Abstract
Fundus fluorescein angiography is useful to evaluate the advancement and effect of treatment of ROP. The findings to focus on are: position and extent of pathologic vascular proliferation, activity of fibrovascular proliferation, angioplany of the retina, absence of capillary network of the retina, extent of photocoagulation, and stabilizing of the vascular activity after treatments.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Fluorescein angiography
- Pathological new vessel
- Plus disease
- Retinal detachment
- Aggressive posterior ROP
- Photocoagulation
- Vitrectomy
Because retinopathy of prematurity is a vascular disease, fundus fluorescein angiography (FFA) is an essential tool to evaluate the condition of the disease. Pathological new vessels, retinal capillary network, and circulation dynamics are much more accurately observed with FFA than routine ophthalmoscopy and fundus photography. Minimal new vessel, capillary dropout, activity, or stabilization of vascular proliferation are often determined by only FFA.
Advantages and points to be evaluated with FFA are as follows:
-
Position and extent of pathologic vascular proliferation
-
Activity of pathologic vascular proliferation, identified by leakage of fluorescein dye
-
Vascular pattern of the retina, including abnormal branching, anastomosis, shunt, tortuosity, circulation speed
-
Capillary network of the retina, which is absent not only in the non-vascularized retina but often partially in the vascularized retina
-
Extent of photocoagulation already applied and spanning area
Use of wide-field digital imaging system is recommended to observe the periphery of the retina. 0.1 ml/kg body weight of 10% fluorescein dye is intravenously injected. Consecutive intravenous injection with 1 ml saline facilitates to put a small amount of dye into the systemic circulation. The intelligibility of images is adjusted by the photographic sensitivity of the CCD image sensor. Monitoring of vital signs by neonatologists or anesthesiologists is necessary during the examination (Fig. 10.1).
To determine the early stage of ROP, vascular shunt and pathological proliferation of primitive vessels in the retina cannot be identified without FFA. Vascular tufts, early intravitreal budding of pathologic new vessels also are easily identified (Figs. 10.2, 10.3, and 10.4). When ROP progresses, the range and extent of fibrovascular proliferation increase (Figs. 10.5, 10.6, 10.7, and 10.8). At an advanced stage, plus disease, tortuosity, and dilation of retinal vessels suggests insufficient retinal circulation. The partial absence of capillary network of retinal vessels is also seen in the retina already vascularized, which needs excessive photocoagulation (Fig. 10.8). When retinal detachment begins to occur, early sign is stretching and tractional elevation of retinal vessels due to dragging of the retina by fibrovascular tissues (Figs. 10.9 and 10.10). Distinctly different from classical ROP that processes in a sequence order of stage 1–4, aggressive posterior ROP rashly develop to stage 4 and 5, which needs early and extensive intervention of photocoagulation and vitreoretinal surgery (Fig. 10.11). Intraretinal vascular proliferation and dropout of the capillary network are important early signs of aggressive posterior ROP (Fig. 10.12). Stabilization of vascular activity in ROP, even spontaneously or after intervention of treatments, is determined by decreasing or disappearance of fluorescein dye leakage (Figs. 10.13 and 10.14).
Stage 1 ROP with demarcation line. The demarcation line is formed at the developing end of retinal vessels. Along the demarcation line, circumferential hyperfluorescence is seen at the developing end of the retinal vessels, just posterior to the demarcation line, where immature vessels form shunts. Retinal vessels at the developing end are multi-branched. Fluorescein dye does not leak from the demarcation line
Stage 2 ROP with ridge. Demarcation line changes to a thick ridge with intraretinal proliferation of primitive vascular endothelia. Posterior to the ridge, immature retinal vessels form shunts. Vascular tufts, and small clumps of intravitreal proliferation of pathological new vessels, arise from the retina posterior to the ridge. Leakage of fluorescein dye does not occur from the ridge and vascular shunt but from the vascular tufts
Stage 3 ROP with severe fibrovascular proliferation. Fibrovascular proliferation at the developing end of retinal vessels and also in its posterior portion. Partial dropout of capillary vessels is seen in the vascularized retina. Tortuosity of retinal arteries and dilation of veins indicates plus disease
Progression of fibrovascular proliferation after photocoagulation. Fibrovascular tissue that contains numerous pathological new vessels and connective tissues develops even after the application of photocoagulation. Retinal vessels posterior to the fibrous tissues begin to be dragged by contraction of the fibrovascular tissues. Significant leakage of fluorescein dye indicates vascular activity in the fibrovascular tissues. Tortuosity of retinal arteries and dilation of veins are the signs of plus disease, also indicating the activity of ROP. These findings are an indication for vitreoretinal surgery
Stage 4A ROP with mild fibrovascular proliferation. Significant fibrovascular proliferation, where connective tissues contract, causing traction retinal detachment, despite the application of photocoagulation. Dragging of the retina by the fibrovascular tissues in anteroposterior and circumferential directions is apprehensible by tractional displacement of retinal vessels
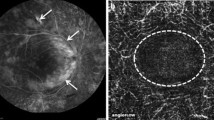
Early phase of aggressive posterior ROP. Although pathological vascular proliferation in the retina is still mild, the capillary network of the retinal vessels is absent in the entire retina, suggesting diffuse obstruction of the capillary vessels. Such finding of severe ischemia is predictive of severe development of vascular proliferation and retinal detachment in the future
Pre- and post-lens sparing vitrectomy for classic ROP. (a) Pre-surgery. Vascular proliferation that shows fluorescein leakage is not stabilized after the application of photocoagulation. Traction retinal detachment has not occurred yet. Born at 23 weeks gestational age, with 495 g body weight. Photocoagulation was applied at 32 weeks corrected gestational age and lens-sparing vitrectomy at 40 weeks. (b) Post-surgery. Part of fibrovascular tissues and vitreous gel around them were removed by vitrectomy, and residual tissues regressed 2 weeks after vitrectomy. Leakage of fluorescein dye decreased, suggesting early stabilization of ROP
Pre- and post-vitrectomy with lensectomy for aggressive posterior ROP. (a) Pre-surgery. Fibrovascular proliferation progresses, which shows prominent leakage of fluorescein dye, even though dense photocoagulation had been applied. Traction retinal detachment occurs underneath the fibrovascular tissues. Dilation of retinal vessels also shows the activity of ROP. Birth at 28 weeks gestational age, with 733gm body weight. Photocoagulation was applied at 33 weeks corrected gestational age and vitrectomy with lensectomy at 36 weeks. (b) Post-surgery. Vitreous gel was mostly removed by vitrectomy. Dissection of the fibrous tissues was minimized to avoid bleeding. Because removal of vitreous gel between the fibrovascular tissue and vitreous base was necessary, lensectomy was simultaneously performed. Fibrovascular tissues regressed 2 weeks after surgery, suggested by minimal leakage of fluorescein dye. The retina underneath was reattached. Improvement of retinal vein dilation also suggests regression of ROP
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Azuma, N. (2021). FA in ROP. In: Wu, WC., Lam, WC. (eds) A Quick Guide to Pediatric Retina. Springer, Singapore. https://doi.org/10.1007/978-981-15-6552-6_10
Download citation
DOI: https://doi.org/10.1007/978-981-15-6552-6_10
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-15-6551-9
Online ISBN: 978-981-15-6552-6
eBook Packages: MedicineMedicine (R0)