Abstract
Atypical mycobacteria (AM) are aerobic, nonspore-forming bacilli that are known to be involved in many ocular infections, most commonly keratitis. The most common AM organisms that cause keratitis are Mycobacterium chelonae, Mycobacterium fortuitum, and Mycobacterium abscessus. While AM keratitis are commonly associated with post-laser in situ keratomilieusis (LASIK) scenarios, AM keratitis is also frequently caused by trauma and contact lens wear. It is difficult to diagnose AM keratitis because of the clinical appearance as well as the slow growth on culture. Hence, initiation of treatment is often delayed, leading to worse outcomes. Specific stains and cultures in addition to standard media are sometimes needed, including acid-fast stains, Ziehl–Neelsen, Lowenstein–Jensen medium, and Middlebrook 7H9 broth. Many treatment options have been studied, but topical amikacin appears to be one of the most commonly used therapies. Occasionally, systemic antibiotics are used, and in recalcitrant cases with risk to continued spread beyond the cornea, surgical intervention is needed. As clinical outcomes are dependent on a high suspicion for this condition and the initiation of prompt treatment, an understanding of and a recognition of AM keratitis is of utmost importance.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
7.1 Introduction
Atypical Mycobacteria (AM), also known as nontuberculous Mycobacteria, are aerobic, nonmotile, nonspore-forming bacilli that can be responsible for multiple ocular infections, most commonly keratitis [1, 2]. Although there are many known species of AM, the majority responsible for keratitis are rapidly growing and non-chromogenic Runyon group IV organisms: Mycobacterium chelonae (42.7%), Mycobacterium fortuitum (14.7%), and Mycobacterium abscessus (11.1%) [3]. In addition to causing keratitis, AM infections have also been known to involve the orbit, periocular skin, and lacrimal system, and they can cause scleritis, conjunctivitis, endophthalmitis, choroiditis, iridocyclitis, and panuveitis [3]. Human infections are acquired from environmental exposure, often from soil, dust, or water [4].
The majority of AM ocular infections are keratitis [4]. Historically, AM have been known to cause corneal ulcers via nonsurgical trauma directly inoculating AM into the corneal stroma [5]. Post-corneal transplant AM keratitis was first reported in the 1980s, followed by multiple cases of post-laser in situ keratomilieusis (LASIK) AM keratitis in the 1990s, both sporadic and epidemic [6,7,8]. Presently, the plurality (47.6%) of AM cases occur post-LASIK, and the most common cause of post-LASIK keratitis is AM. Other common causes of AM include foreign bodies (17.6%), implants (17.3%), trauma (14.8%), and contact lens wear (6.4%) [3].
The common causes of AM keratitis often involve some degree of penetrating trauma to the corneal epithelium [4]. Soft contact lens wearers who develop abrasions are at risk [9], as well as patients who undergo suture removal [10]. Additionally, post-clear corneal cataract surgery wound ulcers secondary to AM have been reported [11, 12]. However, nearly one-third of AM keratitis cases present with no associated epithelial defect and with corneal scrapings showing normal epithelium and no growth of organisms [13] making diagnosis challenging.
Topical corticosteroids may increase the risk of AM keratitis. In a rabbit model, M. fortuitum was cultured from corneal lesions from rabbit corneas treated with subconjunctival methylprednisolone at the time of inoculation with M. fortuitum, whereas controls who did not receive the steroids showed no active disease at 3–4 weeks, and no organisms could be identified from culture [14]. It has been noted that steroids were used in more than half of the cases of reported AM keratitis (57.4%) [3]. Postoperative subconjunctival and topical corticosteroids not only increase the risk of AM keratitis but can also exacerbate AM keratitis and are associated with cases that are recalcitrant to antibiotic therapy [13, 15, 16]. Additionally, corneal grafts require postoperative topical corticosteroids and are therefore also at risk for AM keratitis, and these cases may warrant regraft, or penetrating keratoplasty if the keratitis occurs following endothelial keratoplasty [17,18,19,20]. M. chelonae has even been implicated in AM keratitis after routine phacoemulsification cataract surgery [21].
7.2 Clinical Features
AM keratitis can be difficult to diagnose, and this often results in delayed treatment [22]. In one series, delayed diagnosis was reported in 55.5% of cases, with these being attributed to misdiagnosis, misidentification of the causative organism on culture, delay in taking cultures, or slow or no growth of the organism [3]. Cases have been misidentified as Nocardia and Corynebacterium species or misdiagnosed as herpes keratitis and fungal keratitis. The duration of delay ranged from 1 week to 30 weeks, with an average of 8 weeks.
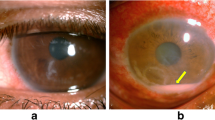
Cases of AM keratitis typically follow an indolent course and are prolonged by the use of topical steroids [14]. Often, cases of AM keratitis are preceded by a history of trauma, both surgical and nonsurgical, including foreign body removal [5, 23]. Pain is variable, and vision is often decreased. A white central ulcer with white satellite infiltrates is characteristic of M. fortuitum and M. chelonae [5, 17, 24]. (Fig. 7.1) These satellite lesions can mimic those seen in fungal keratitis. A hypopyon may develop if untreated (Fig. 7.2), but it is less common with an intact epithelium [25]. A “cracked windshield” appearance of lines radiating from the central area of ulceration is also typical of AM keratitis. Unlike infectious crystalline keratopathy, this sign is seen transiently early in the disease course [4]. Despite the predilection to develop in cases of epithelial breakdown, the infection can be deep in the stroma without an epithelial defect due to direct inoculation, often from prior surgical trauma [13, 26]. These cases are associated with irregular margins and satellite lesions, and usually involve Runyon group I or II AM [27]. AM keratitis can extend to the corneoscleral junction [25, 26] and can appear like any other bacterial, herpetic stromal, or fungal keratitis. Some cases, especially those involving M. marinum, M. chelonae, and M. gordonae, can present with dendritic or geographic epithelial defects with minimal stromal infiltration that simulate a nonsuppurative herpetic keratitis, leading to an incorrect diagnosis [5, 27, 28].
7.3 Diagnosis
A combination of standard and special stains and media are often needed to make a microbiological diagnosis of AM keratitis. Gram and Giemsa stains typically reveal nonspecific polymorphonuclear leukocytes or partially stained bacilli that may be easily misdiagnosed [29]. Specific stains such as Ziehl–Neelsen show gram-variable bacilli which do not decolorize with 20% sulfuric acid (Fig. 7.3). Fluorescein-conjugated stains such as auramine-rhodamine fluorescent acid-fast stain and fluorescent conjugated lectins are also useful [4]. With regards to cultures, blood agar, chocolate agar, MacConkey agar, Lowenstein–Jensen medium, and liquid broth such as Middlebrook 7H9 broth in conjunction with a mycobacterial growth indicator tube are recommended [30]. Multiple culture media increases yield, particularly of slower-growing varieties (Runyon groups I and II). Given variation in growth rates between different species, culture times vary from 2 to 4 days, but they should be maintained for up to 8 weeks [31]. Recently, AM has been further characterized by PCR pulsed-field gel electrophoresis, which can aid in the diagnosis of AM keratitis [32, 33]. Of samples from one series of AM keratitis cases that isolated a causative organism, 64.1% were from superficial corneal scrapings, 9.5% were from corneal biopsy, 13.6% were from flap lift post-LASIK, and 5.5% were from corneal buttons post-transplant [3]. The sample source depends on clinical context.
7.4 Management and Outcomes
Recurrence of AM keratitis is common, despite an initial response to treatment [34], and the prognosis is guarded, as up to 50% of patients may have severe vision loss in cases of post-LASIK AM keratitis [8]. Thus, a high suspicion is needed in post-LASIK keratitis in order to recognize and start treatment promptly [7, 35,36,37,38].
As in all cases of microbial keratitis, antibiotics play a crucial role in AM keratitis, and the choice of antibiotics is based off of sensitivity data and outcomes from the treatment of non-ocular (systemic) AM infections [4]. Slower growing AM are sensitive to agents used to treat M. tuberculosis, such as rifabutin, rifampin, and streptomycin, whereas rapidly growing AM are sensitive to macrolides, fluoroquinolones, and aminoglycosides. Cases often require a combination of two or more antibiotics. Triple therapy with topical clarithromycin or amikacin, azithromycin, and often a fourth generation fluoroquinolone, in conjunction with appropriate corneal debridement has been suggested in the literature [7, 33, 39]. Two antibiotics have been used in 55.2% of eyes, with a plurality of combinations including amikacin (50 mg/mL) (39.4%) [3]. Only in the minority of cases (27.6%) has amikacin been used as monotherapy [3]. Clarithromycin (10 mg/mL) is ideally reconstituted from lyophilized parenteral drug, but it is no longer available in the US [40]. Currently, it is formulated from an oral suspension, which has been shown to have decreased bioavailability in rabbit corneas with intact corneal epithelium [40]. Azithromycin (2 mg/mL) can be reconstituted from lyophilized drug used parenterally or from a mucoadhesive commercial preparation. Azithromycin 1% is a commercially available topical formulation, but its efficacy is not known for AM keratitis. Finally, moxifloxacin or gatifloxacin has been used in AM keratitis with varying success. In vitro susceptibility testing has shown that based on the MIC cut off, fluoroquinolones are less likely to be useful in AM keratitis. In a series of 15 cases of AM keratitis, all isolates were sensitive to amikacin, azithromycin, and clarithromycin, but only 9/15 (60%) were sensitive to gatifloxacin and 6/15 (40%) were sensitive to ciprofloxacin [41].
Systemic antibiotics may be used in recalcitrant cases [39], with oral clarithromycin or oral doxycycline being commonly used. In one series of 203 cases of AM keratitis, topical antibiotic therapy alone was used in 53.2% of cases, whereas systemic and topical antibiotics were combined in 41.9% of cases [3]. There are no set guidelines regarding duration of treatment, but cases of AM keratitis often require 6 weeks to 6 months of topical and systemic antibiotic therapy [4]. Discontinuation of topical corticosteroids is also recommended as reduced local host immune mechanisms prolong the duration of AM keratitis [13].
Surgical management is a mainstay of treatment, and it is required in 55.1% of eyes in one series [3]. Of the 73.1% of eyes that had an initial lack of response to medical therapy, 79.3% required surgical intervention [3]. Surgical debridement is often necessary given the potential for biofilm formation, which may allow for evasion of immune defense mechanisms and an ability to withstand antimicrobial therapy [6, 42]. Surgical intervention varies depending on the location of the infection with corneal scraping, unroofing of abscesses [7], and excimer phototherapeutic keratectomy described [43]. Flap lift and irrigation are commonly performed in post-LASIK cases [44, 45], with removal of corneal flap noted in 17.3% of AM cases [3]. When looking specifically at cases of post-LASIK AM keratitis, removal of the flap for resolution of the infections was needed in 80.3% of cases. Removal of the flap allowed for improved penetration of the topical antibiotics into the cornea and a shortened disease course [36, 46]. Flap removal results in a hyperopic shift, but vision has been shown to return to 20/50 with correction after healing [7, 37, 47].
Lamellar keratectomy or penetrating keratoplasty may be indicated in recalcitrant cases (Fig. 7.3). Penetrating keratoplasty was required in 14.1% of cases [3], in situations where there was no response to medical therapy or there were recurrent, severe exacerbations with attempted weaning of topical antibiotics [13]. Surgically, it is imperative to maintain clean margins as recurrence in the graft can occur if adequate margins are not obtained [10, 48]. In such cases, AM keratitis can recur in the corneal graft, and may ultimately require a regraft [20].
Untreated infections may extend from the deep stroma to the epithelium and cause a secondary breakdown of the corneal epithelium or may spread to internal ocular structures, highlighting the need for timely diagnosis and treatment [13]. Resolution of AM keratitis occurred in 80.9% of cases in one series, with visual acuity of 20/40 or better in 54.9% of cases, and visual acuity of 20/200 or worse in 19.6% [3]. In 1.3% of cases, there is loss of eye, and patients who undergo surgical interventions are more likely to end up with visual impairment [3]. Among cases that resolved without severe vision loss, 25.8% had a prolonged course that required either multiple medical therapies or more than one surgical intervention.
7.5 Conclusion
The majority of AM keratitis is caused by Runyon group IV mycobacteria and poses diagnostic and therapeutic challenges. A high degree of suspicion is needed to diagnose AM keratitis, with close attention in cases of keratitis in the setting of previous trauma or surgery or recalcitrance to traditional antibiotics. Specific cultures in addition to standard media may be needed, including acid-fast stains, Ziehl–Neelsen, auramine-rhodamine fluorescence, and fluorescent conjugated lectins. Special media such as Lowenstein–Jensen medium and Middlebrook 7H9 broth are useful, as well as PCR. Cultures should be kept for at least 2 weeks to detect slow-growing AM, but may need to be kept for up to 8 weeks. While AM keratitis is sensitive to topical antibiotics, it often requires combination therapy including amikacin, clarithromycin, and azithromycin. In addition, systemic antibiotics may need to be used adjunctively in many cases. Due to the development of biofilms, surgical debridement is sometimes needed for definitive management.
References
Garg P. Fungal, Mycobacterial, and Nocardia infections and the eye: an update. Eye. 2012;26(2):245–51.
Girgis DO, Karp CL, Miller D. Ocular infections caused by non-tuberculous mycobacteria: update on epidemiology and management. Clin Exp Ophthalmol. 2012;40(5):467–75.
Kheir WJ, Sheheitli H, Abdul Fattah M, Hamam RN. Nontuberculous mycobacterial ocular infections: a systematic review of the literature. Biomed Res Int. 2015;2015:164989.
Moorthy RS, Valluri S, Rao NA. Nontuberculous mycobacterial ocular and adnexal infections. Surv Ophthalmol. 2012;57(3):202–35.
Dugel PU, Holland GN, Brown HH, Pettit TH, Hofbauer JD, Simons KB, Ullman H, Bath PE, Foos RY. Mycobacterium fortuitum keratitis. Am J Ophthalmol. 1988;105(6):661–9.
Alvarenga L, Freitas D, Hofling-Lima AL, Belfort R Jr, Sampaio J, Sousa L, Yu M, Mannis M. Infectious post-LASIK crystalline keratopathy caused by nontuberculous mycobacteria. Cornea. 2002;21(4):426–9.
Chandra NS, Torres MF, Winthrop KL, Bruckner DA, Heidemann DG, Calvet HM, Yakrus M, Mondino BJ, Holland GN. Cluster of Mycobacterium chelonae keratitis cases following laser in-situ keratomileusis. Am J Ophthalmol. 2001;132(6):819–30.
Chang MA, Jain S, Azar DT. Infections following laser in situ keratomileusis: an integration of the published literature. Surv Ophthalmol. 2004;49(3):269–80.
Khooshabeh R, Grange JM, Yates MD, McCartney AC, Casey TA. A case report of Mycobacterium chelonae keratitis and a review of mycobacterial infections of the eye and orbit. Tuber Lung Dis. 1994;75(5):377–82.
Newman PE, Goodman RA, Waring GO 3rd, Finton RJ, Wilson LA, Wright J, Cavanagh HD. A cluster of cases of Mycobacterium chelonei keratitis associated with outpatient office procedures. Am J Ophthalmol. 1984;97(3):344–8.
Servat JJ, Ramos-Esteban JC, Tauber S, Bia FJ. Mycobacterium chelonae-Mycobacterium abscessus complex clear corneal wound infection with recurrent hypopyon and perforation after phacoemulsification and intraocular lens implantation. J Cataract Refract Surg. 2005;31(7):1448–51.
Mah-Sadorra JH, Cohen EJ, Rapuano CJ. Mycobacterium chelonae wound ulcer after clear-cornea cataract surgery. Arch Ophthalmol. 2004;122(12):1888–9.
Ford JG, Huang AJ, Pflugfelder SC, Alfonso EC, Forster RK, Miller D. Nontuberculous mycobacterial keratitis in south Florida. Ophthalmology. 1998;105(9):1652–8.
Paschal JF, Holland GN, Sison RF, Berlin OG, Bruckner DA, Dugel PU, Foos RY. Mycobacterium fortuitum keratitis. Clinicopathologic correlates and corticosteroid effects in an animal model. Cornea. 1992;11(6):493–9.
Palani D, Kulandai LT, Naraharirao MH, Guruswami S, Ramendra B. Application of polymerase chain reaction-based restriction fragment length polymorphism in typing ocular rapid-growing nontuberculous mycobacterial isolates from three patients with postoperative endophthalmitis. Cornea. 2007;26(6):729–35.
Valenton M. Wound infection after cataract surgery. Jpn J Ophthalmol. 1996;40(3):447–55.
Zimmerman LE, Turner L, McTigue JW. Mycobacterium fortuitum infection of the cornea. A report of two cases. Arch Ophthalmol. 1969;82(5):596–601.
Laflamme MY, Poisson M, Chéhadé N. Mycobacterium chelonei keratitis following penetrating keratoplasty. Can J Ophthalmol. 1987;22(3):178–80.
Busin M, Ponzin D, Arffa RC. Mycobacterium chelonae interface infection after endokeratoplasty. Am J Ophthalmol. 2003;135(3):393–5.
Aylward GW, Stacey AR, Marsh RJ. Mycobacterium chelonei infection of a corneal graft. Br J Ophthalmol. 1987;71(9):690–3.
Martinez JD, Amescua G, Lozano-Cárdenas J, Suh LH. Bilateral Mycobacterium chelonae keratitis after phacoemulsification cataract surgery. Case Rep Ophthalmol Med. 2017;2017:6413160.
Broadway DC, Kerr-Muir MG, Eykyn SJ, Pambakian H. Mycobacterium chelonei keratitis: a case report and review of previously reported cases. Eye. 1994;8(1):134–42.
Turner L, Stinson I. Mycobacterium fortuitum: as a cause of corneal ulcer. Am J Ophthalmol. 1965;60(2):329–31.
Levenson DS, Harrison CH. Mycobacterium fortuitum corneal ulcer. Arch Ophthalmol. 1966;75(2):189–91.
Bullington RH Jr, Lanier JD, Font RL. Nontuberculous mycobacterial keratitis. Report of two cases and review of the literature. Arch Ophthalmol. 1992;110(4):519–24.
Schönherr U, Naumann GO, Lang GK, Bialasiewicz AA. Sclerokeratitis caused by Mycobacterium marinum. Am J Ophthalmol. 1989;108(5):607–8.
Gangadharam PR, Lanier JD, Jones DE. Keratitis due to Mycobacterium chelonei. Tubercle. 1978;59(1):55–60.
Moore MB, Newton C, Kaufman HE. Chronic keratitis caused by Mycobacterium gordonae. Am J Ophthalmol. 1986;102(4):516–21.
Garg P, Athmanathan S, Rao GN. Mycobacterium chelonei masquerading as Corynebacterium in a case of infectious keratitis: a diagnostic dilemma. Cornea. 1998;17(2):230–2.
Katoch VM. Infections due to non-tuberculous mycobacteria (NTM). Indian J Med Res. 2004;120(4):290–304.
Brown-Elliott BA, Wallace RJ Jr. Clinical and taxonomic status of pathogenic nonpigmented or late-pigmenting rapidly growing mycobacteria. Clin Microbiol Rev. 2002;15(4):716–46.
Shrestha NK, Tuohy MJ, Hall GS, Reischl U, Gordon SM, Procop GW. Detection and differentiation of Mycobacterium tuberculosis and nontuberculous mycobacterial isolates by real-time PCR. J Clin Microbiol. 2003;41(11):5121–6.
Chen KH, Sheu MM, Lin SR. Rapid identification of mycobacteria to the species level by polymerase chain reaction and restriction enzyme analysis—a case report of corneal ulcer. Kaohsiung J Med Sci. 1997;13(9):583–8.
Willis WE, Laibson PR. Intractable Mycobacterium fortuitum corneal ulcer in man. Am J Ophthalmol. 1971;71(2):500–4.
Daines BS, Vroman DT, Sandoval HP, Steed LL, Solomon KD. Rapid diagnosis and treatment of mycobacterial keratitis after laser in situ keratomileusis. J Cataract Refract Surg. 2003;29(5):1014–8.
Freitas D, Alvarenga L, Sampaio J, Mannis M, Sato E, Sousa L, Vieira L, Yu MC, Martins MC, Hoffling-Lima A, Belfort R Jr. An outbreak of Mycobacterium chelonae infection after LASIK. Ophthalmology. 2003;110(2):276–85.
Fulcher SF, Fader RC, Rosa RH Jr, Holmes GP. Delayed-onset mycobacterial keratitis after LASIK. Cornea. 2002;21(6):546–54.
Winthrop KL, Steinberg EB, Holmes G, Kainer MA, Werner SB, Winquist A, Vugia DJ. Epidemic and sporadic cases of nontuberculous mycobacterial keratitis associated with laser in situ keratomileusis. Am J Ophthalmol. 2003;135(2):223–4.
John T, Velotta E. Nontuberculous (atypical) mycobacterial keratitis after LASIK: current status and clinical implications. Cornea. 2005;24(3):245–55.
Kuehne JJ, Yu AL, Holland GN, Ramaswamy A, Taban R, Mondino BJ, Yu F, Rayner SA, Giese MJ. Corneal pharmacokinetics of topically applied azithromycin and clarithromycin. Am J Ophthalmol. 2004;138(4):547–53.
Reddy AK, Garg P, Babu KH, Gopinathan U, Sharma S. In vitro antibiotic susceptibility of rapidly growing nontuberculous mycobacteria isolated from patients with microbial keratitis. Curr Eye Res. 2010;35(3):225–9.
Holland SP, Pulido JS, Miller D, Ellis B, Alfonso E, Scott M, Costerton JW. Biofilm and scleral buckle-associated infections. A mechanism for persistence. Ophthalmology. 1991;98(6):933–8.
Fogla R, Rao SK, Padmanabhan P. Interface keratitis due to Mycobacterium fortuitum following laser in situ keratomileusis. Indian J Ophthalmol. 2003;51(3):263–5.
Garg P, Bansal AK, Sharma S, Vemuganti GK. Bilateral infectious keratitis after laser in situ keratomileusis: a case report and review of the literature. Ophthalmology. 2001;108(1):121–5.
Solomon A, Karp CL, Miller D, Dubovy SR, Huang AJ, Culbertson WW. Mycobacterium interface keratitis after laser in situ keratomileusis. Ophthalmology. 2001;108(12):2201–8.
Seo KY, Lee JB, Lee K, Kim MJ, Choi KR, Kim EK. Non-tuberculous mycobacterial keratitis at the interface after laser in situ keratomileusis. J Refract Surg. 2002;18(1):81–5.
Chung MS, Goldstein MH, Driebe WT Jr, Schwartz BH. Mycobacterium chelonae keratitis after laser in situ keratomileusis successfully treated with medical therapy and flap removal. Am J Ophthalmol. 2000;129(3):382–4.
Mirate DJ, Hull DS, Steel JH Jr, Carter MJ. Mycobacterium chelonei keratitis: a case report. Br J Ophthalmol. 1983;67(5):324–6.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Mohammed, I.S.K., Jeng, B.H. (2021). Atypical Mycobacterial Keratitis. In: Das, S., Jhanji, V. (eds) Infections of the Cornea and Conjunctiva. Springer, Singapore. https://doi.org/10.1007/978-981-15-8811-2_7
Download citation
DOI: https://doi.org/10.1007/978-981-15-8811-2_7
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-15-8810-5
Online ISBN: 978-981-15-8811-2
eBook Packages: MedicineMedicine (R0)