Abstract
Myopia is an important risk factor for glaucoma. The prevalence of myopia is increasing dramatically, and thus too, myopic glaucoma will be more often encountered. Recent advances in Spectral-Domain Optical Coherence Tomography (SD-OCT) technology enable fast, objective, and quantitative structural imaging of the optic nerve head (ONH), retinal nerve fiber layer (RNFL), and macula for facilitated and enhanced glaucoma diagnostics. However, myopic eyes have unique structural features, which might cause artifacts in OCT imaging or induce false positivity or negativity in interpreting OCT results. For correct diagnosis of glaucoma, it is essential to understand myopic eyes’ structural features that might affect imaging and interpretation of OCT. The key OCT parameters in glaucoma diagnosis include peripapillary RNFL thickness, macular ganglion cell-inner plexiform layer (GCIPL) thickness, and neuroretinal rim thickness measurements. Here, I review the anatomical features of these structures in myopia, how they affect imaging and the diagnostic performance of OCT, how these structures and tests might be misinterpreted, and how to overcome pitfalls and to make correct diagnoses of myopic eyes with or without glaucoma.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Optical coherence tomography
- Myopia
- Glaucoma
- Retinal nerve fiber layer
- Macular imaging
- Optic nerve head imaging
1 RNFL Imaging in Myopia
1.1 Characteristics of RNFL Thickness in Myopia
Since the era of Stratus OCT, several studies have shown that myopic eyes have thinner-measured RNFL thickness (Choi and Lee 2006; Leung et al. 2006; Vernon et al. 2008; Budenz et al. 2007). Leung et al. (2006) reported that RNFL measurements were lower in highly myopic eyes than in low-to-moderate myopic eyes. Budenz et al. (2007) recruited 328 normal eyes with various refractive errors (−11.75 to +6.75 diopters) and reported that higher myopic eyes had a thinner-measured RNFL with a significant negative correlation between spherical equivalent/axial length and RNFL thickness. Studies utilizing SD-OCT have shown similar results (Kang et al. 2010; Wang et al. 2011; Mohammad Salih 2012). Although many studies have reported thinner RNFL thickness measurements in myopic eyes, it was uncertain if this finding resulted from an actual decrease of RNFL thickness in myopic eyes or if it was caused by factors affecting the accuracy of any OCT measurements.
Traditionally, peripapillary RNFL thickness has been measured along a circle scan of 1.73 mm radius. Because of the magnification effect, the actual scanning radius in a myopic eye could be longer than 1.73 mm. The relationship between the measurement obtained from the OCT image and the actual size of the fundus dimension can be expressed as t = p * q * s, where t is the actual fundus dimension, s is the measurement obtained using OCT, p is the magnification factor for the camera of the imaging system, and q is the magnification factor for the eye (Littmann 1982). The p is a constant in a telecentric system, which is 3.382 in the Cirrus and Stratus OCT systems (Carl Zeiss Meditec, California, USA). The ocular magnification factor q of the eye can be determined with the formula q = 0.01306 * (axial length − 1.82) (Bennett et al. 1994). Therefore, the actual radius of the scan circle on the fundus (mm) equals 3.382 * 0.01306 (axial length − 1.82) * 1.73 (mm). For example, in an eye of 28 mm axial length, the actual scanning radius will increase to 2.00 mm, and in an eye of 20 mm axial length, it will decrease to 1.39 mm (Savini et al. 2012). Thus, in myopic eyes with long axial length, the larger actual scanning circle in OCT might underestimate RNFL thickness.
Kang et al. (2010) first reported RNFL thickness measurement with Cirrus OCT after correction of the magnification effect in myopic eyes. Before adjusting the ocular magnification, the mean RNFL significantly decreased with spherical equivalent and increased with axial length. In contrast, after adjusting the ocular magnification, the mean RNFL thickness showed no correlation with spherical equivalent and only a weak positive correlation with axial length. Savini et al. (2012) also showed that RNFL thickness decreased with longer axial length, but this relationship disappeared with correction of axial-length-induced ocular magnification by the Littmann formula. These findings suggest that many previous studies reporting decreased mean RNFL thickness in myopia may, at least partly, have resulted from underestimation of thickness rather than from any true anatomical difference. Nevertheless, current OCT devices do not have any built-in function for correction of ocular magnification. Therefore, when evaluating OCT results for myopic eyes, it should be considered that even myopic eyes without glaucoma can show decreased peripapillary RNFL thickness.
1.2 Distribution Profile of RNFL Thickness in Myopia
Myopic eyes show different RNFL thickness profiles in OCT imaging. Kim et al. (2010) compared circumpapillary RNFL thickness profiles between a high-myopia group and a low-myopia group. Whereas the highly myopic eyes had significantly thinner RNFLs in the non-temporal sectors, they had significantly thicker RNFLs in the temporal quadrant compared with the low-myopia group. Hong et al. studied the angles of peaks in circumpapillary RNFL thickness profiles for young male adults and found a significant correlation between the angle and the degree of myopia (Hong et al. 2010). With increasing myopia, the peaks of RNFL thickness were closer to the temporal quadrant. Because the normative database of Stratus and Cirrus OCT devices largely comprises data from normal eyes with no or low myopia, both studies raised the possibility of misdiagnosis of glaucoma in myopic eyes without glaucoma. Leung et al. (2012) studied the RNFL distribution profile in myopic eyes using the RNFL thickness map of Cirrus OCT. They found that the RNFL distribution angle also diminished with increasing myopia and that this reduction could lead to false-positive classification of abnormal RNFL measurement on the RNFL thickness deviation map (Fig. 1).
Chung and Yoo (2011) raised the possibility of an inappropriate location of the OCT scan circle to explain the different RNFL thickness profiles in eyes with myopic tilted disc. After automated RNFL analysis by the Cirrus OCT built-in algorithm, the authors manually relocated the calculation circle, centering it based on the contours of the neural canal opening (NCO). They found that in the new circle location, the mean number of abnormal clock-hour sectors as well as the proportion of eyes with abnormal clock-hour sectors at the 5% level of the built-in normative database was significantly lower. This result suggested that the NCO might be a suitable reference for peripapillary RNFL imaging. Lee et al. (2016) investigated circumpapillary RNFL thickness centered on the Bruch’s membrane opening (BMO) with Spectralis OCT (Heidelberg Engineering, Heidelberg, Germany), and showed that the new RNFL scanning algorithm had better diagnostic performance, particularly for myopic eyes with externally oblique border tissue.
In clinical practice, adjusting the circle scan location for every myopic eye might not be practical. However, clinicians, in order to minimize the probability of misdiagnosis, should always evaluate circumpapillary RNFL thickness or an RNFL thickness deviation map with caution considering the different RNFL profiles in myopic eyes.
1.3 High False Positivity in Myopia
The principal analysis of conventional OCT devices for diagnosis of glaucoma is based on measurement of RNFL thickness along the circle scan and comparison of the value to the built-in normative database. Due to the thinner RNFL thickness measurement and different distribution profiles in myopic eyes, high false-positivity rates for diagnostic performance of OCT have been reported from the era of Stratus OCT (Leung et al. 2006; Vernon et al. 2008). Leung et al. (2006) first reported that a significant proportion of myopic eyes were identified as abnormal based on the normative database provided in Stratus OCT. The most frequently abnormal sector was 2 o’clock, where 44.3% of myopic eyes were below the 95% confidence interval of the age-matched normative database. In addition, more eyes were classified as abnormal in the high-myopia group than in the low-to-moderate myopia group. Vernon et al. (2008) reported a similar finding with highly myopic Caucasians, specifically a substantial proportion of false-positivity errors. These studies both concluded that the normative database may not be entirely reliable for the evaluation of RNFL thickness in myopic eyes. Thus, the current in-machine normative database should be used with caution in the case of myopic eyes.
Studies with SD-OCT have shown similar findings (Leung et al. 2012; Aref et al. 2014; Qiu et al. 2011; Kim et al. 2011a). Leung et al. (2012) analyzed abnormal RNFL measurements on the RNFL thickness deviation map in Cirrus OCT and found that the reduction in the RNFL distribution angle with increasing myopia affected the false positivity. Qiu et al. (2011) reported that the frequency of false-positives was even higher in Cirrus OCT than in Stratus OCT. Kim et al. (2011a) studied the factors contributing to false-positive RNFL color-code results in Cirrus OCT. The overall false-positivity rate of 149 healthy eyes was as high as 26.2%, and eyes of longer axial length were more likely to show false-positive results.
1.4 Myopic Normative Database
Although myopic eyes have thinner RNFL thickness measurements and different thickness profiles in OCT, the normative databases of current OCT devices do not include moderate-to-highly myopic eyes. In the Cirrus OCT, the mean refractive error of 271 eyes in the normative database was −0.82D (SD 1.96D) (Knight et al. 2012). In order to decrease the frequency of false-positive errors in myopic eyes, the diagnostic performances of normative databases comprising myopic eyes have been studied. Biswas et al. (2016) obtained a normative database including 180 highly myopic eyes (spherical equivalent, −6.0D or less), and showed that the application of such a myopic normative database improved the specificity for detection of glaucomatous RNFL abnormalities in eyes with high myopia. This improvement in specificities did not trade-off an overall sensitivity for detection of glaucoma; rather, improved sensitivity was observed in certain criteria. Similarly, Seol et al. (2017) obtained a normative database comprising 154 myopic eyes ranging from mild to severe myopia. They found that the myopic normative database showed a higher specificity than did the built-in normative database in quadrant RNFL thickness, clock-hour RNFL thickness, and GCIPL thickness, whereas the sensitivities of the OCT color probability codes after applying the built-in and myopic normative databases were not statistically different. Therefore, the authors of both studies suggested the importance of incorporating a myopic normative database in OCT instruments for the evaluation of RNFL measurements in myopic eyes. Unfortunately, none of the commercially available OCT devices has a built-in normative database of moderate-to-high myopia for RNFL thickness analysis. The retina scan–OCT (Nidek Co, Ltd) has a normative database including myopic eyes of long axial length (26–29 mm), but it is used only for macular map analysis (Nakanishi et al. 2015).
1.5 Imaging Artifacts in RNFL imaging
Proper segmentation of RNFL thickness is a fundamental step in the evaluation of OCT RNFL measurements. However, several studies have reported segmentation errors for myopic eyes. Nakano et al. (2013) reported that circumpapillary RNFL segmentation errors were more prevalent in patients with high myopia than in those without. The authors suggested that the error sources were related to myopic peripapillary changes that had crossed the circumpapillary RNFL circle scan and were of low signal intensity. Correspondingly, Suwan et al. (2018) found that 14% of normal myopic eyes and 44% of myopic glaucoma eyes had RNFL segmentation errors requiring manual correction in OCT. The authors also found that correction of the errors significantly improved the glaucoma-diagnostic capability. Kamal Salah et al. (2015) reported the effect of peripapillary neurosensory retinal detachment on RNFL measurement in high myopia. This study showed that eyes with peripapillary neurosensory retinal detachment had a significantly greater average RFNL thickness relative to those without peripapillary detachment, due to misidentification of the outer profile of the RFNL.
Retinal pathologies along the circle scan as well as segmentation errors of OCT software may affect RNFL thickness measurement of OCT. Thus, careful interpretation is required for the evaluation of OCT reports on myopic eyes. Because manual correction of segmentation errors is currently time-consuming and not provided for in all commercially available OCT devices, the development of a more accurate automated segmentation algorithm is necessary.
2 Macular Imaging in Myopia
2.1 Macular Imaging in Glaucoma
Glaucoma is characterized by selective loss of retinal ganglion cells (RGC). Since the macular area contains more than 50% of all RGCs and the RGC bodies are 10 to 20 times the diameter of their axons, the macular area is important in the detection of glaucomatous RGC damage (Hood et al. 2013). From the idea that macular thickness can reflect glaucomatous damage, the change of macular thickness as a measure of glaucoma had been studied prior to OCT’s introduction (Zeimer et al. 1998). Later, with Stratus OCT, several studies tested whether assessment of macular thickness might outperform circumpapillary RNFL measurement. Initial research investigating total macular thickness showed that circumpapillary RNFL evaluation was superior to that of macular thickness, due probably to the retinal thickness other than the RGC layer masking glaucomatous change (Guedes et al. 2003; Wollstein et al. 2004). Customized segmentation of the inner retinal complex (RGC layer+inner plexiform and nuclear layers) by Stratus OCT showed comparable diagnostic performance to circumpapillary RNFL for glaucoma diagnosis, which served to raise the importance of segmentation analysis (Ishikawa et al. 2005).
Introduction of SD-OCT enabled segmented macular thickness measurements by a built-in segmentation algorithm. Several commercially available SD-OCTs provide different segmented macular thickness measurements. RTVue FD-OCT (Optovue, Fremont, California, USA) provides the macular ganglion cell complex (GCC), which is the sum of the macular RNFL, ganglion cell layer (GCL) and inner plexiform layer (IPL). Cirrus SD-OCT provides macular ganglion cell–inner plexiform layer (GCIPL) thickness via a macular ganglion cell analysis (GCA) algorithm. Topcon 3D-OCT (Topcon, Tokyo, Japan) provides both GCC and GCIPL thickness. Spectralis OCT enables separate measurement of the entire retinal layers. Several studies have shown that the diagnostic ability of the macular thickness parameters is comparable to that of RNFL thickness for diagnosis of manifest glaucoma (Mwanza et al. 2012; Oddone et al. 2016).
Whereas circumpapillary RNFL thickness measurement is affected by several factors such as disc size (Savini et al. 2005), disc tilt (Hwang et al. 2012a), and axial length (Kang et al. 2010; Wang et al. 2011; Mohammad Salih 2012), macular GCIPL thickness is less influenced by them (Lee et al. 2014; Jeong et al. 2016). Therefore, the diagnostic benefit of macular thickness parameters in myopic glaucoma has been studied.
2.2 Diagnostic Ability of Macular Parameters in Myopia
Initial studies evaluating the ability of macular thickness to detect glaucoma in myopia have used RTVue OCT with GCC parameters. Kim et al. (2011b) compared the diagnostic ability to detect glaucoma between macular GCC and peripapillary RNFL thickness in highly myopic eyes using RTVue OCT. They found that the macular GCC thickness measurements had higher AUROC than did peripapillary RNFL thickness in highly myopic eyes for detection of glaucoma, but without statistical significance. Shoji et al. (2011) determined that the diagnostic power of GCC parameters was significantly higher than that of circumpapillary RNFL parameters in high myopia. Another paper by Shoji et al. studied the effects of high myopia on the glaucoma-diagnostic ability of OCT parameters. The ability of circumpapillary RNFL measurement to detect glaucoma in highly myopic eyes was inferior to that in emmetropic eyes, whereas macular GCC measurements showed a good ability to detect glaucoma in both groups. Based on the results of these studies, it can be concluded that macular GCC thickness has a glaucoma-diagnostic ability comparable or superior to that of circumpapillary RNFL thickness for highly myopic eyes.
GCC is the sum of three layers, the RNFL, GCL, and IPL, whereas GCIPL is the sum of the GCL and IPL. Thus, GCPIL is less influenced by RNFL thickness variation than GCC thickness is. Several studies have investigated its glaucoma detection ability for myopic eyes. Choi et al. (2013) compared the glaucoma-diagnostic ability of GCIPL with that of circumpapillary RNFL thickness in high myopia, finding them to be comparable. Seol et al. (2015) studied the glaucoma detection ability of GCIPL in myopic preperimetric glaucoma and determined the inferotemporal macular GCIPL thickness parameter to be the best for detection of PPG in myopic eyes, superior to the RNFL thickness parameters.
Besides measuring GCIPL thickness and comparing the values with a normative database, other approaches to the improvement of glaucoma-diagnostic ability have been reported as well. Kim et al. introduced a MATLAB-based GCIPL hemifield test for detection of GCIPL thickness difference across the temporal horizontal raphe and evaluated its glaucoma-diagnostic ability in highly myopic eyes (Kim et al. 2015a, 2016). They found that the AUC value for the GCIPL hemifield test, as compared with the RNFL and GCIPL thickness parameters, was the best (Kim et al. 2016). Baek et al. (2018) proposed a new scoring system combining the topographic signs of both RNFL and GCIPL analysis. The system had a higher diagnostic ability than the RNFL or GCIPL thickness parameters for myopic eyes and even for highly myopic eyes. The recently introduced swept-source OCT (SS-OCT) provides a wide 12- * 9-mm2 area in a single scan showing the macular and peripapillary structures simultaneously. Kim et al. determined that SS-OCT wide-field imaging, as compared with conventional SD-OCT, had greater diagnostic power for glaucoma with myopia (Kim et al. 2020).
Although the macular parameters in myopic eyes have shown similar or better diagnostic ability relative to the RNFL thickness parameters, GCA maps can miss abnormal findings if the angular distance between the fovea and an RNFL defect is great (Hwang et al. 2014; Kim et al. 2014). In addition, several factors (see below) affect macular thickness in myopia. Therefore, macular thickness parameters cannot substitute for RNFL thickness evaluation by OCT but rather should be considered as complementary to it.
2.3 Imaging Artifacts in Macular Imaging
Several studies have reported that segmentation errors in OCT total retinal thickness measurements could be found in various macular diseases including age-related macular degeneration, epiretinal membrane (ERM), diabetic retinopathy, and retinal vein occlusion (Ray et al. 2005; Ho et al. 2009; Giani et al. 2010; Han and Jaffe 2010). Abnormal segmentations in GCIPL also have been reported in eyes with macular degeneration and ERM (Hwang 2014). Even in eyes without macular disorders, segmentation errors in macular GCA can be found in some cases. Hwang et al. (2016) reported that 9.7% of glaucoma or healthy eyes without macular disorder had segmentation errors and that only higher-degree myopia was associated with the presence of segmentation errors. Therefore, the possibility of segmentation errors as well as accompanying macular pathologies should be considered when evaluating macular analysis in myopic eyes, especially in eyes with high myopia.
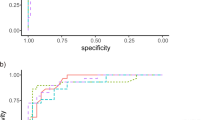
False-positive classification is frequently observed in the case of the color-code GCA map. Kim et al. (2015b) reported that 40.4% of 104 healthy eyes showed abnormal classifications on any of the GCA maps. Abnormal classification was associated with longer axial length and larger fovea-disc angle. Due to the higher frequency of false-positive GCA classification, macular thickness analyses should be carefully evaluated, especially in myopic eyes (Fig. 2).
3 Disc Imaging in Myopia
3.1 Disc Imaging with OCT
Stratus OCT provides various ONH parameters including disc area, cup area, rim area, cup/disc area ratio, cup/disc horizontal ratio, cup/disc vertical ratio, vertical integrated rim area (volume), and horizontal integrated rim width (area). However, owing to the low reproducibility of ONH scans, frequent inappropriate recognition of optic disc margin, and imaging artifacts, ONH analysis has not been widely used in glaucoma diagnostics using Stratus OCT (Iliev et al. 2006; Ortega Jde et al. 2009; Marsh et al. 2010).
SD-OCT provides excellent reproducibility and a low rate of incorrect optic disc margin detection ranging from 0.5 to 2.6% (Mwanza et al. 2010; Cheung et al. 2011; Sung et al. 2012). In addition, studies using Cirrus-OCT have reported that ONH parameters are comparable to RNFL thickness measurements in terms of differentiating normal eyes from glaucoma (Mwanza et al. 2011; Hwang and Kim 2012). In myopic eyes, however, errors in neuroretinal rim measurement by Cirrus HD-OCT were found in 17.6% of myopic eyes, especially eyes with PPA, higher myopia, greater axial length, vitreous opacity, or acute cup slope angle (Hwang et al. 2012b).
3.2 BMO-MRW
Studies comparing the clinical disc margin and structures in SD-OCT have found that the clinical disc margin is neither a single anatomic entity nor a clinical construct that underlies a consistent anatomical structure within or between eyes (Reis et al. 2012a, b). Because RGC axons exit the eye through the BMO from the inner retinal layer, and because axons cannot pass through an intact Bruch’s membrane, neuroretinal rim measurements using the BMO as a reference plane have been proposed (Chauhan and Burgoyne 2013; Chauhan et al. 2013). The BMO-minimum rim width (BMO-MRW), defined as the minimum distance between the BMO and the internal limiting membrane, has shown a higher sensitivity compared with peripapillary RNFL thickness measurements for diagnosis of early glaucoma (Chauhan et al. 2013).
In myopic eyes, evaluation of glaucomatous optic disc changes has been challenging due to the unique optic disc morphology, including large peripapillary atrophy, varying degrees of disc tilt or torsion, and abnormally large or small optic disc size. Therefore, several studies have evaluated the usefulness of BMO-MRW for myopic eyes. Malik et al. (2016) reported that the sensitivity of BMO-MRW was comparable to that of RNFL thickness; however, the sensitivity of both parameters was only 71.4% at 90% specificity. Recent studies have reported that for healthy myopic eyes, the BMO-MRW parameter shows a lower rate of false-positives than does RNFL thickness (Rebolleda et al. 2016; Sastre-Ibañez et al. 2018). Kim et al. studied the diagnostic accuracy of three-dimensional neuroretinal rim (3D-NRR) thickness, which is defined as the distance between the BMO and the vitreoretinal interface, in myopic eyes (Kim and Park 2018). They found that the false-positivity rate was significantly lower for 3D-NRR thickness than for RNFL thickness and that its diagnostic accuracy for glaucoma outperformed RNFL thickness. However, Zheng et al. (2018) demonstrated that in high myopia, 32% of eyes had indiscernible BMO in at least one meridian. Furthermore, the BMO was indistinct most frequently at the temporal, inferotemporal, and superotemporal meridians where glaucomatous neuroretinal rim loss is most common. Therefore, utilization of BMO-MRW might be compromised in highly myopic eyes. Based on these results, it can be concluded that rim evaluation referencing the BMO as an anatomic landmark can be a complementary tool to RNFL thickness measurements for the diagnosis of glaucoma in myopic eyes (Fig. 3).
References
Aref AA, Sayyad FE, Mwanza JC, Feuer WJ, Budenz DL. Diagnostic specificities of retinal nerve fiber layer, optic nerve head, and macular ganglion cell-inner plexiform layer measurements in myopic eyes. J Glaucoma. 2014;23(8):487–93.
Baek SU, Kim KE, Kim YK, Park KH, Jeoung JW. Development of topographic scoring system for identifying glaucoma in myopic eyes: a spectral-domain OCT study. Ophthalmology. 2018;125(11):1710–9.
Bennett AG, Rudnicka AR, Edgar DF. Improvements on Littmann’s method of determining the size of retinal features by fundus photography. Graefes Arch Clin Exp Ophthalmol. 1994;232(6):361–7.
Biswas S, Lin C, Leung CK. Evaluation of a myopic normative database for analysis of retinal nerve fiber layer thickness. JAMA Ophthalmol. 2016;134(9):1032–9.
Budenz DL, Anderson DR, Varma R, et al. Determinants of normal retinal nerve fiber layer thickness measured by Stratus OCT. Ophthalmology. 2007;114(6):1046–52.
Chauhan BC, Burgoyne CF. From clinical examination of the optic disc to clinical assessment of the optic nerve head: a paradigm change. Am J Ophthalmol. 2013;156(2):218–27.e2.
Chauhan BC, O’Leary N, AlMobarak FA, et al. Enhanced detection of open-angle glaucoma with an anatomically accurate optical coherence tomography-derived neuroretinal rim parameter. Ophthalmology. 2013;120(3):535–43.
Cheung CY, Chen D, Wong TY, et al. Determinants of quantitative optic nerve measurements using spectral domain optical coherence tomography in a population-based sample of non-glaucomatous subjects. Invest Ophthalmol Vis Sci. 2011;52(13):9629–35.
Choi SW, Lee SJ. Thickness changes in the fovea and peripapillary retinal nerve fiber layer depend on the degree of myopia. Korean J Ophthalmol. 2006;20(4):215–9.
Choi YJ, Jeoung JW, Park KH, Kim DM. Glaucoma detection ability of ganglion cell-inner plexiform layer thickness by spectral-domain optical coherence tomography in high myopia. Invest Ophthalmol Vis Sci. 2013;54(3):2296–304.
Chung JK, Yoo YC. Correct calculation circle location of optical coherence tomography in measuring retinal nerve fiber layer thickness in eyes with myopic tilted discs. Invest Ophthalmol Vis Sci. 2011;52(11):7894–900.
Giani A, Cigada M, Esmaili DD, et al. Artifacts in automatic retinal segmentation using different optical coherence tomography instruments. Retina. 2010;30(4):607–16.
Guedes V, Schuman JS, Hertzmark E, et al. Optical coherence tomography measurement of macular and nerve fiber layer thickness in normal and glaucomatous human eyes. Ophthalmology. 2003;110(1):177–89.
Han IC, Jaffe GJ. Evaluation of artifacts associated with macular spectral-domain optical coherence tomography. Ophthalmology. 2010;117(6):1177–89.e4.
Ho J, Sull AC, Vuong LN, et al. Assessment of artifacts and reproducibility across spectral- and time-domain optical coherence tomography devices. Ophthalmology. 2009;116(10):1960–70.
Hong SW, Ahn MD, Kang SH, Im SK. Analysis of peripapillary retinal nerve fiber distribution in normal young adults. Invest Ophthalmol Vis Sci. 2010;51(7):3515–23.
Hood DC, Raza AS, de Moraes CG, Liebmann JM, Ritch R. Glaucomatous damage of the macula. Prog Retin Eye Res. 2013;32:1–21.
Hwang YH. Patterns of macular ganglion cell abnormalities in various ocular conditions. Invest Ophthalmol Vis Sci. 2014;55(6):3995–6.
Hwang YH, Kim YY. Glaucoma diagnostic ability of quadrant and clock-hour neuroretinal rim assessment using cirrus HD optical coherence tomography. Invest Ophthalmol Vis Sci. 2012;53(4):2226–34.
Hwang YH, Yoo C, Kim YY. Myopic optic disc tilt and the characteristics of peripapillary retinal nerve fiber layer thickness measured by spectral-domain optical coherence tomography. J Glaucoma. 2012a;21(4):260–5.
Hwang YH, Kim YY, Jin S, Na JH, Kim HK, Sohn YH. Errors in neuroretinal rim measurement by Cirrus high-definition optical coherence tomography in myopic eyes. Br J Ophthalmol. 2012b;96(11):1386–90.
Hwang YH, Jeong YC, Kim HK, Sohn YH. Macular ganglion cell analysis for early detection of glaucoma. Ophthalmology. 2014;121(8):1508–15.
Hwang YH, Kim MK, Kim DW. Segmentation errors in macular ganglion cell analysis as determined by optical coherence tomography. Ophthalmology. 2016;123(5):950–8.
Iliev ME, Meyenberg A, Garweg JG. Morphometric assessment of normal, suspect and glaucomatous optic discs with Stratus OCT and HRT II. Eye (Lond). 2006;20(11):1288–99.
Ishikawa H, Stein DM, Wollstein G, Beaton S, Fujimoto JG, Schuman JS. Macular segmentation with optical coherence tomography. Invest Ophthalmol Vis Sci. 2005;46(6):2012–7.
Jeong JH, Choi YJ, Park KH, Kim DM, Jeoung JW. Macular ganglion cell imaging study: covariate effects on the spectral domain optical coherence tomography for glaucoma diagnosis. PLoS One. 2016;11(8):e0160448.
Kamal Salah R, Morillo-Sánchez MJ, García-Ben A, et al. The effect of peripapillary detachment on retinal nerve fiber layer measurement by spectral domain optical coherence tomography in high myopia. Ophthalmologica. 2015;233(3–4):209–15.
Kang SH, Hong SW, Im SK, Lee SH, Ahn MD. Effect of myopia on the thickness of the retinal nerve fiber layer measured by Cirrus HD optical coherence tomography. Invest Ophthalmol Vis Sci. 2010;51(8):4075–83.
Kim KE, Jeoung JW, Park KH, Kim DM, Kim SH. Diagnostic classification of macular ganglion cell and retinal nerve fiber layer analysis: differentiation of false-positives from glaucoma. Ophthalmology. 2015b;122(3):502–10.
Kim MJ, Lee EJ, Kim TW. Peripapillary retinal nerve fibre layer thickness profile in subjects with myopia measured using the Stratus optical coherence tomography. Br J Ophthalmol. 2010;94(1):115–20.
Kim MJ, Jeoung JW, Park KH, Choi YJ, Kim DM. Topographic profiles of retinal nerve fiber layer defects affect the diagnostic performance of macular scans in preperimetric glaucoma. Invest Ophthalmol Vis Sci. 2014;55(4):2079–87.
Kim NR, Lim H, Kim JH, Rho SS, Seong GJ, Kim CY. Factors associated with false positives in retinal nerve fiber layer color codes from spectral-domain optical coherence tomography. Ophthalmology. 2011a;118(9):1774–81.
Kim NR, Lee ES, Seong GJ, et al. Comparing the ganglion cell complex and retinal nerve fibre layer measurements by Fourier domain OCT to detect glaucoma in high myopia. Br J Ophthalmol. 2011b;95(8):1115–21.
Kim YK, Yoo BW, Kim HC, Park KH. Automated detection of hemifield difference across horizontal raphe on ganglion cell-inner plexiform layer thickness map. Ophthalmology. 2015a;122(11):2252–60.
Kim YK, Yoo BW, Jeoung JW, Kim HC, Kim HJ, Park KH. Glaucoma-diagnostic ability of ganglion cell-inner plexiform layer thickness difference across temporal raphe in highly myopic eyes. Invest Ophthalmol Vis Sci. 2016;57(14):5856–63.
Kim YW, Park KH. Diagnostic accuracy of three-dimensional neuroretinal rim thickness for differentiation of myopic glaucoma from myopia. Invest Ophthalmol Vis Sci. 2018;59(8):3655–66.
Kim YW, Lee J, Kim JS, Park KH. Diagnostic accuracy of wide-field map from swept-source optical coherence tomography for primary open-angle glaucoma in myopic eyes. Am J Ophthalmol. 2020;218:182–91.
Knight OJ, Girkin CA, Budenz DL, Durbin MK, Feuer WJ. Effect of race, age, and axial length on optic nerve head parameters and retinal nerve fiber layer thickness measured by Cirrus HD-OCT. Arch Ophthalmol. 2012;130(3):312–8.
Lee EJ, Lee KM, Kim H, Kim TW. Glaucoma diagnostic ability of the new circumpapillary retinal nerve fiber layer thickness analysis based on Bruch’s membrane opening. Invest Ophthalmol Vis Sci. 2016;57(10):4194–204.
Lee KH, Kim CY, Kim NR. Variations of retinal nerve fiber layer thickness and ganglion cell-inner plexiform layer thickness according to the torsion direction of optic disc. Invest Ophthalmol Vis Sci. 2014;55(2):1048–55.
Leung CK, Mohamed S, Leung KS, et al. Retinal nerve fiber layer measurements in myopia: an optical coherence tomography study. Invest Ophthalmol Vis Sci. 2006;47(12):5171–6.
Leung CK, Yu M, Weinreb RN, et al. Retinal nerve fiber layer imaging with spectral-domain optical coherence tomography: interpreting the RNFL maps in healthy myopic eyes. Invest Ophthalmol Vis Sci. 2012;53(11):7194–200.
Littmann H. Determination of the real size of an object on the fundus of the living eye. Klin Monatsbl Augenheilkd. 1982;180(4):286–9.
Malik R, Belliveau AC, Sharpe GP, Shuba LM, Chauhan BC, Nicolela MT. Diagnostic accuracy of optical coherence tomography and scanning laser tomography for identifying glaucoma in myopic eyes. Ophthalmology. 2016;123(6):1181–9.
Marsh BC, Cantor LB, WuDunn D, et al. Optic nerve head (ONH) topographic analysis by stratus OCT in normal subjects: correlation to disc size, age, and ethnicity. J Glaucoma. 2010;19(5):310–8.
Mohammad Salih PA. Evaluation of peripapillary retinal nerve fiber layer thickness in myopic eyes by spectral-domain optical coherence tomography. J Glaucoma. 2012;21(1):41–4.
Mwanza JC, Chang RT, Budenz DL, et al. Reproducibility of peripapillary retinal nerve fiber layer thickness and optic nerve head parameters measured with cirrus HD-OCT in glaucomatous eyes. Invest Ophthalmol Vis Sci. 2010;51(11):5724–30.
Mwanza JC, Oakley JD, Budenz DL, Anderson DR. Ability of cirrus HD-OCT optic nerve head parameters to discriminate normal from glaucomatous eyes. Ophthalmology. 2011;118(2):241–8.e1.
Mwanza JC, Durbin MK, Budenz DL, et al. Glaucoma diagnostic accuracy of ganglion cell-inner plexiform layer thickness: comparison with nerve fiber layer and optic nerve head. Ophthalmology. 2012;119(6):1151–8.
Nakanishi H, Akagi T, Hangai M, et al. Sensitivity and specificity for detecting early glaucoma in eyes with high myopia from normative database of macular ganglion cell complex thickness obtained from normal non-myopic or highly myopic Asian eyes. Graefes Arch Clin Exp Ophthalmol. 2015;253(7):1143–52.
Nakano N, Hangai M, Noma H, et al. Macular imaging in highly myopic eyes with and without glaucoma. Am J Ophthalmol. 2013;156(3):511–23.e6.
Oddone F, Lucenteforte E, Michelessi M, et al. Macular versus retinal nerve fiber layer parameters for diagnosing manifest glaucoma: a systematic review of diagnostic accuracy studies. Ophthalmology. 2016;123(5):939–49.
Ortega Jde L, Kakati B, Girkin CA. Artifacts on the optic nerve head analysis of the optical coherence tomography in glaucomatous and nonglaucomatous eyes. J Glaucoma. 2009;18(3):186–91.
Qiu KL, Zhang MZ, Leung CK, et al. Diagnostic classification of retinal nerve fiber layer measurement in myopic eyes: a comparison between time-domain and spectral-domain optical coherence tomography. Am J Ophthalmol. 2011;152(4):646–53.e2.
Ray R, Stinnett SS, Jaffe GJ. Evaluation of image artifact produced by optical coherence tomography of retinal pathology. Am J Ophthalmol. 2005;139(1):18–29.
Rebolleda G, Casado A, Oblanca N, Muñoz-Negrete FJ. The new Bruch’s membrane opening – minimum rim width classification improves optical coherence tomography specificity in tilted discs. Clin Ophthalmol. 2016;10:2417–25.
Reis AS, Sharpe GP, Yang H, Nicolela MT, Burgoyne CF, Chauhan BC. Optic disc margin anatomy in patients with glaucoma and normal controls with spectral domain optical coherence tomography. Ophthalmology. 2012a;119(4):738–47.
Reis AS, O’Leary N, Yang H, et al. Influence of clinically invisible, but optical coherence tomography detected, optic disc margin anatomy on neuroretinal rim evaluation. Invest Ophthalmol Vis Sci. 2012b;53(4):1852–60.
Sastre-Ibañez M, Martinez-de-la-Casa JM, Rebolleda G, et al. Utility of Bruch membrane opening-based optic nerve head parameters in myopic subjects. Eur J Ophthalmol. 2018;28(1):42–6.
Savini G, Zanini M, Carelli V, Sadun AA, Ross-Cisneros FN, Barboni P. Correlation between retinal nerve fibre layer thickness and optic nerve head size: an optical coherence tomography study. Br J Ophthalmol. 2005;89(4):489–92.
Savini G, Barboni P, Parisi V, Carbonelli M. The influence of axial length on retinal nerve fibre layer thickness and optic-disc size measurements by spectral-domain OCT. Br J Ophthalmol. 2012;96(1):57–61.
Seol BR, Jeoung JW, Park KH. Glaucoma detection ability of macular ganglion cell-inner plexiform layer thickness in myopic preperimetric glaucoma. Invest Ophthalmol Vis Sci. 2015;56(13):8306–13.
Seol BR, Kim DM, Park KH, Jeoung JW. Assessment of optical coherence tomography color probability codes in myopic glaucoma eyes after applying a myopic normative database. Am J Ophthalmol. 2017;183:147–55.
Shoji T, Sato H, Ishida M, Takeuchi M, Chihara E. Assessment of glaucomatous changes in subjects with high myopia using spectral domain optical coherence tomography. Invest Ophthalmol Vis Sci. 2011;52(2):1098–102.
Sung KR, Na JH, Lee Y. Glaucoma diagnostic capabilities of optic nerve head parameters as determined by Cirrus HD optical coherence tomography. J Glaucoma. 2012;21(7):498–504.
Suwan Y, Rettig S, Park SC, et al. Effects of circumpapillary retinal nerve fiber layer segmentation error correction on glaucoma diagnosis in myopic eyes. J Glaucoma. 2018;27(11):971–5.
Vernon SA, Rotchford AP, Negi A, Ryatt S, Tattersal C. Peripapillary retinal nerve fibre layer thickness in highly myopic Caucasians as measured by Stratus optical coherence tomography. Br J Ophthalmol. 2008;92(8):1076–80.
Wang G, Qiu KL, Lu XH, et al. The effect of myopia on retinal nerve fibre layer measurement: a comparative study of spectral-domain optical coherence tomography and scanning laser polarimetry. Br J Ophthalmol. 2011;95(2):255–60.
Wollstein G, Schuman JS, Price LL, et al. Optical coherence tomography (OCT) macular and peripapillary retinal nerve fiber layer measurements and automated visual fields. Am J Ophthalmol. 2004;138(2):218–25.
Zeimer R, Asrani S, Zou S, Quigley H, Jampel H. Quantitative detection of glaucomatous damage at the posterior pole by retinal thickness mapping. A pilot study. Ophthalmology. 1998;105(2):224–31.
Zheng F, Wu Z, Leung CKS. Detection of Bruch’s membrane opening in healthy individuals and glaucoma patients with and without high myopia. Ophthalmology. 2018;125(10):1537–46.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Kim, S.H. (2021). Imaging in Myopic Glaucoma. In: Park, K.H., Kim, TW. (eds) OCT Imaging in Glaucoma. Springer, Singapore. https://doi.org/10.1007/978-981-16-1178-0_8
Download citation
DOI: https://doi.org/10.1007/978-981-16-1178-0_8
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-16-1177-3
Online ISBN: 978-981-16-1178-0
eBook Packages: MedicineMedicine (R0)