Abstract
Influenza vaccination is the primary strategy for preventing influenza and its severe complications. Because influenza vaccine has been used internationally for a long time, the methodologies used to evaluate influenza vaccine efficacy/effectiveness have also changed over time. In this chapter, we provide an overview of the epidemiological approaches to assess influenza vaccine efficacy/effectiveness with reference to the fundamental principles of epidemiology. We also highlight the test-negative design, a modified case-control study, because it is currently the most desirable epidemiological approach for evaluating influenza vaccine effectiveness against laboratory-confirmed influenza. Evidence of vaccine effectiveness from test-negative design studies, global trends to monitor vaccine effectiveness using test-negative design across the seasons, inherent limitations of the current influenza vaccines in terms of effectiveness, available influenza vaccines worldwide, as well as future perspectives for vaccine development are also discussed.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Influenza is an acute febrile respiratory disease that causes annual epidemics, typically in the winter in Japan. Persons who are known to be at higher risk for severe complications from influenza include young children, the elderly, persons with certain chronic diseases, and pregnant women [1]. Vaccination is the primary strategy for preventing influenza and its severe complications. In the United States, annual influenza vaccination is recommended for all persons aged ≥6 months who do not have contraindications [1]. In Europe, those requiring vaccination vary between countries: the elderly and pregnant women are generally recommended to have the vaccination, whereas guidelines for children and adolescents are variable [2]. In Japan, influenza vaccination is designated as a national immunization program under the Immunization Law. The target population are those aged ≥65 years and those aged 60–64 years with a specific underlying disease. Otherwise vaccination is performed voluntarily.
Influenza vaccine has been used internationally for a long time. Methodologies to evaluate influenza vaccine efficacy/effectiveness have also changed over time. In this chapter, we provide an overview of influenza vaccine efficacy/effectiveness with special reference to current epidemiological methodology. Available vaccines overseas and future perspectives for vaccine development are also discussed.
2 Epidemiological Approaches to Evaluate Influenza Vaccine Efficacy/Effectiveness
The best evidence for vaccine efficacy (i.e., the extent of disease prevention under experimental settings) in a human population comes from randomized controlled trials (RCTs). The study subjects are randomly allocated (or assigned) by the investigator to either the vaccine group or the comparison group, and they are followed up over time to estimate the vaccine efficacy by comparing the incidence of influenza between the groups (Fig. 20.1a). However, RCTs cannot be performed ethically in populations for which vaccination is already recommended. Additionally, even an excellent RCT only provides time-, place-, and subject-specific observations and not conclusive findings because (1) the characteristics of circulating influenza viruses differ by time and place; (2) the proportion of patients with pre-existing immunity differ by time, place, and age group; and (3) vaccine strains differ by time (i.e., season) [3]. In this context, observational studies that assess vaccine effectiveness (i.e., the extent of disease prevention under non-experimental settings) also provide important evidence in a real-world setting. Hereafter, both “vaccine efficacy” and “vaccine effectiveness” are referred to as “VE.”
Outline of intervention trials (including RCTs) or cohort studies (a), and case-control studies (b). General equations to calculate the vaccine effectiveness/efficacy are shown. RCT randomized controlled trial, Flu influenza, RR relative risk, VE vaccine effectiveness/efficacy, OR odds ratio. In epidemiological terms, the VE corresponds to the “prevented fraction” defined as “the extent to the relative reduction of attack rate among vaccinated in comparison to unvaccinated.” In other words, it refers to “the proportion of those who would not become influenza positive among those who actually became influenza positive without vaccination, if they had been vaccinated”
Among observational epidemiological studies, cohort studies have the highest level of evidence for evaluating VE. The concept of calculating VE in cohort studies is the same as that for RCTs as shown in Fig. 20.1a. However, when the outcome measure is defined as laboratory-confirmed influenza, it is difficult for cohort studies to achieve an “equal intensity” of follow-up because of a disparity in healthcare-seeking attitudes between vaccinated and unvaccinated subjects. Furthermore, it is difficult for the investigators to provide active surveillance for outcome confirmation throughout the influenza season (e.g., all subjects are periodically surveyed for the pre-defined influenza-like illness (ILI); once ILI onset is recognized, the researchers have to visit the subjects’ homes to obtain a respiratory specimen for influenza diagnosis) [3].
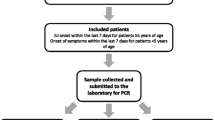
The test-negative design, which was introduced in the mid-2000s, is currently the most desirable epidemiological approach for evaluating influenza VE against laboratory-confirmed influenza. Because the test-negative design is a modified case-control study, the starting point is not identifying vaccinated and unvaccinated individuals, but identifying subjects with the disease (cases) and without the disease (controls). Although VE cannot be directly calculated as shown in Fig. 20.1a, the odds ratio (i.e., ratio of the odds of vaccinations among cases to the odds among controls) can be calculated as an approximation of the relative risk (Fig. 20.1b). In the test-negative design, cases are defined as those with “positive test results for influenza” and controls are defined as those with “negative test results for influenza,” both of which are selected from eligible subjects who visited a clinic or hospital due to pre-defined ILI during the influenza season (Fig. 20.2). A notable feature of the test-negative design is its ability to minimize the misclassification of diseases and confounding by healthcare-seeking attitudes when evaluating influenza VE. Because patients with ILI are expected to visit a clinic or hospital immediately after the onset of symptoms, healthcare-seeking attitude is likely to be similar between cases and controls, which can solve potential problems in cohort studies. The detailed principles of this method have been discussed elsewhere [3,4,5,6,7].
Adapted from [3, 7]. Outline of the test-negative design to evaluate influenza vaccine effectiveness. To avoid selection bias occurring at “recruitment and test” (asterisk), all eligible patients (or a subset of eligible patients selected in a random or systematic manner) have to be recruited in the study and all study subjects (or a subset who are selected in a random or systematic manner) have to be tested
3 Influenza Vaccine Effectiveness Using the Test-Negative Design
After the introduction of the test-negative design, evidence has accumulated regarding influenza VE. A meta-analysis showed that inactivated influenza vaccines provide moderate protection against laboratory-confirmed influenza [8]. They summarized 56 studies that recruited patients (largely outpatients) on the basis of pre-defined ILI criteria and used real-time reverse-transcriptase polymerase chain reaction (PCR) to confirm the influenza diagnosis. Pooled VE according to type or subtype is shown in Table 20.1. A low VE for A(H3N2) was indicated (33%), which might be partly explained by the antigenic mismatch between vaccine strains and circulating strains due to egg-induced mutations in hemagglutinin, particularly for the A(H3N2) strain [9, 10]. However, additional analyses showed that the VE for A(H3N2) was still low (33%) in a season where the vaccine strains and circulating strains were antigenically similar. Furthermore, the VE for A(H3N2) was not uniform across age groups: the highest estimate was for pediatric age groups (43%, 95% confidence interval [CI]: 28–55%) and the lowest estimate was for older adults (24%, 95% CI: −6% to 45%). Recent reports have emphasized the importance of factors other than antigenic match in the interpretation of influenza VE [11, 12]. Another meta-analysis focused on preventing hospitalization with influenza-associated conditions and summarized 30 test-negative design studies [13]. Overall, the pooled VE showed moderate protection against laboratory-confirmed hospitalized influenza among adults (Table 20.2).
In several developed countries, test-negative designs are currently used to “monitor” influenza VE across the seasons, in which influenza is diagnosed by PCR to estimate VE against laboratory-confirmed influenza [14,15,16,17,18]. These studies have contributed to the Global Influenza Vaccine Effectiveness (GIVE) Collaboration, which is led by the World Health Organization (WHO) and provided VE data at a WHO meeting where seasonal influenza vaccine strains were recommended [19]. Factors considered at the meeting included worldwide seasonal influenza activity, antigenic and genetic characteristics of recent circulating influenza viruses, proliferation of candidate vaccine strains, and results from the antigenic analysis of candidate vaccine strains by hemagglutination inhibition assay using post-infection ferret antisera or post-vaccination human antisera. The data from the GIVE Collaboration, although confidential, will be an important indicator of VE in a human population during the latest season.
4 Inherent Limitations of the Current Influenza Vaccine in Terms of Effectiveness
Evidence shows that when inactivated influenza vaccines function at full ability, they reduce the risk of developing influenza by about two-thirds (i.e., VE of 60–70%) and the risk of hospitalization from influenza by about half (i.e., VE of 50%). Although this is “statistically significant,” the public may consider this unsatisfactory. Reasons why the influenza vaccine is “not very effective” include the following: (1) despite yearly vaccine strain selections being based on the best scientific knowledge available, the extent of antigenic matching between the vaccine strains and the epidemic strains varies; (2) the inactivated influenza vaccine induces limited immunity due to its structure and administration route; and (3) from an epidemiological point of view, the most notable limitation is that even unvaccinated persons have a degree of immunity, because influenza epidemics occur every year. As shown in Fig. 20.1a, VE is theoretically the “contrast” of the disease incidence between unvaccinated and vaccinated individuals: the greater the difference, the higher the VE. For influenza, the presence of immunity among unvaccinated individuals results in a lower influenza incidence, which makes it difficult to obtain a clear VE. To achieve a high VE, an influenza vaccine should reduce disease incidence among those vaccinated to almost “zero,” which is challenging.
5 Trends of Influenza Vaccination Worldwide and Future Perspectives
To date, all influenza vaccines currently approved in Japan are quadrivalent, standard-dose, egg-based, unadjuvanted, split-virus inactivated vaccines that contain 15 μg of hemagglutinin (HA) per vaccine virus in a 0.5-mL dose. However, a variety of influenza vaccines are available overseas. Table 20.3 shows the influenza vaccines available in the 2019–2020 season in the USA [1]. The use of a cell culture-based inactivated vaccine and recombinant vaccine can avoid antigenic changes of vaccine strain during egg adaptation. A high-dose trivalent influenza vaccine (containing 60 μg of hemagglutinin per vaccine virus) and adjuvanted influenza vaccine, both of which are approved for the elderly and currently available as a trivalent formulation, can improve immunogenicity. Live attenuated influenza vaccine (LAIV) is administered intranasally and induces mucosal immune responses (secretory IgA) in the upper respiratory tract, which theoretically prevent “infection” by influenza.
Global efforts are also ongoing to achieve a more effective influenza vaccine. Vaccines under development in Japan include an intranasal inactivated influenza vaccine that incorporates the advantages of classical inactivated vaccines and LAIV [20], and a whole virus inactivated influenza vaccine that can provide similar immunity to the natural infection by retaining the original virus structure and components [21]. Overseas, a universal influenza vaccine that provides broad-spectrum cross-protection against influenza A and B by inducing humoral and cell-mediated immunity is under development [22].
6 Conclusions
Influenza vaccines are often criticized, probably because their VE is difficult to understand. However, it should be recognized that most infectious diseases do not have available vaccines. For influenza, it is important to make the best use of the current vaccines, while developing more effective vaccines. Given the current globalization, the benefit of influenza vaccine as a primary prevention tool should be better understood.
References
Grohskopf LA, Alyanak E, Broder KR, Walter EB, Fry AM, Jernigan DB. Prevention and control of seasonal influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices—United States, 2019-20 influenza season. MMWR Recomm Rep. 2019;68(3):1–21.
Sheikh S, Biundo E, Courcier S, Damm O, Launay O, Maes E, et al. A report on the status of vaccination in Europe. Vaccine. 2018;36(33):4979–92.
Fukushima W, Hirota Y. Basic principles of test-negative design in evaluating influenza vaccine effectiveness. Vaccine. 2017;35(36):4796–800.
Jackson ML, Nelson JC. The test-negative design for estimating influenza vaccine effectiveness. Vaccine. 2013;31(17):2165–8.
Foppa IM, Haber M, Ferdinands JM, Shay DK. The case test-negative design for studies of the effectiveness of influenza vaccine. Vaccine. 2013;31(30):3104–9.
Sullivan SG, Tchetgen Tchetgen EJ, Cowling BJ. Theoretical basis of the test-negative study design for assessment of influenza vaccine effectiveness. Am J Epidemiol. 2016;184(5):345–53.
Ozasa K, Fukushima W. Commentary: test negative design reduces confounding by healthcare-seeking attitude in case-control studies. J Epidemiol. 2019;29(8):279–81.
Belongia EA, Simpson MD, King JP, Sundaram ME, Kelley NS, Osterholm MT, et al. Variable influenza vaccine effectiveness by subtype: a systematic review and meta-analysis of test-negative design studies. Lancet Infect Dis. 2016;16(8):942–51.
Skowronski DM, Janjua NZ, De Serres G, Sabaiduc S, Eshaghi A, Dickinson JA, et al. Low 2012-13 influenza vaccine effectiveness associated with mutation in the egg-adapted H3N2 vaccine strain not antigenic drift in circulating viruses. PLoS One. 2014;9(3):e92153.
Zost SJ, Parkhouse K, Gumina ME, Kim K, Diaz Perez S, Wilson PC, et al. Contemporary H3N2 influenza viruses have a glycosylation site that alters binding of antibodies elicited by egg-adapted vaccine strains. Proc Natl Acad Sci U S A. 2017;114(47):12578–83.
Skowronski DM, Chambers C, Sabaiduc S, De Serres G, Winter AL, Dickinson JA, et al. Beyond antigenic match: possible agent-host and immuno-epidemiological influences on influenza vaccine effectiveness during the 2015-2016 season in Canada. J Infect Dis. 2017;216(12):1487–500.
Cobey S, Gouma S, Parkhouse K, Chambers BS, Ertl HC, Schmader KE, et al. Poor immunogenicity, not vaccine strain egg adaptation, may explain the low H3N2 influenza vaccine effectiveness in 2012-2013. Clin Infect Dis. 2018;67(3):327–33.
Rondy M, El Omeiri N, Thompson MG, Levêque A, Moren A, Sullivan SG. Effectiveness of influenza vaccines in preventing severe influenza illness among adults: a systematic review and meta-analysis of test-negative design case-control studies. J Infect. 2017;75(5):381–94.
Ferdinands JM, Fry AM, Reynolds S, Petrie J, Flannery B, Jackson ML, et al. Intraseason waning of influenza vaccine protection: evidence from the US Influenza Vaccine Effectiveness Network, 2011-12 through 2014-15. Clin Infect Dis. 2017;64(5):544–50.
Kissling E, Rondy M, I-MOVE/I-MOVE+ study team. Early 2016/17 vaccine effectiveness estimates against influenza A(H3N2): I-MOVE multicentre case control studies at primary care and hospital levels in Europe. Euro Surveill. 2017;22(7):30464.
Skowronski DM, Zou M, Sabaiduc S, Murti M, Olsha R, Dickinson JA, et al. Interim estimates of 2019/20 vaccine effectiveness during early-season co-circulation of influenza A and B viruses, Canada, February 2020. Euro Surveill. 2020;25(7):2000103.
Cheng AC, Holmes M, Dwyer DE, Senanayake S, Cooley L, Irving LB, et al. Influenza epidemiology in patients admitted to sentinel Australian hospitals in 2018: the Influenza Complications Alert Network (FluCAN). Commun Dis Intell (2018). 2019:43. https://pubmed.ncbi.nlm.nih.gov/31738866/
Fukushima W, Morikawa S, Matsumoto K, Fujioka M, Matsushita T, Kubota M, et al. for the study group funded by Ministry of Health, Labour and Welfare Japan. Influenza vaccine effectiveness in Japanese children under 6 years: summary findings from 2013-14 season to 2017-18 season. IASR. 2019;40:194–5. [in Japanese]
World Health Organization. Recommended composition of influenza virus vaccines for use in the 2020-2021 northern hemisphere influenza season. https://www.who.int/influenza/vaccines/virus/recommendations/202002_recommendation.pdf?ua=1. Accessed 28 Mar 2020.
Sano K, Ainai A, Suzuki T, Hasegawa H. Intranasal inactivated influenza vaccines for the prevention of seasonal influenza epidemics. Expert Rev Vaccines. 2018;17(8):687–96.
Sekiya T, Mifsud EJ, Ohno M, Nomura N, Sasada M, Fujikura D, et al. Inactivated whole virus particle vaccine with potent immunogenicity and limited IL-6 induction is ideal for influenza. Vaccine. 2019;37(15):2158–66.
Pleguezuelos O, Dille J, de Groen S, Oftung F, Niesters HGM, Islam MA, et al. Immunogenicity, safety, and efficacy of a standalone universal influenza vaccine, FLU-v, in healthy adults: a randomized clinical trial. Ann Intern Med. 2020;172(7):453–62.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Fukushima, W. (2021). Influenza Vaccine Efficacy/Effectiveness: With Special Reference to Current Epidemiological Methodology. In: Fujita, J. (eds) Influenza. Respiratory Disease Series: Diagnostic Tools and Disease Managements. Springer, Singapore. https://doi.org/10.1007/978-981-15-9109-9_20
Download citation
DOI: https://doi.org/10.1007/978-981-15-9109-9_20
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-15-9108-2
Online ISBN: 978-981-15-9109-9
eBook Packages: MedicineMedicine (R0)