Abstract
Tissue repair and regeneration are ubiquitous phenomena and are required for the long-term survival of animals in the natural environment. However, the ability of tissue repair and regeneration in different animals varies widely. Some lower invertebrates (such as polyps and worms) can regenerate a new individual. Lower vertebrate animals such as newt can regenerate limbs, retina, lens, spinal cord and tail. Zebrafish can regenerate heart and fins [1, 2]. Higher vertebrates, such as mammals, cannot completely regenerate tissues and organs, and can only undergo simple healing and fibrotic repair [1, 2].
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
2.1 The Prevalence of Cellular Dedifferentiation and the Significance of Tissue Repair and Regeneration
2.1.1 Tissue Regeneration: From Lower Animals to Mammals and Cellular Dedifferentiation
Tissue repair and regeneration are ubiquitous phenomena and are required for the long-term survival of animals in the natural environment. However, the ability of tissue repair and regeneration in different animals varies widely. Some lower invertebrates (such as polyps and worms) can regenerate a new individual. Lower vertebrate animals such as newt can regenerate limbs, retina, lens, spinal cord and tail. Zebrafish can regenerate heart and fins [1, 2]. Higher vertebrates, such as mammals, cannot completely regenerate tissues and organs, and can only undergo simple healing and fibrotic repair [1, 2].
The differences in the regenerative ability of different animals are related to the cellular mechanisms of regeneration. There are two main types of regeneration: morphallaxis and epimorphosis [2]. Morphallaxis relies on the differentiation of existing stem cells into the desired cells in the tissue, which does not require cell division and proliferation. Epimorphosis relies on dedifferentiation by mature cells, which divide, proliferate, and re-differentiate, eventually replacing the cells that have been damaged. Morphallaxis of the polyps and worms are mainly due to the large number of totipotent or pluripotent stem cells in their bodies [2]. The regeneration of newt and zebrafish rely on the dedifferentiation of mature cells [1]. In mammalian adults the number of stem cells remaining in various tissues and organs is extremely small, and therefore repair and regeneration after injury cannot be spontaneously achieved.
In mammals, some mature cells are found to dedifferentiate to participate in tissue repair and regeneration. After peripheral nerve injury, Schwann cells dedifferentiate, proliferate, migrate, and re-differentiate into new Schwann cells, which support the regeneration of peripheral axons [3]. When the kidney suffers from acute injury, the proximal tubular epithelial cells dedifferentiate, proliferate and migrate to the injured site, and participate in the repair of the damaged renal tubules [4]. Unfortunately, mature cells in most tissues and organs of mammals cannot spontaneously dedifferentiate into proliferative stem cells after an injury. Therefore, inducing dedifferentiation of mature cells is an important way to promote tissue repair and regeneration in mammals.
This section outlines the prevalence of cellular dedifferentiation after injury and its role and significance in the repair and regeneration of various tissues and organs.
2.1.2 The Definition of Cell Dedifferentiation
Mature cells have their own morphology, function, and physiological characteristics. In general, mature cells are very stable and cannot divide and proliferate. Dedifferentiation refers to a state in which mature cells return to an undifferentiated or immature state. In terms of morphology, the specific morphology of the original cells is lost, the cells become smaller, and the proportion of nucleus to plasma increases. In terms of gene expression, the expression of differentiation-related genes decreases and gene expression related embryonic development rises. In terms of cell cycle, cells re-enter the cell cycle, undergoing division and proliferation.
The result is that the potential of proliferation and differentiation is regained by dedifferentiation.
2.1.3 Epidermal Cell Dedifferentiation and Skin Regeneration
The skin is the largest organ in the body. Epidermal stem cells exist in the basal layer of the epidermis. Under normal conditions, epidermal stem cell proliferate and differentiate to keratinocytes, maintaining the dynamic balance of the epidermis. When the skin is severely wounded, a large number of epidermal stem cells are destroyed and are insufficient in number to rebuild new skin. Therefore, obtaining epidermal stem cells is the key to skin regeneration. In 2001, Xiaobing Fu et al. were the first to report the phenomenon of epidermal cell dedifferentiation in Lancet [5]. The patient’s leg wound ulcer was treated with recombinant human epidermal growth factor (rhEGF), and tissue biopsy was performed for immunohistochemistry. The authors found that β1-integrin and CK19, the markers of epidermal stem cells, were detected in the spinous and granular layers of the new epidermis. However, in normal skin, the cells in the spinous and granular layers are already differentiated, and these two layers do not express β1-integrin and CK19. This study demonstrates that differentiated epidermal cells dedifferentiate to form new epidermal stem cells under skin injury conditions. Mannik et al. provided strong evidences to confirm the dedifferentiation of epidermal cells [6]. They used the Cre/lox tracing technique to track differentiated epidermal cells after transplantation onto the fascia of mice. In the early stages of transplantation, they found that epidermal cells dedifferentiated. The dedifferentiated epidermal cells subsequently re-differentiate to form epidermis, hair follicles, and sebaceous glands. Therefore, promoting the dedifferentiation of epidermal cells is an important way to improve skin regeneration.
2.1.4 Renal Cell Dedifferentiation and Kidney Regeneration
Acute kidney injury (AKI) is a clinical syndrome of acute renal secretion loss. The disease is often caused by ischemic or toxic irritation, such as vasoconstrictor drugs, contrast agents, hypotension caused by sepsis, surgery, or traumatic bleeding [7]. In these cases, local hypoxia and massive metabolite accumulation lead to damage of renal proximal tubular epithelial cells.
The kidneys of mammals such as humans and mice can return to normal function after mild ischemic or toxic damage. Cell lineage tracing techniques reveal that dedifferentiation of surviving proximal tubular epithelial cells is a major source of regenerating tubular epithelial cells [4, 8]. Surviving tubular epithelial cells dedifferentiate under the stimulation of injury and become proliferative and undifferentiated cells (Fig. 2.1). Normal tubular epithelial cells are columnar or elliptical with rich adhesive connections. After dedifferentiation, the cells lose their apical–basal polarity, become flat and long, and lack tight junctions between cells. At the gene expression level, dedifferentiated tubular epithelial cells re-express the renal mesenchymal cell marker vimentin, while vimentin is not expressed in normal renal tubular epithelial cells [9]. The neural cell adhesion molecule (NCAM) expressed by the renal mesenchymal cells is also re-expressed after injury [10]. After kidney injury, the embryonic transcription factor Pax2 is re-expressed in renal tubular epithelial cells [11]. In addition, dedifferentiated tubular epithelial cells also express undifferentiated markers such as Kim1, Annexin A3, CD44, CD133, and CD24 [4]. After dedifferentiation, renal tubular epithelial cell migration ability is enhanced. The cell proliferation ability is also enhanced, and the expression of proliferation markers PCNA and Ki67 are detected [11]. Dedifferentiated renal tubular epithelial cells migrate to the necrotic or apoptotic area, and then differentiate into functional tubular epithelial cells. Therefore, enhancing the dedifferentiation of renal tubular epithelial cells will benefit patients with acute kidney injury.
2.1.5 Cardiomyocyte Dedifferentiation and Heart Regeneration
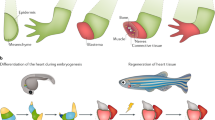
Mammalian heart regeneration is weak, and the infarcted myocardium is basically not regenerated. However, the zebrafish perfectly regenerates the heart. When the ventricle of the zebrafish heart is removed by 20%, the remaining cardiomyocytes near the wound re-grow the lost cardiomyocytes [12]. The zebrafish heart is completely regenerated 60 days after injury (Fig. 2.2). Studies have found that the strong regenerative capacity of the zebrafish heart is attributed to the dedifferentiation of cardiomyocytes [13]. Normal zebrafish cardiomyocytes have well-ordered sarcomere and clear Z-line. After the cardiac injury, the cardiomyocytes near the injury are separated from each other, the sarcomere is dissociated, the Z-line is missing, and the morphology of altered cardiomyocytes is similar to that of the embryonic period. Consistent with this, sarcomere-associated gene expression is downregulated [13]. It is further found that these dedifferentiated cells become more proliferative and expressed proliferation markers such as phosphorylated H3 and PCNA [14]. At the same time, dedifferentiated cardiomyocytes re-express the genes such as Gata4 that is involved in embryonic cardiac development [14]. The expression of the Hand2 gene in embryonic heart is also upregulated, which is beneficial to the proliferative response of dedifferentiated cardiomyocytes [15]. Cre/lox lineage tracing techniques show that proliferative cardiomyocytes are derived from already differentiated cardiomyocytes [13]. These results indicate that differentiated cardiomyocytes undergo dedifferentiation and regain proliferation and differentiation potential, which is the cellular basis for zebrafish heart regeneration.
Adult mammals lose their ability to regenerate their heart, but neonatal mice can regenerate their heart [16]. However, this ability to regenerate is lost 1 week after birth. The study found that the gene Gata4 related to cardiac development, is highly expressed on day 1 after birth, but decreased sharply on the 7th day after birth, corresponding to the period of cardiac regeneration ability lost. Introduction of Gata4 by adenovirus significantly improved the regenerative capacity of the mice heart 7 days after birth [17]. Although mammalian adult cardiomyocytes do not spontaneously initiate dedifferentiation after heart injury, it is found that a very small number of cardiomyocytes re-enter the cell cycle and proliferate under normal physiological or injured conditions [18, 19]. Recently, epicardial cells have been found to contain Follistatin-like 1 (Fstl1) protein that promotes heart regeneration [20]. The human Fstl1 protein is applied to the myocardial infarction by epicardial patch, which can stimulate the survived cells around the infarction to dedifferentiation, ultimately improving heart regeneration. However, this protein is not expressed after myocardial infarction injury, maybe it is an important cause of mammalian loss of myocardial regeneration. Therefore, enhancing dedifferentiation of mammalian cardiomyocytes may improve the ability of heart regeneration.
2.1.6 Visual Cell Dedifferentiation and Retinal Regeneration
Vision is the most important sensory system. The retina is the most important organ for inputting visual information, and it is also the most vulnerable to trauma and diseases such as glaucoma, diabetic retinopathy, and macular degeneration. Death of retinal neurons will severely impair vision and even lead to total blindness.
Mammalian retinas lack the ability to regenerate after injury, but lower animals, such as zebrafish, amphibians, and reptiles, can completely regenerate retinal neurons and restore vision after retinal damage.
The retina of the newt can completely regenerate a new retina after surgical removal, which relies mainly on dedifferentiation of retinal pigmented epithelial cells (RPEC) [21]. When the entire retina is removed, RPEC are separated from each other, re-synthesizing DNA and entering the cell cycle. Reentry of RPEC into the cell cycle is associated with activation of the MEK-ERK pathway after injury [22]. Then, RPEC lose the pigment and begin to express retinal progenitor cell markers. Dedifferentiated RPEC acquire the potential for proliferation and differentiation, then divide along the Bruch membrane and differentiate to form new epithelial cells, including neural retinal progenitor cells and RPEC [23, 24]. The outer cells stop dividing, synthesize pigments, reconstruct the retinal pigment epithelial layer, and the inner cells continue to proliferate and differentiate into various retinal neuronal cells, and finally regenerate the functional retina (Fig. 2.3).
The retina of the zebrafish can also regenerate after injury, mainly relying on dedifferentiation of retinal Müller cells. After surgical removal, neuronal toxic damage, genetic or photodamage, Müller cells can dedifferentiate, proliferate and re-differentiate, regenerating various neurons in the retina. During dedifferentiation, firstly Müller cells decrease expression of glial cell markers and begin to express retinal progenitor cell markers. The Müller cell-specific gene Rlbp1a decreases sharply within 4 h after acute photodamage, completely disappears 1 day after injury, and the glial cell marker GFAP expression is downregulated [25]. In contrast, after 1 h of thermal injury or photodamage, Müller cells begin to express the neural stem cell marker Blbn and the pluripotent retinal progenitor cell marker Rx1 [26, 27], and Müller cells also re-express the retinal progenitor markers Pax6 and Vsx2 [28]. Six3 is a transcription factor which is responsible for the development of vertebrate eyes. After acute photodamage, Müller cells express Six3b and it is found that the expression of Six3b is beneficial to the proliferation of progenitor cells derived from Müller cells [29]. Olig2 is expressed in progenitor cells derived from Müller cells after photodamage [30]. The expression of Olig2 controls the differentiation of retinal progenitor cells into photoreceptor cells and amacrine neurons. Dedifferentiated Müller cells have significant pluripotency and can differentiate into various retinal nerve cells (Fig. 2.4).
Similar to zebrafish, retinal regeneration in neonatal chicken also relies on dedifferentiation of Müller cells. In the N-Methyl-D-aspartate (NMDA)-induced retinal damage model, chicken retinal Müller cells re-enter the cell cycle and proliferate, at the same time, express embryonic retinal progenitor markers Gash-1, Pax6, and Vsx2 [31]. Dedifferentiation-derived retinal progenitor cells migrate to the inner and outer nucleus of the retina, some differentiate into retinal neurons.
The dedifferentiation ability of mammalian retinal Müller cells is even weaker. Generally, after retinal injury, Müller cells can only be activated to form glial scars. The loss of retinal regeneration in mice has recently been found to be associated with the inability of Pax6-positive Müller cells to re-enter cell cycle and proliferate after photoreceptor cell injury [32]. However, when cultured in vitro, after treatment with bFGF, Müller cells are able to form neurospheres and express neural stem cell markers Sox2, Nestin, and Musashi, and also express retinal progenitor markers Pax6 and Rx1 [33].In a mouse or rat model treated with NMDA in vivo, the retina is treated with bFGF or retinoic acid, Müller cells can proliferate and express the progenitor cell marker Pax6, and a small number of dedifferentiated progenitor cells can re-differentiate into bipolar cells, rod-shaped visual receptor cells or amacrine cells [34, 35]. This demonstrates that mammalian Müller cells still retain low levels of dedifferentiation. In vitro, glutamate and alpha-aminoadipate stimulate Müller cells to dedifferentiate into retinal progenitor cells [36]. Subretinal injection of glutamate and alpha- aminoadipate can also induce Müller cells to become retinal progenitor cells in vivo. Similarly, human Müller cells can also dedifferentiate into cells expressing neural stem cell markers and retinal progenitor cell markers after being treated with bFGF or retinoic acid in vitro [37]. In vitro, transfection of the transcription factor Ascl1 or Lin28b into mouse or rat Müller cells also induces dedifferentiation of Müller cells into retinal progenitor cells [38, 39]. In vivo, transfection of the Ascl1 into mouse Müller cells can induce retinal regeneration after retinal damage [40]. Therefore, promoting dedifferentiation of Müller cells will likely improve mammalian retinal regeneration.
2.1.7 Dedifferentiation of Bone Cells and Muscle Cells and Musculoskeletal Regeneration
Mammal limbs cannot be regenerated after amputation, but lower animals such as zebrafish and newt can completely regenerate limbs. The important components of the limb are bones and muscles. The repair of mouse bones mainly depends on the differentiation of bone marrow mesenchymal stem cells into osteoblasts, and bone healing can only be achieved within a certain range of damage. However, the bone components of the zebrafish fins can be completely regenerated, mainly relying on osteoblast dedifferentiation (Fig. 2.5). When the fins are cut off, the mature osteoblasts in the proximal end change in morphology and express cell proliferation markers, suggesting that osteoblasts dedifferentiate. Knof et al. use lineage tracing techniques to label mature osteoblasts and find that when fins are amputated, the mature osteoblasts downregulate markers associated with osteoblast differentiation such as osteocalcin and the osteoblast-specific transcription factor Osterix, and upregulate precursor osteoblast markers such as Runx2b and Tenascin, demonstrating that osteoblasts undergo dedifferentiation after amputation of fins and become osteoblast progenitors [41]. Dedifferentiated osteoblasts participate in the formation of the blastema of the fin, and then differentiate into osteoblasts, regenerate new bone spurs [42]. These suggest that osteoblast dedifferentiation is the major cellular mechanism for the regeneration of the bone components of zebrafish fins.
During the regeneration of adult newt limbs, the regeneration of muscle components also depends on muscle cell dedifferentiation (Fig. 2.6). Muscle cell dedifferentiation participates in muscle regeneration during the regeneration of adult newt. In vivo fluorescent labeling of individual muscle fibers showed that multinucleated muscle fibers near the amputated plane of the limb dedifferentiated to form monocytes [43]. More convincing evidence comes from experiments by Sandoval-Guzman et al. Using Cre/lox tracing technology, they found that multinucleated myofibers break into proliferating Pax7 (a marker of muscle stem cells) negative monocytes, and that these cells are involved in the blastema formation [44]. These dedifferentiated sources of muscle cells then proliferate and differentiate into new muscle fibers, regenerating the muscle parts of the limbs. Interestingly, in different developmental stages, the regeneration of newt limb muscles depends on different ways [45]. In the larval stage of the newt, the muscle regeneration depends on the proliferation and differentiation of muscle stem cells (satellite cells); when the newt is deformed, muscle regeneration depends on dedifferentiation of muscle cells. This may be due to the presence of sufficient muscle stem cells in the body at the larval period. With development, muscle stem cells gradually decrease, and adult newt do not rely on muscle stem cells, instead rely on muscle dedifferentiation.
2.1.8 Schwann Cell Dedifferentiation and Peripheral Nerve Regeneration
Peripheral nerve damage often results in partial or total loss of motor, sensory, and autonomic function. Despite this, mammalian peripheral nerves have a degree of regenerative capacity after injury. When the peripheral nerve is damaged, the axons can be slowly regenerated, generally 1–3 mm per day [46]. In patients with brachial plexus injury, damaged neurons must grow more than 1 m of axons to innervate the muscles and sensory organs of the hand, which can take several years [47].
After peripheral nerve injury, the axons at the distal end of the injury dissociated from the proximal neuron, and Wallerian degeneration occurred. Schwann cells and macrophages are activated to dissolve and clear distal axons and myelin debris, creating a microenvironment that facilitates nerve regeneration [48]. Activation of Schwann cells plays an important role in peripheral nerve regeneration. As the distal axon dissociates, the nearby Schwann cells lose contact with the axons, and the Schwann cells dedifferentiate and lose the myelinating properties, becoming an undifferentiated repairing cell (Fig. 2.7). Dedifferentiated Schwann cells can phagocyte myelin and myelin-associated glycoproteins, secreting cytokines to attract macrophages to function in the nerve stump. Dedifferentiated Schwann cells provide scaffolding and nutritional support for the new axons. When the axon regeneration is over, the Schwann cells contact with the new axons again, encapsulating the new axons to form the myelin sheath, and restoring the electrophysiological conduction of the axons.
During the dedifferentiation of Schwann cells, a series of changes take place in morphology, phenotype, and function. First, the normal morphology of the Schwann cells is lost. At the same time, the characteristic markers of myelinated Schwann cells such as myelin proteins P0, MBP, and Periaxin are downregulated [49]. Dedifferentiated Schwann cells can proliferate and secrete a variety of neurotrophic factors and neurotropic factors [50]. For example, the secreted neurotrophic factors NGF, BDNF, NT-4, GDNF, and IGF-1 promote axonal regeneration; neurotropic factors such as Fibronectin, Laminin, and Tenascin provide matrix support for axonal elongation.
2.1.9 Dedifferentiation or Reprogramming of Somatic Cells into Pluripotent Stem Cells
In 2006, Japanese scientists induced the dedifferentiation of fibroblasts in the skin into embryonic stem cell-like cells, namely induced pluripotent stem cells (iPSCs) [51]. The study introduced four pluripotency-related transcription factors into the fibroblasts through the virus, activating the genetic program of pluripotent stem cells. This process of dedifferentiation to the pluripotent stem cell state is also known as somatic cell reprogramming. Inspired by this research, other somatic cells such as epidermal cells, epithelial cells, mesenchymal stem cells, liver cells, and gastric cells can all be completely dedifferentiated into iPSCs [52]. In addition, non-gene integration methods such as microRNAs, episomal vectors, recombinant proteins, and small molecule compounds can be used to induce iPSCs [53] (Fig. 2.8). iPSCs avoid the ethical issues faced by embryonic stem cells. Currently, iPSCs have been widely used in medical research and clinical treatment, and have rapidly promoted the development of regenerative medicine.
2.1.10 Perspectives
Adults have very few residual stem cells in various tissues and organs, which are insufficient to compensate for the large number of cell loss caused by injury, resulting in the loss of normal function of tissues and organs. Replenishing stem cells is the key to tissue regeneration. Dedifferentiation of differentiated cells that have survived in the damaged site into stem cells is the best source of stem cells, being more compatible with local tissues. Lower animals have significant regenerative capacity due to their strong dedifferentiation potential. Mammalian cells have weak dedifferentiation potential and cannot compensate for cell damage caused by injury. Therefore, how to enhance the dedifferentiation potential of local viable cells in the injured environment is of great significance for the in situ regeneration of tissues. At present, some tissue cells can induce dedifferentiation by biological, physical or chemical stimulation in vitro. How to induce dedifferentiation of mature somatic cells in vivo is challenging in regenerative medicine research. Studies have been conducted to induce in vivo dedifferentiation by transgene, growth factors, or small molecule compounds.
In the regenerative animal model, a variety of tissue cells have the potential to dedifferentiate. Studying their dedifferentiation mechanisms will have important insights for inducing dedifferentiation of mammalian cells. We believe that in the future, by promoting dedifferentiation, it will help to improve the mammalian regeneration.
2.2 Dedifferentiation of Epidermal Cells and Sweat Gland Regeneration
In 2001, during the research of tissue repair and regeneration, we accidentally discovered an important biological phenomenon that skin cells can be reversed into stem cells under the stimulation of certain factors during wound healing [54], which is academically called dedifferentiation and finally confirmed by hard work. On this basis, with the help of the basic biological law of dedifferentiation, we have transformed the human bone marrow mesenchymal stem cells (BM-MSCs) into sweat gland cells for the first time in the world, and successfully regenerated sweat glands in human bodies. After nearly 10 years of follow-up of typical cases, we proved that local wounds have stable and sustained sweating function, and no adverse reactions are observed, thus establishing an innovative method for sweat gland regeneration and providing important innovation theories and key technologies for solving the post-treatment problems of patients with severe trauma and burns.
2.2.1 A Huge Controversy Brought by an Accidental Discovery
In 2000, we cooperated with related gene pharmaceutical companies to study the mechanism of skin wound healing promoted by growth factors. When observing some tissue sections by microscope stained with the markers of epidermal stem cells, we found that the positive cells appeared in areas where epidermal stem cells should not appear. At that time, we felt that this phenomenon was difficult to explain with conventional knowledge, because the classical view was that epidermal stem cells should be located in the basal layer of the epidermis. But our result showed that the positive cell cluster appeared in the prickle cell layer above the basal layer of the epidermis, so we called it “stem cell island.”
In 2001, the results of the study titled “Dedifferentiation of epidermal cells to stem cells in vivo” were published in Lancet (2001, 358: 1067–1068), the internationally renowned medical journal. After that, it caused a relatively large response at home and abroad. About 5% of the experts considered it to be a very important discovery, and suggested further researches. About 85% of scholars disagreed with this discovery and the existence of this phenomenon. The common question at that time was “How can old cells become younger?” Even about 10% of people thought that it was pseudoscience and opposed it.
2.2.2 Giving a Correct Explanation to the Academic World through Hardship
On the basis of the success in the wound model of the nude mice, we have demonstrated the prevalence of this dedifferentiation phenomenon in animal skin and human skin respectively, and also reproduced it in vitro by growth factors and UV stimulation at the cell level. At the same time, we also have basically cleared up the three aspect questions.
First, what signaling pathway induces the dedifferentiation of these cells into stem cells? Through literature research, we found that Wnt [55] and ERK pathways play an important regulatory role in the dedifferentiation of mature cells and maintenance of skin stem cells. It was found that repairing factors in the wound microenvironment can promote the expression of Wnt-1 and Wnt-7α, thereby increasing intranuclear transfer and deposition of β-catenin, then promoting transcription and expression of its target genes Cyclin D1 and c-Myc. We used transgenic technology to obtain epidermal cell lines with high expression of Cyclin D1. After 5 days of culture in vitro, the morphology, phenotype and functional properties of most cells were similar to those of epidermal stem cells. In addition, we also detected the activation of the ERK signaling pathway. This signaling pathway cooperates with Wnt/β-catenin to regulate the dedifferentiation process of the epidermal cells.
The second is whether these epidermal stem cells derived from dedifferentiation have the same or similar biological characteristics of normal epidermal stem cells. Through a series of analysis of cell morphology, structure and phenotypic characteristics, we have found that the morphological characteristics, ultrastructural and phenotypic characteristics of the dedifferentiated epidermal stem cells are consistent with those of normal epidermal stem cells [56].
The third is whether these epidermal stem cells derived from dedifferentiation have the same biological functions as normal epidermal stem cells. Under the condition of in vitro culture, these dedifferentiated cells have a high colony-forming efficiency and long-term proliferative potential. Under the condition of three-dimensional culture, these cells can form a three-dimensional skin that is similar in structure to that constructed by normal epidermal stem cells. Thus, it is confirmed that these dedifferentiated epidermal stem cells can be used as seed cells for skin tissue engineering as well as normal epidermal stem cells to form the relevant skin structure.
In 2006, Cell, the internationally renowned magazine, reported that skin cells were reprogrammed into pluripotent stem cells (iPS) by transfecting Oct4, Sox2, Klf4, and c-Myc [51], which provided direct evidences for the dedifferentiation of epidermal cells into epidermal stem cells by growth factors. Especially, the special review written by Professor T. M. Beardsley, editor of BioScience in 2007, highly appraised our research on dedifferentiation of epidermal cells.
2.2.3 The Original Discovery of Cellular Dedifferentiation Is Used to Guide the Clinical Research on Sweat Gland Regeneration
We know that after several generations of burn medical experts in China, the current success rate of burn treatment in China has reached more than 98%, but unfortunately many skin wounds of rescued patients are repaired by scar without skin attachments, especially sweat glands. Due to the lack of sweat glands, the living and working of many rescued patients are seriously affected and it is difficult to return to society. Based on clinical problems, we have carried out a new round of research on transformation application from basic theory discovery to sweat gland regeneration and achieved the goal of innovation and transformation in 10 steps.
In the first step, we found out the process and regularity of sweat gland development before and after human birth from the perspective of development and summarized the causes of difficulty in regeneration of sweat glands after severe burns in clinical practice. From the point of embryogenesis, the sweat glands are formed by the germinal cells, epidermal stem cells, in the epidermis derived from the ectoderm, which grow into the mesenchyme by developing a cylindrical cell cord. The study finds that human sweat glands appear about 12 weeks, peak around 24 weeks, and end around 36 weeks of embryo. After the scar healing, the non-perspiration function of the wound does not mean that there is no sweat gland tissue in the scar. Studies have shown that in the scar tissue, the presence of CK19 and CK14 positive cells can be detected, the CK19 expression is mainly located at the junction of the deep dermis and the normal subcutaneous tissue at the base of the scar, and the CK14 positive cells are scattered in the scar tissue, showing concentric circles. We need to remove scars, plant sweat gland cells or transplant tissue-engineered skin containing sweat gland cells to solve the sweating problem of severely burned patients. However, the patient’s own sweat gland cells have been severely damaged, so it is necessary to promote sweat gland regeneration by exogenous methods, especially by the induced differentiation of stem cells.
In the second step, we confirmed the feasibility of BM-MSCs as seed cells for regeneration of sweat glands. Since epidermal stem cells and sweat glands have a common origin in auxology, it is possible to induce epidermal stem cells to differentiate into sweat gland cells and regenerate sweat glands. However, the key to the problem is that when a large area of severe burns occurs, the epidermis of the patient is destroyed, and the source of autologous mature epidermal cells or epidermal stem cells is lacking. BM-MSCs, under appropriate conditions, can not only differentiate into mesenchymal tissue cells homologous to mesoderm, but also break through the boundaries of germ layers and differentiate into ectodermal tissue. Therefore, we consider the use of BM-MSCs (or umbilical cord mesenchymal stem cells) as seed cells for the regeneration of sweat glands. Our previous research also found that under severe trauma and burn conditions, the mesenchymal stem cells in circulating blood have actually participated in the repair process of the wound. Recently, we have been able to directly induce fibroblasts into sweat gland cells, which provides a new source of seed cells for sweat gland regeneration and has great clinical application value.
In the third step, we confirmed the feasibility of inducing the BM-MSCs into sweat gland cells by a new generation of in vitro co-culture system [57] and established key induction differentiation techniques. By exploration, we initially established a direct co-culture system of heat shock sweat gland cells and mesenchymal stem cells. This system can successfully induce mesenchymal stem cells into sweat gland-like cells, but the cells obtained from the induction system are heterogeneous, and may contain some residual sweat gland cells. In order to solve this problem, we established an indirect co-culture system of heat shock sweat gland cells and mesenchymal stem cells. This method adopts the transwell culture model. The heat shock sweat gland cells are in the lower chamber, and the mesenchymal stem cells are in the upper chamber, and the active components in the culture system can pass freely, thus effectively solving the problem of heterogeneous cells. With the deepening of research and continuous improvement of induction conditions, we have successfully established a new generation of induction system of sweat gland regeneration, which is composed of the indirect co-culture induction system adding a series of sweat gland lineage development-specific proteins or factors (e.g., EDA-A1 and EGF). This new system effectively improves the trans-differentiation efficiency of mesenchymal stem cells into sweat gland-like cells. Phenotypic analysis showed that 40–60% of the induced cells expressed CEA, CK7, CK8, and CK19, the markers of original sweat gland cells.
In the fourth step, we confirmed the safety and effectiveness of BM-MSC transdifferentiation into sweat gland-like cells under in vitro co-culture conditions. The safety of cell co-culture is a concern for everyone and a prerequisite for regenerating sweat glands. It is also a question that must be answered. In the experiments, we co-cultured sweat gland cells with cells from different germ layers, observed changes in cell behaviors and related indicators, and focused on changes in apoptotic rate and karyotype. Finally, we demonstrated that there is no abnormal change in sweat gland-like cells under co-culture conditions.
In the fifth step, we further confirmed that the sweat gland-like cells transdifferentiated from BM-MSCs have similar electrophysiological properties with sweat gland cells. The electrophysiological index is another indicator for evaluating whether sweat gland-like cells have the characteristics of sweat gland cells. By patch-clamp technique, we confirmed that the induced MSCs have unique ion channels (CFTR Cl- channels and Amiloride-sensitive Na+ channels) and the potential of sweat gland cells, whereas non-induced MSCs do not have this characteristic potential, which further confirmed that the induced MSCs are transformed into sweat gland cells from the electrophysiological point of view.
In the sixth step, we initially identified the signaling pathways that may be involved in the transdifferentiation of BM-MSCs into sweat gland-like cells. In the study on transdifferentiation, we first discovered the role of ERK signaling pathway. During the transdifferentiation of MSCs into sweat gland-like cells, EGF can significantly increase the transdifferentiation rate and the ERK pathway blocker PD98059 can partially block the transdifferentiation of MSCs, which means that there may be other regulatory pathways beside the ERK pathway during this process. Our follow-up studies have also found that the EDA-A1/ EDAR signaling pathway is also involved in the transdifferentiation process of sweat gland regeneration. In the process of transdifferentiation of stem cells into sweat gland-like cells, we found that high expression of EDA-A1 could successfully induce transdifferentiation of BM-MSCs into sweat gland-like cells, and the increase of EDA-A1 and EDAR expression was also observed during the process of UC-MSC transdifferentiation into sweat gland-like cells. In addition, we found that NF-B is the downstream pathway of EDA/EDAR to transfer signals into the nucleus, and promote the expressions of a series of genes that regulate the repair and regeneration of sweat glands, such as Shh and cyclin D1.
In the seventh step, after transplantation of the induced BM-MSCs into the paws of the nude mice, we observed the sweat gland-like structure which had sweating function. We made deep II degree burn wounds on the paws of nude mice, followed by multiple point injection of BrdU-labeled BMSCs at the injured site. After 2 weeks, the wound healing was observed and the iodine-starch sweating experiment was performed. The results showed that the sweating test was positive in the wounds injected with the induced BM-MSCs, while the control was negative. When the sweat-positive site was taken for histological examination, we found the relatively intact sweat gland-like structures in the tissue sections and these sweat gland structures were derived from transplanted induced BM-MSCs.
In the eighth step, on the basis of the success of experiments in vitro and in vivo, we performed the first clinical research on sweat gland regeneration by autologous BM-MSCs with the approval of the ethics committee and informed consent of the patient. This case was burned in 2006 due to careless work, and the area and depth of burns in both upper limbs were basically the same. We used the left upper limb of the patient as a control and the right upper limb as a test. The induced autologous BM-MSCs were transplanted into the right upper limb wound using the method described above, and the left upper limb wound was treated as well as the right upper limb except for the stem cell transplantation. A sweating test was performed 2 months after transplantation. The results demonstrated that the wounds where the induced BM-MSCs were transplanted had sweating function, and the iodine-starch test was positive, while the control was negative for the iodine-starch test. Furthermore, the sweating test at 12 and 24 months after transplantation showed that the wounds had a stable sweating function.
In the ninth step, after the successful regeneration of the sweat glands, another question to be answered is whether the sweat-positive test sites have sweat gland-like structures and whether the sweat produced by transplanted area is consistent with the sweat produced by normal skin. To this end, we conducted in-depth research on these issues. The biopsy specimen of transplanted area was taken for histological examination after informed consent of the patient. We found that there were positive cells stained by the markers of sweat gland cells and cell clusters similar to sweat glands (but did not form the complete structure of sweat glands including secretions and ducts). Furthermore, neuronal-like structures and the capillary structures were found around the sweat gland-like structures, which suggested that the sweat gland-like structures were active and functional. In 2009, the results of the clinical study were published in the form of the cover story and research paper in the Wound Rep Reg [58], an important journal in the field of repair and regeneration. Professor Patricia A. Hebda, editor-in-chief of the magazine, wrote a special review that highly praised the sweat gland regeneration as a “landmark study.”
2.2.4 The Perception and Experience Gained from the Experiment in the Past Ten Years
From the discovery of dedifferentiation of epidermal cells to the initial clinical application of sweat gland regeneration, a total of about 10 years of research was conducted. This experience of 10 years is how we can confirm the discovery in the course of scientific research, how to perfect the discovery and how to turn this discovery into an application process. The main experience can be summarized as follows:
-
1.
Some accidental phenomena and casual discoveries may be the basis of a major innovation. Never give up because of arbitrariness. And whether you can seize this momentary opportunity lies in whether you are ready in the early stage, whether you have a wealth of knowledge accumulation and a correct judgment.
-
2.
To prove these accidental phenomena and discoveries is a long and very difficult process, which involves science and other aspects and is affected by many factors. We must be prepared to be questioned and answer various questions.
-
3.
The final aim of the basic discovery is the application to practice. In terms of medical research, basic discovery should be applied to create new clinical treatment technologies that ultimately benefit patients.
-
4.
From the discovery of basic theory to the establishment of major new technologies for clinical treatment, the key to the success is the grasp of theoretical discovery and innovation and the understanding of clinical treatment needs and difficulties.
-
5.
From the discovery of basic theory to the application of clinical treatment, it is a cyclical process. In this process, clinical needs put forward requirements for basic theoretical research and basic theory innovation provides the possibility to solve these needs. The combination of the two provides a way for this successful transformation.
Scientific research needs constant innovation, and the results of innovation need to be transformed and applied and then its value will be realized. Therefore, we believe that in scientific research, the research without innovation is nonsense, and the research without application is done for nothing. An excellent researcher can neither do nonsense nor do for nothing. This is the true meaning of scientific research.
2.3 Cellular Dedifferentiation and Synchronized Repair and Regeneration of Complex Tissues In Situ
2.3.1 Difficulties and Breakthroughs in Repair and Regeneration of Complex Tissues: Cellular Dedifferentiation
With the development of life science and medicine, significant improvements have been made in the repair and regeneration of single tissues, such as bones, blood vessels, nerves, and muscles. However, multiple tissues cannot be regenerated at the same time. For instance, when the limbs are traumatized, the wound undergo scar healing, without regeneration of distal damaged tissues.
Synchronized repair and regeneration of multiple tissues has more challenges compared to a single tissue. A single tissue, such as the skin, can regenerate the epidermis as long as there are enough epidermal stem cells. However, the complex structure of the limb, including bones, blood vessels, nerves, and connective tissue, requires a variety of tissue-specific stem cells or progenitor cells [59]. Although it is possible to amplify these cells in vitro, these cells are difficult to regrow complex tissues in vitro or spontaneously regrow complex tissues in vivo after transplantation. However, terminally differentiated cells can dedifferentiate into an immature progenitor cells or stem cells in a damaged environment. Lower animals such as salamander and zebrafish lack stem cells in their adult stages, but mature cells have strong potential to undergo cellular dedifferentiation and can provide sufficient stem cell sources for regeneration of complex tissues [1]. By contrast, the potential for cellular dedifferentiation in mammals is very weak, and after tissue damage, mature cells cannot provide stem cells or progenitor cells by regenerating damaged tissues [1]. Therefore, how to induce the dedifferentiation of various mature cells is a key issue to achieve in situ synchronized regeneration of multiple tissues.
Current methods of using transgenes and small molecule compounds can induce adult cells to dedifferentiate into stem cells in vitro [52, 53, 60]. If this technology can be achieved in vivo, it is possible to induce mature cells to become progenitor cells in situ, providing a source of stem cells for regeneration.
The following sections describe animal models with synchronized regeneration of multiple tissues, focusing on the important role of cellular dedifferentiation and the molecular mechanisms underlying dedifferentiation.
2.3.2 The Study Model of In Situ Synchronized Regeneration of Complex Tissues
2.3.2.1 The Fin Regeneration of Zebrafish
Zebrafish is an animal model for studying the regeneration of complex tissues [61]. Zebrafish can regenerate complex tissues, such as the heart and fin, after injury. The fin is a complex limb appendage, like human limbs, containing multiple tissue components such as fish bones, mesenchymal cells, nerve fibers, and blood vessels; 10–14 days after the fins are amputated, the zebrafish can completely regenerate new fins in situ [62]. At the proximal end of the amputated fin, epidermal cells surrounding the wound area migrated to the wound surface, forming a wound epidermis (Fig. 2.9). A mass of proliferating cells is formed under the wound epidermis, called fin blastema. The cells of fin blastema can continue to proliferate and differentiate under the guidance of the wound epidermis, and regrow the distal end of the amputated fins. Because zebrafish is easy to perform genetic manipulation and its genetic spectrum is clear, it is a typical model to study the synchronized regeneration of multiple tissues.
2.3.2.2 Salamanders Limb Regeneration
Salamanders, such as axolotl and newt, are capable of regenerating various organs and tissues [59]. Unlike mammals, their limbs can be completely regenerated after amputation. Along the long axis of the limb, amputation at any position can perfectly regenerate the distal end (Fig. 2.10). The limbs are derived from various germ layers. The epidermis and peripheral nerve tissue are derived from the embryonic ectoderm, and the internal tissue components (such as dermis, muscle, bone, and blood vessels) are derived from the embryonic mesoderm [63]. Therefore, it is necessary to restore the original organized structure of various tissue cells for regeneration. A group of proliferating cells, namely limb blastema, is formed under the wound epidermis. The cells in the limb blastema then grow all the tissue components of the limb. Therefore, salamanders are also important biological models for studying limb regeneration in vertebrates.
2.3.2.3 Mouse Toe Tip Regeneration
Mammalian limbs do not have the same ability to regenerate as zebrafish fin and salamander limbs. After trauma, the limb undergoes wound healing and cannot grow the lost distal part. Although the mammal loses the ability to regenerate the entire limb, the distal toe tip can be completely regenerated [64, 65]. It is clinically found that the tip of the finger and the nail can be regenerated after the finger tip of the child or adult is cut off. Similarly, both neonatal mice and adult mice regenerate the toe and nail after amputation of the distal third toe (Fig. 2.11). It is worth noting that the second toe and the proximal end cannot be regenerated, indicating that the mammalian limb regeneration ability is very limited [66]. The blastema is formed under the wound epidermis and regenerate the toe tip [64]. Therefore, the mouse toe tip regeneration model is an important model for studying the complex tissue regeneration of mammalian limbs.
2.3.2.4 Zebrafish and Neonatal Mouse Heart Regeneration
The mammalian heart lacks the ability to self-repair and regenerate after injury. However, zebrafish have a strong heart regenerative potential [12]. When the ventricles are surgically cut by 20%, the cardiomyocytes near the wound undergo dedifferentiation and proliferate, and about 60 days after amputation, the zebrafish heart can be completely regenerated (Fig. 2.11). This fully demonstrates that zebrafish heart has a strong ability to regenerate and is a classic biological model for studying the heart regeneration.
Adult mammals generally lose the ability to regenerate the heart, but neonatal mice within 1 week of birth can fully regenerate the heart after removal of the apex (Fig. 2.12) [16]. But after the first week, the mouse heart gradually loses its ability to regenerate. This indicates that the mammalian heart retains some of its ability to regenerate early in life. Therefore, using the mouse heart as a model for regenerative research can help reveal the mechanisms that regulate cardiac regeneration in mammals.
2.3.3 The Cellular Basis of Limb Regeneration: Blastema and Cellular Dedifferentiation
The regeneration of the appendages, from lower animals to mammals, shares similar cellular mechanisms. The epidermal cells around the wound cover the wound and form a wound epidermis. Below it, a cell mass having proliferative activity is formed, that is blastema. The blastema is the cellular basis of limb regeneration, and it will proliferate and differentiate all tissue cells of the limb. The blastema formation depends on dedifferentiation of mature cells. Therefore, cellular dedifferentiation is the core mechanisms for limb regeneration.
2.3.3.1 Cellular Dedifferentiation: The Basis of Blastema
The development of cell-lineage tracing techniques provides a powerful tool for studying the cell fate and revealing the cellular mechanisms underlying the blastema formation.
2.3.3.1.1 Salamander Limb Blastema Formation
By using cell-lineage tracing method, Krag et al. revealed the cellular mechanism of Salamander blastema formation. They designed a clever experiment to transplant a transgenic salamander donor expressing GFP into a GFP-negative recipient embryo, such that various tissues express GFP in the limb of the recipient embryo [63]. The limbs expressing GFP were amputated and found that the labeled muscle cells can regenerate new muscles but not cartilage or skin, dermal cells can regenerate cartilage and connective tissue, and Schwann cells can only regenerate Schwann cells. This study demonstrates for the first time that existing tissues in the limb provide their own tissue-specific progenitor cells during regeneration, and then these progenitor cells differentiate into their own cells and do not become other types of cells. However, this study did not demonstrate whether these lineage-specific progenitor cells derive from dedifferentiation of mature cells or activation of resident stem cells. Using Cre/loxP tracing technology, Sandoval-Guzman et al. found that multinucleated myotubes cleave into Pax7-negative (a marker of muscle stem cells) monocytes with proliferative ability, and that these monocytes are involved in the blastema formation of adult newt limbs [44]. Recent studies have found that in the larval stage of the newt, limb muscle regeneration depends on Pax7-positive satellite cells [67]. Therefore, the muscles of the newt rely on muscle stem cells during the larval stage, and rely on the dedifferentiation of muscle cells during the adulthood [45]. In contrast, muscle regeneration in axolotl relies on muscle stem cells participating in the blastema formation [44]. The regeneration of peripheral nerves in the limbs relies on the dedifferentiation of Schwann cells into immature Schwann cells.
2.3.3.1.2 Zebrafish Fin Blastema
The zebrafish fin blastema formation depends mainly on the cellular dedifferentiation mechanisms, but also includes stem cell activation. For the bone component of the fin (i.e., bone spur), Knopf et al. confirmed that the mature osteoblasts undergo transient dedifferentiation, gain proliferative capacity, migrate and participate in the blastema formation, and then proliferate and differentiate to form new osteoblasts [41]. Interestingly, zebrafish can also regenerate fin spurs by de novo osteoblasts [68]. When all of the fin osteoblasts are destroyed by genetic damage, those surviving are still able to re-grow osteoblasts and bone spurs. The authors believe that when osteoblasts are present, the bone regeneration of the fin mainly depends on the dedifferentiation of osteoblasts, and when osteoblasts are absent, the de novo osteoblasts support regeneration. For muscle components, in the larval stage of zebrafish, it was found that the muscle regeneration of the fins was mainly dependent on the activation of satellite cells, and no dedifferentiation of muscle cells was found [69]. In adult zebrafish, there is no reliable evidence that the regeneration of other components of the fins is realized by cellular dedifferentiation or stem cell activation.
2.3.3.1.3 Mouse Toe Blastema
During the toe tip regeneration, the tracing technique shows that the mesoderm bone, tendon, dermis, nail bed, blood vessels are derived from mesodermal progenitor cells or stem cells, while the ectoderm epidermal cells and sweat glands are derived from ectoderm cells [70]. Are the progenitor cells derived from the activation of residual stem cells in the tissues or the dedifferentiation of mature cells? There are toenail stem cells in the mouse toenail, and when the toe tip is amputated, the toenail stem cells depend on the activation of the Wnt pathway to differentiate and regenerate [71]. The regeneration of the toe tip depends not only on the activation of the toenail stem cells, but also on the cellular dedifferentiation (Fig. 2.13). Schwann cell precursors dedifferentiate and secretes oncostatin M and platelet-derived growth factor AA (PDGF-AA) to promote the continuous expansion of the blastema [72].
2.3.3.2 Molecular Mechanisms of Cellular Dedifferentiation, Proliferation, and Blastema Formation
After injury, the damaged microenvironment will release various factors to activate signaling pathways, induce resident to dedifferentiate, proliferate, re-differentiate, and participate in the regeneration and repair of tissues. The microenvironment mainly includes growth factors, cytokines, and extracellular matrices. Among them, growth factors are the most critical components in the microenvironment. Growth factors and related signaling pathways play an important role in the regeneration of complex tissues. The signaling pathways is as follows (Fig. 2.14).
The epidermal growth factor (EGF) binds to the epidermal growth factor receptor (EGFR) and activates downstream signaling pathways to cause expression of target genes. Neuregulin 1 is a member of the EGF family and its expression is significantly increased after the amputation of zebrafish fins [73]. Neuregulin 1 activates the EGFR and PIK/Akt signaling pathways, promoting the proliferation and migration of dedifferentiated progenitor cells to form blastema [73].
Fibroblast growth factor (FGF) acts by activating the four-transmembrane FGF tyrosine kinase receptor (FGFR). During the regeneration of zebrafish fins, it was found that FGF20a is critical for the regeneration of fins [74]. FGF20a was expressed near the wound epidermis and the mesenchymal space within 6–12 days after the fin amputation, and then FGF20a was gradually restricted to the blastema. FGF20a may promote cell dedifferentiation, proliferation, and blastema formation. FGF3/10a expressed in the late blastema regulates the proliferation of blastema progenitor cells [75]. During the regeneration of the salamander limbs, the wound epidermis and peripheral nerves produce FGF1 and FGF2, which promote the blastema formation [76]. FGF2 also promotes the proliferation of the blastema progenitor cells during the mouse toe tip regeneration.
Bone marrow morphogenetic protein (BMP) is a member of transforming growth factor-β (TGF-β) that binds to transmembrane type 1 BMP receptor and type 2 BMP receptor (BMPR). After amputation of zebrafish fins, BMP2 expression was significantly upregulated, and BMP2 was able to induce dedifferentiated osteoblasts to redifferentiate and form spurs [77]. BMP is a growth factor essential for mouse toe regeneration. After the toe tip was amputated, BMP2, BMP4, and BMP7 were upregulated in the stump and blastema. By using exogenous BMP4 to treat Msx-1 mutant mice (gene-deficient mice with loss of regeneration ability of toe tip), the ability to regenerate toe tip can be restored [78]. It cannot be regenerated after the proximal end of the toe tip is cut off, but when exogenous BMP2 and BMP7 magnetic beads are embedded in the proximal end, the regeneration of the proximal part can be promoted [79].
The Notch protein is a single transmembrane receptor that possesses the intracellular domain (NICD). The Notch signal is continuously activated during the formation of fin blastema, which promotes the proliferation of progenitor cells [80].
Wnt protein binds to the Frizzled receptor and promotes nuclear transfer of β-catenin. The Wnt/β-catenin signal axis regulates the regeneration of the limbs. Inhibition of the Wnt signaling pathway will result in the inability of salamander and zebrafish fins to regenerate [81]. However, different Wnt proteins play an opposite role, such as Wnt8 promotes proliferation of blastema progenitor cells, whereas Wnt5b inhibits proliferation [82]. Therefore, different Wnt proteins can coordinate with each other to form a negative feedback loop that regulates the regeneration process of the limb. The expression of Wnt is increased in the wound epidermis of the toe and guides the differentiation of the toenail stem cells [71]. Similarly, the Wnt pathway regulates the proliferation and further differentiation of osteoblast progenitor cells into osteoblasts during the toe tip regeneration [83].
Hedgehog proteins include Sonic Hedgehog (Shh), Desert Hedgehog (Dhh), and Indian Hedgehog (Ihh). The Hedgehog signal regulates the patterning of limb development. Shh and Ihh expression increased during salamander limb regeneration [84]. Blocking the Hedgehog pathway severely impedes limb regeneration. This is because the Hedgehog pathway regulates polarity and promotes proliferation and outgrowth of blastema. Shh and Ihh are activated to regulate the proliferation and growth of fin blastema progenitors [85]. The spurs of zebrafish fins are segmented and bifurcated, and this structural pattern is regulated by the Shh signaling pathway [86]. Before the spur bifurcation, the Shh expression in the fin epidermis is divided into two regions. This expression pattern regulates the proliferative activity of osteoblasts and bifurcations of the regenerated spur. Destroying the expression pattern of Shh, the fins cannot be bifurcated when they are regenerated.
2.3.4 Differences in Cellular Dedifferentiation Potential and Regeneration
Salamander and zebrafish have strong regenerative capacity, mainly due to high dedifferentiation potential of mature cells. However, the dedifferentiation potential in mammals is much lower than that of lower animals such as salamander and zebrafish. To some extent, the dedifferentiation potential reflects the strength of animal regeneration.
Salamander and zebrafish regenerate limbs and fins, and their strong regenerative capacity is closely related to their high degree of dedifferentiation. The terminally differentiated osteoblasts dedifferentiate, regain their proliferative capacity, migrate and differentiate into newly formed osteoblasts, and regenerate fin bone spurs [41]. However, mammalian bones do not regenerate truncated parts after amputation. The lack of dedifferentiation in mammalian osteoblasts may be the underlying cause of weak bone regeneration. The regeneration of the newt limb muscle is related to the strong dedifferentiation potential of the muscle cells. Its mature multinucleated myotubes can dedifferentiate into monocytes, form blastema, and participate in regeneration [87]. In comparison, mammalian muscle has weaker regenerative capacity. Mammalian muscle regeneration relies mainly on a small number of residual muscle satellite cells (muscle stem cells), rather than the dedifferentiation of muscle fibers. However, mammalian muscle fibers can be induced to dedifferentiate to form proliferative progenitor cells. The introduction of the gene msx1 expressed in the blastema of salamander limbs into the muscle fibers of the mouse can turn the mouse muscle fibers to progenitor cells [88]. In addition, the muscle fibers of the mice treated with the substances extracted from the salamander regenerated limbs can also induce dedifferentiation [89]. Even small molecule compounds can induce dedifferentiation of mouse muscle fibers [90]. These studies demonstrate that mammalian muscle cells retain their intrinsic dedifferentiation potential, but this potential cannot be activated by itself after injury and requires external stimulation. Strengthening the dedifferentiation of muscle cells may promote the regeneration of mammalian muscles and various tissues of the limbs.
The zebrafish mature cardiomyocytes rapidly dedifferentiate after injury, re-enter the cell cycle, and regenerate the lost cardiomyocytes. Human has a very small number of mature cardiomyocytes that can re-enter the cell cycle and produce new cardiomyocytes. During normal aging, only 0.0006–1% of cardiomyocytes can re-enter the cell cycle [19]. From this, it can be seen that the dedifferentiation potential of human cardiomyocytes is much weaker than that of zebrafish cardiomyocytes. The heart of the neonatal mouse in the first week has the ability to regenerate [16]. One week later, the mouse heart also lost its ability to regenerate, most likely due to a decrease or loss of cardiomyocyte dedifferentiation. Enhancing the dedifferentiation potential of mammalian cardiomyocytes may be an effective strategy to promote cardiac regeneration.
Although most tissue cells in mammals have lost the potential to dedifferentiate, some tissue cells still have this natural ability. A classic example is that human Schwann cells can be regenerated by dedifferentiation [48]. When the peripheral nerve is injured, the mature Schwann cells dedifferentiate into immature Schwann cells with proliferative capacity. Immature Schwann cells migrate and re-differentiate to new Schwann cells, providing structural and nutritional support for axonal regeneration. Another example is the repair and regeneration of the kidneys after an acute kidney injury [91]. Toxic kidney damage often leads to necrosis of proximal tubular epithelial cells. The renal tubular epithelial cells that survived the injury can dedifferentiate, proliferate and regenerate the necrotic epithelial cells, and reconstruct the integrity of the glomerulus [4]. These indicate that the regenerative capacity is closely related to the dedifferentiation potential.
2.3.5 Cellular Dedifferentiation Potential and Cell Cycle
Why are lower animal cells more likely to dedifferentiate and re-enter into the cell cycle? Although this mechanism has not been fully understood, there is evidence that cell cycle regulators can partially explain the differences in dedifferentiation. In theory, the cell cycle needs to be strictly controlled, otherwise it is carcinogenic. With biological evolution, the more perfect the cell cycle inhibition mechanism develops, the more difficult it is for mature cells to enter the cell cycle again. For example, pocket proteins (Rb, p107, p130) block the transition from G1 to S phase, and p53 blocks the transition from G2 to M phase [92]. Most cancer cells have mutations in Rb or p53 to obtain unlimited proliferation [93]. Salamanders can spontaneously inhibit the expression of cell cycle inhibitors, undergo dedifferentiation, and re-enter the cell cycle [94]. Under normal conditions, the differentiation of muscle cells requires the expression of the tumor suppressor Rb protein, which inhibits cell cycle entry. Although the Rb protein is also retained in the mature myotubes of salamander, the serum released by limb amputation induces the expression of cell cycle-dependent kinase 4 and 6 (CDK4/6) that can phosphorylate Rb and make it inactive [95]. The result is to allow cell cycle-related genes to be re-expressed. In stark contrast, mammalian myotubes cannot phosphorylate Rb protein and cannot re-enter the cell cycle. However, when the Rb gene is knocked out from mature myotubes of mammals, serum can induce dedifferentiation and re-entry into the cell cycle [96]. From this, it can be seen that the mammalian muscle cells cannot be dedifferentiated because the cell cycle inhibitor is not automatically removed after the injury. Interestingly, in mature cardiomyocytes of mammals, tumor suppressor Rb and p130 were also found to block the expression of cell cycle genes and maintain the state of mature cardiomyocytes. Knocking out Rb and p130, mature cardiomyocytes enter the cell cycle again and gain proliferative capacity [97]. During the regeneration of salamander limbs, the expression of tumor suppressor p53 decreases in the early stage of injury, promoting the dedifferentiation of mesenchymal cells and the blastema formation [98]. The expression of p53 increased in the late stage, promoting the differentiation of blastema progenitor cells and regenerating limbs. The homeodomain transcription factor Meis1 is expressed in mouse mature cardiomyocytes, causing cardiomyocytes to exit the cell cycle. When Meis1 was knocked out in mature cardiomyocytes, the cardiomyocytes regained proliferative capacity [99]. Therefore, the elimination of cell cycle inhibitors helps to increase the dedifferentiation potential of mammalian mature cells. The relationship between cell cycle regulation and cellular dedifferentiation is as follows (Fig. 2.15).
2.3.6 Inducing Cellular Dedifferentiation to Promote In Situ Synchronized Regeneration of Complex Mammalian Tissues
Mammals mostly lose the dedifferentiation potential, resulting in weak regenerative capacity. To achieve regeneration of complex tissues, the ideal way is to mimic lower animals, inducing the dedifferentiation of mature cells. It is much more difficult to induce dedifferentiation in the local injury than in vitro culture environment. Currently, based on cell reprogramming technology, reintroduction of exogenous transcription factors in vivo has been found to alter the fate of cells [53, 60]. For example, three transcription factors (Ngn3, Pdx1, and Mafa) are introduced into exocrine cells in the mouse pancreas, and the exocrine cells are reprogrammed into insulin-producing β cells in vivo [100]. The three transcription factors Gata4, Mef2c, and Tbx5 were introduced into the ischemic infarcted myocardium to induce myocardial fibroblasts to cardiomyocyte-like cells and participate in the regeneration of myocardium [101]. In the brain, transcription factors or miRNAs can reprogram astrocytes or radioactive glial cells to participate in neuronal regeneration [102, 103]. The introduction of exogeous gene induces liver myofibroblasts into liver cells in vivo, which reduces liver fibrosis [104]. In addition, the combination of small molecule compounds can induce fibroblasts to dedifferentiate into induced pluripotent stem cell (iPSC), neurons, neural stem cells, or cardiomyocytes [53]. The addition of small molecules SB431542 and XAV939 in vivo increases the efficiency of transcription factors in reprogramming cardiac fibroblasts to cardiomyocytes [105]. If it is effective to use small molecules instead of transcription factors in vivo to induce dedifferentiation of tissue cells in situ, it is possible to achieve in situ regeneration of complex tissues. Accordingly, it is expected to induce in situ dedifferentiation of tissue cells in mammals to achieve the repair and regeneration of complex tissues.
References
Zhao A, Qin H, Fu X. What determines the regenerative capacity in animals? Bioscience. 2016;66(9):735–46.
Tanaka EM, Reddien PW. The cellular basis for animal regeneration. Dev Cell. 2011;21(1):172–85.
Chen Z-L, Yu W-M, Strickland S. Peripheral regeneration. Annu Rev Neurosci. 2007;30:209–33.
Kusaba T, Lalli M, Kramann R, et al. Differentiated kidney epithelial cells repair injured proximal tubule. Proc Natl Acad Sci U S A. 2014;111(4):1527–32.
Fu X, Sun X, Li X, Sheng Z. Dedifferentiation of epidermal cells to stem cells in vivo. Lancet. 2001;358(9287):1067–8.
Mannik J, Alzayady K, Ghazizadeh S. Regeneration of multilineage skin epithelia by differentiated keratinocytes. J Invest Dermatol. 2010;130(2):388–97.
Bellomo R, Kellum JA, Ronco C. Acute kidney injury. Lancet. 2012;380(9843):756–66.
Berger K, Bangen J-M, Hammerich L, et al. Origin of regenerating tubular cells after acute kidney injury. Proc Natl Acad Sci U S A. 2014;111(4):1533–8.
Witzgall R, Brown D, Schwarz C, Bonventre JV. Localization of proliferating cell nuclear antigen, vimentin, c-Fos, and clusterin in the postischemic kidney. Evidence for a heterogenous genetic response among nephron segments, and a large pool of mitotically active and dedifferentiated cells. J Clin Invest. 1994;93(5):2175–88.
Abbate M, Brown D, Bonventre JV. Expression of NCAM recapitulates tubulogenic development in kidneys recovering from acute ischemia. Am J Phys. 1999;277(3):F454–63.
Imgrund M, Gröne E, Gröne HJ, et al. Re-expression of the developmental gene Pax-2 during experimental acute tubular necrosis in mice 1. Kidney Int. 1999;56(4):1423–31.
Poss KD, Wilson LG, Keating MT. Heart regeneration in zebrafish. Science. 2002;298(5601):2188–90.
Jopling C, Sleep E, Raya M, et al. Zebrafish heart regeneration occurs by cardiomyocyte dedifferentiation and proliferation. Nature. 2010;464(7288):606–9.
Kikuchi K, Holdway JE, Werdich AA, et al. Primary contribution to zebrafish heart regeneration by gata4(+) cardiomyocytes. Nature. 2010;464(7288):601–5.
Schindler YL, Garske KM, Wang J, et al. Hand2 elevates cardiomyocyte production during zebrafish heart development and regeneration. Development. 2014;141(16):3112–22.
Porrello ER, Mahmoud AI, Simpson E, et al. Transient regenerative potential of the neonatal mouse heart. Science. 2011;331(6020):1078–80.
Malek Mohammadi M, Kattih B, Grund A, et al. The transcription factor GATA4 promotes myocardial regeneration in neonatal mice. EMBO Mol Med. 2017;9(2):265–79.
Senyo SE, Steinhauser ML, Pizzimenti CL, et al. Mammalian heart renewal by pre-existing cardiomyocytes. Nature. 2013;493(7432):433–6.
Bergmann O, Bhardwaj RD, Bernard S, et al. Evidence for cardiomyocyte renewal in humans. Science. 2009;324(5923):98–102.
Wei K, Serpooshan V, Hurtado C, et al. Epicardial FSTL1 reconstitution regenerates the adult mammalian heart. Nature. 2015;525(7570):479–85.
Mitsuda S, Yoshii C, Ikegami Y, Araki M. Tissue interaction between the retinal pigment epithelium and the choroid triggers retinal regeneration of the newt Cynops pyrrhogaster. Dev Biol. 2005;280(1):122–32.
Mizuno A, Yasumuro H, Yoshikawa T, et al. MEK-ERK signaling in adult newt retinal pigment epithelium cells is strengthened immediately after surgical induction of retinal regeneration. Neurosci Lett. 2012;523(1):39–44.
Islam MR, Nakamura K, Casco-Robles MM, et al. The newt reprograms mature RPE cells into a unique multipotent state for retinal regeneration. Sci Rep. 2014;4:6043.
Susaki K, Chiba C. MEK mediates in vitro neural transdifferentiation of the adult newt retinal pigment epithelium cells: is FGF2 an induction factor? Pigment Cell Res. 2007;20(5):364–79.
Fimbel SM, Montgomery JE, Burket CT, Hyde DR. Regeneration of inner retinal neurons after intravitreal injection of ouabain in zebrafish. J Neurosci. 2007;27(7):1712–24.
Nagashima M, Barthel LK, Raymond PA. A self-renewing division of zebrafish Müller glial cells generates neuronal progenitors that require N-cadherin to regenerate retinal neurons. Development. 2013;140(22):4510–21.
Raymond PA, Barthel LK, Bernardos RL, Perkowski JJ. Molecular characterization of retinal stem cells and their niches in adult zebrafish. BMC Dev Biol. 2006;6:36.
Thummel R, Enright JM, Kassen SC, et al. Pax6a and Pax6b are required at different points in neuronal progenitor cell proliferation during zebrafish photoreceptor regeneration. Exp Eye Res. 2010;90(5):572–82.
Qin Z, Barthel LK, Raymond PA. Genetic evidence for shared mechanisms of epimorphic regeneration in zebrafish. Proc Natl Acad Sci U S A. 2009;106(23):9310–5.
Hafler BP, Surzenko N, Beier KT, et al. Transcription factor Olig2 defines subpopulations of retinal progenitor cells biased toward specific cell fates. Proc Natl Acad Sci U S A. 2012;109(20):7882–7.
Fischer AJ, Reh TA. Müller glia are a potential source of neural regeneration in the postnatal chicken retina. Nat Neurosci. 2001;4(3):247–52.
Joly S, Pernet V, Samardzija M, Grimm C. Pax6-positive Müller glia cells express cell cycle markers but do not proliferate after photoreceptor injury in the mouse retina. Glia. 2011;59(7):1033–46.
Das AV, Mallya KB, Zhao X, et al. Neural stem cell properties of Müller glia in the mammalian retina: regulation by notch and Wnt signaling. Dev Biol. 2006;299(1):283–302.
Ooto S, Akagi T, Kageyama R, et al. Potential for neural regeneration after neurotoxic injury in the adult mammalian retina. Proc Natl Acad Sci U S A. 2004;101(37):13654–9.
Karl MO, Hayes S, Nelson BR, et al. Stimulation of neural regeneration in the mouse retina. Proc Natl Acad Sci U S A. 2008;105(49):19508–13.
Takeda M, Takamiya A, Jiao J-W, et al. Alpha-Aminoadipate induces progenitor cell properties of Müller glia in adult mice. Invest Ophthalmol Vis Sci. 2008;49(3):1142–50.
Lawrence JM, Singhal S, Bhatia B, et al. MIO-M1 cells and similar muller glial cell lines derived from adult human retina exhibit neural stem cell characteristics. Stem Cells. 2007;25(8):2033–43.
Zhao C, Tao Z, Xue L, et al. Lin28b stimulates the reprogramming of rat Müller glia to retinal progenitors. Exp Cell Res. 2017;352(1):164–74.
Pollak J, Wilken MS, Ueki Y, et al. ASCL1 reprograms mouse Muller glia into neurogenic retinal progenitors. Development. 2013;140(12):2619–31.
Ueki Y, Wilken MS, Cox KE, et al. Transgenic expression of the proneural transcription factor Ascl1 in Müller glia stimulates retinal regeneration in young mice. Proc Natl Acad Sci U S A. 2015;112(44):13717–22.
Knopf F, Hammond C, Chekuru A, et al. Bone regenerates via dedifferentiation of osteoblasts in the zebrafish fin. Dev Cell. 2011;20(5):713–24.
Blum N, Begemann G. Osteoblast de- and redifferentiation are controlled by a dynamic response to retinoic acid during zebrafish fin regeneration. Development. 2015;142(17):2894–903.
Echeverri K, Clarke JD, Tanaka EM. In vivo imaging indicates muscle fiber dedifferentiation is a major contributor to the regenerating tail blastema. Dev Biol. 2001;236(1):151–64.
Sandoval-Guzmán T, Wang H, Khattak S, et al. Fundamental differences in dedifferentiation and stem cell recruitment during skeletal muscle regeneration in two salamander species. Cell Stem Cell. 2014;14(2):174–87.
Tanaka HV, Ng NCY, Yang Yu Z, et al. A developmentally regulated switch from stem cells to dedifferentiation for limb muscle regeneration in newts. Nat Commun. 2016;7:11069.
Sunderland S. Rate of regeneration in human peripheral nerves; analysis of the interval between injury and onset of recovery. Arch Neurol Psychiatr. 1947;58(3):251–95.
Chen YY, McDonald D, Cheng C, et al. Axon and Schwann cell partnership during nerve regrowth. J Neuropathol Exp Neurol. 2005;64(7):613–22.
Scheib J, HÖke A. Advances in peripheral nerve regeneration. Nat Rev Neurol. 2013;9(12):668–76.
Navarro X, Vivó M, Valero-Cabré A. Neural plasticity after peripheral nerve injury and regeneration. Prog Neurobiol. 2007;82(4):163–201.
Allodi I, Udina E, Navarro X. Specificity of peripheral nerve regeneration: interactions at the axon level. Prog Neurobiol. 2012;98(1):16–37.
Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126(4):663–76.
Qin H, Zhao A, Zhang C, Fu X. Epigenetic control of reprogramming and Transdifferentiation by histone modifications. Stem Cell Rev. 2016;12(6):708–20.
Qin H, Zhao A, Fu X. Small molecules for reprogramming and transdifferentiation. Cell Mol Life Sci. 2017;74(19):3553–75.
Fu X, Sun X, Li X, et al. Dedifferentiation of epidermal cells to stem cells in vivo. Lancet. 2001;358:1067–8.
Zhang C, Chen P, Fei Y, et al. Wnt/β-catenin signaling is critical for dedifferentiation of aged epidermal cells in vivo and in vitro. Aging Cell. 2012;11(1):14–23.
Zhang C, Fu X, Chen P, et al. Dedifferentiation derived cells exhibit phenotypic and functional characteristics of epidermal stem cells. J Cell Mol Med. 2010;14(5):1135–45.
Li H, Fu X, Ouyang Y, et al. Adult bone marrow derived mesenchymal stem cells contribute to wound healing of skin appendages. Cell Tissue Res. 2006;326(3):725–36.
Sheng Z, Fu X, Cai S, et al. Regeneration of functional sweat gland-like structures by transplanted differentiated bone marrow mesenchymal stem cells. Wound Rep Reg. 2009;17(3):427–35.
Nacu E, Tanaka EM. Limb regeneration: a new development? Annu Rev Cell Dev Biol. 2011;27:409–40.
Qin H, Zhao A, Fu X. Chemical modulation of cell fates: in situ regeneration. Sci China Life Sci. 2018;61(10):1137–50.
Gemberling M, Bailey TJ, Hyde DR, Poss KD. The zebrafish as a model for complex tissue regeneration. Trends Genet. 2013;29(11):611–20.
Tu S, Johnson SL. Fate restriction in the growing and regenerating zebrafish fin. Dev Cell. 2011;20(5):725–32.
Kragl M, Knapp D, Nacu E, et al. Cells keep a memory of their tissue origin during axolotl limb regeneration. Nature. 2009;460(7251):60–5.
Fernando WA, Leininger E, Simkin J, et al. Wound healing and blastema formation in regenerating digit tips of adult mice. Dev Biol. 2011;350(2):301–10.
Borgens RB. Mice regrow the tips of their foretoes. Science. 1982;217(4561):747–50.
Masaki H, Ide H. Regeneration potency of mouse limbs. Develop Growth Differ. 2007;49(2):89–98.
Morrison JI, LÖÖF S, He P, Simon A. Salamander limb regeneration involves the activation of a multipotent skeletal muscle satellite cell population. J Cell Biol. 2006;172(3):433–40.
Singh SP, Holdway JE, Poss KD. Regeneration of amputated zebrafish fin rays from de novo osteoblasts. Dev Cell. 2012;22(4):879–86.
Rodrigues AMC, Christen B, Martí M, Izpisúa Belmonte JC. Skeletal muscle regeneration in Xenopus tadpoles and zebrafish larvae. BMC Dev Biol. 2012;12:9.
Rinkevich Y, Lindau P, Ueno H, et al. Germ-layer and lineage-restricted stem/progenitors regenerate the mouse digit tip. Nature. 2011;476(7361):409–13.
Takeo M, Chou WC, Sun Q, et al. Wnt activation in nail epithelium couples nail growth to digit regeneration. Nature. 2013;499(7457):228–32.
Johnston APW, Yuzwa SA, Carr MJ, et al. Dedifferentiated Schwann cell precursors secreting paracrine factors are required for regeneration of the mammalian digit tip. Cell Stem Cell. 2016;19(4):433–48.
Rojas-Muñoz A, Rajadhyksha S, Gilmour D, et al. ErbB2 and ErbB3 regulate amputation-induced proliferation and migration during vertebrate regeneration. Dev Biol. 2009;327(1):177–90.
Whitehead GG, Makino S, Lien C-L. Keating MT. fgf20 is essential for initiating zebrafish fin regeneration. Science. 2005;310(5756):1957–60.
Shibata E, Yokota Y, Horita N, et al. Fgf signalling controls diverse aspects of fin regeneration. Development. 2016;143(16):2920–9.
Giampaoli S, Bucci S, Ragghianti M, et al. Expression of FGF2 in the limb blastema of two Salamandridae correlates with their regenerative capability. Proc Biol Sci. 2003;270(1530):2197–205.
Stewart S, Gomez AW, Armstrong BE, et al. Sequential and opposing activities of Wnt and BMP coordinate zebrafish bone regeneration. Cell Rep. 2014;6(3):482–98.
Han M, Yang X, Farrington JE, Muneoka K. Digit regeneration is regulated by Msx1 and BMP4 in fetal mice. Development. 2003;130(21):5123–32.
Yu L, Han M, Yan M, et al. BMP signaling induces digit regeneration in neonatal mice. Development. 2010;137(4):551–9.
Grotek B, Wehner D, Weidinger G. Notch signaling coordinates cellular proliferation with differentiation during zebrafish fin regeneration. Development. 2013;140(7):1412–23.
Kawakami Y, Rodriguez Esteban C, Raya M, et al. Wnt/beta-catenin signaling regulates vertebrate limb regeneration. Genes Dev. 2006;20(23):3232–7.
Stoick-Cooper CL, Weidinger G, Riehle KJ, et al. Distinct Wnt signaling pathways have opposing roles in appendage regeneration. Development. 2007;134(3):479–89.
Takeo M, Hale CS, Ito M. Epithelium-derived Wnt ligands are essential for maintenance of underlying digit bone. J Invest Dermatol. 2016;136(7):1355–63.
Singh BN, Doyle MJ, Weaver CV, et al. Hedgehog and Wnt coordinate signaling in myogenic progenitors and regulate limb regeneration. Dev Biol. 2012;371(1):23–34.
Iovine MK. Conserved mechanisms regulate outgrowth in zebrafish fins. Nat Chem Biol. 2007;3(10):613–8.
Zhang J, Jeradi S, Strähle U, Akimenko M-A. Laser ablation of the sonic hedgehog- a-expressing cells during fin regeneration affects ray branching morphogenesis. Dev Biol. 2012;365(2):424–33.
Wang H, Simon A. Skeletal muscle dedifferentiation during salamander limb regeneration. Curr Opin Genet Dev. 2016;40:108–12.
Odelberg SJ, Kollhoff A, Keating MT. Dedifferentiation of mammalian myotubes induced by msx1. Cell. 2000;103(7):1099–109.
Mcgann CJ, Odelberg SJ, Keating MT. Mammalian myotube dedifferentiation induced by newt regeneration extract. Proc Natl Acad Sci U S A. 2001;98(24):13699–704.
Duckmanton A, Kumar A, Chang Y-T, Brockes JP. A single-cell analysis of myogenic dedifferentiation induced by small molecules. Chem Biol. 2005;12(10):1117–26.
Guo J-K, Cantley LG. Cellular maintenance and repair of the kidney. Annu Rev Physiol. 2010;72:357–76.
Bertoli C, Skotheim JM, De Bruin RAM. Control of cell cycle transcription during G1 and S phases. Nat Rev Mol Cell Biol. 2013;14(8):518–28.
Sherr CJ, McCormick F. The Rb and p53 pathways in cancer. Cancer Cell. 2002;2(2):103–12.
Tanaka Em GAA, Gates PB, Brockes JP. Newt myotubes reenter the cell cycle by phosphorylation of the retinoblastoma protein. J Cell Biol. 1997;136(1):155–65.
Tanaka EM, Drechsel DN, Brockes JP. Thrombin regulates S-phase re-entry by cultured newt myotubes. Curr Biol. 1999;9(15):792–9.
Schneider JW, Gu W, Zhu L, et al. Reversal of terminal differentiation mediated by p107 in Rb−/− muscle cells. Science. 1994;264(5164):1467–71.
Sdek P, Zhao P, Wang Y, et al. Rb and p130 control cell cycle gene silencing to maintain the postmitotic phenotype in cardiac myocytes. J Cell Biol. 2011;194(3):407–23.
Yun MH, Gates PB, Brockes JP. Regulation of p53 is critical for vertebrate limb regeneration. Proc Natl Acad Sci U S A. 2013;110(43):17392–7.
Mahmoud AI, Kocabas F, Muralidhar SA, et al. Meis1 regulates postnatal cardiomyocyte cell cycle arrest. Nature. 2013;497(7448):249–53.
Zhou Q, Brown J, Kanarek A, et al. In vivo reprogramming of adult pancreatic exocrine cells to beta-cells. Nature. 2008;455(7213):627–32.
Qian L, Huang Y, Spencer CI, et al. In vivo reprogramming of murine cardiac fibroblasts into induced cardiomyocytes. Nature. 2012;485(7400):593–8.
Guo Z, Zhang L, Wu Z, et al. In vivo direct reprogramming of reactive glial cells into functional neurons after brain injury and in an Alzheimer's disease model. Cell Stem Cell. 2014;14(2):188–202.
Niu W, Zang T, Zou Y, et al. In vivo reprogramming of astrocytes to neuroblasts in the adult brain. Nat Cell Biol. 2013;15(10):1164–75.
Song G, Pacher M, Balakrishnan A, et al. Direct reprogramming of hepatic Myofibroblasts into hepatocytes in vivo attenuates liver fibrosis. Cell Stem Cell. 2016;18(6):797–808.
Mohamed TMA, Stone NR, Berry EC, et al. Chemical enhancement of in vitro and in vivo direct cardiac reprogramming. Circulation. 2017;135(10):978–95.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 Hubei Scientific and Technical Publishers
About this chapter
Cite this chapter
Qin, H., Zhao, A., Fu, X., Zhang, C. (2021). Cellular Basis for Tissue Regeneration: Cellular Dedifferentiation. In: Fu, X. (eds) Regenerative Medicine in China. Springer, Singapore. https://doi.org/10.1007/978-981-16-1182-7_2
Download citation
DOI: https://doi.org/10.1007/978-981-16-1182-7_2
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-16-1181-0
Online ISBN: 978-981-16-1182-7
eBook Packages: MedicineMedicine (R0)