Abstract
Introduction, Review of literature, Pathophysiology of venous thromboembolism (VTE) with cancer and Incidence of VTE in malignancy, Diagnosis of VTE, Diagnostic value, Clinical features of VTE, Cancer surgical risk groups, Caprini Score Model, and Preventive measures.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Introduction, Review of literature, Pathophysiology of venous thromboembolism (VTE) with cancer and Incidence of VTE in malignancy, Diagnosis of VTE, Diagnostic value, Clinical features of VTE, Cancer surgical risk groups, Caprini Score Model, and Preventive measures.
14.1 Introduction
To tell the truth that relationship between malignancy and thromboembolism has been a well-established fact but unfortunately pathophysiology is still not fully cleared. Trousseau is the person who reported migratory thrombophlebitis in gastric cancer patients in 1865 [1]. Since then a large number of evidence has been identified to showing the relationship between venous thromboembolism (VTE) and cancer. Despite significant advancement in the prevention of VTE, however it remains the most common preventable cause of hospital death in surgical patients [2]. It is well-known that Asian population is genetically quite different from US and European group. A large number of trails supports that Asians have low risk for DVT [3]. There is no Indian data from any major cancer centre reporting the incidence of post-operative cancer patients; hence there is no uniform policy to practice thromboprophylaxis in onco-surgery patients.
The very few literature available in India only two RCTs showed very low incidence of DVT after major abdominal surgeries [4, 5]. A prospective observational study conducted in 250 patients at Surgical Oncology Department at IRCH, AIIMS from 2013 to 2016 showed none of the patients who underwent complete resection (RO) for various cancers showed any evidence of VTE both clinically and radiologically. Post operatively patients were monitored closely for any signs of DVT. Bilateral Colour Doppler should be done by using all modes, on the post op day 7, 28 and earlier if VTE is suspected clinically. But without any doubt VTE is the captain of post-surgical death worldwide. Effective and newer prophylactic methods are now available for high-risks patients [6, 7] and different evidence based guidelines have been showing the way of preventing VTE [8, 9]. But most audits demonstrated that appropriate thromboprophylaxis is not being offered to large number of cancer surgical patients [10, 11].
14.2 Review of Literature
The complications of deep vein thrombosis (DVT), pulmonary embolism (PE), i.e. VTE and the post-thrombotic syndrome are important not only as the most common preventable cause of post-operative death in hospital but also important cause for long-term morbidity [12]. Proper understanding of underlying epidemiology, pathophysiology, and natural history of VTE is important in guiding appropriate prophylaxis for cancer surgery patients. National Comprehensive Cancer Network (NCCN) guidelines divided venous thromboembolism (VTE) broadly into deep vein thrombosis (DVT), pulmonary embolism (PE), superficial vein thrombosis (SVT), and thrombosis in other vascular territories (portal vein, mesenteric vein, inferior vena cava, and superior vena cava) [13].
A thrombus is a semisolid mass formed from the components of fibrin and red blood cells with a variable platelet and leukocyte component. A clot is nothing but blood which has coagulated in vitro (i.e. in a test tube). A DVT is a thrombus, which has formed in the deep veins beneath the deep fascia of the lower limb. Thrombus within the pelvic or abdominal veins that carry blood from the legs is also commonly classified as “deep vein thrombosis” and some would include thrombus in the communicating veins of the lower limb within the definition [12].
14.3 Pathophysiology of DVT
Over a period of time it has been realized that the formation of DVT is multifactorial, with components of Virchow’s triad as depicted below.
Virchow’s triad is well-known for this regard. It consists of (i) Stasis—abnormalities in blood flow such as immobilization, obesity, pregnancy, malignancies, paralysed patients (ii) Vessel wall injury—vascular endothelial injury due to surgery or venepuncture, hypertension, atherosclerosis, chronic inflammation, infection, etc., and (iii) Hypercoagulability—post-operative period, malignancies,pregnancy, etc.
14.4 Pathophysiology of VTE and Cancer
Various studies suggest the prothrombotic pathways of cancer molecular biology [14].
The thrombus formation in cancer is involving of multiple complicated pathways [15].
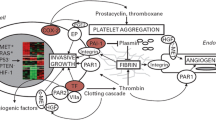
The association between cancer and thrombosis is well-known but pathophysiology remains poorly understood. Trousseau was first to recognize the association between thrombosis and malignancy and later his work was supported by Sack [14]. The genesis of thrombosis in onco-surgery is critical and reflects of multiple pathways including activation of procoagulants, inhibition of anticoagulant or fibrinolytic pathways and cytokine release [15].
14.5 Cell–Cell Interactions and Procoagulants
It is known that cancer cells express important factors for platelet adhesion. Studies have shown that tumour cells express glycoprotein Ib and glycoprotein IIb/IIIa (GP IIb/IIIa), which are key platelet adhesions molecules likewise, cancer has been associated with high levels of von Willebrand factor. Platelet adhesion to tumour cells via GP IIb/IIIa could play a key function in tumour spread. The main pathway for activation of the coagulation involves exposure of the tissue factor (TF) and endothelium. TF subsequently activates coagulation factor VIIa, which leads to conversion of prothrombin to thrombin.
It has been defined that tumour cells not only express TF, but also express normal cells, such as vascular endothelial cells, monocyte, and macrophages. Tumour cells also express a cysteine protease, cancer procoagulants that directly split factor X to Xa. Studies that used enzyme linked immunoabsorbent assay have shown increased cancer procoagulants levels in 81% of malignant patients. Accordingly, cancer procoagulant has been identified as a potential tumour marker.
Adhesions of platelet to tumour cells play an important role in tumour spreading. The main pathway for activation of coagulation pathway involves exposure of sub-endothelium and tissue factor (TF). Ultimately VIIa activated by this tissue factor which is responsible for the conversion of prothrombin to thrombin. Normal cells also can express TF. Tumour cells also express cancer procoagulants, cysteine protease which directly convert factor X to Xa (34,35). As per the literature around 81% of malignant patients having increased level of procoagulant in their blood and thus procoagulants have been acting as tumour markers.
14.6 Fibrinolysis
Fibrinolytic pathway playing the most important part in maintaining hemostatic balance. Tissue plasminogen activities and urokinase type plasminogen (UPA) activities converting plasminogen to plasmin. High levels of UPA (Urokinase type Plasminogen Activities) and its corresponding receptors (UPAR) and PAI are associated with malignancies. When the normal coagulation fibrinolytic balance in malignancy are affeted, bleeding in leukaemia patients and VTE episode occur in solid organ tumours.
14.7 Cytokines and Angiogenesis
The role of cytokines in tumour genesis is well-known. It is also established, angiogenesis plays very important role in tumour growth. These cytokines predispose to develop thrombosis.
VEGF, TNFα, IL1 all stimulate the expression of tissue factor on vascular endothelium leading to formation of thrombosis. Both TNFα and IL1 down regulate the expression of thrombomodulin. The thrombin and thrombomodulin complexes lead to activation of protein c, a strong anticoagulation!
Thus both the upregulation and downregulation of tissue factor produce a prothrombotic effect. On the other hand, IL1 and TNFα produce PAI by stimulating vascular endothelium, thereby there is increase propensity to form the clots.
14.8 Incidence of VTE in Malignancy
To tell the truth true incidence of VTE in cancer patients is not well understood still.
Silvertein et al. estimated a yearly incidence of VTE of 117 per 100,000 population but as far as cancer patients are concerned VTE rate increased 1 in 200 per year. It is more than 4 folds compared to non malignant patients [16]. Stein et al. showed that incidence of VTE is doubled in cancer patient (2% versus 1%) [17].
A study in Netherlands of 66,329 patients showed the cumulative incidence of DVT is 12.3 per 1000 population in initial 6 months [18].
Certain tumours like haematological and metastatic diseases are more prone to develop VTE [19]. Mucin producing cancers like ovarian carcinoma, colorectal cancers, and lung cancers are likely to be associated with VTE than other solid tumours [15].
Levitan et al. reported systematically the incidence of VTE with different cancers, e.g.—Ovarian ca (12 per 1000 patients), lymphoma (9.8 per 1000), pancreatic ca (11 per 1000 pts), brain tumour (11.7 per 1000 patients). They described lowest rates of VTE in Ca breast (2.2 per 1000 pts), Ca bladder (2.2 per 1000 pts), and in head and neck malignancy (1.6 per 1000 pts).
14.9 Diagnosis of DVT
Only 25% of patients of DVT present with compatible symptoms. Maximum patients may have minimal or atypical symptoms and clinical features.
Harmon’s test itself 30% sensitive only. So, a proper clinical assessment, history of varieties of risk factors, and sensitive diagnostic tests may confirm the diagnosis of DVT.
14.9.1 Symptoms
The symptoms that are commonly produced by deep vein thrombosis are pain, swelling, and a faint red blue discoloration of the skin. Profound cyanotic discoloration (phlegmasia cerulea dolens), or pallor (phlegmasia alba dolens) and frank venous gangrene are much less common. The more proximal and occlusive thrombus leads to more marked symptoms and physical signs. Deep vein thrombosis may also present as pyrexia of unknown origin or with the symptoms of pulmonary embolism without any leg symptoms.
14.9.2 Signs
The physical signs of a deep vein thrombosis may be as ephemeral as the symptoms, and often there are none. Diagnostic values of clinical features have been summarized in Table 14.1 and show that the clinical evaluation may imply the need for further evaluation but cannot, by itself, be relied on to confirm or exclude the diagnosis of DVT.
14.10 Diagnostic Value of Clinical Features of VTE
Clinical feature | Sensitivity (%) | Specificity (%) |
|---|---|---|
Calf pain | 66–91 | 3–87 |
Calf tenderness | 56–82 | 26–74 |
Homans’ sign | 13–48 | 39–84 |
Swelling of calf or leg | 35–97 | 8–88 |
14.11 Wells Clinical Probability Score (Table 14.1)
This is a scoring method that categorizes patients into high, intermediate, and low risk of DVT according to numerous defined criteria (outlined below). A score of ≥3 indicates high probability of DVT, 1 or 2 a moderate probability, and ≤ 0 indicates low probability.
Diagnostic Tests for DVT
-
1.
Non-invasive diagnostic tests include Duplex Ultrasound, Impedance Plethysmography, CT venography, MRI/MR venography.
-
2.
Invasive diagnostic tests: Contrast venography.
-
3.
Fibrinogen uptake test.
14.12 Biomarkers for the Diagnosis of Deep Vein Thrombosis
Gold standard for DVT diagnosis is compression ultrasound. Biomarkers and making a serological diagnosis are desirable. D-dimer, a highly sensitive biomarker, is very useful to exclude VTE. But it lacks of specificity.
The upcoming plasma biomarkers in the diagnosis of VTE are selectins, microparticles, IL 10, and other inflammatory markers. These inflammatory markers may also predict recurrence rate, thrombi which resolve spontaneously, and determine the therapy either standard anticoagulation or aggressive therapies.
14.13 Risk Factors for DVT
DVT occurring in the setting of a known risk factor is defined as secondary, whereas that occurring in the absence of risk factors is defined as primary or idiopathic. Risk factors can be further classified into acquired or congenital risk factors. (Table 14.2).
Cancer Surgical risk groups are:
-
1.
Increasing age.
-
2.
Past history of VTE.
-
3.
Family h/o VTE.
-
4.
H/o inherited or acquired hyper coagulable state.
-
5.
Obese patient.
-
6.
History of Chemotherapy 6.5 fold, Presence of mucin secreting cancer like ovary, colorectal, lung other like brain, pancreatic ca, pelvic malignancies 3–5 fold.
-
7.
More co-morbidities (like heart disease, infection, sepsis, chronic inflammatory disease, recent stroke, etc., more prone to develop VTE).
The risk of developing post-operative VTE also depends upon degree of invasiveness type and duration of surgery, Anaesthesia and requirement for immobilization [20]. As per world literature, in the absence of appropriate prophylaxis incidence of asymptomatic DVT is widely varied from 10–80% and total pulmonary embolism is 0.1 to 0.8 percent after effective general surgery [21]. In high-risk patient with Caprini score 5 or more and undergoing abdomino-pelvic surgery without prophylaxis, chance of VTE is approximately 6%.
14.14 Caprini Risk Scoring Method for the Risk Assessment (Table 14.3)
The Caprini score is calculated by adding the scores of all risk factors. The Caprini score is calculated in the following method:
-
Score 0–1: Low risk.
-
Score 2: Moderate.
-
Score 3–4: High risk.
-
Score ≥ 5: Highest risk.
14.15 Preventive Measures of VTE
There are two standard approaches to prevent VTE and PE.

Primary prophylaxis is a preferred method as it is safe, effective, and no need or limited need for laboratory monitoring.
And the secondary prevention is advised for patients in whom primary prophylaxis is either contraindicated or ineffective.
14.16 Primary Prophylaxis
-
1.
Early and frequent ambulation for all patients, maintenance of hydration, and prevention of sepsis.
-
2.
Mechanical methods are preferred in low-risk group (Caprini score 1 to 2) and patients with a contraindication to pharmacologic prophylaxis.
-
3.
Pharmacologic prophylaxis is preferred in surgical patients at moderate- and high-risk patients (Caprini score ≥ 3).
-
4.
Combined pharmacologic and mechanical methods (usually intermittent pneumatic compression) are considered for very high-risk patients (Caprini score ≥ 5).
Pharmacologic Agents for VTE Prevention
Various drugs are now available for VTE prevention, including unfractionated heparin, the LMW heparins, fondaparinux, the vitamin K antagonists, and the newer antithrombotic agents rivaroxaban, dabigatran, and apixaban. These will be discussed below. When available, meta-analyses of the comparative effectiveness among these various agents will also be discussed.
Pharmacological agents usually used:
-
1.
UFH: LMW (Low Molecular Weight) heparins, Daltaparin and Fondaparinux, are preferred than UFH. UFH is safe in renal function derangement and cost is Rs 100–150/dose (5000 IU).
-
(a)
Daltaparin (Fragmin) in moderate- to high-risk cancer patients—2500 IU subcutaneously 1–2 hr. before surgery followed by 2500 IU 8–12 h later and then.
Once daily post-operative dose of 5000 IU subcutaneously for 5–10 days or till the time of discharge depending upon the risk score. Contraindication: Renal insufficiency.
Coagulation profiles like PT and APTT are relatively insensitive measures of Daltaparin, therefore, unsuitable for monitoring the anticoagulant effect. Creatinine clearance should be monitored and the cost for single dose (5000 IU) is Rs 400–600/− in Indian currency.
-
(b)
Fondaparinux: Prophylactic dose 2.5 mg s/c to be started 6–8 h after surgery. Once daily for 5–10 days or till discharge of the patient. Cost Rs 1050–1300/dose (2.5 mg s/c).
-
(c)
Enoxparin: 30–40 mg s/c once daily starting 12 h after surgery. Next time Low dose unfractionated Heparin (UFH) is used where LMW is contraindicated, i.e. in renal insufficiency and where cost is an issue. Cost 175–250/dose (40 mg).
Thrombocytopenia to be monitored routinely.
-
(d)
Warferin may be advised as an alternative to LMWH and UFH when delayed prophylaxis is planned.
14.17 Comparison among all Type of Heparins (Table 14.3)
Comparison of Agents
To compare the agents across studies of hip or knee surgery have been difficult, since the drugs under investigation and the dosing schedules have varied between trials. Even in the similar clinical trial there can be considerable variability. In addition, bleeding rates have varied across the trails, at least in part because different definitions for bleeding have been used.
A number of randomized trials have compared LMW heparin with UFH, warfarin or acenocoumarol, or fondaparinux in patients undergoing total hip replacement (THR), and to a lesser extent total knee replacement (TKR). A meta-analysis compared vitamin K antagonists versus LMW heparin for the prevention of VTE in orthopaedic surgery revealed that the vitamin K antagonists are less effective than LMW heparin, without any remarkable difference in bleeding risk (Table 14.4).
In general, LMW heparin has been shown to be superior to UFH or warfarin, but inferior to fondaparinux in terms of efficacy, with similar bleeding rates in patients undergoing THR OR TKR surgeries.
The use of LMW heparin enoxaparin differs between regions. Thus:
-
In North America, enoxaparin at a dose of 30 mg twice daily started after 12 to 24 h after surgery.
-
In Europe, enoxaparin at a dose of 40 mg is started 12 h after surgery and is then given once daily.
-
Other LMW heparin preparations have usually been given in a once daily dose, started after surgical procedure.
A meta-analysis in patients underwent surgery for cancer concluded that there was no difference between LMWH and UFH in terms of efficacy, DVT location, or bleeding complications.
A Cochrane review of the use of LMW heparin to prevent VTE in surgical patients with lower immobilization concluded that LMW heparin in outpatients effectively reduced the VTE incidence. A further meta-analysis reviewed the use of intermittent pneumatic compression (IPC) with or without pharmacologic prophylaxis. It was shown that, compared with IPC alone, combined prophylactic methods reduced the VTE incidence.
In neurosurgical procedures, LMW heparin was shown to be effective then IPC. In major trauma management, LMW heparin was effective than UFH in the prevention of DVTs.
Timing of Prophylaxis
Recommended either before or immediately after surgical procedure and continued until the patient is mobile.
In moderate- and high-risk patients LMWH started either 12 h before surgery or 18 to 24 h after surgery.
The term extended prophylaxis is used by ACCP & NCCN and ASCO—for a very high-risk patients (Caprini score > 5) where prophylaxis may be extended 10–35 days. It is recommended for a period of four weeks.
Other drugs used are—direct thrombin & Xa inhibitor, Rivaroxaban, DabigationXilate, Apixaban, Endoxaban, etc.
Mechanical Methods
-
1.
Intermittent pneumatic compression (IPC)—It enhances blood flow in the deep veins in the leg, thereby preventing venous stasis.
It reduces plasminogen activator inhibitor-1 (PAI-1) thereby increasing endogenous fibrinolytic activity [22].
Among all mechanical devices, efficacy of IPC appears best [23].
-
2.
A graded compression stocking (GCS)—GCS when combined with other prophylactic modalities appears to improve rate of DVT prevention.
-
3.
Venous foot pump (VFP)—Like GCS, it is used combined with other prophylactic methods.
Inferior vena cava (IVC) filters: In general IVC filters should be avoided as primary prophylaxis. The indication for filter placement as a therapy for DVT. (Figs. 14.1 and 14.2).
14.18 Summary and Recommendations
Every hospital may develop a formal strategy for the prevention of VTE for their surgical patients. Strategies should be developed with proper thromboprophylaxis recommendations, including authorized order sets, periodic audit, follow-up with feedback.
The article “Prevention of venous Thromboembolism” ACCP evidenced based clinical practice guidelines (eighth Edition) is a recommended guideline for the prevention of VTE which may be useful in formulating these policies [8].
Number of attempt has been made to develop risk assessment models for VTE in individual patients. At this time, none of the risk stratification models has been validated in prospective trials, although this subject is under active study.
In patients with additional risk factors (e.g. previous H/O VTE, advanced age particularly >75 years, active cancer or a history of cancer, a more extensive surgical procedure) consideration should be given to more aggressive prophylaxis in the form of increased intensity or duration of a pharmacologic agent, or the addition of intermittent pneumatic compression (IPC) [8].
In patients from specific ethnic groups in which the incidence of post-surgical venous thromboembolism is low (e.g. Asian populations), consideration may be given to less aggressive prophylaxis.
Assignment of surgical risk groups: The risk of post-operative VTE depends upon the surgical procedure (e.g. type and duration of anaesthesia and surgery, requirement for post-operative immobilization), as well as patient-related factors (e.g. increasing age, prior VTE, presence of cancer or obesity, presence of an inherited or acquired hypercoagulable state). Patients have been generally divided into low-, moderate-, and high-risk categories.
Low-risk general and abdominal-pelvic surgery: For low-risk surgery (Caprini score 1 to 2) the use of mechanical prophylaxis is preferred, over no prophylaxis or prophylactic anticoagulation.
Moderate-risk general and abdominal-pelvic surgeries: For moderate-risk general and abdominal-pelvic surgery (Caprini score 3 to 4) the recommend method is the use of prophylactic anticoagulation over no prophylaxis.
High-risk general and abdominal-pelvic surgery: For high-risk general and oncologic abdominal-pelvic surgery (Caprini score 5 or more) the use of prophylactic anticoagulation is recommended. Reasonable choices include LMW heparin, UFH in renal insufficiency, or Fondaparinux.
Length of treatment: For moderate-risk patients undergoing major general and abdominal-pelvic surgeries, the recommendation is that continue thromboprophylaxis until hospital discharge, rather than for a shorter or longer period.
14.19 Timing of Regional Anaesthesia/Analgesia: (Cork University Hospital, Version 1 Guideline 2015)
UFC (subcutaneous)
-
Should wait minimum four hours after a dose prior to block or catheter removal.
-
Should wait minimum one hour prior to dosing after procedure (catheter insertion or withdrawal).
UFH (intravenous)
-
Infusion should be stopped 2–4 h prior to block.
-
Start infusion > one hr. after block.
-
Remove epidural catheter not before 2–4 h after discontinuation of infusion.
LMWH
-
Should wait minimum twelve hours after a prophylaxis dose before block.
-
Should wait minimum 24 h after a therapeutic dose before block.
-
Should wait minimum ten hours after dose before removing catheter.
-
After catheter removal wait 2–4 h before next dose.
Conclusion
For selected high-risk general and abdominal-pelvic surgery patients, the suggestion that continuing thromboprophylaxis after hospitalization with LMW heparin for up to 4 weeks be considered, minimum till the patient discharges from the hospital and ask the patient to keep on moving at home, not to be at bed always.
Further trails involving various regional cancer centres with huge sample size may provide further epidemiological data related to VTE in onco-surgery patients. So continue efforts to be made to find the most effective and safest method to prevent and manage VTE.
References
Trousseau A. Phlegmasia alba dolens. In: Clinique Medicale de L’Hotel-Dieu Paris. London: New Sydenham Society; 1865. p. 94–6.
Lindblad B, Eriksson A, Bergqvist D. Autopsy-verified pulmonary embolism in a surgical department: analysis of the period from 1951 to 1988. Br J Surg. 1991;78:849.
Klatsky AL, Baer D. What protects Asians from venous thromboembolism? Am J Med. 2004 Apr 1;116(7):493–5.
Murugesan A, Srivastava DN, Ballehaninna UK, Chumber S, Dhar A, Misra MC, et al. Detection and prevention of post-operative deep vein thrombosis [DVT] using Nadroparin among patients undergoing major abdominal operations in India; a randomised controlled trial. Indian J Surg. 2010 Aug;72(4):312–7.
Shukla PJ, Siddachari R, Ahire S, Arya S, Ramani S, Barreto SG, et al. Postoperative deep vein thrombosis in patients with colorectal cancer. Indian J Gastroenterol Off J Indian Soc Gastroenterol. 2008 Apr;27(2):71–3.
Clagett GP, Reisch JS. Prevention of venous thromboembolism in general surgical patients. Results of meta-analysis. Ann Surg. 1988;208:227.
Nurmohamed MT, Rosendaal FR, Büller HR, et al. Low-molecular-weight heparin versus standard heparin in general and orthopaedic surgery: a meta-analysis. Lancet. 1992;340:152.
Geerts WH, Bergqvist D, Pineo GF, et al. Prevention of venous thromboembolism: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th edition). Chest. 2008;133:381S.
Lyman GH, Khorana AA, Falanga A, et al. American Society of Clinical Oncology guideline: recommendations for venous thromboembolism prophylaxis and treatment in patients with cancer. J Clin Oncol. 2007;25:5490.
Cohen AT, Tapson VF, Bergmann JF, et al. Venous thromboembolism risk and prophylaxis in the acute hospital care setting (ENDORSE study): a multinational cross-sectional study. Lancet. 2008;371:387.
Muntz J. Duration of deep vein thrombosis prophylaxis in the surgical patient and its relation to quality issues. Am J Surg. 2010;200:413.
Rathbun S. The surgeon General’s call to action to prevent deep vein thrombosis and pulmonary embolism. Circulation. 2009 Apr 21;119(15):e480–2.
National. Cancer-associated venous thromboembolic disease [Internet]. NCCN version 2.2014; [cited 2015 Apr 26]. Available from http://www.nccn.org
Nijziel MR, van Oerle R, Hillen HFP, Hamulyák K. From Trousseau to angiogenesis: the link between the haemostatic system and cancer. Neth J Med. 2006 Dec;64(11):403–10.
Caine GJ, Stonelake PS, Lip GYH, Kehoe ST. The hypercoagulable state of malignancy: pathogenesis and current debate. Neoplasia N Y N. 2002 Dec;4(6):465–73.
Heit JA, Silverstein MD, Mohr DN, Petterson TM, O’Fallon WM, Melton LJ. Risk factors for deep vein thrombosis and pulmonary embolism: a population-based case-control study. Arch Intern Med. 2000 Mar 27;160(6):809–15.
Stein PD, Beemath A, Meyers FA, Skaf E, Sanchez J, Olson RE. Incidence of venous thromboembolism in patients hospitalized with cancer. Am J Med. 2006 Jan;119(1):60–8.
Blom JW, Vanderschoot JPM, Oostindiër MJ, Osanto S, van der Meer FJM, Rosendaal FR. Incidence of venous thrombosis in a large cohort of 66,329 cancer patients: results of a record linkage study. J ThrombHaemost. 2006 Mar;4(3):529–35.
Barbui T, Finazzi G, Falanga A. The impact of all-trans-retinoic acid on the coagulopathy of acute promyelocytic leukemia. Blood. 1998 May 1;91(9):3093–102.
Donzé JD, Ridker PM, Finlayson SR, Bates DW. Impact of sepsis on risk of postoperative arterial and venous thromboses: large prospective cohort study. BMJ. 2014;349:g5334.
Geerts WH, Pineo GF, Heit JA, et al. Prevention of venous thromboembolism: the seventh ACCP conference on antithrombotic and thrombolytic therapy. Chest. 2004;338S:126.
Comerota AJ, Chouhan V, Harada RN, et al. The fibrinolytic effects of intermittent pneumatic compression: mechanism of enhanced fibrinolysis. Ann Surg. 1997;226:306.
Gould MK, Garcia DA, Wren SM, et al. Prevention of VTE in nonorthopedic surgical patients: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141:e227S.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Ray, M.D. (2021). DVT: Prophylaxis and Management. In: Ray, M.D. (eds) Multidisciplinary Approach to Surgical Oncology Patients. Springer, Singapore. https://doi.org/10.1007/978-981-15-7699-7_14
Download citation
DOI: https://doi.org/10.1007/978-981-15-7699-7_14
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-15-7698-0
Online ISBN: 978-981-15-7699-7
eBook Packages: MedicineMedicine (R0)