Abstract
Microorganisms of planktonic types have emerged into biofilm structures by acquiring the ability to attach to the surfaces. The extracellular polymeric substances may play a vital role in anchoring the biofilm onto the surface and also protect it from various antibiotics making it highly resistant. This type of survival is procured by species of bacteria, fungi and protists. These biofilms pose numerous hazards to the human health and environment. Over 80% of the diseases are caused by the biofilms which include colorectal cancer, oral infections, periodontitis, cystic fibrosis and many others. Different strategies have been developed to eliminate the biofilms like the use of nano-sized particles. These nanoparticles provide antimicrobial properties and can also be used as a delivery system of the antibiotics with high stability and good biocompatibility. The high surface-area-to-volume ratio makes the nanoparticles to have high contact surface to the bacterial cell membrane and hence plays an efficient therapeutic way in treating the biofilms. In this review, we discuss the various diseases caused by the biofilm and different antibiofilm strategies using the nanomaterials. There is no doubt that the upcoming antibiofilm strategy which uses nanoscience can gain an ultimately new hope in advancements of coping with bacterial infections and resistance.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
9.1 Introduction
Biofilm is an organized arrangement of microbial cells which are irreversibly attached to a surface contained in a matrix made of self-produced extracellular polymeric substances. It is responsible for many diseases infecting human health and sometimes has a positive influence like the mutualistic bacteria, namely, Staphylococcus epidermidis, which can hold back the formation of colony by pathogenic bacteria through triggering the host immune system and thereby preventing the attachment of microbes (Donlan 2002). Cystic fibrosis (CF), an inherited genetic disorder, is the most common human disease infecting the lungs seen in Western Europe. Patients with cystic fibrosis experience persistent infection caused by P. aeruginosa. So when this P. aeruginosa bacterium invades the CF lung, it starts to acclimatize with the CF lung and survives for decades. The reason is the excess production of matrix polysaccharide alginate which makes up the mucoid biofilm that resists antibiotics, elements of natural and acquired immunity, and thus resists phagocytosis. This whole process again develops noticeable antibody responses in the form of granulocytes and gives rise to chronic inflammation resulting in harsh lung tissue damage in the CF patients. The other negative effects of biofilms include dental caries or cavities which are caused by overproduction of organic acids mainly when sugary drinks are consumed or while frequent snacking. These acids breakdown the teeth enamel and lead to dental caries. It is approximated that 65 out of 100 infections are related to bacterial biofilms (Lewis 2001). They are the device- and non-device-linked infections. Data estimated for the device-related infections are 2% each for breast implants and joint prostheses; 4% each for mechanical heart valves, defibrillators and pacemakers; 10% for ventricular shunts; and 40% for ventricular-assisted. Non-device-related biofilm infections include periodontitis, osteomyelitis and many to list out. It is important to have oral hygiene since a person is susceptible to suffer from periodontitis which infects the gums and damages the soft tissues (Kokare et al. 2007). Osteomyelitis is a disease in bones caused by bacteria or the fungal cells. When the bacteria enter the bloodstream and infect the growth plate of the bone which is the metaphysic portion of the bone, the white blood cells gather at the site and try to phagocytose or kill the pathogen by releasing enzymes. So, these enzymes may break the bone and form pus spreading throughout the blood vessels. This leads to the dysfunctioning of the affected bone areas (Ziran 2007).
The antibiotics which are available to date have shown low efficacy in treating the biofilms associated with infections because of their high values of minimum inhibitory concentration (MIC) and minimum bacterial concentration (MBC) leading to in vivo toxicity. Sometimes, biofilms prevent the phagocytosis of the invading bacteria by impairing the phagocytes and complement system. Hence, it is important to focus on the MBCs, MICs, mechanism of action and chemical structures of the antibiofilm substances which include chelating agents, peptide antibiotics, etc. Different strategies can be implemented to combat with the biofilms like replacing the infected foreign bodies such as stents, implants with the sterile ones, blocking the quorum sensing pathway or by altering the c-di-GMP. LP 3134, LP 3145, LP 4010 and LP 1062 can inhibit the formation of biofilm in P. aeruginosa and Acinetobacter baumannii (Ranita Roy et al. 2018). Nanoscale materials are widely applied in the field of medicine because of their high reactivities with the large surface-area-to-volume ratio. In fact, the physicochemical properties are controllable when it comes to the nanoparticles. The other most important factor why nanotechnology has been more adapted is because of its low toxicity to the host and thus can gain a positive hope over the conventional treatments of biofilms (Ramasamy and Lee 2016). These nanoparticles (NPs) are able to find a way out to prevent the drug resistance mechanisms and other important processes. The combinations of plant-based antimicrobials with the nanoparticles are also studied (Baptista et al. 2018).
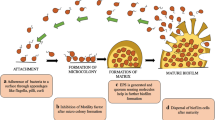
In this chapter, we are going to discuss the different stages leading to the formation of biofilms (Fig. 9.1). It is also important to study the types of pathogens involved in biofilm synthesis and the pathogenesis related to the biofilm in humans. As these biofilms give rise to dangerous diseases leading to chronic infections, there is a necessary need to combat the microbes, and also depending on the applications of nanoscience, we will be reviewing various strategies incorporated till now in treating the biofilms.
Stages of biofilm formation. (Adapted from Vasudevan 2014)
9.2 Biofilms
In 1947, Antonie van Leeuwenhoek examined the aggregates of “animalcules” scrapped from surfaces of human tooth under the microscope. Almost 100 years later, microbiologists studied the formation of biofilms and also concluded that bacteria grow differently when they attach to a specific surface in immobilized populations. Studies also show that the bacteria eliminated from the native ecosystem grow predominantly as planktonic cells (Costerton 1999). Biofilms are the organized structures comprising of microorganisms where they stick to each other producing extracellular polymers to cohesion. Biofilms are formed due to various parameters like nutritional cues; identification of specific or non-specific binding sites on a surface etiofilms can bind to the surfaces like the tooth, rock or any single species or a diverse group of microbes (Karatan and Watnick 2009; Hoffman et al. 2005). This shift of survival from a planktonic growth to biofilm keeps them safe from toxic factors like antibiotic desiccation and host body’s immune system (Tortora et al. 2015).
9.2.1 Bacterial Biofilms
In order to survive, the wild bacterial strains rely on fimbriae which protrude from the thick layer of exopolysaccharides (EPS). Fimbriae facilitate specific adhesion to the surfaces, and non-specific adhesion to inert surfaces is provided by EPS. Fimbriae interaction with the surfaces is not strong as it can be removed easily by simple sonication. Firm adhesion of the bacterial strains needs the elastic polymers of the EPS for effective non-specific interaction in aquatic ecosystem (Costerton 1999). The bacteria release protons and signalling molecules radially diffusing away from the cell. We observe a sharp increase in the concentration of the diffusing molecules finding itself near a surface or interface which would let the cell recognize that it is near the surface because diffusion became limited on that side. Thus, once the bacterial cell has sensed a surface, they start to form colonies in monolayer fashion. and active adhesion starts leading to biofilm formation. The cells aggregate to form microcolonies at a specific location. Now in order to make this reversible attachment to irreversible attachment, the bacteria should synthesize new exopolysaccharide to cement other bacterial cells in developing biofilm. In this process, the attached cells upregulate the genes required for EPS synthesis itself (Costerton 1999).
The basic structural unit of biofilm is the microcolony. It consists of different types of species. Depending on the species, microcolony (mushroom-like shape) may have composed of 10–20% cells and remaining 75–90% EPS matrix (Costerton 1999). Regarding biofilm formation potentials of pure species bacteria, it is interesting that the quantity of biofilms produced is not only different between the genera but also vary among the species of same genus (Maddela and Meng 2020); this could be attributed to multiple factors, such as metabolic properties, quorum sensing properties (Maddela et al. 2019), functional groups of exopolysaccharides (Maddela et al. 2018), etc. In general, cells attach to the surfaces which are rougher and hydrophobic in nature. The presence of fimbriae, flagella and EPS helps an organism when a mixed community is involved (Table 9.1).
9.2.2 Fungal Biofilm
The important fungal species which produce biofilms are Aspergillus, Candida, Cryptococcus, Trichosporon, Coccidioides and Pneumonia (Table 9.2). Factors involving the fungal biofilm resistivity are structural complexity, existence of extracellular matrix (ECM), metabolic heterogeneity intrinsic to biofilms and upregulation of efflux pump genes (Fanning and Mitchell 2012). The biofilms of C. albicans are composed of yeast form and hyphal cells which are important for biofilm formation. Steps involving biofilm formation are attachment to the substrate and multiplication of yeast cells on the surface followed by triggering of hyphal formation (Finkel and Mitchell 2011). As the biofilm matures, the cohesivity appears due to ECM aggregation (Al-Fattani and Douglas 2006). Other species of Candida like C. tropicalis, C. glabrata, though contain ECM, fail to produce true hyphae (Silva et al. 2011).
Biofilms of the cells of Aspergillus called conidia bind to the substrate and mycelia forms with biofilm maturation. Hyphae can be differently organized in two forms of A. fumigatus biofilm infection. For example, hyphae form into a intertwined ball in Aspergilloma and in aspergillosis show hyphae in separated form (Loussert et al. 2010). There are species which do not produce hyphae as part of their biofilm. Some of the species are Cryptococcus neoformans and Pneumocystis jirovecii (Cushion et al. 2009).
9.3 Biofilm-Related Pathogenesis
Back in the 1970s, Nils Høiby perceived the connection between the causes of relentless infection and the clusters of bacteria in cystic fibrosis patients (Høiby 2017). It was then noted that biofilms are involved in clinical infections (Costerton et al. 1999; Hall‐Stoodley and Stoodley 2009). Bacteria in biofilm mode of survival protect itself by staying in the dormant state from the immune system, thereby causing local tissue damage. In the later stages, it leads to acute infection (Table 9.3 gives an overview of the biofilm-related diseases in humans) (Vestby et al. 2020).
9.3.1 Native Valve Endocarditis
Native valve endocarditis is caused when the vascular endothelium of the four valves, namely, mitral, aortic, tricuspid and pulmonic valves, interacts with the microbes travelling in the blood stream. The species responsible for NVE are Pneumococci, Candida, Aspergillus and some Gram-negative bacteria. The route of infection of these organisms is in blood stream via the oropharynx, gastrointestinal tract and genitourinary tract. Since the microbes bind very poorly to the endothelium, nonbacterial thrombotic endocarditis (NBTE) is established when the endothelium is disrupted. This accumulates platelets, fibrin and red blood cells. Fibronectin is released by the endothelium cells with the result of vascular injury. This fibronectin can adhere to collagen, human cells and also the bacteria which leads to biofilm formation. Multiple medications are followed specific to the species involved like the administration of penicillin for streptococcal endocarditis and fluconazole for Candida endocarditis (Donlan and Costerton 2002; Stickler 1996.).
9.3.2 Otitis Media
This is a painful ear infection specific in the middle ear located behind the eardrum. The organisms causing otitis media are Streptococcus pneumoniae, Haemophilus influenzae, Moraxella catarrhalis, Staphylococcus epidermidis and Pseudomonas aeruginosa. Since only low amounts of antibiotics are penetrated in the middle ear, effective drugs are better used to treat otitis media like amoxicillin, cefaclor and erythromycin (Donlan and Costerton 2002; Wells et al. 1995).
9.3.3 Chronic Bacterial Prostatitis
The infection is seen in the prostate gland which moved from the urethra (Domingue and Hellstrom 1998). Once the bacteria occupy the prostate duct, proliferation occurs very rapidly forming spores of microcolonies and thus forming the biofilms in the dust system. Microbes infecting chronic bacterial prostatitis are E.coli, P. aeruginosa and species of Proteus, Serratia, etc. (Donlan and Costerton 2002).
9.3.4 Cystic Fibrosis
This is a type of lower respiratory tract infection. Cystic fibrosis is the absence of cystic fibrosis transmembrane conductance regulatory protein (CFTR). The thickening of the respiratory epithelium is due to the elevated absorption of electrolytes. Staphylococcus aureus, H. influenzae and P. aeruginosa are the microbes involved. The infection can be delayed for several years if treated in the early stages with ciprofloxacin and colistin (Donlan and Costerton 2002).
9.3.5 Periodontitis
This disease is the infection in teeth tissues, gums and periodontal tissues. Subgingival crevice is the first site for infection (Govan and Deretic 1996). The bacteria start colonizing the surfaces of the tooth and mucus and also regulate calcium flux releasing the toxins. Once the bacterial plaque becomes significant with minerals of calcium and phosphate ions (calculus or tartar), the defensive mechanism of the saliva can no longer support the enamel of the tooth and thus cause periodontal diseases (Overman 2000). The suggested treatment is through the elimination of biofilms from the subgingival areas along with addition of antimicrobial substances (Donlan and Costerton 2002).
9.4 Antibiofilm Strategies
With respect to the previous studies, there is an easy and effective way to eliminate premature biofilms with the use of antibiotic than the mature biofilms (Ranita Roy et al. 2018). Despite of it, undetectable nature of the premature biofilms in the body leads to development of clinical conditions which involves most of the mature biofilms (Hoiby et al. 2011; Cramton et al. 1999; Götz 2002; McKenney et al. 1998). Implementation of combinational therapy is more appreciable than the antibiotic monotherapy (Aaron et al. 2002). The biofilm-grown bacteria are more resistant to planktonic bacteria. The other strategy to eliminate biofilm is by finding antifouling or antimicrobial surfaces (Brandl et al. 2008). Nanoparticles made of silver and many other metal nanoparticles display antimicrobial properties which seem to be an effective approach to remove the biofilms (Hoyle and Costerton 1991; Moreau-Marquis et al. 2008; Prasad et al. 2020). The antibiofilm molecules block the signalling pathways in almost all types of bacteria; these molecules can be an enzyme, a peptise, an antibiotic, polyphenols, etc. (Parsek and Singh 2003). Membrane technology has been developed for wastewater treatment. But the formation of biofilm on the membrane is deteriorating the life of the membrane and reducing the water flux. This is the critical problem in the membrane technology development (Ding et al. 2019; Xu and Liu 2011).
The metabolic uncouplers are introduced to disrupt oxidative phosphorylation suppressing the microbial attachment and also reducing the extracellular polymeric substance (EPS) secretions (Chen et al. 2002; Jiang and Liu 2012). The study aimed at inducing the uncoupler, 3,3′,4′,5′ tetrachlorosalicylanilide (TCS), to reduce the EPS and aerobic granulation formation. The optimal level of TCS concentration effectively inhibits the cell binding to the membrane. Bacterial motility is also an important factor in determining the initial cell attachment (O’Toole and Kolter 1998). When the concentration of TCS was increased to 100 μg/L, it showed that the reduced motility ultimately resulted in decreased cell attachment (Feng et al. 2020). The effective way to target the destruction of biofilm is quorum sensing (Sambanthamoorthy et al. 2014; Kareem et al. 2017; Yu et al. 2018). Cells communicate through different signalling molecules but the way by which the expression of virulence genes is controlled by quorum sensing. The molecules or the compounds which interrupt these communications are called quorum quenchers. These quenchers suppress the virulence gene expression which make the proteases, siderophores, toxic compounds and biofilm formation (Antunes et al. 2010; Ali et al. 2020).
Virstatin was employed which cuts the pili binding by Acinetobacter baumannii in order to avoid biofilm production (Chabane et al. 2014). Nanoparticles always showed a better path to inhibit the microbes and so put forth (Ansari et al. 2014). It has been experimented that silver nanoparticles inhibited the growth and occupancy of E. coli and Klebsiella pneumoniae and also eliminated the exopolysaccharides formation. An inhibitor of multidrug resistant of A. baumannii biofilm, 5-episinnuleptolide, attenuated the genes expressing the EPS-producing enzymes which completely vanished in exposure to these compounds (Tseng et al. 2016). Sometimes, the binding of bacteria to the surfaces also depends on the physical properties. Therefore, an effective approach to inhibit the biofilm formation is by changing the surface of several nanostructures of multiwalled carbon nanotubes (Malek et al. 2016).
Bacterial biofilms are a huge threat to the humankind when discussing its growth in water distribution pipelines. These pipes are mostly made of iron stainless steel and galvanized steel or copper-based materials, so biofilms growing on these metals corrode the equipments used in the industry and thus deteriorate the quality of water leading to infectious diseases. The bacteria multiplied in number on stainless steel and titanium. The growth is decreased on copper and nickel substrates as a result of oxidative stress and protein dysfunction. The growth of bacteria in the initial stages is much low on copper, Cu and nickel and Ni substrates when compared to stainless steel (SS) and titanium (Ti); it may be because of varied interactions of microbes with various materials. For example, observe a green coloured material on the copper surface; the possibility is that the surface gave copper ions by getting oxidized. This ceases cellular protein or enzyme activity resulting in the prevention of bacterial attachment to Cu substrate (Santo et al. 2011). In his actual work, the bacterial growth restored after a period of incubation; this occurred as the cells experienced extreme membrane damage, though the DNA is not injured as it is protected by the periplasm (Grosse et al. 2014). Studies showed that the E.coli was chiefly destroyed through membrane damage, and also they are wide open to toxic portion of cu (II) which upregulates the genes responsible for ROS (reactive oxygen species) elimination (Wang et al. 2020).
In the recent years, “green antimicrobials” derived from green medicinal plants have been a promising replacements to the conventional ones to eradicate biofilms. These include essential oils which are of high essence due to its cheaper cost, biocompatibility and the ability to fight the bacteria without impelling drug resistance. To critical mechanism of its bactericidal effects is that they can separate the lipid layer from the cell membrane, which increases the permeability of the membrane troubling the cell structures (Wang et al. 2019). This is due to the hydrophobic nature leading them to indissoluble and unsteady in aqueous media limiting its application in therapeutics. Studies have displayed that enveloping essential oils into a surface-active colloidal transport channel enhances their stability in aqueous medium and also the antibiofilm activity in the food and beverages (Arfat et al. 2014; Chen and Zhong 2015; Landis et al. 2017). Therefore, according to Zhaojie Wang et al. (2018), prepared regulatable thymol (essential oil made of oxygenated compound, phenol) contains chitosan micelles for treating bacterial biofilms. Here, the chitosan is a well-known polycationic polysaccharide made of dispersed structures of beta-(1-4)-linked D-glucosamine and N=acetyl-D-glucosamine with the best biocompatibility and antimicrobial properties. The micelles were produced through spontaneous assembly by amphipathic copolymer comprising of toluidine blue O (TBO)-implanted chitosan (CHI-TBO) and poly(propylene sulphide) (PPS). And now the chitosan, external region of the micelle, easily sticks to the oppositely charged that are the negatively charged biofilms. The ROS (reactive oxygen species) generator is the TBO which is associated with chitosan acting as a photosensitizer destroying various bacteria. This ROS can alter the hydrophobic sulphide to invariable oxidized hydrophilic sulfoxide which is much needed to kill the bacteria. So, ROS is generated from the TBO from thymol-loaded TBO-CHI-PPS micelles (T-TCP) with a simultaneous release of thymol from it (Wang et al. 2018).
9.5 Nanoscience
Nowadays, the nanoparticles are widely used in the fields of biomedical and physiology. Nanoparticle, the name itself, suggests it to be a tiny particle of nanoscale having the size of 1–100 nm. These nanoparticles also have a specific wavelength which is less than that of light. This property allows them to deploy in cosmetics, packaging and coatings. The physical, optical properties, etc. of nanoparticles make them play a unique role in the daily life when compared to the bulk materials. Sometimes nanoparticles of the desired shape and size can be obtained by controlling the parameters like salt concentration, pH value, temperature, aeration, etc. The most common shapes produced are spherical, triangular and hexagonal. Usually, nanoparticles work best when the size is less than the critical value, i.e. 10–20 nm (Singh et al. 2016). Metal nanoparticles are purely made of the metal precursors. Due to well-known localized surface plasmon resonance (LSPR) characteristics, these nanoparticles possess unique optoelectrical properties. The alkali and noble metal nanoparticles whose absorption band is in the visible region of the electromagnetic solar spectrum are Cu, Ag and Au. The facet-, size- and shape-controlled synthesis of metal nanoparticles is important in present-day cutting-edge materials (Dreaden et al. 2012). Metal nanoparticles with the advanced optical properties find applications in many research fields.
9.5.1 Classification of Silver Nanoparticles
Classification of nanoparticles is based on their morphology, size and chemical properties. Some of the well-known classes of NPs are listed below.
9.5.1.1 Carbon-Based NPs
Fullerenes and carbon nanotubes (CNTs) represent two major classes of carbon-based NPs. Fullerenes contain nanomaterial that are made of globular hollow cage such as allotropic forms of carbon. They have created noteworthy commercial interest due to their electrical conductivity, high strength, structure, electron affinity and versatility (Aliana Astefanei 2015). These materials possess arranged pentagonal and hexagonal carbon units, while each carbon is sp2 hybridized, shows some of the well-known fullerenes consisting of C60 and C70 with the diameter of 7.114 and 7.648 nm, respectively. CNTs are elongated, tubular structures, 1–2 nm in diameter (El-Sherbiny et al. 2013). These are structurally resembling to graphite sheet rolling upon itself. The rolled sheets can be single, double or multiwalled, and therefore they are named as single-walled (SWNTs), double-walled (DWNTs) or multiwalled carbon nanotubes (MWNTs), respectively. Deposition of carbon precursors especially the atomic carbons, vaporized from graphite by laser or by electric arc onto metal particles, is widely required for their synthesis. Lately, they have been synthesized via chemical vapour deposition (CVD) technique (Elliott et al. 2013). Due to their unique physical, chemical and mechanical characteristics, these materials are not only used in pristine form but also in nanocomposites for many commercial applications such as fillers (Saeed and Khan 2014, 2016), as support medium for different inorganic and organic catalysts (Mabena et al. 2011), and for environmental remediation, these can be used as efficient gas adsorbents (Ngoy et al. 2014).
9.5.1.2 Metal NPs
Metal NPs are purely made of metal precursors. Due to well-known localized surface plasmon resonance (LSPR) characteristics, these NPs possess unique optoelectrical properties. NPs of the alkali and noble metals, i.e. the broad absorption band of Cu, Ag and Au, lie in the visible zone of the electromagnetic solar spectrum.
The facet-, size- and shape-controlled synthesis of metal NPs is important in present-day cutting-edge materials (Dreaden et al. 2012). Due to their advanced optical properties, metal NPs find applications in many research areas. Gold NP coating is widely used for the sampling of SEM, to enhance the electronic stream, which helps in obtaining high-quality SEM images.
9.5.1.3 Ceramic NPs
Ceramic NPs are inorganic nonmetallic solids, synthesized via heat and successive cooling. They can exist in the form of amorphous, polycrystalline, dense, porous or hollow structures (Sigmund et al. 2006). With their use in applications such as catalysis, photocatalysis, photodegradation of dyes and imaging applications, nanoparticles are getting great attention of researchers (Thomas et al. 2015).
9.5.1.4 Semiconductor NPs
Semiconductor NPs possess characteristics of metals and nonmetals and therefore found various applications in the literature due to this property (Ali et al. 2017; Khan et al. 2017).
Semiconductor NPs possess wide band gaps and therefore showed significant alteration in their properties with band gap tuning. Therefore, they play a very important role in photocatalysis, photo-optics and electronic devices (Sun et al. 2000). Because of their suitable band gap and band edge positions, a variety of semiconductor NPs are found exceptionally efficient in water-splitting applications (Hisatomi et al. 2014).
9.5.1.5 Polymeric NPs (PNPs)
Polymeric nanoparticles are generally organic based and are mostly found in nanospheres or nanocapsular shaped (Mansha et al. 2017; Prasad et al. 2017). The overall mass of the matrix particles is generally solid, and the other molecules attach to the outer boundary of the spherical surface using the phenomenon called adsorption. In the following case, the solid mass is completely encapsulated within the particle (Rao and Geckeler 2011). The PNPs can readily functionalize and thus find bundles of applications in the literature.
9.5.1.6 Lipid-Based NPs
The lipid nanoparticles contain lipid moieties and have effective applications in biomedicine. Generally, a lipid NP is characteristically spherical with diameter ranging from 10 to 1000 nm. In a similar way to polymeric NPs, lipid NPs also possess a solid core built of lipid and a matrix containing soluble lipophilic molecules. Surfactants or emulsifiers stabilized the external core of these NPs (Rawat et al. 2011). Lipid nanotechnology (Mashaghi et al. 2013) is a special field, focusing on the designing and synthesis of lipid NPs for various applications in drug delivery and as drug carriers (Puri et al. 2009) and RNA release in cancer therapy.
9.5.2 Synthesis of Nanoparticles
Nanoparticles can be synthesized in two following approaches: (1) top-down approach and (2) bottom-up approach. In simple words, the way in which smaller particles assemble into complex particles operated either by physical or chemical forces is called bottom-up approach, while the process in which bulk material is turned into simply smaller particles preferably nano-scaled materials is called top-down approach. Nanoparticles can be synthesized using three different methods. They are:
(1) Physical method: nanoparticles are synthesized by physical means such as by mechanical means and vaporization.
(2) Chemical method: nanoparticles are synthesized using chemicals via sol-gel process, aerosol process, etc. This method is the widely used and accepted method.
(3) Biological method: other name for this method is the green synthesis because it makes use of plant extracts or parts of the leaves, root, flower and fruits and biological species like fungi, yeast, algae and bacteria (Prasad 2014, 2016, 2017; Prasad et al. 2016, 2018a,b; Thangadurai et al. 2020; Srivastava et al. 2021).
There are multiple ways by which nanoparticles exhibit antimicrobial activity; they are (a) interacting directly with the bacterial cell wall, (b) prevention of biofilm formation, (c) evoking the natural and acquired immune responses, (d) production of reactive oxygen species (ROS) and (e) triggering of intracellular effects (Fig. 9.2) (Prasad and Swamy 2013; Joshi et al. 2018; Inamuddin et al. 2021). Since they do not follow similar mechanism of action of standard antibiotic drugs, it can be widely used against resistant bacteria (Singh et al. 2014; Aderibigbe 2017; AlMatar et al. 2017; Hemeg 2017; Natan and Banin 2017; Rai et al. 2017; Slavin et al. 2017; Zaidi et al. 2017; Bassegoda et al. 2018; Katva et al. 2018; Siddiqi et al. 2018). Gómez-Gómez et al. (2020) used metalloid based NP like tellurium nanoparticles (TeNPs) to investigate its effect on Staphylococcus aureus and Escherichia coli. These nanoparticles inhibited biofilm formation with reducing nearly 90% of biofilm volume; another exciting findings revealed structure change from sphere to rod-shaped as a consequence of the nanoparticle-biofilm interaction. While the increasing, other co-polymers (for instance polylactic-co-glycolic acid, PLGA) other polymer has emerged to be used in the food and drug administration; PLGA shows significant uses like better compatibility, good stability while preparation (Danhier et al. 2012; Sharma et al. 2016; Swider et al. 2018). So, enclosure of antibiotics into these PLGA nanoparticles helps in safe transport and release at the infection site which in general get degraded by enzymes (Huang et al. 2020). But also the relative low drug packing capability and premature or initial burst release limited the use of PLGA-based nanomaterials in in vivo studies. Therefore, quantum dots appeared as an efficient way to combine with polymer-based nanoparticles for added advantage (Huang et al. 2020).
Different mechanisms of action of NPs in bacterial cells. The combination in a single nanomaterial of a multitude of cellular effects may have a tremendous in fighting MDR bacteria. DNA, deoxyribonucleic acid; ROS, reactive oxygen species; AuNPs, gold NPs; CuONPs, copper oxide NPs; Ag NPs, silver NPs; Fe3O4 NPs, iron oxide NPs; ZnONPs, zinc oxide NPs. (Adapted from Pedro Bapista et al. 2018)
Carbon quantum dots (CQDs) showed an outstanding drug packing capacity because it possesses a large surface area and stable π-π stacking, water repelling and electrostatic interactions or physisorption (Liu et al. 2012; Wang et al. 2017b). The method used for incorporating the CDQs into the PLGA nanoparticles is microvotex-based microfluid which accurately controls the CQD-PLGA hybrid nanoparticle formation with a fine antimicrobial efficacy to fight with P. aeruginosa biofilms (Huang et al. 2020). The enclosure efficacy and the packing capacity of the CQDs can be changed by altering the mass ratio of PLGA to CQDs which helps us facilitate a sufficient space for photothermal effect optimization and reducing the toxic effects triggered by the CQDs. The photothermal effect of both CQDs with and without the PLGA nanoparticles was studied by inducing laser of 808 nm with different power densities. The consequences are the increase in temperature from 37 degrees Celsius to 43 degree Celsius which is seen only in the PLGA with CQDs (Huang et al. 2020). Another interesting finding was that the CQD-encapsulated PLGA nanoparticles are able to transform NIR light into thermal energy which lead us to enhance the photothermal effects. In this study, they used azithromycin (AZI) as an antibiotic and loaded into CQD-PLGA hybrid nanoparticles which eliminated more bacteria of P. aeruginosa than the ones with the only AZI. This may be due to increased concentration of antibiotic at the reach of the biofilm site (Huang et al. 2020).
9.6 Knowledge Gaps and Future Directions
The purpose of using nanoparticles in preference to antibiotics is because nanoparticles can inhibit microbial drug resistance effectively in specific cases (Wang et al. 2017a). The highly preventable measures of biofilms are accomplished by smaller size with high surface-area-to-mass ratio. The shape of the nanoparticle also has a noticeable effect on biofilm elimination, for example, rod-shaped nanoparticles show a high impact over the spherical-shaped nanoparticles (Slomberg et al. 2013). With high available research studies, developing the effects of nanoparticles is the starting step for any researcher to try his best (Wang et al. 2017a). In spite of it, a research study reported that there was a spread of multiple drug resistance (MDR) not just in the same species of bacteria but also across the genera when it was the positive hope in promoting conjugative transfer of RP4, PK2 and PCF10 plasmids by aluminium nanoparticles (Qiu et al. 2012). The underlying factors may be the range of damage caused to the cell membranes by the aluminium NPs, the amounts of aluminium NPs and the breeding cells, parameters like temperature and pH and selective expression of particular genes (trfAp, trfA and trbB) which is crucial in transferring and replicating RP4 plasmids. The negative effects are also considered to avoid the MDR which may lead to health hazards.
There are limitations regarding the use of NPs in inhibiting the biofilms. As there are various bacterial strains, with different action times, it’s difficult to examine the comparative studies of the antibacterial mechanisms. The complexity of the cell membrane structures can be seen as a critical drawback in in vitro studies. Size can become a limitation in transporting all NPs into the bacterial porins which is generally <600 Da. Further research focusing on the intracellular inhibitory mechanisms is left unattended. Huge attention is made towards the NPs which induced oxidative stress, synthesis of proteins and metabolism of bacterial cells. In the view of increasing resistance of the biofilms, nanoparticles are considered to having the greater potential to resolve with low toxic effects (Wang et al. 2017a). However, key factors like NP resistance and surface associations between NP biofilms and hosts need to be sorted out to guarantee fortunate clinical applications (Ramasamy and Lee 2016).
9.7 Conclusions
As of the resistance posed by the biofilms against the conventional antibiotics, there evolved multiple pathogenesis in humans which lead to chronic infections over decades. It has been evident that the therapeutic use of nanoparticles had tremendous antibiofilm effects as far studied. The influence of nanoparticles on bacterial cell membrane permeability, generation of reactive oxygen species and cellular metabolism and reproduction are of high priority. The bacterial attachment and EPS secretions can be reduced by using metabolic uncouplers like 3,3′,4′,5′ tetrachlorosalicylanilide (TCS). Among numerous types of nano-based materials used and studied in antibiofilm strategy, using silver nanoparticles is one of the best possible way in eliminating the microbes. Quorum quenchers can be used to suppress the expression of virulence genes like proteases, siderophore, etc. which is a way to block quorum sensing characteristic. Other compounds like virstatin and 5-episinnuleptolide are also found to be the key inhibitors of some of the bacterial species.
References
Aaron SD, Ferris W, Ramotar K, Vandemheen K, Chan F, Saginur R (2002) Single and combination antibiotic susceptibilities of planktonic, adherent, and biofilm-grown Pseudomonas aeruginosa isolates cultured from sputa of adults with cystic fibrosis. J Clin Microbiol 40:4172–4179
Aderibigbe BA (2017) Metal-based nanoparticles for the treatment of infectious diseases. Molecules 22(8):1370
Al-Fattani MA, Douglas LJ (2006) Biofilm matrix of Candida albicans and Candida tropicalis: chemical composition and role in drug resistance. J Med Microbiol 55:999–1008
Ali S, Khan I, Khan SA, Sohail M, Ahmed R, Atteq UR, Ansari MS, Morsy MA (2017) Electrocatalytic performance of Ni@ Pt core–shell nanoparticles supported on carbon nanotubes for methanol oxidation reaction. J Electroanal Chem 795:17–25
Ali SG, Ansari MA, Alzohairy MA, Alomary MN, Jalal M, AlYahya S, Asiri SMM, Khan HM (2020) Effect of biosynthesized ZnO nanoparticles on multi-drug resistant Pseudomonas aeruginosa. Antibiotics 9(5):260
Aliana Astefanei (2015) Analysis and characterization of fullerene nanoparticles. University of Barcelona, julio de
AlMatar M, Makky EA, Var I, Koksal F (2017) The role of nanoparticles in the inhibition of multidrug-resistant bacteria and biofilms. Curr Drug Deliv 15:470–484
Ansari MA, Khan HM, Khan AA, Cameotra SS, Pal R (2014) Antibiofilm efficacy of silver nanoparticles against biofilm of extended spectrum β-lactamase isolates of Escherichia coli and Klebsiella pneumoniae. Appl Nanosci 4:859–868
Antunes LCM, Ferreira RB, Buckner MM, Finlay BB (2010) Quorum sensing in bacterial virulence. Microbiology 156(8):2271–2282
Arfat YA, Benjakul S, Prodpran T, Sumpavapol P, Songtipya P (2014) Properties and antimicrobial activity of fish protein isolate/fish skin gelatin film containing basil leaf essential oil and zinc oxide nanoparticles. Food Hydrocoll 41:265–273
Bapista PV et al (2018) Nano-strategies to fight multidrug resistant bacteria- “A Battle of the titans”. Front Microbiol 9:1441
Baptista PV, McCusker MP, Carvalho A, Ferreira DA, Mohan NM, Martins M, Fernandes AR (2018) Nano-strategies to fight multidrug resistant bacteria—“A Battle of the Titans”. Front Microbiol 9:1441
Bassegoda A, Ivanova K, Ramon E, Tzanov T (2018) Strategies to prevent the occurrence of resistance against antibiotics by using advanced materials. Appl Microbiol Biotechnol 102:2075–2089
Brandl K, Plitas G, Mihu CN, Ubeda C, Jia T, Fleisher M, Schnabl B, DeMatteo RP, Pamer EG (2008) Vancomycin-resistant enterococci exploit antibiotic-induced innate immune deficits. Nature 455:804–807
Chabane YN, Mlouka MB, Alexandre S, Nicol M, Marti S, Pestel-Caron M et al (2014) Virstatin inhibits biofilm formation and motility of Acinetobacter baumannii. BMC Microbiol 14:62
Chen H, Zhong Q (2015) A novel method of preparing stable zein nanoparticle dispersions for encapsulation of peppermint oil. Food Hydrocoll 43:593–602
Chen G-H et al (2002) Utilization of a metabolic uncoupler, 3,3′,4′,5-tetrachlorosalicylanilide (TCS) to reduce the sludge growth in activated sludge cultures. Water Res 36:2077–2083
Costerton JW (1999) Introduction to biofilm. Int J Antimicrob Agents 11(3–4):217–221
Costerton JW, Stewart PS, Greenberg EP (1999) Bacterial biofilms: a common cause of persistent infections. Science 284:1318–1322
Cramton SE, Gerke C, Schnell NF, Nichols WW, Götz F (1999) The intercellular adhesion (ica) locus is present in Staphylococcus aureus and is required for biofilm formation. Infect Immun 67(10):5427–5433
Cushion MT, Collins MS, Linke MJ (2009) Biofilm formation by Pneumocystis spp. Eukaryot Cell 8(2):197–206
Danhier F, Ansorena E, Silva JM, Coco R, Le Breton A, Préat V (2012) PLGA-based nanoparticles: an overview of biomedical applications. J Control Release 161(2):505–522
Ding A, Lin D, Zhao Y, Ngo HH, Guo W, Bai L, Liang H (2019) Effect of metabolic uncoupler, 2, 4-dinitrophenol (DNP) on sludge properties and fouling potential in ultrafiltration membrane process. Sci Total Environ 650:1882–1888
Domingue GJ, Hellstrom WJ (1998) Prostatitis. Clin Microbiol Rev 11(4):604–613
Donlan RM (2002) Biofilms: microbial life on surfaces. Emerg Infect Dis 8(9):881
Donlan RM, Costerton JW (2002) Biofilms: survival mechanisms of clinically relevant microorganisms. Clin Microbiol Rev 15(2):167–193
Dreaden EC, Alkilany AM, Huang X, Murphy CJ, El-Sayed MA (2012) The golden age: gold nanoparticles for biomedicine. Chem Soc Rev 41(7):2740–2779
Elliott R, Watson J, Greenberg LS, Timulak L, Freire E (2013) Research on humanistic-experiential psychotherapies. In: Lambert MJ (ed) Bergin & Garfield‘s handbook of psychotherapy and behavior change, 6th edn. Wiley, New York, pp 495–538
El-Sherbiny IM, Salih E, Reicha FM (2013) Green synthesis of densely dispersed and stable silver nanoparticles using myrrh extract and evaluation of their antibacterial activity. J Nanostructure Chem 3(1):8
Fanning S, Mitchell AP (2012) Fungal biofilms. PLoS Pathog 8(4):e1002585
Feng X, Wu Q, Che L, Ren N (2020) Analyzing the inhibitory effect of metabolic uncoupler on bacterial initial attachment and biofilm development and the underlying mechanism. Environ Res 185:109390
Finkel JS, Mitchell AP (2011) Genetic control of Candida albicans biofilm development. Nat Rev Microbiol 9:109–118
Gómez-Gómez B, Sanz-Landaluce J, Pérez-Corona MT, Madrid Y (2020) Fate and effect of in-house synthesized tellurium based nanoparticles on bacterial biofilm biomass and architecture. Challenges for nanoparticles characterization in living systems. Sci Total Environ 719:137501
Götz F (2002) Staphylococcus and biofilms. Mol Microbiol 43(6):1367–1378
Govan JR, Deretic V (1996) Microbial pathogenesis in cystic fibrosis: mucoid Pseudomonas aeruginosa and Burkholderia cepacia. Microbiol Rev 60(3):539–574
Grosse C, Schleuder G, Schmole C, Nies DH (2014) Survival of Escherichia coli cells on solid copper surfaces is increased by glutathione. Appl Environ Microbiol 80(22):7071–7078
Hall‐Stoodley L, Stoodley P (2009) Evolving concepts in biofilm infections. Cell microbiol 11(7):1034–1043
Hemeg HA (2017) Nanomaterials for alternative antibacterial therapy. Int J Nanomedicine 12:8211–8225
Hisatomi T, Kubota J, Domen K (2014) Recent advances in semiconductors for photocatalytic and photoelectrochemical water splitting. Chem Soc Rev 43(22):7520–7535
Hoffman LR, D’Argenio DA, MacCoss MJ, Zhang Z, Jones RA, Miller SI (2005) Aminoglycoside antibiotics induce bacterial biofilm formation. Nature 436(7054):1171–1175
Høiby N (2017) A short history of microbial biofilms and biofilm infections. APMIS 125(4):272–275
Hoiby N, Ciofu O, Johansen HK, Song ZJ, Moser C, Jensen PØ, Bjarnsholt T (2011) The clinical impact of bacterial biofilms. Int J Oral Sci 3(2):55–65
Hoyle BD, Costerton JW (1991) Bacterial resistance to antibiotics: the role of biofilms. Prog Drug Res/Fortschritte der Arzneimittelforschung/Progrès des recherches pharmaceutiques 37:91–105
Huang Z, Zhou T, Yuan Y, Kłodzińska SN, Zheng T, Sternberg C, Wan F (2020) Synthesis of carbon quantum dot-poly lactic-co-glycolic acid hybrid nanoparticles for chemo-photothermal therapy against bacterial biofilms. J Colloid Interface Sci 577:66–74
Inamuddin, Ahamed MI, Prasad R (2021) Advanced antimicrobial materials and applications. Springer, Singapore. ISBN: 978-981-15-7098-8. https://www.springer.com/gp/book/9789811570971
Jiang B, Liu Y (2012) Roles of ATP-dependent N-acylhomoserine lactones (AHLs) and extracellular polymeric substances (EPSs) in aerobic granulation. Chemosphere 88:1058–1064
Joshi N, Jain N, Pathak A, Singh J, Prasad R, Upadhyaya CP (2018) Biosynthesis of silver nanoparticles using Carissa carandas berries and its potential antibacterial activities. J Sol-Gel Sci Techn 86(3):682–689. https://doi.org/10.1007/s10971-018-4666-2
Karatan E, Watnick P (2009) Signals, regulatory networks, and materials that build and break bacterial biofilms. Microbiol Mol Biol Rev 73(2):310–347
Kareem SM, Al-Kadmy IM, Al-Kaabi MH, Aziz SN, Ahmad M (2017) Acinetobacter baumannii virulence is enhanced by the combined presence of virulence factors genes phospholipase C (plcN) and elastase (lasB). Microb Pathog 110:568–572
Katva S, Das S, Moti HS, Jyoti A, Kaushik S (2018) Antibacterial synergy of silver nanoparticles with gentamicin and chloramphenicol against Enterococcus faecalis. Pharmacogn Mag 13:S828–S833
Khan I et al (2017) Synthesis of hierarchical WO3 and Bi2O3/WO3 nanocomposites for solar-driven water splitting applications. Int J Hydrog Energy 42:3431–3439
Kokare CR, Kadam SS, Mahadik KR, Chopade BA (2007) Studies on bioemulsifier production from marine Streptomyces sp. S1. IJBT 6:78–84
Landis RF, Gupta A, Lee YW, Wang LS, Golba B, Couillaud B, Rotello VM (2017) Cross-linked polymer-stabilized nanocomposites for the treatment of bacterial biofilms. ACS Nano 11(1):946–952
Lewis K (2001) Riddle of biofilm resistance. Antimicrob Agents Chemother 45(4):999–1007
Liu C, Zhang P, Zhai X, Tian F, Li W, Yang J, Liu W (2012) Nano-carrier for gene delivery and bioimaging based on carbon dots with PEI-passivation enhanced fluorescence. Biomaterials 33(13):3604–3613
Loussert C, Schmitt C, Prevost MC, Balloy V, Fadel E et al (2010) In vivo biofilm composition of Aspergillus fumigatus. Cell Microbiol 12:405–410
Mabena LF et al (2011) Nitrogen-doped carbon nanotubes as a metal catalyst support. Appl Nanosci 1(2):67–77
Maddela NR, Meng F (2020) Discrepant roles of a quorum quenching bacterium (Rhodococcus sp. BH4) in growing dual-species biofilms. Sci Total Environ 713:136402
Maddela NR, Zhou Z, Yu Z, Zhao S, Meng F (2018) Functional determinants of extracellular polymeric substances in membrane biofouling: experimental evidence from pure-cultured sludge bacteria. Appl Environ Microbiol 84(15). https://doi.org/10.1128/AEM.00756-18
Maddela NR, Sheng B, Yuan S, Zhou Z, Villamar-Torres R, Meng F (2019) Roles of quorum sensing in biological wastewater treatment: a critical review. Chemosphere 221:616–629
Malek L et al (2016) Vertically aligned multi walled carbon nanotubes prevent biofilm formation of medically relevant bacteria. J Mater Chem B 4:5228–5235
Mansha M, Khan I, Ullah N, Qurashi A (2017) Synthesis, characterization and visible-light-driven photoelectrochemical hydrogen evolution reaction of carbazole-containing conjugated polymers. Int J Hydrog Energy 42(16):10952–10961
Mashaghi S, Jadidi T, Koenderink G, Mashaghi A (2013) Lipid nanotechnology. Int J Mol Sci 14(2):4242–4282
McKenney D, Hübner J, Muller E, Wang Y, Goldmann DA, Pier GB (1998) The ica locus of Staphylococcus epidermidis encodes production of the capsular polysaccharide/adhesin. Infect Immun 66(10):4711–4720
Moreau-Marquis S, Stanton BA, O’Toole GA (2008) Pseudomonas aeruginosa biofilm formation in the cystic fibrosis airway. Pulm Pharmacol Ther 21:595–599
Natan M, Banin E (2017) From nano to micro: using nanotechnology to combat microorganisms and their multidrug resistance. FEMS Microbiol Rev 41:302–322
Ngoy JM et al (2014) A CO2 Capture technology using multi-walled carbon nanotubes with polyaspartamide surfactant. Energy Procedia 63:2230–2248
O’Toole GA, Kolter R (1998) Flagellar and twitching motility are necessary for Pseudomonas aeruginosa biofilm development. Mol Microbiol 30(2):295–304
Overman PR (2000) Biofilm: a new view of plaque. J Contemp Dent Pract 1(3):18–29
Parsek MR, Singh PK (2003) Bacterial biofilms: an emerging link to disease pathogenesis. Annu Rev Microbiol 57:677–701
Prasad R (2014) Synthesis of silver nanoparticles in photosynthetic plants. J Nanoparticles, Article ID 963961. https://doi.org/10.1155/2014/963961
Prasad R (2016) Advances and applications through fungal nanobiotechnology. Springer International Publishing, Cham. ISBN 978-3-319-42989-2
Prasad R (2017) Fungal nanotechnology: applications in agriculture, industry, and medicine. Springer Nature Singapore Pte Ltd., Singapore. ISBN 978-3-319-68423-9
Prasad R, Swamy VS (2013) Antibacterial activity of silver nanoparticles synthesized by bark extract of Syzygium cumini. J Nanoparticles. https://doi.org/10.1155/2013/431218
Prasad R, Pandey R, Barman I (2016) Engineering tailored nanoparticles with microbes: quo vadis. WIREs Nanomed Nanobiotechnol 8:316–330. https://doi.org/10.1002/wnan.1363
Prasad R, Pandey R, Varma A, Barman I (2017) Polymer based nanoparticles for drug delivery systems and cancer therapeutics. In: Kharkwal H, Janaswamy S (eds) Natural polymers for drug delivery. CAB International, London, pp 53–70
Prasad R, Jha A, Prasad K (2018a) Exploring the realms of nature for nanosynthesis. Springer International Publishing, Cham. ISBN 978-3-319-99570-0. https://www.springer.com/978-3-319-99570-0
Prasad R, Kumar V, Kumar M, Wang S (2018b) Fungal nanobionics: principles and applications. Springer Nature Singapore Pte Ltd., Singapore. ISBN 978-981-10-8666-3. https://www.springer.com/gb/book/9789811086656
Prasad R, Siddhardha B, Dyavaiah M (2020) Nanostructures for antimicrobial and antibiofilm applications. Springer International Publishing, Cham. ISBN 978-3-030-40336-2. https://www.springer.com/gp/book/9783030403362
Puri A et al (2009) Lipid-based nanoparticles as pharmaceutical drug carriers: from concepts to clinic. Crit Rev Ther Drug Carrier Syst 26:523–580
Qiu Z, Yu Y, Chen Z, Jin M, Yang D, Zhao Z, Huang A (2012) Nanoalumina promotes the horizontal transfer of multiresistance genes mediated by plasmids across genera. Proc Natl Acad Sci 109(13):4944–4949
Rai M, Ingle AP, Pandit R, Paralikar P, Gupta I, Chaud MV et al (2017) Broadening the spectrum of small-molecule antibacterials by metallic nanoparticles to overcome microbial resistance. Int J Pharm 532:139–148
Ramasamy M, Lee J (2016) Recent nanotechnology approaches for prevention and treatment of biofilm-associated infections on medical devices. Biomed Res Int 2016:1851242
Rao JP, Geckeler KE (2011) Polymer nanoparticles: preparation techniques and size-control parameters. Prog Polym Sci 36:887–913
Rawat MK et al (2011) Studies on binary lipid matrix based solid lipid nanoparticles of repaglinide: in vitro and in vivo evaluation. J Pharm Sci 100:2366–2378
Roy R, Tiwari M, Donelli G, Tiwari V (2018) Strategies for combating bacterial biofilms: a focus on anti-biofilm agents and their mechanisms of action. Virulence 9(1):522–554
Saeed K, Khan I (2014) Preparation and properties of single-walled carbon nanotubes/poly(butylene terephthalate) nanocomposites. Iran Polym J 23:53–58
Saeed K, Khan I (2016) Preparation and characterization of single-walled carbon nanotube/nylon 6,6 nanocomposites. Instrum Sci Technol 44:435–444
Sambanthamoorthy K, Luo C, Pattabiraman N, Feng X, Koestler B, Waters CM, Palys TJ (2014) Identification of small molecules inhibiting diguanylate cyclases to control bacterial biofilm development. Biofouling 30(1):17–28
Santo CE, Lam EW, Elowsky CG, Quaranta D, Domaille DW, Chang CJ, Grass G (2011) Bacterial killing by dry metallic copper surfaces. Appl Environ Microbiol 77(3):794–802
Sharma S, Parmar A, Kori S, Sandhir R (2016) PLGA-based nanoparticles: a new paradigm in biomedical applications. TrAC Trends Anal Chem 80:30–40
Siddiqi KS, Husen A, Rao RA (2018) A review on biosynthesis of silver nanoparticles and their biocidal properties. J Nanobiotechnol 16(1):14
Sigmund W et al (2006) Processing and structure relationships in electrospinning of ceramic fiber systems. J Am Ceram Soc 89:395–407
Silva S, Negri M, Henriques M, Oliveira R, Williams DW et al (2011) Adherence and biofilm formation of non-Candida albicans Candida species. Trends Microbiol 19:241–247
Singh K, Panghal M, Kadyan S, Chaudhary U, Yadav JP (2014) Green silver nanoparticles of Phyllanthus amarus: as an antibacterial agent against multi drug resistant clinical isolates of Pseudomonas aeruginosa. J Nanobiotechnology 12:40
Singh P, Kim YJ, Zhang D, Yang DC (2016) Biological synthesis of nanoparticles from plants and microorganisms. Trends Biotechnol 34(7):588–599
Slavin YN, Asnis J, Hafeli UO, Bach H (2017) Metal nanoparticles: understanding the mechanisms behind antibacterial activity. J Nanobiotechnology 15:65
Slomberg DL, Lu Y, Broadnax AD, Hunter RA, Carpenter AW, Schoenfisch MH (2013) Role of size and shape on biofilm eradication for nitric-oxide-releasing silica nanoparticles. ACS Appl Mater Interfaces 5(19):9322–9329
Srivastava S, Usmani Z, Atanasov AG, Singh VK, Singh NP, Abdel-Azeem AM, Prasad R, Gupta G, Sharma M, Bhargava A (2021) Biological nanofactories: using living forms for metal nanoparticle synthesis. Mini-Rev Med Chem 21(2):245–265
Stickler DJ (1996) Bacterial biofilms and the encrustation of urethral catheters. Biofouling 9(4):293–305
Sun S, Murray CB, Weller D, Folks L, Moser A (2000) Monodisperse FePt nanoparticles and ferromagnetic FePt nanocrystal superlattices. Science 287(5460):1989–1992
Swider E, Koshkina O, Tel J, Cruz LJ, de Vries IJM, Srinivas M (2018) Customizing poly (lactic-co-glycolic acid) particles for biomedical applications. Acta Biomater 73:38–51
Thangadurai D, Sangeetha J, Prasad R (2020) Functional bionanomaterials. Springer International Publishing, Cham. ISBN 978-3-030-41464-1. https://www.springer.com/gp/book/9783030414634
Thomas S, Kumar Mishra P, Talegaonkar S (2015) Ceramic nanoparticles: fabrication methods and applications in drug delivery. Curr Pharm Des 21(42):6165–6188
Tortora GJ, Funke BR, Case CL (2015) Microbiology: an introduction. Pearson Higher Ed
Tseng SP, Hung WC, Huang CY, Lin YS, Chan MY, Lu PL, Sheu JH (2016) 5-Episinuleptolide decreases the expression of the extracellular matrix in early biofilm Formation of multi-drug resistant Acinetobacter baumannii. Mar Drugs 14(8):143
Vasudevan R (2014) Biofilms: microbial cities of scientific significance. J Microbiol Exp 1(3):00014
Vestby LK et al (2020) Bacterial biofilm and its role in the pathogenesis of disease. Antibiotics (Basel) 9(2):59
Wang L, Hu C, Shao L (2017a) The antimicrobial activity of nanoparticles: present situation and prospects for the future. Int J Nanomedicine 12:1227–1249
Wang H, Wang K, Mu Q, Stephen ZR, Yu Y, Zhou S, Zhang M (2017b) Mesoporous carbon nanoshells for high hydrophobic drug loading, multimodal optical imaging, controlled drug release, and synergistic therapy. Nanoscale 9(4):1434–1442
Wang H, Qian J, Ding F (2018) Emerging chitosan-based films for food packaging applications. J Agri food Chem 66(2):395–413
Wang Z, Bai H, Lu C, Hou C, Qiu Y, Zhang P, Mu H (2019) Light controllable chitosan micelles with ROS generation and essential oil release for the treatment of bacterial biofilm. Carbohydr Polym 205:533–539
Wang J, Li G, Yin H, An T (2020) Bacterial response mechanism during biofilm growth on different metal material substrates: EPS characteristics, oxidative stress and molecular regulatory network analysis. Environ Res 185:109451
Wells CJ, Leech GJ, Lever AML, Wansbrough-Jones MH (1995) Treatment of native valve Candida endocarditis with fluconazole. J Infect 31(3):233–235
Xu H, Liu Y (2011) Control and cleaning of membrane biofouling by energy uncoupling and cellular communication. Environ Sci Technol 45:595–601
Yu S, Zhu X, Zhou J, Cai Z (2018) Biofilm inhibition and pathogenicity attenuation in bacteria by Proteus mirabilis. R Soc Open Sci 5(4):170702
Zaidi S, Misba L, Khan AU (2017) Nano-therapeutics: a revolution in infection control in post antibiotic era. Nanomed Nanotechnol Biol Med 13:2281–2301
Ziran BH (2007) Osteomyelitis. J Trauma Acute Care Surg 62(6):S59–S60
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Pabbati, R., Aerupula, M., Shaik, F., Kondakindi, V.R. (2021). Nanoparticles for Biofilm Control. In: Maddela, N.R., Chakraborty, S., Prasad, R. (eds) Nanotechnology for Advances in Medical Microbiology. Environmental and Microbial Biotechnology. Springer, Singapore. https://doi.org/10.1007/978-981-15-9916-3_9
Download citation
DOI: https://doi.org/10.1007/978-981-15-9916-3_9
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-15-9915-6
Online ISBN: 978-981-15-9916-3
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)