising biomaterial due to its characteristic properties and has found applications in many fields like biomedical, engineering, wastewater treatment, cosmetic, food industry, and packaging. Chitin is of great economic significance as it costs 220 dollars per kilo (Jaganathan et al. 2016). The commercial value of chitin and its derivatives is accounted for 100 billion tons per year (Ioelovich 2014). The global research statistics have concluded that the chitin market is expected to rise to 53 million US dollars in 2024 (Global Chitosan Derivatives Market 2019).
15.3 Chitin Structure and their Properties
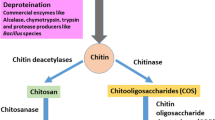
Chitin is a linear semi-crystalline polymer with high molecular weight comprising N-acetyl glucosamine units bonded by β-glycosidic bonds. They resemble cellulose polysaccharide with the C-2 position of the hydroxyl group replaced by the acetamido group. To be distinguished as a chitin, their degree of acetylation is greater than 50% (Anitha et al. 2014). Chitin is tough, inert, and insoluble in water and other organic solvents. The other characteristics of chitin are its ability to chelate metal ions and form films and polyoxy salts. Chitin is consists of three allomorphs containing α-, β-, and γ-forms. The α-chitin is abundantly found in shrimps, lobsters, and crabs with antiparallel chains with strong intra- and intermolecular bonds. The β-form consists of parallel chains bonded by intrasheet hydrogen bonding, which are of weak bonds, hence unstable, and are mainly found in squid (Ioelovich 2014), whereas γ-chitin is an amalgamation of α- and β-chitin forms comprising parallel and antiparallel chains, e.g., Ptinus beetles and Loligo squids (Ramirez-Coutino et al. 2006; Casadidio et al. 2019). The characteristics of pure chitin are dependent on their molecular weight, degree of acetylation, purity, and polydispersity index (Kaur and Dhillon 2015). The characteristics like biodegradability, bioactivity, non-toxicity, and biocompatibility have made these marine polymers useful for various versatile applications. Factors like the degree of deacetylation (DD) are used to determine the number of glucosamine units present in a chitin structure. If the degree of deacetylation exceeds 50%, it improves the solubility of chitin, by changing into chitosan. The molecular weight of chitin is based on the emergence of the source, acid and base concentration used in demineralization and deproteination, duration for incubation, and temperature required for the processes (No and Meyers 1995). The average molecular weight of chitin is reported to have a range of 0.4 to 2.5 × 106 (No and Meyers 1995; Ravi Kumar 2000). Chitin portrays biological properties like antimicrobial, antiulcer, hemostatic, wound healing, fungistatic, antiacid, anticholesterolemic, etc.; hence, it can be used for biomedical applications (Dutta et al. 2004; Zargar et al. 2015; Lim and Hudson 2003; Cheba 2011). Processes involved in synthesizing chitin are (a) demineralization (DM), (b) deproteination (DP), and (c) depigmentation.
15.4 Chemical Methods in the Extraction of Chitin
Traditional methods in chitin extraction from shrimp shells involved the usage of chemicals (Table 15.1 and Fig. 15.1). The usage of a strong alkali like NaOH and acids like HCl affects the ecosystem as the water obtained after processing chitin is highly acidic or basic, which are dumped into the water bodies. The process is expensive as the costs involved in neutralizing the dumped wastes are high.
15.4.1 Chemical Demineralization
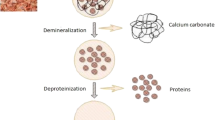
The chitin entrapped in the shrimp exoskeleton can be extracted by the removal of the process of demineralization and deproteination. In demineralization, the inorganic minerals like calcium carbonate from the crustacean exoskeleton are removed using inorganic acids, like HCl, HNO3, and H2SO4 (Younes and Rinaudo 2015; Kumar Gadgey and Bahekar 2017), and organic acids like HCOOH and CH3COOH (Regis et al. 2015). Predominantly, hydrochloric acid is used for higher removal rate of minerals from shell wastes. HCl combines with calcium carbonate (CaCO3) to form calcium chloride (CaCl2) that can be removed by using activated carbon (Fadli et al. 2018) (15.1).
15.4.2 Chemical Deproteination
The next step for the extraction of chitin is deproteination, which involves the removal of proteins. Proteins are removed from the shell wastes using chemicals like NaOH, KOH, Ca(OH)2, CaHSO4, NaHSO4, NaHCO3, Na3PO4, Na2CO3, Na2S, and K2CO3 (Younes and Rinaudo 2015). NaOH is mostly preferred for deproteination. A higher concentration of NaOH at elevated temperature causes deacetylation of chitin to chitosan (40% NaOH incubated at 100–130 °C) (Hülsey 2018).
Deproteination and demineralization can be reversed based on the quality of chitin produced with less incubation time and temperature.
15.4.3 Depigmentation
The process of demineralization and deproteination cannot completely remove the carotenoid pigments like astaxanthin, lutein, β-carotene, and astacene. In order to obtain colorless chitin, the pigments are removed using organic solvents like glacial acetone (Soon et al. 2018) and inorganic solvent like sodium hypochlorite (Srinivasan et al. 2018; Devi and Dhamodharan 2018). Duan et al. (2012) decolorized colored chitin from shrimp wastes with potassium permanganate followed by incubating in oxalic acid (1%). Through the process of decolorization, colorless chitin is obtained which improves their commercial value and utilization for various industrial applications.
15.5 Microbial Action on Shrimp Shells for Chitin Recovery
Shrimp shell waste biofermentation is probably the ideal environmentally friendly method that is cost-effective and sustainable. Although shrimp shells are insoluble and not easily degraded by natural degradation, they contain chitin, a natural polymer resembling cellulose in chemical structure. Chitin and its derivative chitosan have been used widely for commercial applications in agriculture, biomedicine, biotechnology, waste treatment, food industry, etc. Biofermentation of shrimp shell wastes is advantageous over chemical methods. The usage of chemicals release effluents into the soil and water body and are harmful that biological methods using microorganisms. Khanafari et al. (2008) found out that the quality of chitin obtained from the biological methods was better than chemical methods. Chitin with high molecular weight was produced by the deproteination of shrimp shells using proteolytic microorganisms (Bustos and Healy 1994). Shrimp shells are fermented by single-stage fermentation, cofermentation, or two-stage fermentation processes, which involve lactic acid bacteria and non-lactic acid bacteria that assist in demineralization and deproteination (Table 15.2).
15.5.1 Lactic Acid Bacteria
Conventional methods of demineralization used HCl which affected the quality of chitin altering their molecular weight and intrinsic properties (Percot et al. 2003). Lactic acid is used as an alternative instead of HCl for demineralization, and it was found that a) usage of lactic acid was less toxic to the environment due to the release of acid and alkali liquid obtained after chitin processing, b) it was also cost-effective, and c) calcium lactate (Ca(C3H5O3)2) formed by the action of lactic acid (C3H6O3) (15.2) with calcium carbonate can be used as anti-icing agents (Mahmoud et al. 2007). Lactic acid is naturally produced by lactic acid-producing bacteria, which is preferred over commercial lactic acid considering their cost (Ghaffar et al. 2014). Lactic acid-fermenting bacteria can be isolated from the shrimp shell itself (Duan et al. 2012). Lactic acid fermentation converts sugars to form lactic acid, which reduces the pH of the fermentation broth, reducing the growth of unwanted bacteria (Vandenbergh 1993).
For lactic acid fermentation using shrimp shells, different parameters have been considered; these are various sugar sources and their optimal concentrations, the concentration of inoculum used, and incubation time to produce lactic acid (Mathew and Nair 2006; Healy et al. 2003; Rao et al. 2000; Bhaskar et al. 2007). Lactic acid fermentation of shrimp wastes is optimized using different parameters like type of lactic acid bacteria used, sugar concentration, incubation time, etc. using response surface methodology (RSM), a statistical method that uses a sequence of designed experiments with different variables to obtain an optimal condition (Bhaskar et al. 2007). The addition of glucose in shrimp shell fermentation leads to the formation of lactic acid that lowers the pH causing demineralization (Khanafari et al. 2008). Different concentrations of glucose were added to test the demineralization efficiency. It was observed that the presence of glucose inhibited the protease activity of non-lactic acid bacteria; hence, other sugar sources were also considered (Aytekin and Elibol 2009). Some of the commonly used sugar sources that were added along with shrimp waste to enhance lactic acid production included sucrose (Cira et al. 2002), molasses (Fagbenro 1996; Evers and Carroll 1998), date juice (Khorrami et al. 2011), cassava starch (Francisco et al. 2015), fruit peels, etc. (Tan et al. 2020).
LAB can undergo single fermentation or cofermentation for shrimp shell degradation. Shrimp shells were fermented with Lactobacillus plantarum 541 resulting in a demineralization value of 90% (Rao et al. 2000). Natural curd containing lactic acid bacteria (LAB) was used for shrimp biofermentation having a demineralization value of 69% and deproteination of 89% (Prameela et al. 2010). Pacheco et al. (2011) isolated Lactobacillus strain B2 from the shellfish waste, and through fermentation, it resulted in 92% demineralization and 94% deproteination, respectively. Lactic acid bacteria can be combined with other non-lactic acid-producing bacteria that aid in protease activity causing deproteination. Some LAB organisms can carry both demineralization and deproteination and hence be used as a single strain for the biofermentation of shrimp shells. Chitin was obtained using Lactobacillus plantarum from fresh shrimp shell wastes by batch fermentations adjusting the pH, incubation time, and inoculum to obtain a deproteination of 99% and demineralization of 87% (Neves et al. 2017). The chitin produced by biological fermentation was observed to be 40% better than the chemical produced chitin. Lactic acid bacteria are used for deproteination of shrimp shells (Woods 1998).
Lactic acid bacteria were co-cultured with other lactic acid bacteria/non-lactic acid bacteria to enhance the demineralization and deproteination efficiency in shrimp shells. Co-culturing of Lactobacillus isolates T1 and L137 in the presence of sugar sources like glucose and cassava starch led to DM efficiency of 82–83% and deproteination value of 84.4% (Francisco et al. 2015). Evers and Carroll (1998) co-cultured Lactobacillus plantarum and Enterococcus faecium for shrimp shell biofermentation using dry molasses. Ploydee and Chaiyanan (2014) co-cultured Lactobacillus pentosus and Bacillus thuringiensis for shrimp shell processing resulting in calcium carbonate removal efficiency of 98.1 ± 0.3% with a protein removal efficiency of 96.8 ± 0.7% (w/w). Junianto and Setyahadi (2013) demonstrated three different strategies for the pretreatment of shrimp shells using Lactobacillus acidophilus FNCC 116 and Bacillus licheniformis F11.1 by two-stage fermentation processes. 99.6% of minerals were removed when 100% of the medium was replaced by fresh media after 24 h of incubation with Lactobacillus acidophilus FNCC 116. 95.37% of protein was removed after subsequent fermentation and 100% media removal and replaced with fresh media after 24 h. Co-culturing of L. plantarum subsp. plantarum ATCC14917 and B. subtilis subsp. subtilis ATCC 6051 in the presence of fruit peels enhanced the shrimp biofermentation to produce good-quality chitin (Tan et al. 2020). Zhang et al. (2012) demonstrated two-stage fermentation of shrimp shells using Lactobacillus plantarum and Serratia marcescens. For the deproteination, S. marcescens was cultured with the shrimp shells at 30 °C for 4 days. The solid mass obtained after drying was further demineralized at 37 °C for 2 days. Their deproteination efficiency was 93% and demineralization 94.5% resulting in a chitin yield of 18.9% (Zhang et al. 2012). Similarly, heterofermenting Lactobacillus brevis was cultured with Rhizopus oligosporus for the biological shrimp shell processing (Aranday-García et al. 2017). In this study, L. brevis was cultured first followed by R. oligosporus to yield 66.45 ± 2.14% demineralization and 96 ± 0.43% of deproteination efficiency. Aytekin and Elibol (2009) studied the fermentative action of Lactococcus lactis and Teredinobacter turnirae on shrimp shell wastes for demineralization and deproteination. From their studies, co-culturing of Lactococcus lactis and Teredinobacter turnirae showed the best results, especially when proteolytic T. turnirae was cultured first followed by the demineralization with L. lactis displaying a DP and a DM value of 95%.
15.5.2 Non-lactic Acid Bacteria
Non-lactic acid bacteria produce proteases responsible for the deproteination process. The non-lactic acid bacteria produce protein hydrolysates, which help in the growth of lactic acid bacteria that help in demineralization. The proteolytic activities of the microorganisms are responsible for the deproteination of the shrimp shells (Table 15.3). Wang and Chio (1998) observed that the deproteination efficiency of Pseudomonas aeruginosa K-187 grown with shrimp and crab shell wastes was 82%. Shimahara et al. (1984) used P. maltophilia LC 102 for the protein removal of shrimp shells of Penaeus japonicus supplemented with EDTA. Paul et al. (2015) deproteinized the shrimp shells of P. monodon with Paenibacillus woosongensis TKB2 containing NaCl and chicken feather leading to 80% deproteination efficiency.
Bacillus species were used in shrimp shell deproteination. The proteolytic activities of six Bacillus species namely, B. amyloliquefaciens, B. subtilis A26, Bacillus pumilus A1, B. licheniformis RP1, and B. cereus SV1 strain, were studied for deproteination (Ghorbel-Bellaaj et al. 2012a). The deproteination of shrimp shells enzymatically was optimized by Box-Behnken design using Bacillus mojavensis A21 crude protease resulting in 88% deproteination (Younes et al. 2012). A chitinase-free extracellular protease was isolated from Brevibacillus parabrevis TKU046 which was used for the deproteination study against shrimp shell wastes (Doan et al. 2019a). It was observed that maximum deproteination of 96.44 ± 0.72% was observed on cooked tiger shrimp shell by liquid fermentation.
In a single reactor, the concurrent production of chitin was initiated by adding shrimp shell with Aspergillus niger. The proteases produced from A. niger caused deproteination releasing protein hydrolysates that were of low pH. Lower pH of the supernatant facilitated the demineralization process aiding in chitin separation (Teng et al. 2001). Cofermentation of non-lactic acid-producing microorganisms also helped in shrimp shell degradation. Successive cofermentation of proteolytic B. licheniformis and Gluconobacter oxydans produced a DP efficiency of 87% followed by a DM value of 93.5%, and the chitin content was 90.8%.
15.6 Other Green Methods for Chitin Synthesis
Biological fermentation can be combined with other greener approaches to extract chitin. Some methods are ionic liquid extraction, the usage of protease enzymes for deproteination, micro-irradiation, and ultrasonication before or after the demineralization and deproteination in shrimp shell biofermentation (Qin et al. 2010; Mao et al. 2017; Suryawanshi et al. 2020; El Knidri et al. 2016). Extraction of chitin using ionic liquids is a one-pot method using ionic liquids (ILs) like hydroxyl ammonium acetate that has low inflammability, low vapor pressure, and highly soluble nature (Shamshina et al. 2016). Apart from using ionic liquids in chitin extraction, deep eutectic solvents (DESs) are preferred over ionic liquids in chitin extraction for their better solubility and economical and simple extraction process. In a two-step chitin extraction process, shrimp shells were pretreated first using citric acid leading to a DM value of 98% followed by the addition of DESs with the microwave irradiation causing deproteination with an efficiency of above 88% (Zhao et al. 2019). High-quality chitin (DESs-chitin) was produced in this method and matched the standards of chemically produced chitin. Huang et al. (2018a, b) devised a chitin extraction method from shrimp shells with Natural Deep Eutectic Solvent (NADES) along with microwave irradiation. Demineralization was attained by the adding malic acid, which removed 99% calcium chloride. The deproteination efficiency was dependent on the microwave radiation, the incubation time, and the shrimp shell-to-NADES ratio. Maximum deproteination efficiency was obtained at 93.8% with a shrimp shell-to-NADES ratio of 1:20 and microwave irradiation for 9 min. The chitin obtained through this process had a high crystallinity index of 71%. Devi and Dhamodharan (2018) developed a green and facile process to obtain chitin nanofibers from prawn shell wastes. The prawn shells were pretreated in hot glycerol (at 200 °C, for 4 min) that caused deproteination leading to the release of low molecular weight water-soluble proteins. The deproteinated shells were demineralized using citric acid forming calcium citrate salt and chitin of high crystallinity index (80.9%). From this process, the glycerol could be reused by using charcoal. Ultrasonication is another method for enhancing the pretreatment processes involved in deproteination and demineralization (Suryawanshi et al. 2019). In an ultrasonication-assisted method, a mild concentration of HCl (0.6 M HCl) and NaOH (O.6 M NaOH) was employed for demineralization and deproteination of shellfish wastes (Suryawanshi et al. 2020). Through ultrasonication, microbubbles are generated leading to an increase in the reaction rate with temperatures of 5000 K and 1000 atmospheric pressure.
For the deproteination of shrimp shells, commercial enzymes like pepsin, papain, bluefin trypsin, Alcalase®, and protease are used. Shrimp shell wastes of Penaeus indicus were demineralized with 1.75 N glacial acetic acid and papain (1:100 papain to shrimp shells) incubated at 72 h room temperature to obtain a deproteination value of 73.1%, and the degree of acetylation (DA) of the chitin produced was 19.37% (Gopalakannan et al. 2000). Pepsin enzyme was incubated with white shrimp shells for 16 h at 40 °C, and it resulted in 92% deproteination efficiency (Duong and Nghia 2014).
Hongkulsup et al. (2016) used commercial protease enzyme from Streptomyces griseus for deproteination of L. vannamei shells and effectively removed 91.1% proteins, and the chitin produced had a DA of 90.83% with a crystallinity index of 82.56%, with lactic acid as the demineralization agent. Another enzyme like Alcalase® was used in the removal of proteins from shrimp heads to recover chitin (Valdez-Peña et al. 2010). Hence, commercial proteolytic enzymes can be used in shrimp shell degradation to obtain chitin, but are expensive compared to using proteolytic microorganisms.
15.7 Functional Aspects of Chitin
Due to the insoluble nature of chitin, chitin is deacetylated to chitosan, which has pleiotropic applications in the field of agriculture, food, waste management, and biomedical sectors (Table 15.4). In the wastewater management, green chitin nanoadsorbents were developed for the removal of carmine dyes (Meshkat et al. 2019). Adsorption of anionic dyes was initiated using a chitin biopolymer (Longhinotti et al. 1998). Chitin derivatives are used in heavy metal removal of lead (Zhou et al. 2005), chromium (Baran et al. 2007), cadmium (Benguella and Benaissa 2002), copper, and arsenic (Kartal and Imamura 2005). Biological denitrification and sulfate reduction in groundwater were initiated using crab shell chitin (CS-20) (Robinson-Lora and Brennan 2009). Chitin is also used for coagulating and flocculating activated sludge (Kurita 2006).
In the biomedical application, chitin fabrics (non-woven) and chitin threads are used in the development of artificial skin and sutures for wound dressing because of their biocompatibility and degradability (Nishimura 2001). The mechanical strength of pure chitin sutures can be improved by incorporating graphene oxide with chitin monofilament (Zhang et al. 2019).
In the field of agriculture, chitin is used for developing resistance against plant diseases and develops elicitor activity in fruits and vegetables (Parada et al. 2018; Pusztahelyi 2018). Nanochitin, derived from shrimp shells, is used to improve the quality and quantity of winter wheat: multi-spike wheat and large spike wheat, respectively (Xue et al. 2018). To improve soil fertility, chitin can be used as a fertilizer due to their rich nitrogen content (Malerba and Cerana 2019).
In the food sector, chitin derivatives are utilized as a food preservative (Hu and Gänzle 2019). They are also used as thickener mixed with vegetable oil for developing bio-lubricants (Sánchez et al. 2011). As a stabilizer/emulsifier, chitin is used in food, cosmetics, and biomedical applications (Casadidio et al. 2019; İlyasoğlu et al. 2018). Lipophilized chitin as chitin fatty esters (chitin laurate, chitin palmate, chitin stearate, chitin octanoate) is used for developing novel stabilizers with oil in water emulsions (İlyasoğlu et al. 2018). Chitin materials are replacing petroleum-based packaging materials as they are eco-friendly and biodegradable (Srinivasa and Tharanathan 2007). Chitin-based packaging materials, in the form of antimicrobial films and composite materials, are used in preserving fruits and vegetables after post-harvest to maintain their freshness and enhance the shelf life (Srinivasa and Tharanathan 2007; Suryawanshi et al. 2019). In paper finishing, hydroxyl methyl chitin is added to improve the wet strength characteristics of paper (Allan et al. 1980; Song et al. 2018). For cosmeceutical applications, chitin was used as a skin conditioner, moisturizer, emollient, and surfactant, shows antimicrobial activity against skin acne, was used as an ingredient in hair care products, and in oral health-care acts as a carrier for herbal extracts in toothpaste, mouthwash, and chewing gums (Aranaz et al. 2018). In the field of nanotechnology, chitin nanoparticles developed from shrimp wastes of P. semisulcatus are used in developing iron/chitin nanocomposite with aqueous leaf extract of Corchorus olitorius that were analyzed for their antimicrobial activity and heavy metal and dye adsorption (Gomaa 2018). In the textile industry, chitin can be used to prevent the wear and tear of fabrics while weaving and can be used to improve properties like water resistance and antimicrobial resistance to the fabric (Hahn et al. 2019). Chitin is used in textile dyeing as anti-wrinkle, anti-static, and anti-bacterial finishing by blending chitosan with cotton, silk, wool, etc., thus enhancing the value of the fabric and utilizing the natural polymers (Huang et al. 2018a, b). Hence, chitin can be used for various pleiotropic applications that can benefit humankind.
15.8 Conclusion
The production of chitin from shrimp wastes involving microorganisms is beneficial over other chemical methods. Although there are several reports on microbial shrimp shell degradation, the usage of the environmentally safe microorganisms (GRAS status) for shrimp shell biofermentation is beneficial, as the byproducts like protein hydrolysate derived from them can be used in animal, fish, and poultry feed, without causing risk of any infection. The derived protein hydrolysates from such GRAS organisms can be attempted to cultivate beneficial fungi that produce SCP and other enzymes like chitinases, cellulases, etc. Lactic acid bacteria, being GRAS microorganisms, can be used directly in the demineralization process in shrimp shell processing, producing beneficial products like calcium lactate and lactic acid. Thus, the chitin derived by microbial action of shrimp shell wastes is a safer approach that can resolve the problem of environmental pollution and be beneficial for innumerable applications in various industries.
References
Abdel-Ghany HM, Salem ME-S (2020) Effects of dietary chitosan supplementation on farmed fish; a review. Rev Aquac 12(1):438–452. https://doi.org/10.1111/raq.12326
Adour L, Arbia W, Amrane A, Mameri N (2008) Combined use of waste materials – recovery of chitin from shrimp shells by lactic acid fermentation supplemented with date juice waste or glucose. J Chem Technol Biotechnol 83:1664–1669. https://doi.org/10.1002/jctb.1980
Akkaya G, Uzun I, Güzel F (2009) Adsorption of some highly toxic dyestuffs from aqueous solution by chitin and its synthesized derivatives. Desalination 249:1115–1123. https://doi.org/10.1016/j.desal.2009.05.014
Allan G, Crospy GD, Lee JH, Miller ML, Reif WM (1980) Proceedings of a symposium on man made polymers in papermaking. In. Helsinki, Finland
Anastopoulos I, Bhatnagar A, Bikiaris DN, Kyzas GZ (2017) Chitin adsorbents for toxic metals: a review. Int J Mol Sci 18(1):114. https://doi.org/10.3390/ijms18010114
Anitha A, Sowmya S, Kumar PTS, Deepthi S, Chennazhi KP, Ehrlich H, Jayakumar R (2014) Chitin and chitosan in selected biomedical applications. Prog Polym Sci 39(9):1644–1667. https://doi.org/10.1016/j.progpolymsci.2014.02.008
Aranaz I, Acosta N, Civera C, Elorza B, Mingo J, Castro C et al (2018) Cosmetics and cosmeceutical applications of chitin, chitosan and their derivatives. Polymers 10:213. https://doi.org/10.3390/polym10020213
Aranday-García R, Román Guerrero A, Ifuku S, Shirai K (2017) Successive inoculation of Lactobacillus brevis and Rhizopus oligosporus on shrimp wastes for recovery of chitin and added-value products. Process Biochem 58:17–24. https://doi.org/10.1016/j.procbio.2017.04.036
Aranday-García R, Saimoto H, Shirai K, Ifuku S (2019) Chitin biological extraction from shrimp wastes and its fibrillation for elastic nanofiber sheets preparation. Carbohydr Polym 213:112–120. https://doi.org/10.1016/j.carbpol.2019.02.083
Aytekin O, Elibol M (2009) Cocultivation of Lactococcus lactis and Teredinobacter turnirae for biological chitin extraction from prawn waste. Bioprocess Biosyst Eng 33:393–399. https://doi.org/10.1007/s00449-009-824
Babu CM, Chakrabarti R, Sambasivarao KRS (2008) Enzymatic isolation of carotenoid-protein complex from shrimp head waste and its use as a source of carotenoids. LWT- Food Sci Technol 41:227–235
Bahasan SHO, Satheesh S, Ba-akdah MA (2017) Extraction of chitin from the Shell wastes of two shrimp species Fenneropenaeus semisulcatus and Fenneropenaeus indicus using microorganisms. J Aquat Food Prod Technol 26:16. https://doi.org/10.1080/10498850.2016.1188191
Bajaj M, Freiberg A, Winter J et al (2015) Pilot-scale chitin extraction from shrimp shell waste by deproteination and decalcification with bacterial enrichment cultures. Appl Microbiol Biotechnol 99:9835–9846. https://doi.org/10.1007/s00253-015-6841-5
Baran A, Biçak E, Baysal H, Önal S (2007) Comparative studies on the adsorption of Cr(VI) ions on to various sorbents. Bioresour Technol 98:661–665. https://doi.org/10.1016/j.biortech.2006.02.020
Barros FCF, Vasconcellos LCG, Carvalho TV, Nascimento RF (2014) Removal of petroleum spill in water by chitin and chitosan. Orbital: The Electronic J Chem 6(1):70–74
Beaney P, Lizardi-Mendoza J, Healy M (2005) Comparison of chitins produced by chemical and bioprocessing methods. J Chem Technol Biotechnol 80:145–150. https://doi.org/10.1002/jctb.1164
Benguella B, Benaissa H (2002) Cadmium removal from aqueous solutions by chitin: kinetic and equilibrium studies. Water Res 36(10):2463–2474. https://doi.org/10.1016/S0043-1354(01)00459-6
Bhaskar N, Suresh PV, Sakhare PZ, Sachindra NM (2007) Shrimp biowaste fermentation with Pediococcus acidolactici CFR2182: optimization of fermentation conditions by response surface methodology and effect of optimized conditions on deproteination/demineralization and carotenoid recovery. Enzym Microb Technol 40:1427–1434. https://doi.org/10.1016/j.enzmictec.2006.10.019
Bough WA, Salter WL, Wu ACM, Perkins BE (1978) Influence of manufacturing variables on the characteristics and effectiveness of chitosan products. Chemical composition, viscosity, and molecular-weight distribution of chitosan products. Biotechnol Bioeng 20:1931–1943
Bustos RO, Healy MG (1994) Microbial deproteinization of waste prawn shell. In: proceedings of the second international symposium on environmental biotechnology, Brighton, UK. pp. 15–25
Casadidio C, Peregrina DV, Gigliobianco MR, Deng S, Censi R, Di Martino P (2019) Chitin and Chitosans: characteristics, eco-friendly processes, and applications in cosmetic science. Mar Drugs 17(6):369. https://doi.org/10.3390/md17060369
Charoenvuttitham P, Shi J, Mittal GS (2006) Chitin extraction from black tiger shrimp (Penaeus monodon) waste using organic acids. Sep Sci Technol 41:1135–1153. https://doi.org/10.1080/01496390600633725
Cheba BA (2011) Chitin and chitosan: marine biopolymers with unique properties and versatile applications. Biotechnol Biochem 6:149–153
Choorit W, Patthanamanee W, Manurakchinakorn S (2008) Use of response surface method for the determination of demineralization efficiency in fermented shrimp shells. Bioresour Technol 99:6168–6173. https://doi.org/10.1016/j.biortech.2007.12.032
Cira LA, Huerta S, Hall GM, Shirai K (2002) Pilot scale lactic acid fermentation of shrimp wastes for chitin recovery. Process Biochem 37:1359–1366
Damodarasamy A, Baby S, Ramachandran R (2012) Microbial deproteinization of shrimp shell waste for chitin production by wild strains of Serratia marcescens. Electronic J Environ Agri Food Chem 11(5):469–476
Değim Z, Celebi N, Sayan H, Babül A, Erdoğan D, Take G (2002) An investigation on skin wound healing in mice with a taurine-chitosan gel formulation. Amino Acids 22(2):187–198. https://doi.org/10.1007/s007260200007
Devi R, Dhamodharan R (2018) Pretreatment in hot glycerol for facile and green separation of chitin from prawn shell waste. ACS Sustain Chem Eng 6:846–853. https://doi.org/10.1021/acssuschemeng.7b03195
Díaz-Rojas E, Argüelles-Monal WM, Higuera-Ciapara I, Hernández J, Lizardi-Mendoza J, Goycoolea FM (2006) Determination of chitin and protein contents during the isolation of chitin from shrimp waste. Macromol Biosci 6:340–347. https://doi.org/10.1002/mabi.200500233
Doan CT, Tran TN, Wen I-H, Nguyen VB, Nguyen AD, Wang S-L (2019a) Conversion of shrimp head waste for production of a Thermotolerant, detergent-stable, Alkaline Protease by Paenibacillus sp Catalysts. (9):798. doi:https://doi.org/10.3390/catal9100798
Doan CT, Tran TN, Nguyen VB, Vo TPK, Nguyen AD, Wang SL (2019b) Chitin extraction from shrimp waste by liquid fermentation using an alkaline protease-producing strain, Brevibacillus parabrevis. Int J Biol Macromol 131:706–715. https://doi.org/10.1016/j.ijbiomac.2019.03.117
Duan S, Zhang Y, Lu T, Cao D, Chen J (2011) Shrimp waste fermentation using symbiotic lactic acid bacteria. Adv Mater Res 196:2156–2163
Duan S, Li L, Zhuang Z, Wu W, Hong S, Zhou J (2012) Improved production of chitin from shrimp waste by fermentation with epiphytic lactic acid bacteria. Carbohydr Polym 89(4):1283–1288. https://doi.org/10.1016/j.carbpol.2012.04.051
Duong NTH, Nghia ND (2014) Kinetics and optimization of the Deproteinization by pepsin in chitin extraction from white shrimp Shell. J Chitin Chitosan Sci 2:21–28. https://doi.org/10.1166/jcc.2014.1054
Dutta PK, Dutta J, Tripathi V (2004) Chitin and chitosan: chemistry, properties and applications. JSIR, Delhi, India
El Knidri H, El Khalfaouy R, Laajeb A, Addaou A, Lahsini A (2016) Eco-friendly extraction and characterization of chitin and chitosan from the shrimp shell waste via microwave irradiation. Process Saf Environ Prot 104:395–405. https://doi.org/10.1016/j.psep.2016.09.020
Elhussieny A, Faisal M, D’Angelo G, Aboulkhair NT, Everitt NM, Fahim IS (2020) Valorisation of shrimp and rice straw waste into food packaging applications. Ain Shams Eng J. https://doi.org/10.1016/j.asej.2020.01.008
Evers D, Carroll D (1998) Ensiling salt-preserved shrimp waste with grass straw and molasses. Anim Feed Sci Technol 71(3–4):241–249. https://doi.org/10.1016/s0377-8401(97)00145-4
Fadli A, Maulana S, Drastinawati (2018) Shrinking core model of demineralization of chitin isolation from shrimp shell. MATEC Web of Conferences 154:01014. https://doi.org/10.1051/matecconf/201815401014
Fagbenro OA (1996) Preparation, properties and preservation of lactic acid fermented shrimp heads. Food Res Int 29:595–599. https://doi.org/10.1016/s0963-9969(96)00077-4
FAO (2020) The State of World Fisheries and Agriculture, Food and Agricultural Organization of the United Nations. Food and Agriculture Organization of the United Nations
Francisco FC, Simora RMC, Nuñal SN (2015) Deproteination and demineralization of shrimp waste using lactic acid bacteria for the production of crude chitin and chitosan. AACL Bioflux 8:107–115
Ghaffar T, Irshad M, Anwar Z, Aqil T, Zulifqar Z, Tariq A, Kamran M, Ehsan N, Mehmood S (2014) Recent trends in lactic acid biotechnology: A brief review on production to purification. J Radiat Res Appl Sci 7(2):222–229. https://doi.org/10.1016/j.jrras.2014.03.002
Ghorbel-Bellaaj O, Younes I, Maalej H, Hajji S, Nasri M (2012a) Chitin extraction from shrimp shell waste using Bacillus bacteria. Int J Biol Macromol 51:1196–1201. https://doi.org/10.1016/j.ijbiomac.2012.08.034
Ghorbel-Bellaaj O, Jridi M, Khaled HB, Jellouli K, Nasri M (2012b) Bioconversion of shrimp shell waste for the production of antioxidant and chitosan used as fruit juice clarifier. Int J Food Sci Technol 47:1835–1841. https://doi.org/10.1111/j.1365-2621.2012.03039.x
Global Chitosan Derivatives Market (2019) By manufacturers, regions, type and application. Global Info Research
Gomaa EZ (2018) Iron nanoparticles α-chitin nanocomposite for enhanced antimicrobial, dyes degradation and heavy metals removal activities. J Polym Environ 26:3638–3654. https://doi.org/10.1007/s10924-018-1247-y
Gopalakannan A, Indra Jasmine G, Shanmugam SA, Sugumar G (2000) Application of proteolytic enzyme, papain for the production of chitin and chitosan from shrimp waste. J Mar Biol Assoc India 42:167–172
Hahn T, Bossog L, Hager T, Wunderlich W, Breier R, Stegmaier T, Zibek S (2019) Chitosan application in textile processing and fabric coating. In: Broek LA, Boeriu CG (eds) chitin and chitosan. doi:https://doi.org/10.1002/9781119450467.ch16
Hamdi M (2017) Chitin extraction from blue crab (Portunus segnis) and shrimp (Penaeus kerathurus) shells using digestive alkaline proteases from P. segnis viscera. Int J Biol Macromol 101:455–463. https://doi.org/10.1016/j.ijbiomac.2017.02.103
Harkin C, Mehlmer N, Woortman DV, Brück TB, Brück WM (2019) Nutritional and additive uses of chitin and chitosan in the food industry, Sustainable agriculture reviews, vol 36. Springer, Cham. https://doi.org/10.1007/978-3-030-16581-9_1
Hayes M, Carney B, Slater J, Brück W (2008) Mining marine shellfish wastes for bioactive molecules: chitin and chitosan and ash; part A: extraction methods. Biotechnol J 3(7):871–877. https://doi.org/10.1002/biot.200700197
Healy M, Green MH, A. (2003) Bioprocessing of marine crustacean shell waste. Acta Biotechnol 23:151–160
Hongkulsup C, Khutoryanskiy VV, Niranjan K (2016) Enzyme assisted extraction of chitin from shrimp shells (Litopenaeus vannamei). J Chem Technol Biotechnol 91:1250–1256. https://doi.org/10.1002/jctb.4714
Hossain MS, Iqbal A (2014) Production and characterization of chitosan from shrimp waste. J Bangladesh Agril Univ 12(1):153–160
Hu Z, Gänzle MG (2019) Challenges and opportunities related to the use of chitosan as a food preservative. J Appl Microbiol 126(5):1318–1331. https://doi.org/10.1111/jam.14131
Huang L, Xiao L, Yang G (2018a) Chitosan application in textile processing. Mini-review. 4(2):0032-0034. doi:10.19080/CTFTTE.2018.04.555635
Huang WC, Zhao D, Guo N, Xue C, Mao X (2018b) Green and facile production of chitin from crustacean shells using a natural deep eutectic solvent. J Agric Food Chem 66:11897–11901. https://doi.org/10.1021/acs.jafc.8b03847
Hülsey MJ (2018) Shell biorefinery: A comprehensive introduction. Green energy Environ 3:318–327. https://doi.org/10.1016/j.gee.2018.07.007
İlyasoğlu H, Anankanbil S, Nadzieja M (2018) Lipophilization of chitin as novel polymeric stabilizer for improved oil-in-water emulsions. Colloid Polym Sci 296:1841–1848. https://doi.org/10.1007/s00396-018-4410-z
Ioelovich M (2014) Crystallinity and Hydrophility of chitin and chitosan. J Chem 3(3):7–14
Jaafarzadeh N, Mengelizadeh N, Takdastan A, Farsani MH, Niknam N, Aalipour M, Hadei M, Bahrami P (2015) Biosorption of heavy metals from aqueous solutions onto chitin. Int J Environ Health Eng 4:1–7
Jaganathan K, Raffi SM, Soundarapandian P (2016) Extraction and characterization of chitin from marine bycatch crustaceans employing fermentation method. World J Pharm Pharm Sci 5(1):1290–1301
Junianto WB, Setyahadi S (2013) Selection of methods for microbiological extraction of chitin from shrimp shells. Microbiol Indonesia 7(2):75–83. https://doi.org/10.5454/mi.7.2.5
Kartal SN, Imamura Y (2005) Removal of copper, chromium, and arsenic from CCA-treated wood onto chitin and chitosan. Bioresour Technol 96:389–392. https://doi.org/10.1016/j.biortech.2004.03.004
Kaur S, Dhillon GS (2015) Recent trends in biological extraction of chitin from marine shell wastes: a review. Crit Rev Biotechnol 35(1):44–61. https://doi.org/10.3109/07388551.2013.798256
Kaya M, Baran T, Karaarslan M (2015) A new method for fast chitin extraction from shells of crab, crayfish and shrimp. Nat Prod Res 29:1477–1480
Khanafari A, Marandi R, Sanatei SH (2008) Recovery of chitin and chitosan from shrimp waste by chemical and microbial methods. J Environ Health Sci 5:19–24
Khempaka S, Mochizuki M, Koh K, Karasawa Y (2006) Effect of chitin in shrimp meal on growth performance and digestability in growing broilers. J Poult Sci 43(4):339–343
Khorrami M, Najafpour GD, Younesi H, Amini GH (2011) Growth kinetics and demineralization of shrimp Shell using Lactobacillus plantarum PTCC 1058 on various carbon sources. Iran J Energy Environ 2:320–325. https://doi.org/10.5829/idosi.ijee.2011.02.04.2391
Kjartansson GT, Zivanovic S, Kristbergsson K, Weiss J (2006) Sonication-assisted extraction of chitin from North Atlantic shrimps (Pandalus borealis). J Agr Food Chem 54:5894–5902. https://doi.org/10.1021/jf060646w
Kumar Gadgey K, Bahekar A (2017) Studies on extraction methods of chitin from crab shell and investigation of its mechanical properties. Int J Mech Eng Technol 8:220–231
Kurita K (2006) Chitin and chitosan: functional biopolymers from marine crustaceans. Mar Biotechnol 8(3):203–226. https://doi.org/10.1007/s10126-005-0097-5
Lertsutthiwong P, How NC, Chandrkrachang S, Stevens WF (2002) Effect of chemical treatment on the characteristics of shrimp chitosan. J Met Mat Miner 12(1):11–18
Lim S-H, Hudson SM (2003) Review of chitosan and its derivatives as antimicrobial agents and their uses as textile chemicals. J Macromol Sci Part C Polym Rev 43:223–269. https://doi.org/10.1081/MC-120020161
Liu P, Liu S, Guo N, Mao X, Lin H, Xue C, Wei D (2014) Cofermentation of Bacillus licheniformis and Gluconobacter oxydans for chitin extraction from shrimp waste. Biochem Eng J 91:10–15. https://doi.org/10.1016/j.bej.2014.07.004
Longhinotti E, Pozza F, Furlan L, Sanchez MNM, Klug M, Laranjeira MCM, Fávere VT (1998) Adsorption of anionic dyes on the biopolymer chitin. J Braz Chem Soc 9:435–440. https://doi.org/10.1590/S0103-50531998000500005
Madhavan P, Nair KGR (1974) Utilisation of prawn waste-isolation of chitin and its conversion to chitosan. Fish Technol 11:50–53
Mahmoud NS, Ghaly AE, Arab F (2007) Unconventional approach for demineralization of Deproteinized crustacean shells for chitin production. American J Biochem Biotechnol 3(1):1–9. https://doi.org/10.3844/ajbbsp.2007.1.9
Malerba M, Cerana R (2019) Recent applications of chitin- and chitosan-based polymers in plants. Polymer (Basel) 11:839. https://doi.org/10.3390/polym11050839
Mao X, Guo N, Sun J, Xue C (2017) Comprehensive utilization of shrimp waste based on biotechnological methods: A review. J Clean Prod 143:814–823. https://doi.org/10.1016/j.jclepro.2016.12.042
Maruthiah T, Palavesam A (2017) Characterization of Haloalkalophilic organic solvent tolerant protease for chitin extraction from shrimp Shell waste. Int J Biol Macromol 97:552–560. https://doi.org/10.1016/j.ijbiomac.2017.01.021
Mathew P, Nair KGR (2006) Ensilation of shrimp waste by Lactobacillus fermentum. Fish Technol 43:59–64
Meshkat SS, Nezhad MN, Bazmi MR (2019) Investigation of Carmine Dye Removal by Green Chitin Nanowhiskers Adsorbent Emerg Sci J. 3(3):187–194. doi:10.28991/esj-2019-01181
Mizani M, Aminlari M, Khodabandeh M (2005) An effective method for producing a nutritive protein extract powder from shrimp head waste. Food Sci Tech Int 11:49–54. https://doi.org/10.1177/1082013205051271
Moorjani MN, Achutha V, Khasim DI (1975) Parameters affecting the viscosity of chitosan from prawn waste. J Food Sci Technol 12:187–189
Narayan B, Velappan SP, Zituji SP, Manjabhatta SN, Gowda LR (2010) Yield and chemical composition of fractions from fermented shrimp biowaste. Waste Manag Res 28(1):64–70. https://doi.org/10.1177/0734242X09337658
Neves AC, Zanette C, Grade ST, Schaffer JV, Alves HJ, Arantes MK (2017) Optimization of lactic fermentation for extraction of chitin from freshwater shrimp waste. Acta Scientiarum Technol 39(2):125–133
Nishimura S (2001) Chemical biology and biomedicine: general aspects. In: Fraser-Reid BO, Tastuta K, Thiem J (eds) Glycoscience: chemistry and chemical biology. Springer, New York
No HK, Meyers SP (1995) Preparation and characterization of chitin and chitosan—A review. J Aquat Food Prod Technol 4:27–52. https://doi.org/10.1300/J030v04n02_03
Oh Y-S, Shih I-L, Tzeng Y-M, Wang S-L (2000) Protease produced by Pseudomonas aeruginosa K-187 and its application in the deproteinization of shrimp and crab shell wastes. Enzym Microb Technol 27(1–2):3–10. https://doi.org/10.1016/s0141-0229(99)00172-6
Pachapur VL, Guemiza K, Rouissi T, Sarma SJ, Brar SK (2016) Novel biological and chemical methods of chitin extraction from crustacean waste using saline water. J Chem Technol Biotechnol 91:2331–2339
Pacheco N, Garnica-Gonzalez M, Gimeno M, Bárzana E, Trombotto S, David L, Shirai K (2011) Structural characterization of chitin and chitosan obtained by biological and chemical methods. Biomacromolecules 12:3285–3290. https://doi.org/10.1021/bm200750t
Parada RY, Egusa M, Aklog YF, Miura C, Ifuku S, Kaminaka H (2018) Optimization of nanofibrillation degree of chitin for induction of plant disease resistance: elicitor activity and systemic resistance induced by chitin nanofiber in cabbage and strawberry. Int J Biol Macromol 118:2185–2192. https://doi.org/10.1016/j.ijbiomac.2018.07.089
Paul T, Halder SK, Das A (2015) Production of chitin and bioactive materials from black tiger shrimp (Penaeus monodon) shell waste by the treatment of bacterial protease cocktail. 3. Biotech 5:483–493
Percot A, Viton C, Domard A (2003) Optimization of chitin extraction from shrimp shells. Biomacromolecules 4:12–18. https://doi.org/10.1021/bm025602k
Ploydee E, Chaiyanan S (2014) Production of high viscosity chitosan from biologically purified chitin isolated by microbial fermentation and deproteinization. Int J Polymer Sci 2014:1–8. https://doi.org/10.1155/2014/162173
Prameela K, Mohan CM, Smitha PV, Hemalatha KPJ (2010) Bioremediation of shrimp biowaste by using natural probiotic for chitin and carotenoid production an alternative method to hazardous chemical method. IJABPT 1:903–910
Pusztahelyi (2018) Chitin and chitin-related compounds in plant–fungal interactions. Mycology 9(3):189–201. https://doi.org/10.1080/21501203.2018.1473299
Qin Y, Lu X, Sun N, Rogers RD (2010) Dissolution or extraction of crustacean shells using ionic liquids to obtain high molecular weight purified chitin and direct production of chitin films and fibers. Green Chem 12(6):968–971. https://doi.org/10.1039/C003583A
Ramirez-Coutino L, Marin-Cervantes MDC, Huerta S, Revah S, Shirai K (2006) Enzymatic hydrolysis of chitin in the production of oligosaccharides using Lecanicillium fungicola chitinases. Process Biochem 41:1106–1110. https://doi.org/10.1016/j.procbio.2005.11.021
Rao MS, Stevens WF (2006) Fermentation of shrimp biowaste under different salt concentrations with amylolytic and non-amylolytic Lactobacillus strains for chitin production. Food Technol Biotechnol 44:83–87. https://doi.org/10.1007/s002530000449
Rao MS, Muñoz J, Stevens WF (2000) Critical factors in chitin production by fermentation of shrimp biowaste. Appl Microbiol Biotechnol 54:808–813. https://doi.org/10.1007/s002530000449
Ravi Kumar MNV (2000) A review of chitin and chitosan applications. React Funct Polym 46:1–27. https://doi.org/10.1016/S1381-5148(00)00038-9
Regis B, Marius S, Sandrine B, Roux KL, Del Pino RJ, Jean-Pascal B et al (2015) Kinetic study of solid phase demineralization by weak acids in one-step enzymatic bio-refinery of shrimp cuticles, vol 50. Elsevier Ltd, pp 2215–2223. https://doi.org/10.1016/j.procbio.2015.09.017
Robinson-Lora MA, Brennan RA (2009) The use of crab-shell chitin for biological denitrification: batch and column tests. Bioresour Technol 100:534–541. https://doi.org/10.1016/j.biortech.2008.06.052
Sahu BB, Sahu U, Nagesh Kumar Barik AA, Paikaray A, Mohapatra S, Sahu JK (2017) Bio-refinery products from shell fish processing waste: application of chitin, chitosan, Chitooligosaccharides and derivatives in organic agriculture. Int J Fish Aquat Res 2:27–31
Salaberria AM, Labidi J, Fernandes SCM (2015) Different routes to turn chitin into stunning nano-objects. Eur Polym J 68:503–515. https://doi.org/10.1016/j.eurpolymj.2015.03.005
Sánchez R, Stringari GB, Franco JM, Valencia C, Gallegos C (2011) Use of chitin, chitosan and acylated derivatives as thickener agents of vegetable oils for bio-lubricant applications. Carbohydr Polym 85(3):705–714. https://doi.org/10.1016/j.carbpol.2011.03.049
Sedaghat F, Yousefzadi M, Toiserkani H, Najafipour S (2017) Bioconversion of shrimp waste Penaeus merguiensis using lactic acid fermentation: an alternative procedure for chemical extraction of chitin and chitosan. Int J Biol Macromol 104:883–888. https://doi.org/10.1016/j.ijbiomac.2017.06.099
Setyahadi S, Hermansyah H, Aruan JB (2014) Chitin extraction fermentation Penaeus vannamei Shell wastes with high density cell by recycle culture cells. J Chitin Chitosan Sci 2:209–215. https://doi.org/10.1166/jcc.2014.1061
Shamshina JL, Barber PS, Gurau G, Griggs CS, Rogers RD (2016) Pulping of crustacean waste using ionic liquids: to extract or not to extract. ACS Sustain Chem Engin 4(11):6072–6081. https://doi.org/10.1021/acssuschemeng.6b01434
Shamshina JL, Oldham T, Rogers RD (2019) Applications of chitin in agriculture. In: Sustainable agriculture reviews. vol 36. pp. 125–146
Shimahara K, Takiguchi Y, Ohkouchi K, Kitamura K, Okada O (1984) Chemical composition and some properties of crustacean chitin prepared by use of proteolytic activity of Pseudomonas maltophilia LC102. Chitin, chitosan, and related enzymes. Academic press, New York
Sini TK, Santhosh S, Mathew PT (2007) Study on the production of chitin and chitosan from shrimp shell by using Bacillus subtilis fermentation. Carbohydr Res 342:2423–2429. https://doi.org/10.1016/j.carres.2007.06.028
Sluyanarayana Rao SV, Yashodha KP, Mahendrakar NSP (1987) Deacetylation of chitin at low temperature by a novel alkali impregnation technique. Indian J Technol 25:194–196
Song Z, Li G, Guan F, Liu W (2018) Application of chitin/chitosan and their derivatives in the papermaking industry. Polymers 10(4):389. https://doi.org/10.3390/polym10040389
Soon CY, Tee YB, Tan CH, Rosnita AT, Khalina A (2018) Extraction and physicochemical characterization of chitin and chitosan from Zophobas morio larvae in varying sodium hydroxide concentration. Int J Biol Macromol 108:135–142. https://doi.org/10.1016/j.ijbiomac.2017.11.138
Sorokulova I, Krumnow A, Globa L, Vodyanoy V (2009) Efficient decomposition of shrimp shell waste using Bacillus cereus and Exiguobacterium acetylicum. J Ind Microbiol Biotechnol 36:1123–1126. https://doi.org/10.1007/s10295-009-0587-y
Srinivasa PC, Tharanathan RN (2007) Chitin/chitosan — safe, ecofriendly packaging materials with multiple potential uses. Food Rev Int 23(1):53–72. https://doi.org/10.1080/87559120600998163
Srinivasan H, Kanayairam V, Ravichandran R (2018) Chitin and chitosan preparation from shrimp shells Penaeus monodon and its human ovarian cancer cell line, PA-1. Int J Biol Macromol 107:662–667. https://doi.org/10.1016/j.ijbiomac.2017.09.035
Sumardiono S, Siqhny ZD (2018) Production of fish feed from soy residue and shrimp waste using tapioca as binding agent. In: the 3rd international conference of chemical and materials engineering, Semarang, Indonesia. J Phys Conf Ser
Suryawanshi N, Jujjavarapu SE, Ayothiraman S (2019) Marine shell industrial wastes–an abundant source of chitin and its derivatives: constituents, pretreatment, fermentation, and pleiotropic applications-a revisit. Int J Environ Sci Technol 16:3877–3898. https://doi.org/10.1007/s13762-018-02204-3
Suryawanshi N, Ayothiraman S, Jujjavarapu SE (2020) Ultrasonication mode for the expedition of extraction process of chitin from the maritime shrimp shell waste. Indian J Biochem Bio 57:431–438
Tan YN, Lee PP, Chen WN (2020) Microbial extraction of chitin from seafood waste using sugars derived from fruit waste-stream. AMB Expr 10:17. https://doi.org/10.1186/s13568-020-0954-7
Teng WL, Khor E, Tan TK, Lim LY, Tan SC (2001) Concurrent production of chitin from shrimp shells and fungi. Carbohydr Res 332(3):305–316. https://doi.org/10.1016/s0008-6215(01)00084-2
The Marine Products Export Development Authority [MPEDA] (2013) Annual report
Valdez-Peña AU, Espinoza-Perez JD, Sandoval-Fabian GC (2010) Screening of industrial enzymes for deproteinization of shrimp head for chitin recovery. Food Sci Biotechnol 19:553–557. https://doi.org/10.1007/s10068-010-0077-z
Vandenbergh PA (1993) Lactic acid bacteria, their metabolic products and interference with microbial growth. FEMS Microbiol Rev 12:221–238
Verma ML, Kumar S, Das A, Randhawa JS, Chamundeeswari M (2020) Chitin and chitosan-based support materials for enzyme immobilization and biotechnological applications. Environ Chem Lett 18:315–323
Wang SL, Chio SH (1998) Deproteinization of shrimp and crab shell with the protease of Pseudomonas aeruginosa K-187–waste pretreatment, enzyme production, process design, and economic analysis. Enzym Microb Technol 22:629–633
Woods B (1998) Microbiology of fermented foods, vol 1. Blackie, London
Wu ACM, Bough WA (1977) A study of variables in the chitosan manufacturing process in relation to molecular-weight distribution, chemical characteristics and waste-treatment effectiveness. In: Muzzarelli RAA, Pariser ER (eds) Proceedings of the 1st International Conference on Chitin/Chitosan, Boston, USA, 11–13, April, 1978. pp 88–102
Ximenes JCM, Hissa DC, Ribeiro LH, Rocha MVP, Oliveira EG, Melo VMM (2019) Sustainable recovery of protein-rich liquor from shrimp farming waste by lactic acid fermentation for application in tilapia feed. Braz J Microbiol 50(1):195–203. https://doi.org/10.1007/s42770-018-0024-3
Xu Y, Gallert C, Winter J (2008) Chitin purification from shrimp wastes by microbial deproteination and decalcification. Appl Microbiol Biotechnol 79:687–697. https://doi.org/10.1007/s00253-008-1471-9
Xue W, Han Y, Tan J, Wang Y, Wang G, Wang H (2018) Effects of nanochitin on the enhancement of the grain yield and quality of winter wheat. J Agric Food Chem 66:6637–6645. https://doi.org/10.1021/acs.jafc.7b00641
Younes I, Rinaudo M (2015) Chitin and chitosan preparation from marine sources. Structure, properties and applications. Mar Drugs 13:1133–1174. https://doi.org/10.3390/md13031133
Younes I, Ghorbel-Bellaaj O, Nasri R, Chaabouni M, Rinaudo M, Nasri M (2012) Chitin and chitosan preparation from shrimp shells using optimized enzymatic deproteinization. Process Biochem 47(12):2032–2039. https://doi.org/10.1016/j.procbio.2012.07.017
Zakaria Z, Hall GM, Shama G (1998) Lactic acid fermentation of scampi waste in a rotating horizontal bioreactor for chitin recovery. Process Biochem 33(1):1–6. https://doi.org/10.1016/S0032-9592(97)00069-1
Zargar V, Asghari M, Dashti A (2015) A review on chitin and chitosan polymers: structure, chemistry, solubility, derivatives, and applications. Chem Bioeng Rev 2:204–226. https://doi.org/10.1002/cben.201400025
Zhang H, Yafang Jin Y, Yun Deng Y, Danfeng Wang D, Zhao Y (2012) Production of chitin from shrimp shell powders using Serratia marcescens B742 and Lactobacillus plantarum ATCC 8014 successive two-step fermentation. Carbohydr Res 362:13–20
Zhang W, Yin B, Xin Y et al (2019) Preparation, mechanical properties, and biocompatibility of graphene oxide-reinforced chitin monofilament absorbable surgical sutures. Mar Drugs 17(4):210. https://doi.org/10.3390/md17040210
Zhao D, Huang WC, Guo N, Zhang S, Xue C, Mao X (2019) Two-step separation of chitin from shrimp shells using citric acid and deep eutectic solvents with the assistance of microwave. Polym (Basel) 11:409. https://doi.org/10.3390/polym11030409
Zhou D, Zhang L, Guo S (2005) Mechanisms of lead biosorption on cellulose/chitin beads. Water Res 39:3755–3762. https://doi.org/10.1016/j.watres.2005.06.033
Acknowledgments
Gincy Marina Mathew thanks and acknowledges the Women Scientists Division, Kerala State Council for Science, Technology and Environment, for the financial assistance under the “Back-to-Lab” Post-Doctoral Fellowship Programme, Kerala, India. Raveendran Sindhu acknowledges the Department of Science and Technology for sanctioning a project under DST WOS-B scheme.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Mathew, G.M., Sukumaran, R.K., Sindhu, R., Binod, P., Pandey, A. (2022). Microbes for the Synthesis of Chitin from Shrimp Shell Wastes. In: Inamuddin, Ahamed, M.I., Prasad, R. (eds) Application of Microbes in Environmental and Microbial Biotechnology. Environmental and Microbial Biotechnology. Springer, Singapore. https://doi.org/10.1007/978-981-16-2225-0_15
Download citation
DOI: https://doi.org/10.1007/978-981-16-2225-0_15
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-16-2224-3
Online ISBN: 978-981-16-2225-0
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)