Abstract
Environment plays an indispensable role in the way an organism responds and behaves. Our body responds to different environment cues and handles them by producing various kinds of signal molecules. The behavior of an organism is in fact controlled by the individual cells which make up the organism and the behavior of the cells ultimately reflects in the behavior of the organism. The same is true for stem cells which make up the entire organism and also reside in niche of each organ. This chapter aims to summarize the existing knowledge and research conducted in the field of stem cells response to surrounding macro- and micro-environment. Micro-environment or “the niche” plays an important role in controlling the behavior of cells which is further affected by the macro-environment.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
1 Brief History of Stem Cells
Robert Hooke in the year 1665 for the first time discovered and coined the term “cell.” This discovery further led to the development of multifaceted scientific approaches aiming to unravel the secrets of cell division, growth, and organization into complete organisms.
In 1868, Ernst Haeckel, for the first time used Stammzelle to describe unicellular ancestral organism as the source of evolution for all the existing multicellular organisms. In 1877, he further postulated that the fertilized egg is also a stem cell as it gives rise to all the cells of an organism. Following this in 1892, Theodor Boveri and Valentin Häcker conducted experiments on Ascaris and Cyclops, respectively, and postulated that the cells between the fertilized egg and committed germ cells can be considered as stem cells. It was during the nineteenth century that the pathologists Durante and Conheim for the first time hypothesized the existence of stem cells in adults as “embryonal rests” in order to include the survival of embryonic-like stem cells in adult tissues (Sell 2004). In 1981, Martin Evans and group set an important milestone by establishing an in vitro culture for non-malignant pluripotent cells derived from mouse embryos (Evans and Kaufman 1981). During the same year Gail Martin too isolated cells from the mouse embryo and named “stem cells,” since it seemed that the isolated cells could grow into nearly all cell types (Martin 1981).
The fertilized egg, which is considered to be the ultimate stem cell, is formed from the fusion of haploid germinal stem cells. In adults, the tissues are renewed by proliferation of the stem cell pool, which divide stem cells. In adults, the tissues are renewed by proliferation of the stem cell pool, which divide asymmetrically yielding an adult stem cell and a progenitor cell. The progenitor cell divides further to ultimately produce terminally differentiated cells of the tissue with a limited lifespan in the tissues which are lost by wear or through apoptosis.
The differentiating progeny of the stem cells leads to tissue renewal. It has been demonstrated that stem cells have the ability to produce progenitor cells for tissue repair and renewal which opens up vast panorama of applications in the regenerative biology. Determination or lineage commitment is a specialized function acquired by stem cells during differentiation. As the stem cells differentiate to give rise to specialized cells, with decreasing differentiation potential and finally to the terminally differentiated cells. Classic embryology considers determination as an irreversible process; once a cell is differentiated into adult, it is completely stable and not able to revert to stemness (Surani 2001). However, it has been demonstrated by the researchers that this differentiated cells can be reversed to stemness a process called “de-differentiation.” One way to do de-differentiation is through Somatic Cell Nuclear Transfer (SCNT) where a nucleus is transferred from differentiated adult cell into an enucleated oocyte which results in restoration of totipotency of the nucleus in the oocyte (Hakelien and Collas 2002; Hochedlinger and Jaenisch 2002; Surani 2001; Wilmut et al. 1997).
More recently Shinya Yamanaka has developed the concept of iPSCs (Induced Pluripotent Stem Cells). His lab has developed a methodology to reprogram the differentiated cells into stem cells which they have referred to as—induced pluripotent stem cells (iPSCs) (Takahashi and Yamanaka 2006). iPSCs have the potential to present themselves as an ideal source of stem cells source for cell-based therapy and regenerative medicine in future (Novak et al. 2010).
Embryologists and developmental biologists have known the power of stem cells for tissue development, regeneration, and renewal for many years (Sell 2004). In the recent years, a lot of research and development work has been focussed on understanding the biology of stem cells derived from different sources. Researchers are working on different aspects of stem cells including differentiation, trans-differentiation, and de-differentiation and more recently the work is initiated on iPSCs and their role in regenerative medicine. All these developments have brought a lot of hope and opened new avenues for stem cell applications in treatment for different degenerative diseases. The field is developing at a rapid pace; we hope to see a lot of cell-based treatment modalities for degenerative diseases in near future, which is altogether set to change our current perception about a disease scenario.
2 Micro-environment of Stem Cell: The Niche
The word “niche” has different meaning in different contexts, it refers to a habitat where an organism can reside and reproduce in ecology (Scadden 2006).
Schofield for the first time in 1978 used the term “stem cell niche” describing the whole picture of stem cells survival and their interaction with the neighboring cells (Wang et al. 2016; Wang and Kingshott 2016). Stem cells produce their own matrix and milieu with the specialized micro-environment. The microenvironment of the stem cells is very complex and dynamic structure which is very much required for the self-renewal and differentiation of these cells. Due to complex structural organization of the stem cell niche it has not been characterized completely. In Drosophila and Caenorhabditis elegans the first germline stem cell (GSC) niche has been defined (De Cuevas and Matunis 2011). Several stem cell niches have been identified in different tissues including the hematopoietic stem cells (HSCs), the germline stem cells (GSCs) in ovaries and testes and MSCs in bone marrow, stem cells in the epithelia muscle satellite cells in the skeletal muscle, neural stem cells in the brain, dental pulp stem cells in the teeth, and even some cancer stem cell niches (Lane et al. 2014; Xie and Li 2007). Bone marrow is considerably the most nourishing niche for hematopoietic (HSCs) and mesenchymal stem cells (MSCs), but human HSCs can be alternatively isolated from the peripheral blood, umbilical cord, and umbilical cord blood. HSCs differentiate to form all the cells of the myeloid and lymphoid lineage (Zhang et al. 2003), whereas MSCs can differentiate into plethora of cell types including osteocytes, chondrocytes, stromal cells, cells of myotube, and connective tissue. In vitro, MSCs can be differentiated outside their lineage, which means stem cells of mesodermal origin can give rise to the cells of the endodermal or ectodermal origin, this is called trans-differentiation or stem cell plasticity (Lv et al. 2014).
One of the most important limiting steps in stem cells culture in vitro is to mimic the stem cell micro-environment in vivo. Absence of proper culture systems and protocols, normal growth of stem cells in in vitro is difficult and their differentiation potential is compromised which leads to altered state or even apoptosis (De Cuevas and Matunis 2011).
There are several structural features common between the stem cell niches of different tissues and species, however, there are difference in the functional aspects of these niches (Lane et al. 2014). Structurally the stem cell niche is highly dynamic, with multiple cell types involving complex biochemical and biophysical interactions (Ema 2012). There are niches which consist of one or few cell types which locally regulate the stem cells, whereas there are niches which consist of multiple cell types which are involved in systemic regulation. These niches act as stem cells shelters where they maintain quiescence and self-renewal (Ema 2012).
3 Components of Stem Cell Niche
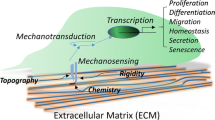
3.1 Extra-Cellular Matrix (ECM)
ECM is the essential component of the stem cell niche and plays an important role in regulating the stem cell fate (Frantz et al. 2010; Xie and Li 2007). ECM provides space and physical support to stem cells to anchor and orient (Kerever et al. 2007). Understanding and analyzing the structure and composition of ECM is important to mimic its function to make the products for synthetic cell culture. One approach to study the ECM structure and its organization is through tissue decellularization process. Decellularization eliminates the cellular components from the tissue leaving the ECM intact which can be further studied microscopically (Badylak et al. 2011; Baptista et al. 2011; Batchelder et al. 2015; Moorman et al. 2003; Sabetkish et al. 2014).
3.2 Surface Receptors Proteins
Anchorage dependent cells interact with their ECM by means of cell surface receptors proteins, namely integrins, selectins, and cadherins. Integrins are heterodimeric transmembrane molecules (α and β subunits) involved in cell adhesion, migration, differentiation, and even in apoptosis (Le Gall et al. 1998). The commonly expressed subunits in most human cells are β1, α5, and αV. Integrin are expressed ubiquitously in almost all the stem cells. Integrins have the ability to bind directly to the ECM proteins such as laminin, collagen, vitronectin, and fibronectin. (Assoian 1997).
Cadherins, the second type of surface receptor proteins, are regulate cell–cell interactions (Chen et al. 2013). The subunit E (epithelial) cadherin (CDH1) is best studied and is involved in adhesion of stem cell niche. Maintenance of the self-renewal potential of stem cells and retention of nondifferentiated cells in the niche are mediated via interaction with E-cadherin (Moore and Lemischka 2006).
N (neural)-cadherin (CDH2) which is another subtype of cadherins is expressed during the neuronal plate development. In the HSC niche, both HSCs and osteoblasts express N-cadherin. N-cadherin in multiple studies has also been reported to affect the self-renewal potential of HSCs in the niche (Moore and Lemischka 2006). However, few studies are emerging which counter indicate this by showing that the expression of N-cadherin in HSCs is not necessary for niche function (Kiel et al. 2009).
The satellite stem cells in the skeletal muscle niche express a third subtype of cadherin molecule M (myotubule)-cadherin (CDH15). Which is expressed at the site facing the muscle fibers (Moore and Lemischka 2006).
Apart from integrins, cadherins, selectins, and cadherins, other cell surface receptors have also been described to be crucial for stem cell niche interactions which include EGF, frizzled, Notch, TGF-ß, c-kits, gap junctions, VCAM 1, and CD44 (Chen et al. 2013)
3.3 Basement Membrane
Basement membrane is thin and densely organized sheet like structure consisting of self-organized ECM surrounding most of the animal tissues. Recent studies have demonstrated that basement membrane is a component of stem cell niches in many tissues including testis of Drosophila, mammary gland, epidermis, ovary and gut, and prostate (Hall et al. 2006; Kendrick et al. 2008; Lawson et al. 2007; O’Reilly et al. 2008; Ohlstein and Spradling 2006; Shackleton et al. 2006; Tanentzapf et al. 2007; Tumbar et al. 2004).
Laminins are the key proteins forming a component of the basement membrane proteins and consist of α6-containing integrin dimers which serve as receptors for the laminin protein. Studies have proven that stem cell properties are altered on disruption of the stem cell niche. For example, Lechler and Fuchs in 2005 demonstrated that on deleting β1 integrin or α-catenin from basal keratinocytes the orientation of the cell division plane changes resulting in altered epidermal homeostasis. Research has shown that in some mammalian tissues which include brain, intestine, mammary glands, and prostate gland, stem cells are in close contact to the basement membrane. In mammalian intestine these stem cells are localized at the base of the crypts and are in direct contact with the basement membrane (Barker et al. 2008; Haegebarth and Clevers 2009; Walker and Stappenbeck 2008). Similarly, stem cells in mammary and prostate glands reside in the basal compartment of the epithelium and interact with the underlying basement membrane directly (Lawson et al. 2007). Basement membrane-like structures in the neurogenic zones of the brain have been shown by Krever et al (2007).
4 Stem Cell a Target for Environmental Pollution
Extensive studies on the effect of environmental pollution on stem cells properties and accumulation of vast information has led to the development of new branch of toxicology, i.e. stem cell toxicology.
4.1 Stem Cell Toxicology
It is the branch of toxicology aiming to provide solution to evaluate the cellular, developmental, reproductive, and functional toxicity of the pollutant of interest using stem cells as a model system mimicking the human physiology in vitro (Faiola et al. 2015). Several pollutants such as industrial waste, pesticides, drugs, cosmetics, food additives, radiations, smoking, and atmospheric fine particles are continuously produced in surrounding environment and adversely affects human health (Yao et al. 2016). Most of these pollutants are either slow degrading or stable in nature as a result of which they tend to accumulate in the environment. The harmful effects of these toxic chemicals on health of humans have raised a growing concern about the urgency and necessity to implement rapid, sensitive, cost-effective, novel, and high-throughput screening tests which can assess toxicity of these toxic pollutants. (Yao et al. 2016). Earlier most of the toxicity screening test for pollutants or drugs were dependent on the animal models. In 1959 Russell and Burch postulated the theory of “high fidelity fallacy,” which states that experimental animals based toxicity assays are not always translatable to human health (Russell and Burch 1959) due to inter-specific variations. For example, numerous drugs which passed the animal testing during the development process failed during the clinical trials. The principles of alternative toxicology are mainly based on in vitro studies, i.e. 3Rs (Replacement, Reduction, and Refinement) may be more important today than ever before (Gibb 2008; Krewski et al. 2008; Russell and Burch 1959).
Though animal models and in vitro cell culture systems pose several limitations including time consumption, intensive resourcing, and ethical concerns (Krewski et al. 2008), most of the toxicity screenings and research in industries and research institutes are still relying on it. Although toxicological screening systems based on in vitro human models of fast growing immortalized or cancer cell lines present a solution to this problem, these are not true representatives of the native tissue due to accumulation of mutations or altered cell functions during expansion. Toxicity screening system based on primary human cell cultures present a better option but has limitations as these cells have limited growth and proliferative potential in culture (Yao et al. 2016). Together these issues can significantly restrict the reproducibility of the tests, generation of data and its interpretation (McNeish 2004). Generally, these in vitro assays rely on the response of a single cell type and are unable to indicate correct information about the toxicological responses at the level of tissues or even whole organism where there is a heterogenous population of cells (Krewski et al. 2008).
Recently, Faiola et al. (2015) demonstrated that toxicology studies based on stem cells could be a quick, powerful, and cost-effective screening system in detecting the developmental toxicity of environmental pollutants (Jennings 2015). Human stem cell-based toxicology studies present an efficient, quick, and specific toxicity screening, establishing it as an effective model to animal experimental testing or conventional toxicity assays because it utilizes the potential of stem cells to differentiate into various cell types and tissues present in body relating it more closely to humans (Yao et al. 2016). Other advantages include less time consumption, low cost, and higher accuracy than tests using animals.
4.2 Somatic Stem Cell Toxicology
Apart from embryonic stem cell testing, adult stem cells also called somatic stem cells are utilized in the toxicological studies in vitro. Somatic cells represent the entire cell pool of an organism excluding the germline cells, while somatic stem cells (SSCs) are the stem cells located in the adult tissues. In the adult tissue niches, these SSCs tend to remain quiescent and maintain homeostasis at very low turnover rate. After tissue injury these cells get triggered to repair damaged tissues through multiple fold turnover of self-renewal and differentiation (Blau et al. 2015; Engstrom et al. 2015; Wang et al. 2011). With physiological aging of the body, there is a progressive disruption in the tissue homeostasis and gradual loss of the ability of SSCs to repair the damaged differentiated cells (Blau et al. 2015). With reduced repair potential of the SSCs the environmental pollutants can either cause an irreversible damage to the tissues which cannot be repaired sufficiently by proliferation and differentiation of SSCs or they can target SSCs directly, causing their exhaustion resulting their premature aging/or pathological state, including cancer and eventually death (Wilhelm Engström et al. 2015). Due to the limited differentiation potential of SSCs in comparison to ESCs, they are not utilized in embryotoxicity studies and teratogenic experiments. However, SSCs have the ability differentiate and self-renew into somatic stem cells during the periods of infancy and adolescence, thus finding their use in evaluating the detrimental effects of environmental toxins and pollutants during post-natal development of an adult organism. It was during 1980 that Robert M. Pratt and his colleagues for the first time demonstrated the application of SSCs in toxicological testing by using human embryonic palate derived for prescreening of environmental teratogens (Pratt et al. 1982). Further, Cao and colleagues first time evaluated the use of hMSCs for in vitro screening of cytotoxic chemicals for assessing the LD50 (Lethal Dose, 50%) values and in categorizing the hazardous status of the tested chemicals in accordance to the globally harmonized system of classification (GHS) (Scanu et al. 2011). Findings from these studies demonstrated that hMSCs could serve as precise model for mimicking the in vivo conditions in comparison to the previously established tests such as Normal Human Keratinocyte/Neutral Red Uptake methods and validated 3T3 cell test. Thus, primary tissue-derived or PSC-derived (ESC or iPSC) SSCs can be utilized in vitro for assessing the harmful effects of pollutants on the development of infants and adolescents into adults. SSC-based injury or disease models can also be used in specific applications where the toxic effects of chemical during tissue repair following injury or degenerative diseases are to be assessed. They can also be used in determining the effects of pollutants on stem cell aging and exhaustion.
Akhavan and group (Akhavan et al. 2012) evaluated the toxicity of graphene based materials on SSCs. They demonstrated that reduced graphene oxide nanoplatelets at low concentrations (0.1 mg/mL) exerted genotoxic effects on hMSCs as a result of chromosomal aberrations and DNA fragmentation. Later, Strong and colleagues studied the toxic effect of endocrine-disrupting chemical dichlorodiphenyltrichloroethane (DDT) on SSCs. The groups treated hMSCs with DDT and revealed significant modifications in the stem cell properties including proliferation, self-renewal, differentiation (adipogenic and osteogenic lineages), and in gene expression, which could partly justify the homeostatic imbalance caused and increased risk of cancer occurrence among the individuals exposed to the chemical (Amy et al. 2014).
In another study, Tamm and his group demonstrated that primary human embryonic cortical stem cells and C17.2, a neural stem cell line expressed high sensitivity on exposure to methylmercury (MeHg) at low levels. Their findings indicated the effects of MeHg on survival, proliferation and differentiation of these cells, offers new avenues for studying the biological outcomes of low level exposure of this chemical using highly sensitive and reliable in vitro models (Tamm et al. 2006). In 2009, Buzanska and group (Buzanska et al. 2009) established an umbilical cord blood derived neural stem cell line and used it for testing the neural development based toxicity by analyzing parameters such as cellular proliferation, neuronal and glial differentiation, and apoptosis.
It can be widely accepted that, by employing these stem cell model for extensive in vitro testing for identifying the significant toxicity mechanisms at the cellular and molecular levels on human biology, we would be able to eliminate the requirement of animal testing and would be able to cater decent environment-friendly and healthy decision-making in future.
5 Stem Cells, Environment, and Cancer Risk
One of the key factors in the battle against cancer is understanding and determining the underlying cause for this devastating disease. The potential target of cancer and site of occurrence of cancer is usually defined with the help of environmental and genetic factors (Zhao 2015). Recently, a study by Wu and group has confirmed the role of environmental factors including ionizing radiations, ultraviolet radiations, and carcinogens in causing cancer (Wu et al. 2015)
According to their calculations, intrinsic (random errors in DNA replication) risk can be effectively determined by the lower bound risk accounting for total stem cell divisions and the internal processes are not adequate to account for the cancer risks observed. Also, there have been overwhelming scientific evidences which establish genetic and environmental factors as critical players in the development of cancer (Zhao 2015).
6 Embryonic Stem Cell Test
Embryonic Stem Cell Test uses mouse embryonic stem cells (mESCs) as model system to (Seiler et al. 2004; Seiler and Spielmann 2011) test the embryotoxicity of pharmaceutical compounds in vitro. This technique was pioneered by Horst Spielmann in 1991. During the initial stages of development of the assay it was not precise with poor rate of prediction. It was only during 1995–2004 that the European Centre for the Validation of Alternative Methods (ECVAM) nominated, described, and approved (Genschow et al. 2002; Spielmann et al. 2008) EST as the in vitro assay for embryotoxicity. Since then, this assay has been utilized for drug screening (Paquette et al. 2008; Whitlow et al. 2007) to study the toxicity of a panels of related compounds on development (de Jong et al. 2009) and even for assessing embryotoxicity of nanomaterials (Di Guglielmo et al. 2010). Establishment of EST is considered a milestone in the history of stem cell toxicology testing because of its advantage over in vitro and pre-clinical tests (Genschow et al. 2002).
Even though considered as a very effective assay for toxicology testing, EST, did have several weaknesses such as for assessing myocardial differentiation, microscopic observation was the sole means for studying the beating areas whereas no metabolic tests for analysis were not available (Spielmann et al. 2006). Further, limitation in the EST prediction model was highlighted by another study which was sponsored by a ECVAM and ReProTect where EST based analysis was able to classify only 15% of previously untested compounds correctly (Marx-Stoelting 2009).
With the development and technological advancements in the field of biotechnology accompanied with the emergence of new molecular techniques and tools, modifications in the EST assay were proposed which could overcome the existing limitations. For evaluating beating cardiomyocytes, the visual microscopic evaluation was replaced by highly sensitive and specific techniques such as fluorescence activated cell sorting (FACS) and reverse transcription quantitative PCR (RT–qPCR) providing more quantitative molecular endpoints analysis in terms of gene and protein expression of myocardial markers (Bigot et al. 1999; Buesen et al. 2009; Pellizzer et al. 2004b; Riebeling et al. 2011; Seiler et al. 2004; Seiler 2006; Zur Nieden et al. 2001).
Further, to assess the toxicity of pollutants on various tissue and organs tests were developed on the basis of the potential of stem cells to differentiate into multiple lineages including pancreatic, neuronal, osteogenic, and skeletal muscle lineages (Schmidt et al. 2001; Mori and Hara 2013; Pellizzer et al. 2004a, b; Rolletschek et al. 2004). Based on the new advancements, in 2004 a new EST subtype referred as molecular multiple-endpoint EST developed, which not only included traditional cardiomyocyte differentiation as an assay parameter but also incorporated quantitative analysis using RT-qPCR and multilineage stem cell differentiation-based assessment as additions (Zur Nieden et al. 2004).
Above described ESTs used differentiated fibroblasts (3T3 cell line) and ESCs as assay model. For simplifying and improving the reproducibility of EST based testing, development of advanced procedures relying exclusively on ESCs were targeted. High-throughput toxicological analysis techniques such as miRNA and whole genome profiling using techniques like microarrays and mass spectrometry were incorporated.
Moreover, the advantage of ESCs over cancerous and other cell types is that they can be used in the development of toxicity assay. For instance, ESCs have ability to grow into three-dimensional cell aggregates the so-called organoids. These organoids or embryoid bodies (EBs) can be considered as miniature organs as these are useful in mimicking the early stages of embryonic development occurring in vivo (Wobus and Loser 2011) (Liu et al. 2013; Mori and Hara 2013).
As the results of toxicological analysis performed using mESCs could not be applied to humans directly due to inter-generic variations, Cezar and group introduced use human embryonic stem cells (hESCs) as model in toxicology testing (Gabriela et al. 2007) to overcome the drawbacks associated with mESCs. They performed small metabolite-based profiling using mass spectrometry and demonstrated that hESCs and hESC-derived cell types (for example, neural precursors) exposed to pollutants could be helpful in elucidating the molecular mechanisms associated with toxicity. On performing metabolomic profiling using hESC model, biomarkers for developmental toxicity were identified and it was demonstrated that the teratogenicity was correctly predicted for 88% of drugs and 83% of environmental toxicants.
Further, fibroblasts derived from hESCs were utilized for in vitro toxicology screening and it was shown that hESCs derived fibroblasts displayed more sensitive dose response to mitomycin C treatment in comparison to other in vitro models such as human lung fibroblast L929 cells (Cao et al. 2008). Several studies utilized hESCs for specifically assessing toxic effects of drugs or compounds on neural differentiation only.
7 Artificial Niche
Harrison et al. in 1907 reported the first cell culture. Since then, with the advent of new technology and scientific advancements there has been a tremendous improvement in the techniques of cell culture. These advancement culture methods include mouse ESCs culture established by Evans (Evans and Kaufman 1981), culture of human ESC by Thomson (Thomson et al. 1998), mouse iPSC culture establishment of by Shinya Yamanaka (Takahashi and Yamanaka 2006), and recent establishment of human iPSC culture by Yamanaka and Thomson (Takahashi et al. 2007; Yu et al. 2007).
With establishment of new and advanced culture techniques it has been accepted that culture protocols require standardization depending upon the cell type. For example, in case of umbilical cord blood and bone marrow derived MSCs culture, tissue culture plate surface (TCPS) using a specific cell culture medium is sufficient for expansion of MSCs. The TCPS properties allow for the adsorption of the serum proteins present in medium (e.g. fibronectin, vitronectin, etc.) on the TCPS thereby supporting cellular adherence. Alternatively, while using human ESCs, iPSC and several other sensitive cultures including neural stem cells, an ECM protein pre-coated surface is required.
Although TCPS with various surface treatments are commercially available which provide charged surfaces and low cellular attachment, these surfaces do not replicate the true physical and structural cues which are important for determining the correct cell fate. More complicated culture protocols are required involving early progenitor cells (e.g. ESCs or GSCs) where stem cell niche interactions are important for regulating their fate in vitro. Though stem cells can be conveniently cultured and expanded on 2D synthetic surfaces, there is a gradual loss in their ability to self-renew, proliferate, and differentiate into tissue specific cells. Hence, to mimic the stem cell niches in vitro the minimum requirement is a complex micro-environment including a cell specific surface with topographical distribution of physical cues to support cell attachment and movement such as matrigel for 3D organoid culture or flexible surfaces for differentiation into contractile muscle cells or even complex cell culture approaches like 3D culture, co-culture, dynamic cultures, physical stimulation, or multiple combinations of the described approaches. It is clear that the existing tools and strategies for culture are limited and require improvement for the progress of our understanding about the cell functions on different surface and strategies for controlled differentiation into clinically relevant cell types. The key factors regulating cells fate in vitro are medium composition, surface chemistry, and substrate surface topography. A plethora of reports have been published describing the standardization of the above mentioned factors to effectively mimic stem cell niches in vitro (Ding et al. 2016; Schuldiner et al. 2000).
Recent development of recombinant proteins have proven to be an improvement over the currently used mouse embryonic fibroblasts (MEF) supported cultures (e.g., M of human PSCs. Being sensitive, PSCs require complex medium with additional growth factors and ECM to support their self-renewal in vitro compared to other more differentiated cells. Although several serum free media compositions have been developed and available commercially in conjunction with modified TCPS with ECM or cell binding coatings but they still might not be able to maintain the native state of these PSCs (Theunissen et al. 2014).
References
Akhavan O, Ghaderi E, Akhavan A (2012) Size-dependent genotoxicity of graphene nanoplatelets in human stem cells. Biomaterials 33:8017–8025
Amy LS, Zhenzhen S, Michael JS, David FBM, Douglas BR, Aaron MB, Erik KF, John AM, Kenneth PN, Matthew EB, Bruce AB (2014) Effects of the endocrine-disrupting chemical DDT on self-renewal and differentiation of human Mesenchymal stem cells. Environ Health Perspect 123:42–48
Assoian RK (1997) Anchorage-dependent cell cycle progression. J Cell Biol 136:1–4
Badylak SF, Taylor D, Uygun K (2011) Whole-organ tissue engineering: decellularization and recellularization of three-dimensional matrix scaffolds. Annu Rev Biomed Eng 13:27–53
Baptista PM, Siddiqui MM, Lozier G, Rodriguez SR, Atala A, Soker S (2011) The use of whole organ decellularization for the generation of a vascularized liver organoid. Hepatology 53:604–617
Barker N, van de Wetering M, Clevers H (2008) The intestinal stem cell. Genes Dev 22:1856–1864
Batchelder CA, Martinez ML, Tarantal AF (2015) Natural scaffolds for renal differentiation of human embryonic stem cells for kidney tissue engineering. PLoS One 10:e0143849
Bigot K, de Lange J, Archer G, Clothier R, Bremer S (1999) The relative semi-quantification of mRNA expression as a useful toxicological endpoint for the identification of Embryotoxic/Teratogenic substances. Toxicol In Vitro 13:619–623
Blau HM, Cosgrove BD, Ho AT (2015) The central role of muscle stem cells in regenerative failure with aging. Nat Med 21:854–862
Buesen R, Genschow E, Slawik B, Visan A, Spielmann H, Luch A, Seiler A (2009) Embryonic stem cell test remastered: comparison between the validated EST and the new molecular FACS-EST for assessing developmental toxicity in vitro. Toxicol Sci 108:389–400
Buzanska L, Sypecka J, Nerini-Molteni S, Compagnoni A, Hogberg HT, del Torchio R, Domanska-Janik K, Zimmer J, Coecke S (2009) A human stem cell-based model for identifying adverse effects of organic and inorganic chemicals on the developing nervous system. Stem Cells 27:2591–2601
Cao T, Lu K, Fu X, Heng BC (2008) Differentiated fibroblastic progenies of human embryonic stem cells for toxicology screening. Cloning Stem Cells 10:1–10
Chen S, Lewallen M, Xie T (2013) Adhesion in the stem cell niche: biological roles and regulation. Development 140:255–265
De Cuevas M, Matunis EL (2011) The stem cell niche: lessons from the Drosophila testis. Development 138:2861–2869
de Jong E, Louisse J, Verwei M, Blaauboer BJ, van de Sandt JJ, Woutersen RA, Rietjens IM, Piersma AH (2009) Relative developmental toxicity of glycol ether alkoxy acid metabolites in the embryonic stem cell test as compared with the in vivo potency of their parent compounds. Toxicol Sci 110:117–124
Di Guglielmo C, Lopez DR, De Lapuente J, Mallafre JM, Suarez MB (2010) Embryotoxicity of cobalt ferrite and gold nanoparticles: a first in vitro approach. Reprod Toxicol 30:271–276
Ding S, Kingshott P, Thissen H, Pera M, Wang PY (2016) Modulation of human mesenchymal and pluripotent stem cell behavior using biophysical and biochemical cues: a review. Biotechnol Bioeng 114:260–280
Ema HS, Suda T (2012) Two anatomically distinct niches regulate stem cell activity. Blood 120:2174–2181
Engstrom W, Darbre P, Eriksson S, Gulliver L, Hultman T, Karamouzis MV, Klaunig JE, Mehta R, Moorwood K, Sanderson T, Sone H, Vadgama P, Wagemaker G, Ward A, Singh N, Al-Mulla F, Al-Temaimi R, Amedei A, Colacci AM, Vaccari M, Mondello C, Scovassi AI, Raju J, Hamid RA, Memeo L, Forte S, Roy R, Woodrick J, Salem HK, Ryan EP, Brown DG, Bisson WH (2015) The potential for chemical mixtures from the environment to enable the cancer hallmark of sustained proliferative signalling. Carcinogenesis 36(Suppl 1):S38–S60
Engström W, Darbre P, Eriksson S, Gulliver L, Hultman T, Karamouzis MV, Klaunig JE, Mehta R, Moorwood K, Sanderson T, Sone H, Vadgama P, Wagemaker G, Ward A, Singh N, Al-Mulla F, Al-Temaimi R, Amedei A, Colacci AM, Vaccari M, Mondello C, Scovassi AI, Raju J, Hamid RA, Memeo L, Forte S, Roy R, Woodrick J, Salem HK, Ryan EP, Brown DG, Bisson WH (2015) The potential for chemical mixtures from the environment to enable the cancer hallmark of sustained proliferative signalling. Carcinogenesis 36(Suppl 1):S38–S60
Evans MJ, Kaufman MH (1981) Establishment in culture of pluripotential cells from mouse embryos. Nature 292:154–156
Faiola F, Yin N, Yao X, Jiang G (2015) The rise of stem cell toxicology. Environ Sci Technol 49:5847–5848
Frantz C, Stewart KM, Weaver VM (2010) The extracellular matrix at a glance. J Cell Sci 123:4195–4200
Gabriela GC, Jessica AQ, Alan MS, Guilherme JMR, Marian SP, James FB, Fred HG, Alysson RM (2007) Identification of small molecules from human embryonic stem cells using metabolomics. Stem Cells Dev 16:869–882
Genschow E, Spielmann H, Scholz G, Seiler A, Brown N, Piersma A, Brady M, Clemann N, Huuskonen H, Paillard F, Bremer S, Becker K (2002) The ECVAM international validation study on in vitro embryotoxicity tests: results of the definitive phase and evaluation of prediction models. European Centre for the Validation of alternative methods. Altern Lab Anim 30:151–176
Gibb S (2008) Toxicity testing in the 21st century: a vision and a strategy. Reprod Toxicol 25:136–138
Haegebarth A, Clevers H (2009) Wnt signaling, lgr5, and stem cells in the intestine and skin. Am J Pathol 174:715–721
Hakelien AM, Collas P (2002) Novel approaches to transdifferentiation. Cloning Stem Cells 4:379–387
Hall PE, Lathia JD, Miller NG, Caldwell MA, Ffrench-Constant C (2006) Integrins are markers of human neural stem cells. Stem Cells 24:2078–2084
Hochedlinger K, Jaenisch R (2002) Nuclear transplantation: lessons from frogs and mice. Curr Opin Cell Biol 14:741–748
Jennings P. (2015). "The future of in vitro toxicology". Toxicol In Vitro 29:1217-1221.
Kendrick H, Regan JL, Magnay FA, Grigoriadis A, Mitsopoulos C, Zvelebil M, Smalley MJ (2008) Transcriptome analysis of mammary epithelial subpopulations identifies novel determinants of lineage commitment and cell fate. BMC Genomics 9:591
Kerever ASJ, Vellinga D, Ichikawa N, Moon C, Arikawa-Hirasawa E, Efird JT, Mercier F (2007) Novel extracellular matrix structures in the neural stem cell niche capture the neurogenic factor fibroblast growth factor 2 from the extracellular milieu. Stem Cells 25(9):2146–2157
Kiel MJAM, Radice GL, Morrison SJ (2009) Hematopoietic stem cells do not depend on N-cadherin to regulate their maintenance. Cell Stem Cell 4:170–179
Krewski D, Acosta D Jr, Andersen M, Anderson H, Bailar JC 3rd, Boekelheide K, Brent R, Charnley G, Cheung VG, Green S Jr, Kelsey KT, Kerkvliet NI, Li AA, McCray L, Meyer O, Patterson RD, Pennie W, Scala RA, Solomon GM, Stephens M, Yager J, Zeise L (2008) Toxicity testing in the 21st century: a vision and a strategy. J Toxicol Environ Health B Crit Rev 13:51–138
Lane SW, Williams DA, Watt FM (2014) Modulating the stem cell niche for tissue regeneration. Nat Biotechnol 32:795–803
Lawson DA, Xin L, Lukacs RU, Cheng D, Witte ON (2007) Isolation and functional characterization of murine prostate stem cells. Proc Natl Acad Sci U S A 104:181–186
Le Gall M, Grall D, Chambard JC, Pouyssegur J, Van Obberghen-Schilling E (1998) An anchorage-dependent signal distinct from p42/44 MAP kinase activation is required for cell cycle progression. Oncogene 17:1271–1277
Liu W, Y Deng, Y Liu, W Gong and W Deng. (2013). Stem cell models for drug discovery and toxicology studies. J Biochem Mol Toxicol 27:17-27.
Lv FJ, Tuan RS, Cheung KM, Leung VY (2014) Concise review: the surface markers and identity of human mesenchymal stem cells. Stem Cells 32:1408–1419
Martin GR (1981) Isolation of a pluripotent cell line from early mouse embryos cultured in medium conditioned by teratocarcinoma stem cells. Proc Natl Acad Sci U S A 78:7634–7638
Marx-Stoelting P (2009) A review of the implementation of the embryonic stem cell test (EST). The report and recommendations of an ECVAM/ReProTect workshop. Altern Lab Anim 37:313–328
McNeish J (2004) Embryonic stem cells in drug discovery. Nat Rev Drug Discov 3:70–80
Moore KA, Lemischka IR (2006) Stem cells and their niches. Science 311:1880–1885
Moorman A, Webb S, Brown NA, Lamers W, Anderson RH (2003) Development of the heart: (1) formation of the cardiac chambers and arterial trunks. Heart 89:806–814
Mori H, Hara M (2013) Cultured stem cells as tools for toxicological assays. J Biosci Bioeng 116:647–652
Novak A, Shtrichman R, Germanguz I, Segev H, Zeevi-Levin N, Fishman B, Mandel YE, Barad L, Domev H, Kotton D, Mostoslavsky G, Binah O, Itskovitz-Eldor J (2010) Enhanced reprogramming and cardiac differentiation of human keratinocytes derived from plucked hair follicles, using a single excisable Lentivirus. Cell Reprogram 12:665–678
O’Reilly AM, Lee HH, Simon MA (2008) Integrins control the positioning and proliferation of follicle stem cells in the Drosophila ovary. J Cell Biol 182:801–815
Ohlstein B, Spradling A (2006) The adult Drosophila posterior midgut is maintained by pluripotent stem cells. Nature 439:470–474
Paquette JA, Kumpf SW, Streck RD, Thomson JJ, Chapin RE, Stedman DB (2008) Assessment of the embryonic stem cell test and application and use in the pharmaceutical industry. Birth Defects Res B Dev Reprod Toxicol 83:104–111
Pellizzer C, Adler S, Corvi R, Hartung T, Bremer S (2004a) Monitoring of teratogenic effects in vitro by analysing a selected gene expression pattern. Toxicol In Vitro 18:325–335
Pellizzer C, Bello E, Adler S, Hartung T, Bremer S (2004b) Detection of tissue-specific effects by methotrexate on differentiating mouse embryonic stem cells. Birth Defects Res B Dev Reprod Toxicol 71:331–341
Pratt RM, Grove RI, Willis WD (1982) Prescreening for environmental teratogens using cultured mesenchymal cells from the human embryonic palate. Teratog Carcinog Mutagen 2:313–318
Riebeling C, Pirow R, Becker K, Buesen R, Eikel D, Kaltenhãuser J, Meyer F, Nau H, Slawik B, Visan A, Volland J, Spielmann H, Luch A, Seiler A (2011) The embryonic stem cell test as tool to assess structure-dependent teratogenicity: the case of Valproic acid. Toxicol Sci 120:360–370
Rolletschek A, Blyszczuk P, Wobus AM (2004) Embryonic stem cell-derived cardiac, neuronal and pancreatic cells as model systems to study toxicological effects. Toxicol Lett 149:361–369
Russell WMS, Burch RL (1959) The principles of humane experimental technique. Methuen, In. London
Sabetkish S, Kajbafzadeh AM, Sabetkish N, Khorramirouz R, Akbarzadeh A, Seyedian SL, Pasalar P, Orangian S, Beigi RS, Aryan Z, Akbari H, Tavangar SM (2014) Whole-organ tissue engineering: decellularization and recellularization of three-dimensional matrix liver scaffolds. J Biomed Mater Res A 103:1498–1508
Scadden D (2006) The stem-cell niche as an entity of action. Nature 441(7097):1075–1079
Scanu M, Mancuso L, Cao G (2011) Evaluation of the use of human Mesenchymal stem cells for acute toxicity tests. Toxicol In Vitro 25:1989–1995
Schmidt MM, Guan KAOMEI, Wobus AM (2001) Lithium influences differentiation and tissue-specific gene expression of mouse embryonic stem (ES) cells in vitro. Int J Dev Biol 45:421–429
Schuldiner M, Yanuka O, Itskovitz-Eldor J, Melton DA, Benvenisty N (2000) Effects of eight growth factors on the differentiation of cells derived from human embryonic stem cells. Proc Natl Acad Sci U S A 97:11307–11312
Seiler AE (2006) Use of murine embryonic stem cells in embryotoxicity assays: the embryonic stem cell test. Methods Mol Biol 329:371–395
Seiler AE, Spielmann H (2011) The validated embryonic stem cell test to predict embryotoxicity in vitro. Nat Protoc 6:961–978
Seiler A, Visan A, Buesen R, Genschow E, Spielmann H (2004) Improvement of an in vitro stem cell assay for developmental toxicity: the use of molecular endpoints in the embryonic stem cell test. Reprod Toxicol 18:231–240
Sell S (2004) Stem cells what are they? Where do they come from? Why are they Here? When do they go wrong? Where are they going? In: Stem cell handbook. Humana Press Inc., Totowa, pp 1–18
Shackleton M, Vaillant F, Simpson KJ, Stingl J, Smyth GK, Asselin-Labat ML, Wu L, Lindeman GJ, Visvader JE (2006) Generation of a functional mammary gland from a single stem cell. Nature 439:84–88
Spielmann H, Seiler A, Bremer S, Hareng L, Hartung T, Ahr H, Faustman E, Haas U, Moffat GJ, Nau H, Vanparys P, Piersma A, Sintes JR, Stuart J (2006) The practical application of three validated in vitro embryotoxicity tests. The report and recommendations of an ECVAM/ZEBET workshop (ECVAM workshop 57). Altern Lab Anim 34:527–538
Spielmann H, Grune B, Liebsch M, Seiler A, Vogel R (2008) Successful validation of in vitro methods in toxicology by ZEBET, the National Centre for alternatives in Germany at the BfR (Federal Institute for Risk Assessment). Exp Toxicol Pathol 60:225–233
Surani MA (2001) Reprogramming of genome function through epigenetic inheritance. Nature 414:122–128
Takahashi K, Yamanaka S (2006) Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 126:663–676
Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S (2007) Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 131:861–872
Tamm C, Duckworth J, Hermanson O, Ceccatelli S (2006) High susceptibility of neural stem cells to methylmercury toxicity: effects on cell survival and neuronal differentiation. J Neurochem 97:69–78
Tanentzapf G, Devenport D, Godt D, Brown NH (2007) Integrin-dependent anchoring of a stem-cell niche. Nat Cell Biol 9:1413–1418
Theunissen TW, Powell BE, Wang H, Mitalipova M, Faddah DA, Reddy J, Fan ZP, Maetzel D, Ganz K, Shi L, Lungjangwa T, Imsoonthornruksa S, Stelzer Y, Rangarajan S, D’Alessio A, Zhang J, Gao Q, Dawlaty MM, Young RA, Gray NS, Jaenisch R (2014) Systematic identification of culture conditions for induction and maintenance of naive human pluripotency. Cell Stem Cell 15:524–526
Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, Jones JM (1998) Embryonic stem cell lines derived from human blastocysts. Science 282:1145–1147
Tumbar T, Guasch G, Greco V, Blanpain C, Lowry WE, Rendl M, Fuchs E (2004) Defining the epithelial stem cell niche in skin. Science 303:359–363
Walker MR, Stappenbeck TS (2008) Deciphering the ‘black box’ of the intestinal stem cell niche: taking direction from other systems. Curr Opin Gastroenterol 24:115–120
Wang YZ, Plane JM, Jiang P, Zhou CJ, Deng W (2011) Concise review: quiescent and active states of endogenous adult neural stem cells: identification and characterization. Stem Cells 29:907–912
Wang P-Y, Thissen H, Kingshott P (2016) Modulation of human multipotent and pluripotent stem cells using surface nanotopographies and surface-immobilised bioactive signals: a review. Acta Biomater 45:31–59
Whitlow S, Burgin H, Clemann N (2007) The embryonic stem cell test for the early selection of pharmaceutical compounds. ALTEX 24:3–7
Wilmut I, Schnieke AE, McWhir J, Kind AJ, Campbell KH (1997) Viable offspring derived from fetal and adult mammalian cells. Nature 385:810–813
Wobus AM, Loser P (2011) Present state and future perspectives of using pluripotent stem cells in toxicology research. Arch Toxicol 85:79–117
Wu S, Powers S, Zhu W, Hannun YA (2015) Substantial contribution of extrinsic risk factors to cancer development. Nature 529:43–47
Xie T, Li L (2007) Stem cells and their niche: an inseparable relationship. Development 134:2001–2006
Yao X, Yin N, Faiola F (2016) Stem cell toxicology: a powerful tool to assess pollution effects on human health. Natl Sci Rev 3:430–450
Yu J, Vodyanik MA, Smuga-Otto K, Antosiewicz-Bourget J, Frane JL, Tian S, Nie J, Jonsdottir GA, Ruotti V, Stewart R, Slukvin II, Thomson JA (2007) Induced pluripotent stem cell lines derived from human somatic cells. Science 318:1917–1920
Zhang J, Niu C, Ye L, Huang H, He X, Tong WG, Ross J, Haug J, Johnson T, Feng JQ, Harris S, Wiedemann LM, Mishina Y, Li L (2003) Identification of the haematopoietic stem cell niche and control of the niche size. Nature 425:836–841
Zhao AH (2015) Stem cells, environment, and cancer risk. Stem Cell Invest 2:24
Zur Nieden NI, Ruf LJ, Kempka G, Hildebrand H, Ahr HJ (2001) Molecular markers in embryonic stem cells. Toxicol In Vitro 15:455–461
Zur Nieden NI, Kempka G, Ahr HJ (2004) Molecular multiple endpoint embryonic stem cell test--a possible approach to test for the teratogenic potential of compounds. Toxicol Appl Pharmacol 194:257–269
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Kumar, A., Jain, K.G., Arora, V. (2021). Environmental Interaction and Impact on the Life Span of Stem Cells. In: Singh, A., Srivastava, S., Rathore, D., Pant, D. (eds) Environmental Microbiology and Biotechnology. Springer, Singapore. https://doi.org/10.1007/978-981-15-7493-1_12
Download citation
DOI: https://doi.org/10.1007/978-981-15-7493-1_12
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-15-7492-4
Online ISBN: 978-981-15-7493-1
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)