Abstract
Epidemiological data on birth defects is usually derived from surveillance systems. Systematic surveillance for birth defects is yet to be established in India. Data on the magnitude and trends of birth defects have to be derived from other sources, primarily research studies. This review provides an overview of sources of data on congenital anomalies in India, and the magnitude, mortality and disability associated with these conditions in the country. Available data indicate a birth prevalence of 18.44–23.05 per 1000 births, which translates into 472,177 and 581,899 affected births occurring annually in the country. Estimates of congenital anomaly neonatal mortality range from 54,000 to 99,000. Epidemiological data identify that as congenital anomaly mortality is averted, the numbers of disability survivors increase. Data from limited studies suggest that 70% of birth defects are non-fatal. The Modell Global Database of Congenital Disorders estimates that there would be over 150,000 survivors with congenital disability at five years of age per birth cohort. The review identifies the need for a nation-wide birth defects surveillance system for monitoring these large numbers in India. The Rashtriya Bal Swasthya Karyakram, a community-based screening and early identification programme for common childhood diseases, nutritional deficiencies, birth defects and developmental delays and disabilities, is a potential opportunity for collecting nation-wide data on birth defects. Till surveillance is established, robust primary studies are needed, that can inform the true prevalence of congenital anomaly affected births, mortality and survivors with disability. Strategically established population-based surveillance can be utilized to monitor the types and prevalence of congenital anomalies in India.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
Epidemiological data describe the person, place and time of occurrence of a disease or a health condition. The data provide information on the magnitude and impact of the condition in terms of mortality and disability, morbidity patterns and trends, and risk and causative factors for the disease or health condition. Such data inform public health action by identifying healthcare needs, the required services and the financial investment for implementing these services. Epidemiological data can be obtained through several sources. Typically, health information systems provide information on the numbers affected, and the consequences in terms of morbidity, mortality and disability. For example, HIV surveillance determines the number of people affected. Healthcare usage by patients with HIV can be determined from hospital in-patient or out-patient data. Vital statistics provide data on cause-specific mortality, for example, the number of AIDS-related deaths. Routinely available data from public health information systems and vital records provide information for understanding the epidemiology of the health condition, and the impact of implemented interventions.
The challenge of describing the epidemiology of diseases/health conditions arise when data collection systems are unavailable, or the data lack in quality and timeliness, or are incomplete. The latter is especially relevant in India, where 70% of healthcare providers belong to the private health sector. These providers do not report health data to the public health information system, resulting in a large gap in the understanding of the epidemiology of prevalent conditions. In such instances, surveys and research studies form the major source of data.
Obtaining data on birth defects is especially challenging, as these are conditions with low prevalence. Visible structural defects (congenital anomalies, congenital malformations) like spina bifida, orofacial clefts or limb defects are relatively easy to recognize at birth. Some common chromosomal anomalies or single gene disorders may be suspected in very sick newborns, or those with abnormal physical appearance. The signs and symptoms of some common conditions, especially congenital heart defects may not be immediately apparent at birth, and may be detected later during childhood.
Data on congenital anomalies are reported as birth prevalence. Birth prevalence of congenital anomalies is the number of congenital anomaly affected newborns per 1000 population. This measure is distinct from population prevalence, which reports the number of individuals with congenital anomalies resident in a population. Due to the higher mortality associated with severe birth defects, birth prevalence of congenital anomalies is higher than population prevalence. However, if prevalence is measured at later ages (for example, among children at 15 years of age), then population prevalence may be higher than the birth prevalence due to cumulative cohorts of surviving children. Measurement of birth prevalence has the advantage that denominator data, that is the number of births occurring in the population, are routinely collected by health information systems. Thus, the relative ease of recognition of visible structural defects, as well as the availability of denominator data on numbers of births makes birth prevalence the most appropriate indicator to report the prevalence of congenital anomalies.
In industrialized countries, and some Latin American countries, birth defects surveillance systems report data on congenital anomalies [1,2,3,4,5,6,7,8]. Birth defects surveillance systems were established after the thalidomide incident, as described in Chap. 3. The understanding that drugs could cross the placental barrier and cause foetal malformations, led to the establishment of birth defects surveillance, with the specific purpose of monitoring teratogenic exposures. Birth defect surveillance is a resource intensive activity that requires careful planning and funding [9]. The essential steps of establishing a surveillance are summarized in Box 6.1 and Chap. 4. The key point to note is that in order to produce quality data, the surveillance system has to be supported by sufficient resources.
Key considerations while establishing a birth defects surveillance system (from Ref. [9])

The birth prevalence of congenital anomalies is typically 2–3%, that is, it ranges between 20 and 30 per 1000 births. However, this prevalence is influenced by the way the data is reported, that is, the number of conditions included, methods of coding and categorization used, and the age of inclusion, that is, the age till which the registry collects and reports data. For example, the South Australian Birth Defects Register which includes data on congenital anomalies, chromosomal conditions, cerebral palsy and haematological/immunological conditions in children till five years of age and includes data on pregnancy terminations and stillbirths, reports a prevalence of around 6% [3]. It is, however, important to remember that birth defect rates are usually constant and show rare and limited fluctuations unless there is a teratogenic exposure in the population. Box 6.2 illustrates the utility of investing in birth defects surveillance, as the data provides a rich source of information on the epidemiology and required healthcare interventions.
Utility of birth defects surveillance (data from South Australia birth defects registry [3])

Where such surveillance systems are not in place, such as in India, other sources of data provide information on congenital anomalies. This article reviews the sources of data and describes the magnitude of congenital anomaly mortality and disability in India, beginning with an overview of child health in the country, which underlines the need for determining the trends of congenital anomalies in India.
Child Health in India
India reported over 24 million births in 2016. In 2017, the Neonatal Mortality Rate (NMR) was 23 per thousand live births. The Infant Mortality Rate (IMR) was 33, while the under-5 mortality rate was 37. There are large variations in indicators across states of the country. IMR, for example, ranged from 10 in the state of Kerala to 47 in Madhya Pradesh, while mortality rates among children younger than five years of age ranged from 12 in Kerala to 55 in Madhya Pradesh [10].
Indian national data reflected major decline in child deaths. Deaths among children below five years of age reduced by 37% since 2012, from 1.4 million to 882,000. Infant deaths reduced by 34% from 1.09 million to 721,000 between 2015 and 2017. Neonatal deaths reduced by 29%, from 779,000 to 549,000. Although child mortality from common infectious causes such as pneumonia, diarrhoea, and measles, and neonatal mortality due to sepsis, birth asphyxia or trauma declined significantly, neonatal deaths due to prematurity and low birth weight remained a major concern, and significant focus of public health action [11].
Rigorous data on congenital anomalies are not collected in India, but estimated data indicated that in 2017, congenital anomalies were not insignificant contributors to child mortality. The data suggested that 35% of neonatal deaths were associated with preterm births, 24% with intra-partum events, 14% were due to sepsis and meningitis, while 11% were associated with congenital anomalies [12]. Trends indicated that the proportion of deaths due to prematurity and congenital anomalies increased, as other causes of child deaths reduced [12]. Such data signal the urgency of monitoring data on birth defects in the country.
Sources of Data
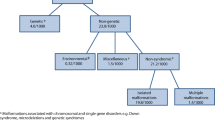
In lieu of birth defects surveillance in India, data on congenital anomalies have to be obtained from different sources of data (Fig. 6.1).
Sources of data to describe the epidemiology of congenital anomalies in India. MGDb = Modell Global Database of Congenital Disorders, RBSK = Rashtriya Bal Swasthya Karyakram, SEARO-NBDD = South East Asia Regional Office of the World Health Organization—Neonatal-Birth Defects Database, MCCD = Medical Certification of Cause of Death, MDS = Million Death Study, MCEE = Maternal and Child Epidemiology Estimation Group, GBD = Global Burden of Disease, MGDb = Modell Global Database of Congenital Disorders, NSSO = National Sample Survey
-
1.
Prevalence (magnitude)
Congenital anomaly birth prevalence data are available from different hospital-based cross-sectional studies, and a single cohort study. Population prevalence data are available from limited community-based cross-sectional studies. Estimated data on the magnitude of congenital anomalies are available from the Modell Global Database of Congenital Disorders (MGDb). Routine data on prevalence of birth defects are available from public health information systems. Data on birth defects among beneficiaries of the Rashtriya Bal Swasthya Karyakram (RBSK) child screening service are infrequently reported. There is and have been attempts to establish birth defects surveillance, but the data are not systematic and based on voluntary reporting.
-
2.
Congenital anomaly mortality
These data are available from cause-specific child mortality estimates from the Million Death Study (MDS), estimates from the Maternal and Child Epidemiology Estimation Group (MCEE), from of the MGDb and the Global Burden of Disease (India) data.
-
3.
Data on congenital disability at 5 years of age are available from a study, the MGDb, and from a national disability survey that enquired about the time of onset of disability. Onset of disability since birth provides data on the magnitude of functional impairments caused by birth defects (and developmental disabilities).
Congenital Anomaly Prevalence
Individual Research Studies
In India, estimates of congenital anomaly prevalence are available from cross-sectional studies, and a single cohort study. Cross-sectional studies are standard epidemiological methods for measuring prevalence. However, unlike other diseases or health conditions, population-based cross-sectional studies are challenged by the low prevalence of congenital anomalies. Reporting birth prevalence is the most appropriate method, as the surveys can be set in maternity hospitals. The number of congenital anomalies among all births occurring at the hospital will provide data on the proportion of congenital anomaly affected births. In India, hospital-based prevalence will not provide true estimates of congenital anomalies. Selection bias will occur if a major referral hospital is selected. Data will be over-estimates, due to referral of complicated cases. Hence, hospital-based cross-sectional studies, although feasible, will only report proportion of birth defects among all births occurring at the selected hospitals.
Population-based surveys of congenital anomalies require very large populations, which make these projects expensive. Data may be collected through door-to-door survey of households in a defined population. Although expensive, this approach is likely to yield the most accurate population prevalence rates. The major limitation is recall or willingness to share data on birth defects, and the availability of accurate clinical records. Certain key considerations for conducting studies to measure the prevalence of congenital anomalies are summarized in Box 6.3.
Box 6.3
Cross-sectional studies for measurement of congenital anomalies in India and other mixed healthcare settings*
-
Characteristics of cross-sectional studies
-
Observational study, that is, the investigator does not alter exposure variables.
-
Outcome and exposure variables are measured at the same time. For example, a study to measure the prevalence of spina bifida (outcome variable) can simultaneously measure data on exposure variables such preconception folate supplementation, age of mother, socio-economic status, access to antenatal care, etc.
-
Can be used to measure association between outcome and exposures, but cannot be used to establish causality.
-
Useful method to know the magnitude of congenital anomalies when surveillance systems are not in place.
-
Necessary to keep in mind that the prevalence of a disease/disorder is dependent on incidence (new cases) and duration of survival. Congenital anomalies are rare conditions, and survival is likely to be low in LMICs. As such, prevalence will be low.
-
Low prevalence indicates the necessity to survey large populations, making the research expensive.
-
Important points to keep in mind while planning the study
-
1.
Population
Population where the study is being conducted should be the population at risk and should have the same characteristics as the general population. Otherwise, it will not be possible to generalize the estimates to the general population. For example, women delivering at referral facilities for complicated cases would not represent the general population of women delivering at all birthing facilities.
-
2.
Study setting: Hospital based or population based?
-
Hospital based: Will yield data on proportion of affected births among all births occurring at the hospital(s). Selection bias has to be avoided by ensuring representativeness of study hospitals in the sample. For example, the selection of a general hospital versus a hospital providing care for complicated cases will influence prevalence, as the numbers of cases of congenital anomalies are likely to be higher in the second type of facility. Prevalence measurements will be over-estimates.
-
Population based: Challenging as large populations will have to be surveyed. Timing (newborn, infants or older ages) will be an important determinant of prevalence. For older ages, it is necessary to ensure proper recall of events. Under-estimates are likely due to reduced survival, out-migration for treatment, lack of availability of clinical records for surviving children, recall/willingness to disclose details of termination of pregnancy or death or disability, sufficient knowledge about the birth defect in case of a stillbirth, availability and accessibility to antenatal care (ANC), type of ANC service, etc.
-
-
3.
Measurement of outcome
Measurement of outcome (congenital anomaly) has to keep in mind measurement bias arising from misclassification (misdiagnosed or undiagnosed cases). It is necessary to follow globally used descriptions being used by international registries. The WHO/CDC/ICBDSR manual [9] is an excellent source to understand coding and classification. An online course is available on the ICBDSR website. Steps to consider are:
-
Selection of type of malformations to be studied: all/selected congenital anomalies?
-
Case definitions.
-
-
4.
Defining exposure variables
Depending on the objectives of the study, these may be characteristics such as teratogenic exposures, habits and health status, family history and folic acid use.
-
5.
Sampling
-
For hospital-based studies, it is necessary to draw a random selection of hospitals.
-
Denominator will be all women delivering at selected hospitals.
-
For population-based studies, all birth occurring to women resident in a geographic area are included.
-
Age needs to be defined, that is, will the data be collected for measuring the prevalence of congenital anomalies among neonates, infants and children under five years of age or older.
-
Denominator will be all pregnant women resident in the area for the duration of the survey.
-
Data will be incorrect if data on deliveries of resident women occurring at facilities outside the study area (e.g. maternal residence of the woman) are excluded, and women from outside the area, delivering at one of the hospitals is included.
-
-
6.
Data reporting
-
1.
Live birth prevalence of congenital anomalies = live birth cases/total live births × 10,000.
-
2.
Birth prevalence of congenital anomalies = live birth cases + foetal death (stillbirths) cases/total live births + foetal deaths (stillbirths) × 10,000 [9].
-
3.
Total prevalence of congenital anomalies = live birth cases + foetal death (stillbirths) cases + ETOPFA cases/total live births + total foetal deaths (stillbirths) + total ETOPFA × 10,000 [9].
-
4.
Data may be reported as point prevalence (prevalence as measured at any given period of time) or as period prevalence (prevalence as measured over a period of time).
-
1.
-
*Mixed healthcare settings are typically those where there are government and private health facilities. There is no compulsory data reporting from private health facilities, which are used by the majority of the population. As a significant proportion of health care is financed through out of pocket expenditure, there is population mobility. In context of these population characteristics, it is critically important to ensure appropriate study designs for estimating prevalence.
Several studies have reported the magnitude of congenital anomalies in India. A systematic review and meta-analysis pooled the data from these studies to derive a national estimate [13]. The systematic review conducted in 2015 identified 52 hospital-based and 3 population-based studies. The quality of studies varied. Hospitals were selected in a non-random manner, with most being large referral hospitals where high risk women were likely to be referred. None of the studies used (or mentioned) case definitions and case ascertainment methods. ICD codes were rarely used/reported. Physical examination for visible birth defects was the method of detection of malformations in all studies. None of the studies included data on pregnancy terminations.
The pooled national prevalence among 802,658 births estimated a congenital anomaly birth prevalence of 184.48 per 10,000 births (95% CI 164.74–204.21) [13]. The pooled live birth prevalence from hospital-based studies was 203.33 per 10,000 live births (95% CI 171.32–235.34) for 44,392 live births. Population-based studies reported a higher pooled prevalence of 261.05 per 10,000 live births (95% CI 199.13–322.96) among 10,193 live births. Table 6.1 shows the system-wise prevalence of anomalies, and the differences due to the inclusion of stillbirths among hospital-based studies (community-based studies reported data on live births only). Central nervous system anomalies were most frequently reported in both hospital and community-based studies, followed by anomalies of the musculoskeletal system (75.85 per 10,000 births, (95% CI 58.80–92.90) and 65.64 per 10,000 births (95% CI 52.97–78.31), respectively). Cardiovascular system anomalies, the most prevalent type of congenital anomaly, had the lowest birth prevalence across both hospital and community settings [13].
Table 6.2 shows the prevalence of selected congenital anomalies. Anencephaly was the most commonly reported anomaly with a birth prevalence of 21.1 per 10,000 births (95% CI 16.91–25.29). Talipes equinovarus was the next most prevalent congenital anomaly (birth prevalence 17.9 per 10,000 births, 95% CI 15.09–20.71), followed by orofacial clefts (birth prevalence 14.94 per 10,000 births, 95% CI 12.64–17.24) and hypospadias (birth prevalence 12.20 per 10,000 births, 95% CI 9.79–14.60). The analysis found that among live births, the pooled prevalence of clubfoot was highest (35.08 per 10,000 live births, 95% CI 16.88–53.29).
PUBOs Study Estimates
None of the hospital or community-based studies included data on pregnancy terminations. In order to circumvent, the methodological issues associated with a cross-sectional study, the Pune Urban Birth Outcome study (PUBOs) recruited a cohort of 2107 women at 9 ± 3 weeks of gestation and followed them up till outcome (stillbirths, pregnancy termination, live births, neonatal deaths) [14]. The advantage of a cohort design was that all pregnancy outcomes could be measured. There was a 9% loss to follow-up, so that data was available for 1910 women. The characteristics of the cohort were similar to that of the general population, and the pregnancy indicators matched those that were reported for the general population. The total prevalence of congenital anomalies was 230.51 (95% CI 170.99–310.11) (Table 6.3). The congenital anomaly neonatal mortality rate was 3.93 per 1000 live births. There were 4.39 pregnancy terminations for foetal malformation per 1000 births [14].
Table 6.4 shows the rates per 10,000 for specific congenital anomalies identified during the neonatal period. Due to the small size of the study, it was not possible to compare the data with those reported by large registries. However, the prevalence of 230.51 per 10,000 births indicated over 530,000 congenital anomaly affected births per year in India. There are likely to be 151,488 cases of congenital heart defects, 75,000 with talipes equinovarus, over 60,000 neural tube defects, 88,000 with congenital urogenital disorders, over 25,000 hypospadias, and over 10,000 cases of orofacial clefts (Table 6.4).
The data from the PUBOs cohort indicated that while one in five births were low birth weight, one in nine were preterm births and one in 20 pregnancies resulted in a miscarriage, one in 44 births were affected with a major congenital anomaly, which was similar to the number of stillbirths. In terms of type of anomaly, the cohort data suggested that one in 152 births would be affected with a congenital heart defect, one in 304 births would be affected with clubfoot or a renal anomaly, while one in 364 births would present with a neural tube defect.
Estimates from the Modell Database
The Modell Global Database of Congenital Disorders (MGDb) is a database of modelled estimates of congenital anomaly prevalence, mortality and disability [15]. The work was initiated in 1980 by Prof. Bernadette Modell. The first estimates were reported in the highly cited March of Dimes Report of 2006 [16]. Updated estimates for all countries are freely available (https://discovery.ucl.ac.uk/1532179/). Table 6.5 shows that the estimated baseline birth prevalence (total affected stillbirths and live births per 1000 births) for India was 20.83 per 1000, similar to that reported by the meta-analysis of congenital anomaly magnitude in India. In absolute numbers, this would indicate 540,421 affected births. Data are available for neural tube defects, orofacial clefts, congenital heart defects and other lethal and sub-lethal congenital anomalies. However, these estimates are based on existing primary data. The MGDb estimates 14,087 pregnancy terminations for foetal malformations. Most importantly, the MGDb is the only source of data on survivors with disability, a crucial public health indicator for birth defects. The MGDb estimated that for each birth cohort, there would be 156,218 survivors with disability at the age of five years (Table 6.5).
Rashtriya Bal Swasthya Karyakram Data
Population-based data on the magnitude of birth defects are available from the Rashtriya Bal Swasthya Karyakram (RBSK) [17], further explained in Chap. 12. Children are screened in the community and referred to District Early Intervention Centres for confirmatory diagnosis and treatment for nine common birth defects (neural tube defects, Down syndrome, cleft lip/palate, clubfoot, developmental dysplasia of hip, congenital cataract, congenital deafness, congenital heart defects, retinopathy of prematurity, congenital hypothyroidism, sickle cell anaemia and thalassemia) and selected developmental disabilities. In an early publication, the prevalence of congenital anomalies among children between the age of 2 and 18 years was reported to be 1% of 42 million children screened by this programme. Among 135,000 congenital anomaly affected births, 39% were congenital heart defects and 13% were cases of neural tube defects [18]. The latest available data mentions that in 2016, of 187 million screened children, 346,000 were diagnosed with a birth defect [19] (Table 6.6). These data suggest that children with birth defects make up less than 0.2% of cases.
The RBSK data has several limitations. There is a possibility that screening children above the age of two years may miss sick children or children with disabilities, as they may not attend schools or play centres. The RBSK does not screen children attending private schools. Nevertheless, the RBSK is a promising option for surveillance for birth defects in India.
Data from Birth Defects Surveillance Systems in India
There are two attempts at establishing birth defects surveillance in India. The Birth Defects Registry of India (BDRI) was a private initiative by the Fetal Care Research Foundation, located in Chennai, India [20]. Between 2000 and 2015, nearly 278 hospitals voluntarily reported data on birth defects. These data were reported to the ICBDSR for a brief period. Due to the voluntary nature of these activities, and the passive method of surveillance, the data were not systematic. The WHO-SEARO has established a Newborn-Birth Defects Database (SEAR-NBDD) [21]. The network includes 170 hospitals from seven countries of the region. The purpose of this network is to develop capacity for birth defects surveillance, with the purpose of providing data on the epidemiology of birth defects in these countries. However, this registry is also limited by the passive method of surveillance, and the lack of specific funding to support the activity.
Congenital Anomaly Mortality
Data on congenital anomaly neonatal and child mortality are available. They identify that congenital anomalies cause considerable numbers of neonatal and child deaths in India.
Data from the Medical Certification of Cause of Death
Data on vital statistics in India are available from the Registrar General of India. Medical certification of cause of death is available for only 22% of total registered deaths across all ages occurring in the country. Most of these medically certified deaths are reported from hospitals located in urban areas. The most recent data from the Medical Certification of Cause of Death (MCCD) is available for 2017, which covered 1,411,060 deaths from across the country [22]. A total of 105,605 infant deaths were recorded, among which 8829 (8.4%) were certified as being caused by ‘congenital malformations, deformations, and chromosomal abnormalities’. According to the MCCD data, congenital malformation, deformation and chromosomal abnormalities caused 5.3% of deaths. In contrast, 77% of infant mortality was due to ‘certain conditions originating in the perinatal period’, followed by infectious and parasitic diseases (5.6%) (Table 6.7).
Congenital malformations of the circulatory system (4802, 54%) were the major type of congenital anomalies, followed by ‘other’ congenital malformations (40% of mortality). Spina bifida caused 464 (5.3%) of deaths, with more deaths (327) occurring in the age group above one year than among infants (137). The MCCD recorded only 51 (0.7%) deaths due to cleft lip/palate. Majority of congenital anomaly deaths occurred in the first year of life (5595, 63%). The MCDD data are obviously not systematically collected, and therefore, cannot be used to determine congenital anomaly mortality in India.
National Child Mortality Estimates
Data on congenital anomaly mortality are available from studies that have estimated cause-specific child mortality in India.
Cause-Specific Mortality Estimates
Data on congenital anomaly mortality is available from the Million Death Study (MDS), a study that estimated cause-specific mortality trends from among 1.3 million households in India [23]. These households were randomly selected from the Sample Registration System (SRS), an ongoing surveillance system that was established in 1971 by the Registrar General of India to collect vital statistics data. The SRS includes randomly selected villages, and urban blocks, from which data on approximately 140,000 births and 460,000 deaths are recorded annually. The MDS used the WHO verbal autopsy questionnaire to collect information on cause-specific mortality. The field data were assigned underlying cause of death by trained physicians using ICD (International Statistical Classification of Diseases and Related Health Problems) coding guidelines.
Between 2000 and 2015, the MDS reported 696,000 neonatal deaths and 505,000 deaths among children between 1 and 59 months [23]. The congenital anomaly neonatal mortality rate per 1000 live births was 1.1 and the rate for death among children between 1 and 59 months was 1.0 per 1000 live births. In absolute numbers, the MDS data indicated 28,000 neonatal 26,000 congenital anomaly deaths in the 1–59 month age group, respectively, for 2015. The MDS reported that between 2000 and 2015, average annual neonatal congenital anomaly mortality had decreased by 5.1%, while child (1–59 months) congenital anomaly mortality had reduced by 3.5%.
The Maternal and Child Epidemiology Estimation Group (MCEE) reported cause-specific under-5 mortality in India [24]. The methodology adopted by this group differs from that used by the MDS. The study estimated 71,802 (Uncertainty Interval (UI) 56,681–92,640) congenital anomaly neonatal deaths, and 28,035 (UI 21,820–33,777) deaths in the age group of 1–59 months. According to these estimates, in 2015, congenital anomalies were the fourth major cause of neonatal death, with prematurity accounting for 44%, intra-partum complications for 19%, sepsis/meningitis for 14% and congenital anomalies for 10% of mortality. Congenital anomalies were the fourth-largest cause of child deaths in the 1–59 month age group (31% mortality due to pneumonia, 21% due to diarrhoea, 7% due to injuries and 6% due to congenital anomalies).
The study reported a relationship between under 5 mortality rate and congenital anomalies [24]. In regions of the country that had achieved the rate of <25 deaths per 1000 live births, the leading causes were preterm birth complications (26.4%), followed by congenital anomalies (17.1%). In contrast, in regions with high under 5 mortality (>65 deaths per 1000 live births), preterm birth complications (27.4%), pneumonia (18.7%) and diarrhoea (11.2%) were the leading causes of mortality. The two leading causes of child death across the country were preterm birth complications and pneumonia, but in the southern regions (with the lowest under-5 mortality rates), preterm birth complications and congenital anomalies were the leading causes of mortality.
Global Burden of Disease Study
The India State-Level Disease Burden Initiative as part of the Global Burden of Diseases, Injuries and Risk Factors Study (GBD) 2017 has reported national and sub-national trends of cause-specific under-5 mortality [25]. This study reported that congenital anomalies were the fourth leading cause of neonatal deaths, causing 8.6% of neonatal deaths, after mortality caused by preterm birth 27.7%, encephalopathy due to birth asphyxia and trauma 14.5%, and lower respiratory tract infections, 11%. The study reported a decline in the death rate for all major causes of child deaths, but the smallest declines were observed for congenital anomalies. For example, the decline was 82 and 69% for measles and diarrhoeal diseases, while it was the least (15%) for congenital anomalies. Although the study observed a strong inverse correlation between infectious diseases like measles and socio-demographic index (SDI), no such relationship was observed for congenital birth defects.
GBDI Congenital Birth Defect Data
A more focussed analysis on congenital anomaly mortality was conducted using the data available from the Global Burden of Disease (India) (GBDI) database [26]. The GBD uses the term congenital birth defects to include a number of congenital anomalies (neural tube defects, congenital heart anomalies, orofacial clefts, congenital musculoskeletal and limb anomalies, urogenital congenital anomalies, digestive congenital anomalies, other congenital birth defects), and chromosomal abnormalities (i.e. Down syndrome, Turner syndrome, Klinefelter syndrome, other chromosomal abnormalities). Condition-specific mortality data are available from the GBD India Visualization Hub (IHME GBD India Compare 2017).
The GBD modelled data estimated a total of 501,764 congenital birth defect deaths globally among children below five years of age in 2017, with over 70% of these deaths occurring in low and low-middle SDI countries (Fig. 6.2a). Estimated data indicated that birth defects caused over 82,436 deaths among children below five years of age in India in 2017. Within the neonatal period, birth defect mortality was highest in the early and late neonatal periods (7842 and 846 per 100,000 population, respectively) (Fig. 6.2b).
Birth defect mortality. a Birth defect mortality by SDI regions showing that the largest numbers of deaths occur in lower SDI regions. b Proportion of birth defect mortality by age (early neonatal, late neonatal, post-neonatal and 1–4 years). The magnitude of the problem in lower SDI regions is apparent
In comparison with other causes of child mortality which showed considerable decline between 1990 and 2017, trend analysis indicated a relatively smaller decline in birth defect mortality between 1990 and 2017 (Fig. 6.3a). In the early neonatal period, for example, neonatal encephalopathy deaths reduced by 52%, preterm birth complications reduced by 45%, and neonatal sepsis reduced by 30%, while there was only 11% reduction in birth defect mortality between 1990 and 2017. The proportion of birth defect mortality in children below five years of age increased from 4.0% in 1990 to 7.9% in 2017 (Fig. 6.3b). The proportionate increase in congenital anomaly mortality was caused by reduction in other major causes of mortality in India [26].
Decline in cause-specific neonatal mortality in India. a The decline in neonatal preterm birth complications, sepsis and encephalopathy has been more rapid than that of birth defect deaths. b shows the small decline in birth defect mortality rate between 1990 and 2017 (solid bars). The proportion of birth defect mortality among all-cause mortality shows an increase between 1990 and 2017 (line)
The modelled estimates of the GBDI suggest that congenital anomalies are emerging as significant causes of mortality in the more developed states of India. Among the states, birth defects were the second largest cause of mortality in Kerala. In 17 out of 31 states/regions of the country, birth defects were the third leading cause of neonatal mortality [26]. Although the GBD data are modelled estimates, and they do not include data on pregnancy terminations, they provide a best-available source of data to understand the epidemiology of selected birth defects in India.
Mortality Data from MGDb
In addition to providing baseline estimates of the prevalence of congenital anomalies, the MGDb provides data on foetal deaths, and mortality in children under five years of age [15]. The data are estimated for two scenarios, if interventions were available, and if interventions were unavailable. The effect of two interventions, folic acid fortification and pregnancy termination are used for estimation. For India, the MGDb computed 37,887 foetal and 314,602 deaths in children less than five years of age without either of these interventions (Table 6.5).
Disability
One of the significant findings from the PUBOs cohort was that 70% of congenital anomaly affected neonates were live born (congenital anomaly live born rate 168.44 per 10,000 births) [14]. Congenital anomaly live births included neonates with congenital heart defects, congenital talipes equinovarus, congenital hydronephrosis, hypospadias, undescended testicles and ear anomalies. The data reflect the magnitude of children surviving with disabilities.
The MGDb is the only source that estimates survivors with disability [15]. The data predicts over 150,000 survivors with congenital disability at five years of age per birth cohort (Table 6.8). One of the most important issues highlighted in the MGDb is that as congenital anomaly deaths are averted, the numbers of children surviving with disabilities increase. The MGDb offers three indicators, decrease in under-5 mortality, increase in disability, and the ratio of increase in disability to decrease in under- 5 mortality. The MGDb estimates that existing interventions would result in 36,543 less congenital anomaly deaths among children below five years of age in India, but this would increase the numbers of children with disability by 25,749.
Another source of data on congenital disability is from the National Sample Survey, a nation-wide disability survey [27]. The survey covered 1172.86 million individuals across the country, of which 2.2% were persons with disability (25.802 million). The proportion of individuals with disability since birth was 30% among all persons with disabilities (approximately 7.74 million). The survey data are not yet available, but the results of an earlier survey, conducted in 2002, also reported a 30% prevalence of disability since birth (Chap. 8).
Summary
A number of observations can be made from this review. Firstly, estimates on the magnitude of congenital anomalies in India are challenged by the lack of reliable data. The potential of the RBSK surveillance remains under-utilized, as data are unavailable for children less than 2 years of age. If made functional, the RBSK would be a useful system to monitor birth defects across the country. The RBSK collects data from a diversity of settings, both urban and rural. As RBSK teams monitor areas frequently, the RBSK has the potential for quickly detecting a teratogenic exposure.
In lieu of any such surveillance systems, data on the magnitude of congenital anomalies in India are available from studies and estimates. Individual studies suffer from poor methodology, with little consistency between the findings of different studies. Cardiovascular defects, the most common anomalies had the lowest prevalence in reported studies, but visible anomalies like anencephaly, talipes, orofacial clefts and hypospadias were commonly reported. These studies, however, remain the main source of data on the magnitude of congenital anomalies in India, yielding a national birth prevalence of 184.48 per 10,000 births. These numbers suggest that annually congenital anomalies affect 472,177 births in India. The MGDb estimated the total birth prevalence (live births and stillbirths) of congenital anomalies to be 20.83 per 1000, which would indicate 540,421 affected births. The total birth prevalence (pregnancy termination, live birth and stillbirths) reported from the PUBOs cohort was higher at 230.51 per 10,000 births (95% CI 170.99–310.11). In absolute numbers, these data indicated 581,899 (431,647–782,841) annual congenital anomaly affected births in India.
The data on congenital anomaly child mortality reported by the MDS, MCEE and the India State-Level Disease Burden Initiative differ in their methodology. But the data indicate that anywhere between 54,000 (MDS), over 99,000 (MCEE) and 82,436 (GBD) deaths are likely to have been caused by congenital anomalies in India, among children below five years of age. The Global Burden of Disease data indicate that the decline in the numbers of congenital anomaly deaths has been smaller than the decline in other common causes of neonatal deaths. Such data suggest that congenital anomalies will account for increasing numbers of deaths, as is being observed in the southern parts of India.
A major knowledge gap is in the magnitude of disability survivors. PUBOs reported congenital anomaly live birth prevalence of 168.44 per 10,000 live births. These data on survival till the end of the neonatal period, when extrapolated would suggest 415,630 disability survivors (till the end of the neonatal period) annually. The MGDb estimates that per birth cohort, there would be 156,218 children surviving with disabilities at the age of 5 years. Cumulative cohorts could explain the nearly 8 million persons with congenital disabilities in India.
Based on the challenges of interpreting hospital-based surveillance data from India, population-based birth defects surveillance, among carefully selected communities remains a possibility that needs to be explored. India has several high risk situations and areas. Industrial catastrophes like the Bhopal gas tragedy or reports of children with severe birth defects in areas where banned pesticides are being used are examples of potential sites for long-term surveillance of communities. The other alternative is to support ongoing academic study sites, where cohorts are being followed up for different types of maternal and child health studies [28]. Such sites could not only provide data on congenital anomalies, but also data on risk factors and outcomes (such as morbidity, mortality and hospitalizations) [29]. India already has in place a maternal health surveillance system, the Mother and Child Tracking System. Although there are questions on the data quality [30, 31], this system can be used to have an ongoing surveillance on birth defects. Overall, till systems are put in place for collecting data on birth defects, rigorously performed studies still remain the main source of birth defects data in India.
References
Mai CT, Isenburg JL, Canfield MA et al (2019) National population‐based estimates for major birth defects, 2010–2014. Birth Defects Res 111(18):1420–1435
Lanzoni M, Morris J, Garne E et al (2017) European monitoring of congenital anomalies. JRC-EUROCAT report on statistical monitoring of congenital anomalies (2006–2015). EUR 29010 EN. Publications Office of the European Union, Luxembourg. ISBN 978-92-79-77305-1. https://doi.org/10.2760/157556, PUBSY No. JRC109868. https://publications.jrc.ec.europa.eu/repository/bitstream/JRC109868/kjna29010enn.pdf. Accessed 3 Sept 2018
Gibson CS, Scott H, Rice R, Scheil W (2017) Birth defects in South Australia 2013. SA Birth Defects Register, Women’s and Children’s Health Network, Adelaide. Available at https://www.wch.sa.gov.au/services/az/other/phru/documents/2013_sabdr_annual_report.pdf. Accessed 3 Sept 2018
Correa‐Villaseñor A, Cragan J, Kucik J, O’Leary L, Siffel C, Williams L (2003) The metropolitan Atlanta congenital defects program: 35 years of birth defects surveillance at the centers for disease control and prevention. Birth Defects Res Part A Clin Mol Teratol 67(9):617–624
Mai CT, Kirby RS, Correa A, Rosenberg D, Petros M, Fagen MC (2016) Public health practice of population-based birth defects surveillance programs in the United States. J Public Health Manag Pract 22(3):E1–E8
Botto LD, Robert-Gnansia E, Siffel C, Harris J, Borman B, Mastroiacovo P (2006) Fostering international collaboration in birth defects research and prevention: a perspective from the International Clearinghouse for Birth Defects Surveillance and Research. Am J Public Health 96(5):774–780
Irgens LM (2000) The medical birth registry of Norway. Epidemiological research and surveillance throughout 30 years. Acta Obstet Gynecol Scand Spec Issue Rev 79(6):435–439
Groisman B, Bidondo MP, Gili JA, Barbero P, Liascovich R (2013) Strategies to achieve sustainability and quality in birth defects registries: the experience of the National Registry of Congenital Anomalies of Argentina. J Registry Manag 40(1):29–31
Birth defects surveillance: a manual for programme managers, second edition. Geneva: World Health Organization; 2020. Licence: CC BY-NC-SA 3.0 IGO
Office of the Registrar General & Census Commissioner, India. SRS statistical report 2017. In: Estimates of mortality indicators, Chap 4. Available at https://censusindia.gov.in/vital_statistics/SRS_Report_2017/11.%20Chap%204-Estimates%20of%20Mortality%20Indicators-2017.pdf. Accessed April 2021
UNICEF, WHO, World Bank, UN-DESA Population Division. Levels and trends in child mortality report 2019. Estimates developed by the UN Inter-Agency Group for Child Mortality Estimation. Available at https://www.unicef.org/media/60561/file/UN-IGME-child-mortality-report-2019.pdf. Accessed Sept 2018
Hug L, Alexander M, You D, UN Inter-agency Group for Child Mortality Estimation (2019) National, regional, and global levels and trends in neonatal mortality between 1990 and 2017, with scenario-based projections to 2030: a systematic analysis. Lancet Glob Health 7(6):e710–e720
Bhide P, Kar A (2018) A national estimate of the birth prevalence of congenital anomalies in India: systematic review and meta-analysis. BMC Pediatr 18(1):175
Bhide P, Gund P, Kar A (2016) Prevalence of congenital anomalies in an Indian maternal cohort: healthcare, prevention, and surveillance implications. PLoS ONE 11(11):e0166408
Modell B, Darlison MW, Moorthie S, Blencowe H, Petrou M, Lawn J (2016) Epidemiological methods in community genetics and the Modell Global Database of Congenital Disorders (MGDb). Available at https://discovery.ucl.ac.uk/id/eprint/1532179/17/Epidemiological%20Methods%20in%20Community%20Genetics%20and%20the%20Modell%20Global%20Database%202017-04.pdf. Accessed 5 Sept 2018
Christianson A, Howson CP, Modell B (2006) March of Dimes global report on birth defects: the hidden toll of dying and disabled children. March of Dimes Birth Defects Foundation, White Plains
Government of India, Ministry of Health and Family Welfare, National Health Mission (2013) Rashtriya Bal Swasthya Karyakram. A child health screening and early intervention services under NRHM. Ministry of Health and Family Welfare. Available at https://nhm.gov.in/images/pdf/programmes/RBSK/Operational_Guidelines/Operational%20Guidelines_RBSK.pdf. Accessed 6 Aug 2019
Singh AK, Kumar R, Mishra CK, Khera A, Srivastava A (2015) Moving from survival to healthy survival through child health screening and early intervention services under Rashtriya Bal Swasthya Karyakram (RBSK). Indian J Pediatr 82(11):1012–1018
Government of India, Ministry of Health and Family Welfare (2019) Answers data of Rajya Sabha questions for session 240/year wise physical status Rashtriya Bal Swasthya Karyakram (RBSK) during 2014–15 and 2015–16. Available at https://data.gov.in/node/3978901/download. Accessed 18 Nov 2019
Birth Defects Registry of India. Available at https://fcrf.org.in/bdri_abus.asp. Accessed 11 Oct 2017
SEAR NBBD newborn and birth defects surveillance initiative. https://origin.searo.who.int/entity/child_adolescent/nbbd/web/en/#:~:text=In%202014%2C%20WHO%2DSEARO%20created,for%20submission%20to%20the%20system. Accessed 6 Aug 2019
Office of the Registrar General India. Report on medical certification of cause of death 2017 Available at https://censusindia.gov.in/2011-Documents/mccd_Report1/MCCD_Report-2017.pdf. Accessed Dec 2018
Fadel SA, Rasaily R, Awasthi S, Begum R, Black RE, Gelband H et al (2017) Changes in cause-specific neonatal and 1–59-month child mortality in India from 2000 to 2015: a nationally representative survey. Lancet 390(10106):1972–1980
Liu L, Chu Y, Oza S, Hogan D, Perin J, Bassani DG et al (2019) National, regional, and state-level all-cause and cause-specific under-5 mortality in India in 2000–15: a systematic analysis with implications for the Sustainable Development Goals. Lancet Glob Health 7(6):e721–e734
Dandona R, Kumar GA, Henry NJ et al (2020) Subnational mapping of under-5 and neonatal mortality trends in India: the Global Burden of Disease Study 2000–17. Lancet 395(10237):1640–1658
Ujagare D, Kar A (2021) Birth defect mortality in India 1990–2017: estimates from the Global Burden of Disease data. J Community Genet 12(1):81–90. https://doi.org/10.1007/s12687-020-00487-z. Epub 2020 Oct 15. PMID: 33063164; PMCID: PMC7846616
Ministry of Statistics and Programme Implementation. Persons with disabilities in India. NSS report no. 583 (76/26/1), July–Dec 2018. Available at https://www.mospi.gov.in/sites/default/files/publication_reports/Report_583_Final_0.pdf. Accessed 5 Sept 2018
Fall CHD (2018) Nutrition in fetal life and childhood and its linkage with adult non-communicable disease: lessons from birth cohort studies in India. Proc Indian Natl Sci Acad 84(4):881–889
Botto LD, Mastroiacovo P (2018) Triple surveillance: a proposal for an integrated strategy to support and accelerate birth defect prevention. Ann N Y Acad Sci 1414(1):126–136
Nagarajan TJP, Goel S (2016) Is mother and child tracking system (MCTS) on the right track? An experience from a northern state of India. Indian J Public Health 60(1):34–39
Gera R, Muthusamy N, Bahulekar A, Sharma A, Singh P, Sekhar A, Singh V (2015) An in-depth assessment of India’s mother and child tracking system (MCTS) in Rajasthan and Uttar Pradesh. BMC Health Serv Res 15(1):315
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Kar, A., Ujagare, D. (2021). Magnitude of Congenital Anomalies in India. In: Kar, A. (eds) Birth Defects in India. Springer, Singapore. https://doi.org/10.1007/978-981-16-1554-2_6
Download citation
DOI: https://doi.org/10.1007/978-981-16-1554-2_6
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-16-1553-5
Online ISBN: 978-981-16-1554-2
eBook Packages: MedicineMedicine (R0)