Abstract
6-Benzylaminopurine, meta-topolin and their derivatives have been and are being used in and tested for the micropropagation of plants using tissue culture techniques. Meta-topolin and meta-methoxy-topolin were synthesized and tested for cytokinin activity in the late 1960s, but interest in these substances has accelerated since the 1990s. The family of topolin and methoxy-topolin cytokinins was first discovered in plants. Synthetic derivatives were then prepared, and this enabled the cytokinin activity of a wide variety of aromatic cytokinins to be evaluated in various bioassays. The latter revealed that aromatic cytokinins and most notably their N9-ribosides were very effective in retarding chlorophyll degradation, i.e. possessing strong senescence delaying effect. This led to the development of topolin derivatives with other N9-substituents. Such substances, in addition to their anti-senescence activity, show a milder negative effect on root growth and development than classical cytokinins. A combination of these two properties was crucial for choosing topolins for use and testing in plant tissue cultures. The uniqueness of the aromatic cytokinins is being gradually revealed using the molecular techniques of today. This chapter chronologically overviews what has been discovered so far about the cytokinin activity of topolins and compounds related to them.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
3.1 The History of Meta-Topolin Activity Discovery
The history of the discovery of meta-topolin is closely related to that of kinetin and its cell division stimulatory activity (Miller et al. 1955). When this was first described, Japanese researchers synthesized a wide range of kinetin analogues which included among others the ortho-, meta- and para-substituted 6-benzylaminopurine (BAP) derivatives (Kuraishi 1959; Okumura et al. 1959). Their series contained meta-hydroxy-BAP which was later called meta-topolin (mT, Strnad et al. 1997). The compounds were tested as possible cytokinetic agents in the radish (Raphanus sativus L.) leaf disc expansion assay (Kuraishi and Okumura 1956). Ortho-methyl-BAP and ortho-chloro-BAP were slightly more active than unsubstituted BAP which was a little more active than ortho- and meta-hydroxy-BAP (this refers to ortho- topolin (oT) and mT, respectively, Okumura et al. 1959). The effect of different substituents introduced into the benzyl ring of BAP on the promotion of the growth of tobacco callus was analysed by Iwamura et al. (1980), but no significant effect of the substitution was found. These results appeared to be consistent with the results reported by Keim et al. (1981) who showed that BAP, oT and mT all bind to CBF-1 protein with similar high affinity, although the affinity of oT was a little lower. CBF-1 is a soluble protein isolated from wheat germ which binds cytokinins with relatively high affinity and specificity (Fox and Erion 1975).
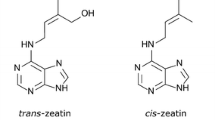
It was not until 28 years later from the first mT synthesis that Kaminek et al. (1987) reported significant biological activity of meta-hydroxy-benzyladenosine (mTR) over the standard N6-benzyladenosine (BAPR) in the tobacco callus and wheat leaf senescence assay (WLSA). In the discussion section of their paper, the authors noted that mT was slightly more active than mTR in the Amaranthus assay, while the two compounds exhibited about the same activity in WLSA. Ortho- and para-hydroxy-BAPRs showed significantly lower activity than BAPR in all bioassays (Kaminek et al. 1987). The latter was the first report showing that meta-hydroxylation of BAPR leads to a significant increase in cytokinin activity, while ortho- and para-hydroxylation of BAPR have the opposite effect. These results contrasted sharply with the results of the radish leaf disc expansion assay (Kuraishi and Okumura 1956). Interestingly, the cytokinin activity of mTR was as high as that of trans-zeatin in all tested bioassays, including the Amaranthus assay and pea bud formation assay (Kaminek et al. 1987) apart from the two aforementioned. In this report, mTR was first introduced and tested for cytokinin activity, which was the beginning of mTR exploration. In the same year, Kaminek and Vaněk (1987) patented mT and mTR for use in agriculture and horticulture as shooting- and branching-stimulating plant growth regulators. As is so far apparent from the overview, since the discovery of mT and its cytokinin properties, very little attention had been paid to this molecule for almost three decades. This was about to change in the 1990s.
Meta-topolin riboside (mTR) has been tested by Czech scientists as a crop yield-increasing substance in barley (Hradecká and Petr 1992a) and wheat (Trčková et al. 1992; Hradecká and Petr 1992b; Borkovec and Prochazka 1995). Relative plant growth rate, 1000-grain weight, number of grains per plant and other yield-forming parameters were improved after application of mTR. In 1991, Holub presented his teams’ findings at the 14th IPGSA conference in Amsterdam that mT is slightly more active than mTR in the tobacco callus and WLSA, making it the most active compound of the hydroxylated aromatic cytokinins studied so far in these assays (Holub et al. 1991). Such observation led to the idea whether mT could be an alternative to BAP in tissue culture (Werbrouck et al. 1996). Strnad and his co-workers further developed an analytical HPLC-ELISA screening system specific for BAP, oT and mT and their N9-substituted derivatives (Strnad et al. 1992a; Strnad 1996). This enabled the screening of plant tissue extracts for these types of cytokinins. Shortly afterwards, a brand new family of endogenous aromatic cytokinins, including mT, was discovered in plants, characterized and labelled as topolins (Strnad et al. 1992b, 1994, 1997; Jones et al. 1996).
The structure-activity relationships of natural cytokinins, namely, trans-zeatin, BAP, oT, mT and their N9-ribosides and N9-glucosides, were then examined and compared in three cytokinin bioassays (Holub et al. 1998). The bioassays were based on the stimulation of tobacco callus growth (tobacco callus assay), retention of chlorophyll in excised wheat leaves (wheat leaf senescence assay, WLSA) and dark induction of betacyanin synthesis in Amaranthus cotyledons (Amaranthus assay). The results showed what could have been read between the lines, but was never shown in one publication. Hydroxylation of the benzyl ring in the meta-position significantly increased the activity of BAP and BAPR in WLSA. In this assay, the activity of mT as well as mTR was doubled compared to BAP and BAPR, respectively. In the tobacco callus assay, the activity was increased only in the case of the mT free base but not in the case of mTR. Interestingly, in the Amaranthus assay, meta-hydroxylation led to a decrease in BAP and BAPR activity. Ortho-hydroxylation reduced the activity of all compounds in all bioassays, and the N9-glucosylation inactivated all the compounds completely (Holub et al. 1998). The authors further reported that the N9-ribosylation had no significant effect on the cytokinin activity of the compounds; however, N9-ribosylation slightly diminished their activity in tobacco callus and Amaranthus assays but markedly improved their activity in WLSA. Even from the graphs in Holub’s work, it is clear that N9-ribosides retained more of the chlorophyll in the detached wheat leaves than cytokinin free bases. This effect was later observed by other authors and further investigated. It can be definitively affirmed that the WLSA played an irreplaceable role in the future development of meta-topolin derivatives, their analogues and all aromatic cytokinins in general.
3.2 Meta-Topolin and Related Compounds in a New Millennium
For the family of topolin compounds, a new millennium began with the discovery of their very close relatives—methoxy-topolins, 6-(2-methoxybenzylamino)purine (ortho-methoxy-topolin, MeoT) and 6-(3-methoxybenzylamino)purine (meta-methoxy-topolin, MemT). Their N9-ribosides (MeoTR and MemTR, respectively) were found again in Populus x canadensis leaves and in Arabidopsis thaliana plants (Tarkowská et al. 2003). The latter evaluated the cytokinin activity of the four new hormones in the three abovementioned cytokinin bioassays. It is not very surprising that MemT and MemTR were highly active in the WLSA. Their ortho-analogues were highly active as well. All derivatives exhibited plus/minus double activity relative to BAP (Table 3.1). In the other two bioassays, the compounds had an equal or a slightly lower activity than BAP (Tarkowská et al. 2003). These results demonstrated that the cytokinin activity of the benzyl ring-substituted BAPs and BAPRs is not limited to their meta-derivatives. The need for a comprehensive review of the cytokinin activity of individual BAP and BAPR derivatives was accomplished by Doležal et al. (2006, 2007). Their systematic approach made it possible to identify key substituents for achieving high cytokinin and particularly high anti-senescence activity of BAP and BAPR derivatives. Of the substituted BAPs, two fluorinated compounds, 3F-BAP and 2F-BAP, showed the highest cytokinin activities (Doležal et al. 2006 and Table 3.1). Apropos BAPR derivatives, many of them showed high activity in WLSA (Doležal et al. 2007). From the available results (Holub et al. 1998; Doležal et al. 2007; Hönig et al. 2018), it is clear that the presence of ribose at the N9-position alone significantly increased the compound’s anti-senescence activity. This is very well demonstrated in Table 3.1. Based on the disproportion in activity of aromatic cytokinins in WLSA and in the other cytokinin bioassays, Doležal et al. suggested that the effect of aromatic cytokinins including topolins is predominantly associated with protection of the photosynthetic apparatus. This is consistent with the work of other authors. It was shown that in Rosa hybrida cuttings, MemTR exhibited the best anti-senescing effects of all tested cytokinins (Bogaert et al. 2006). On the other hand, the compounds 3F-BAP and 3F-BAPR showed the best shoot multiplication rate. MemTR was also superior to BAP in the multiplication of Petunia hybrida (Bogaert et al. 2006). Further, mT was evaluated as the most effective cytokinin in stimulating chlorophyll and protein biosynthesis at low concentrations in cucumber cotyledons (Cucumis sativus L., Çağ et al. 2007). Meta-topolin was also very efficient in retarding the leaf senescence of Pelargonium cuttings with only marginal negative effect on the root development. This was in contrast with the use of thidiazuron which severely affected root growth (Mutui et al. 2012).
These observations correspond very well with the ability of each compound to activate cytokinin receptors in plants. Three cytokinin receptors, AHK2, AHK3 and CRE1/AHK4, have been described in Arabidopsis (Inoue et al. 2001; Suzuki et al. 2001; Yamada et al. 2001). It has been reported that the receptor CRE1/AHK4 is responsible for the root inhibitory activity of cytokinins in Arabidopsis (Riefler et al. 2006), while the AHK3 receptor is the key element in cytokinin-mediated leaf longevity (Riefler et al. 2006; Kim et al. 2006). Spíchal et al. (2004) showed that TDZ is a strong activator of both mentioned cytokinin receptors, while mT activates preferably the receptor AHK3. The relative activity of mT at AHK3 and CRE1/AHK4 receptors was 80% and 30% of tZ activity, respectively. Meta-topolin riboside did not activate these receptors, but it was able to activate the expression of ARR5 (Arabidopsis cytokinin response regulator 5) in Arabidopsis plants (Spíchal et al. 2004). Recently, it has also been shown that cytokinin receptors from Brassica napus bind mT and with higher affinity than BAP (Kuderová et al. 2014).
The continuous search for compounds with cytokinin activity but with limited root growth inhibitory activity led to the development of topolin derivatives with N9-substituents, such as tetrahydropyran-2-yl, 9-tetrahydrofuran-2-yl and 4-chlorobutyl (Szüčová et al. 2009; Plíhal et al. 2013; Podlešáková et al. 2012). Such compounds are reported to maintain high anti-senescence activity (Szüčová et al. 2009) while exhibiting negligible negative effects on root growth and development. It is assumed that the reasons for this are (1) the compounds do not activate the CRE1/AHK4 cytokinin receptor; and (2) they are preferably transported from roots to shoots, (3) where the free base is slowly released and takes action (Plíhal et al. 2013). It has been also speculated that such compounds may act as CRE1/AHK4 receptor antagonists, which would further favour their indifference to the root system (Plíhal et al. 2013). This is feasible given that two other derivatives of BAP were shown to be cytokinin receptor antagonists. A compound known as PI-55, which is (6-(2-hydroxy-3-methylbenzylamino)purine), is an antagonist of the CRE1/AHK4 receptor (Spíchal et al. 2009). The compound LGR-991 (2,5-dihydroxy-BAP) showed antagonistic effects on AHK3 and CRE1/AHK4 receptors (Nisler et al. 2010).
For the reasons described above, it is clear that the N9-substituted derivatives of aromatic cytokinins have great potential for improving plant in vitro regeneration techniques.
References
Bogaert I, Cauter S, Werbrouck SPO et al (2006) New aromatic cytokinins can make the difference. In: Fári MG, Holb I, Bisztray GD (eds) Acta horticulturae, vol 725. International Society for Horticultural Science (ISHS), Leuven, pp 265–270
Borkovec V, Prochazka S (1995) Proceedings of the Plant Growth Regulator Society of America, vol 22, pp 360–364
Çağ S, Gören-Sağlam N, Çıngıl-Barış Ç et al (2007) The effect of different concentration of epibrassinolide on chlorophyll, protein and anthocyanin content and peroxidase activity in excised red cabbage (Brassica oleracea L.) cotyledons. Biotechnol Biotechnol Equip 21:422–425
Doležal K, Popa I, Kryštof V et al (2006) Preparation and biological activity of 6-benzylaminopurine derivatives in plants and human cancer cells. Bioorg Med Chem 14:875–884
Doležal K, Popa I, Hauserová E et al (2007) Preparation, biological activity and endogenous occurrence of N6-benzyladenosines. Bioorg Med Chem 15:3737–3747
Fox JE, Erion JL (1975) A cytokinin binding protein from higher plant ribosomes. Biochem Biophys Res Commun 64:695–700
Holub J, Hanuš J, Vaněk T et al (1991) Cytokinin activities of some 9-substituted ortho- and meta-hydroxybenzyladenines. In: Abstract 14th IPGSA Conference. Agricultural University, Wageningen, Amsterdam, p 70
Holub J, Hanuš J, Hanke DE et al (1998) Biological activity of cytokinins derived from Ortho- and Meta-Hydroxybenzyladenine. Plant Growth Regul 26:109–115
Hönig M, Plíhalová L, Husičková A et al (2018) Role of cytokinins in senescence, antioxidant defence and photosynthesis. Int J Mol Sci 19:4045
Hradecká D, Petr J (1992a) Spring barley kernel weight after treatment with a cytokinin. In: SbornikVysokeSkolyZemedelske v Praze, FakultaAgronomicka, Rada A: RostlinnaVyroba, vol 54, pp 153–162
Hradecká D, Petr J (1992b) The effect of cytokinins on the yield of some cereal. In: Kaminek M, Mok WS, Zažímalová E (eds) Physiology and biochemistry of cytokinins in plants. SPB Academic, The Hague, pp 245–247
Inoue T, Higuchi M, Hashimoto Y et al (2001) Identification of CRE1 as a cytokinin receptor from Arabidopsis. Nature 409:1060–1063
Iwamura H, Fujita T, Koyama S et al (1980) Quantitative structure-activity relationship of cytokinin-active adenine and urea derivatives. Phytochemistry 19:1309–1319
Jones LH, Martinková H, Strnad M et al (1996) Occurrence of aromatic cytokinins in oil palm (Elaeis guineensis Jacq.). J Plant Growth Regul 15:39
Kaminek M, Vaněk T (1987) Agricultural and horticultural plant growth regulator compositions containing N6-(m-hydroxybenzyl)adenosine and -adenine. From Czech.CS 238548.B1 19851113
Kaminek M, Vaněk T, Motyka V (1987) Cytokinin activities of N6-Benzyladenosine derivatives hydroxylated on the side-chain phenyl ring. J Plant Growth Regul 6:113–120
Keim P, Erion J, Fox JE (1981) The current status of cytokinin-binding moieties. In: Guern J, Péaud-Lenoël C (eds) Metabolism and molecular activities of cytokinins. Proceedings in life sciences. Springer, Berlin
Kim HJ, Ryu H, Hong SH et al (2006) Cytokinin-mediated control of leaf longevity by AHK3 through phosphorylation of ARR2 in Arabidopsis. Proc Natl Acad Sci U S A 103:814–819
Kuderová A, Gallová L, Kuricová K et al (2014) Identification of AHK2- and AHK3-like cytokinin receptors in Brassica napus reveals two subfamilies of AHK2 orthologues. J Exp Bot 66:339–353
Kuraishi S (1959) Effect of kinetin analogs on leaf growth. Sci Pap Coll Gen Educ Univ Tokyo 9:67–104
Kuraishi S, Okumura FS (1956) Bot Mag (Tokyo) 69:300
Miller CO, Skoog F, Von Saltza MH et al (1955) Kinetin, a cell division factor from deoxyribonucleic acid. J Am Chem Soc 77:1392
Mutui TM, Mibus H, Serek M (2012) Effect of meta-topolin on leaf senescence and rooting in Pelargonium × hortorum cuttings. Postharvest Biol Technol 63:107–110
Nisler J, Zatloukal M, Popa I et al (2010) Cytokinin receptor antagonists derived from 6-benzylaminopurine. Phytochemistry 71:823–830
Okumura FS, Kotani Y, Ariga T, Kuraishi S (1959) Syntheses of kinetin-analogues. II. Bull Chem Soc Jpn 32:883–886
Plíhal O, Szüčová L, Galuszka P (2013) N9-substituted aromatic cytokinins with negligible side effects on root development are an emerging tool for in vitro culturing. Plant Signal Behav 8:6–9
Podlešáková K, Zalabák D, Čudejková M et al (2012) Novel cytokinin derivatives do not show negative effects on root growth and proliferation in submicromolar range. PLoS One 7:e39293
Riefler M, Novák O, Strnad M et al (2006) Arabidopsis cytokinin receptor mutants reveal functions in shoot growth, leaf senescence, seed size, germination, root development, and cytokinin metabolism. Plant Cell 18:40–54
Spíchal L, Rakova NY, Riefler M et al (2004) Two cytokinin receptors of Arabidopsis thaliana, CRE1/AHK4 and AHK3, differ in their ligand specificity in a bacterial assay. Plant Cell Physiol 45:1299–1305
Spíchal L, Werner T, Popa I et al (2009) The purine derivative PI-55 blocks cytokinin action via receptor inhibition. FEBS J 276:244–253
Strnad M (1996) Enzyme immunoassays of N6-benzyladenine and N6-(meta-hydroxybenzyl)adenine cytokinins. J Plant Growth Regul 15:179–188
Strnad M, Veres K, Hanus J et al (1992a) Immunological methods for quantification and identification of cytokinins. In: Kaminek M, Mok DWS, Zazimalova E (eds) Physiology and biochemistry of cytokinins in plants. SPB Academic, The Hague, pp 437–446. ISBN 90-5103-066-5
Strnad M, Peters W, Beck E et al (1992b) Immunodetection and identification of N6-(o-hydroxybenzylamino)purine as a naturally occurring cytokinin in Populus x canadensis Moench cv. Robusta leaves. Plant Physiol 99:74–80
Strnad M, Peters W, Hanuš J et al (1994) Orthotopolin-9-glucoside, an aromatic cytokinin from Populus x canadensis cv. Robusta leaves. Phytochemistry 37:1059–1062
Strnad M, Hanuš J, Vaněk T et al (1997) Meta-Topolin, a highly active aromatic cytokinin from poplar leaves (Populus x canadensis Moencho cv. Robusta). Phytochemistry 42:213–218
Suzuki T, Miwa K, Ishikawa K et al (2001) The Arabidopsis sensor his-kinase, AHK4, can respond to cytokinins. Plant Cell Physiol 42:107–113
Szüčová L, Spíchal L, Doležal K et al (2009) Synthesis, characterization and biological activity of ring-substituted 6-benzylamino-9-tetrahydropyran-2-yl and 9-tetrahydrofuran-2-ylpurine derivatives. Bioorg Med Chem 17:1938–1947
Tarkowská D, Doležal K, Tarkowski P et al (2003) Identification of new aromatic cytokinins in Arabidopsis thaliana and Populus X canadensis leaves by LC-ESI-MS and capillary liquid chromatography/frit–fast atom bombardment mass spectrometry. Physiol Plant 117:579–590
Trčková M, Kamínek M, Zmrhal Z (1992) Grain formation and distribution of nutrients in wheat plants after application of synthetic cytokinin N6-(meta-hydroxybenzyl)adenosine. In: Kaminek M, Mok WS, Zažímalová E (eds) Physiology and biochemistry of cytokinins in plants. Kluwer Academic, The Hague, pp 241–244
Werbrouck SPO, Strnad M, Van Onckelen HA et al (1996) Meta-topolin, an alternative to benzyladenine in tissue culture? Physiol Plant 98:291–297
Yamada H, Suzuki T, Terada K et al (2001) The Arabidopsis AHK4 histidine kinase is a cytokinin-binding receptor that transduces cytokinin signals across the membrane. Plant Cell Physiol 42:1017–1023
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Nisler, J. (2021). Cytokinin Properties of Meta-Topolin and Related Compounds. In: Ahmad, N., Strnad, M. (eds) Meta-topolin: A Growth Regulator for Plant Biotechnology and Agriculture. Springer, Singapore. https://doi.org/10.1007/978-981-15-9046-7_3
Download citation
DOI: https://doi.org/10.1007/978-981-15-9046-7_3
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-15-9045-0
Online ISBN: 978-981-15-9046-7
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)