Abstract
Parkinson’s disease (PD) is a multifactorial neurodegenerative disease that disturbs the dopamine neural circuit by affecting the basal nuclei. It is symptomized as motor and cognitive disturbances. Standard therapeutic regimen exhibits improvement in motor symptoms but is ineffectual in reversing the condition and delaying the progression of the disease. Thus, alternative approaches have been pursued in various areas. This perspective analyzes the various strategies studied and researched for treatment of PD along with contributions in the treatment of PD.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
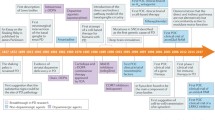
Parkinsonism or Parkinson’s disease (PD) is a progressive condition related to aging. After Alzheimer’s disease, PD is the second most common neurodegenerative disease. It is considered a complex multifactorial disease in which unknown environmental factors, genetic alterations, and/or oxidative stress subsequently cause premature neuronal death mainly in catecholamine (dopamine, noradrenaline, and adrenaline) neurons, especially in dopaminergic neurons in substantia nigra [1] (Fig. 1). The pathological features in patients with PD show retardation of dopaminergic neurons and presence of increased levels of α-synuclein protein, which is accumulated in Lewy bodies [2]. Typical symptoms of PD are tremor, bradykinesia, rigidity, and postural instability, as well as non-motor symptoms such as constipation, insomnia, and depression [3]. The therapeutic strategy of patients with PD is focused on the strengthening of dopaminergic transmission with exogenous l-dihydroxy-phenylalanine (l-dopa) in combination with carbidopa and dopamine agonists [4]. Drugs such as monoamine oxidase B (MAO B) and catechol O-methyltransferase (COMT) inhibitors prevent the catabolism of brain dopamine [5]. Several anticholinergic medications such as benztropine and trihexyphenidyl are administered in addition to carbidopa-levodopa therapy and dopamine agonists so as to alleviate the tremor associated with PD [6, 7]. Apart from these, amantadine [8], an NMDA (N-methyl-d-aspartate) receptor antagonist; droxidopa, a prodrug of norepinephrine; pimavanserin, a 5-HT inverse agonist; and rivastigmine, an acetylcholinesterase inhibitor, are administered to provide relief from symptoms of PD [1]. The stated drug regimen facilitates symptomatic relief to maintain the dopamine levels. Nevertheless, these agents have failed to show an impact on the recovery/reestablishment of the dopaminergic neurons. Moreover, the benefits of these agents are offset by unwanted side effects [5]. Besides the loss of dopaminergic neurons, several other elements that contribute to PD progression include mitochondrial dysfunction, oxidative stress, proteasome dysfunction, ubiquitination, and neuroinflammation [9]. Based on these facts, there is a need that calls for alternative approaches that can impart better therapy with minimal side effects.
In recent times, major discovery and development have been achieved in the field so as to counteract and slow down the progression of PD. Some of the achievements are in the field of gene therapy, surgical interventions, cell-based treatments, and immune-based therapy. The introduction and/or application of these strategies have paved the floor for possible alternatives to the existing/developing therapeutic systems.
2 Drug Therapy
Several drug candidates, under clinical and/or preclinical trials for PD, have centered the attention on neuroprotection as well as symptomatic treatment. Nondopaminergic targets/systems are also potentially beneficial in the therapeutic approach for PD. These agents may help in dose reduction of levodopa or may be useful in treatments where levodopa is not responsive. These targets include adenosine, adrenergic, serotonergic, opioid, glutamatergic, and cholinergic pathways [10].
Among these, istradefylline (brand name—Nourianz) has been effective in treating the “off” episodes in patients with PD. “Off” episodes are identified when symptoms of PD are rapidly increasing. The drug is the first adenosine A2A receptor antagonist, administered as an add-on medication to levodopa/carbidopa [11]. Two other A2A receptor antagonists, preladenant and tozadenant, did not contribute significant results in Phase III clinical trials and hence were ceased from further investigations [12, 13].
Neurogenic orthostatic hypotension is commonly found in patients with PD. The response to postural changes to regulate blood pressure is usually maintained through the autonomic nervous system (ANS). Due to the insufficient release of noradrenaline, the ANS fails to regulate blood pressure and thus leads to orthostatic hypotension [14]. Pharmacological treatments used for orthostatic hypotension include pyridostigmine, fludrocortisone, midodrine, l-DOPS, octreotide, yohimbine, and erythropoietin. Pyridostigmine acts through cholinesterase inhibition to improve cholinergic transmissions in ganglia [15]. Studies have shown the effects of fludrocortisone on volume expansion by increasing reabsorption of sodium in the kidneys and also by sensitization of α-adrenoreceptors [15]. Midodrine is a short-acting α1-adrenergic agonist used for symptomatic relief of neurogenic orthostatic hypotension. However, the clinical trial study results of the use of midodrine in PD are not yet published [16]. Droxidopa or (l-DOPS) is a prodrug which gets converted to noradrenaline via decarboxylation. Reports have demonstrated the effects of l-DOPS on improvement of dizziness due to orthostatic hypotension in PD [14]. Yohimbine is an α2-adrenergic antagonist which has shown better control in regulating blood pressure than pyridostigmine [15]. Other drugs such as octreotide and erythropoietin have also been beneficial in the management of neurogenic orthostatic hypotension in patients with PD when given in combination than monotherapy [15].
Glutamate antagonists are vital nondopaminergic targets of interest because of the integral role of glutamatergic system in PD. Apart from amantadine [8], a clinically NMDA (N-methyl-d-aspartate) receptor antagonist, the other glutamate targets investigated for PD therapy include inotropic α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor antagonists and metabotropic glutamate receptor (mGluR5) antagonists. Drugs such as perampanel (AMPA receptor antagonist), mavoglurant, and dipraglurant (selective mGluR5 inhibitors) have revealed no significant difference in symptoms with poor tolerability and efficacy [10].
Loss of serotonergic neurons and subsequent dysfunction in the serotonin system are also described in PD. Alterations in the 5HT2 receptors also add to the serotonin dysfunction in mood variations and psychosis in PD [17]. Drugs with serotonin receptor binding property such as clozapine (5-HT2A/2C receptor antagonist), buspirone (5-HT1A and α1-adrenergic receptor agonist), eltoprazine (a 5-HT1A and 5-HT1B dual agonist), and SYN120 (a dual 5-HT6/5-HT2 antagonist) are under evaluation for their possible benefits in patients with PD [10].
Famotidine (histamine receptor antagonist) has displayed antidyskinetic property in animal models. Nevertheless, there are no clinical trials under evaluation with histamine-based targets for PD [10]. Opioids have been studied for the management of nonmotor symptoms such as pain associated with PD. A Phase II/III is currently under investigation with prolonged-release formulation of oxycodone–naloxone [18].
Safinamide is a selective, reversible MAO-B inhibitor with both dopaminergic and nondopaminergic (glutamatergic) properties. The drug safinamide is approved as an add-on medication to levodopa alone or in combination with other drugs approved. It is mainly used for treatment in mid to late stages of PD [19].
Another add-on therapy accepted for new drug application by the US FDA is opicapone, which is a sustained COMT inhibitor. Nearly 30 clinical studies have been conducted which include two Phase III trials. Reports have revealed that the new treatment approach would be effective in prolonging the beneficial effects of levodopa with better control over motor symptoms [20].
A thiol-based antioxidant, N-acetyl cysteine (NAC), helps to restore the natural antioxidant, glutathione. Since oxidative stress also adds to the pathophysiology of PD, studies have shown that injectable administration of NAC has increased glutathione levels. The boost in glutathione may protect dopaminergic neurons from death by reducing oxidative damage through prevention of the accumulation of reactive oxygen species (ROS) and by increasing the activities in mitochondrial complexes [21, 22]. Furthermore, NAC administration has also shown to improve dopamine modulation which may enhance dopaminergic viability and functionality [21]. However, the potential improvement in symptomatic treatment of PD is yet to be realized.
3 Surgical Interventions for PD
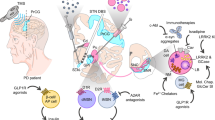
Surgical procedures and ablative processes within the basal ganglia are currently being performed for relief in tremor and rigidity. Pallidotomy and thalamotomy were the standard techniques for treatment of motor symptoms of PD until the effectiveness of levodopa was initiated [23]. The emergence of deep brain stimulation (DBS) brought the recurrence of surgical interventions. DBS involves the implantation of electric stimulators which have the ability to discharge high-frequency electric waves to areas in the brain. DBS into the subthalamic nucleus has been applied to treat PD patients with motor dysfunctions such as tremor, rigidity, and dyskinesia [24]. Areas such as pedunculopontine nucleus and caudal zona incerta are currently under clinical trials as possible targets for DBS for management of PD. No results are posted as yet [25,26,27].
4 Immune-Based Therapy
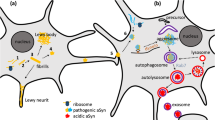
The pathological accumulation of α-synuclein (α-syn) within the astrocytes, neurons, and oligodendrocytes causes the degeneration of these cells and subsequently leads to neuronal loss or degeneration. Recent studies have demonstrated the focus on therapeutic interventions to decrease the α-syn levels and its clearance and further to prevent its propagation [28]. As an alternative to the known therapeutic strategies, immunotherapy, either as active or passive immunization, is the focused rationale for tackling the protein. In active immunization, the immunity gets developed through production of antibodies against the toxic α-syn protein. However, the administration of antibodies against α-syn protein renders protection in case of passive immunization. In both the types, the microglial cells get stimulated by the antibodies to engulf or scavenge the protein. Thus, the protein transfer and propagation get prevented [29].
Prothena Biosciences Inc., in collaboration with Roche, has developed a humanized IgG1-based monoclonal antibody, named as PRX002 [1] (prasinezumab). Single dose and multiple doses of PRX002 were tested in healthy volunteers and were found to be safe and tolerable. A randomized clinical trial of patients with PD treated with multiple ascending doses of PRX002 was also safe and well tolerated. The study also displayed the noticeable reduction in α-syn protein. The Phase II clinical trials are currently under investigation [30].
An advanced monoclonal antibody, BIIB054, was developed by Biogen to target α-syn [1]. This antibody also demonstrated encouraging results with safety and tolerability. The pharmacokinetic study was also safe in volunteers as well as participants with PD [31]. A single ascending dose study is also under evaluation [32].
Affiris AG had developed α-syn-conjugated vaccines named AFFITOPE® PD01A and PD03A [33]. These synthetic peptides would mimic the effect of natural α-syn and thus would stimulate the production of α-syn antibody. The Phase I clinical trials conducted on the vaccines demonstrated clear immune response with safety and tolerability [34]. However, no peer-reviewed reports are available.
5 Gene Therapy
Gene therapy for PD involves the viral vectors that are capable of transferring gene for the expression of specific proteins. The targets for gene therapy are put into two: disease modifying or non-disease modifying. The disease-modifying approaches use the strategy of ceasing neuronal death with or without regeneration of lost neurons. The research has been focused on glial cell-based family of ligands (GFL) which transmits information for activation of the MAP-kinase and PI3-kinase pathways. These pathways are crucial in the survival of neurons and neuritogenesis. Moreover, the genes that encode for the proteins tyrosine hydroxylase (TH), dopamine transporter (DT), vesicular monoamine transporter 2 (VMAT2), and aromatic l-amino acid decarboxylase (AADC) are also activated [35]. The non-disease-modifying strategies aim to alleviate the symptoms by regulating the abnormal firing in the basal ganglia. This is also by expressing enzymes of the dopaminergic or GABAgenic systems. These approaches do affect or alter the disease pathological system and hence demonstrate therapy based on symptoms. Gene therapy using opto- and chemogenetics is sorted as a crucial alternate for treating symptoms of PD. In optogenetics, the expression of opsins (light-sensitive ion channels) could be activated using light of wavelength specific for opsins. This could be beneficial in the deconstruction of parkinsonian neuronal circuitry and also improvement of motor symptoms [36]. Chemogenetics, based on the technology called designer receptors exclusively activated by designer drugs (DREADDs), allows the signaling of G-protein-coupled receptor (GPCR) and regulates the downstream neuronal functions. Another form of personalized gene therapy is genome editing with CRISPR-CAS9 technology, which may be used for genes with known mutation(s) causing PD. Even though there are several investigations and studies on therapy for PD using gene therapy, the true treatment of gene therapy is yet to be realized [36].
A potential gene therapy for targeting the α-syn gene is antisense oligonucleotide (ASO). ASOs are synthetically derived fragments of DNA which are able to bind to mRNAs so as to interfere in the intermediary stage between the DNA and the protein. A study has reported the use of an amido-bridged nucleic acid (AmNA)-modified antisense oligonucleotide (ASO) to target the α-syn protein in PD mouse model that expressed human wild type of the protein. The modified ASO was found to improve the neurological defects in the PD mouse model with improved stability and cellular uptake [37]. Numerous reports have demonstrated different outcomes with the administration of different oligonucleotides in various animal models. AAV-ribozyme, AAV-shRNA, miRNA, exosomal siRNA, and PEI/siRNA complex are other oligonucleotides reported [38].
Leucine-rich repeat kinase 2 (LRRK2) is another protein which imparts to the etiology of PD. Mutations in LRRK2 are proved to be associated with familial type of PD. Oligonucleotides like shRNA are studied for their action by targeting for mutations in LRRK2 in human embryonic kidney (HEK)-derived 293FT cells [39]. Moreover, an intracerebroventricular injection of ASOs aimed to target LRRK2 was found to decrease levels of mRNA, LRRK2, as well as α-syn proteins in the substantia nigra [39]. It is evident that the potential of ASOs is varying based on the different targeting approach. Thus, the safety and efficacy need to be established in the upcoming clinical studies.
6 Targeting α-Synuclein (α-Syn)
Other than immunotherapy and antisense oligonucleotides, the different approaches to block α-syn protein include degradation of the protein and aggregation inhibition. Autophagy or degradation involves multiple and complex pathways to break down the protein and unnecessary organelles that cause protein folding and aggregation. Mammalian target of rapamycin (mTOR) signaling pathway plays a key regulatory role in autophagy by controlling several processes of autophagy including initiation, process, and termination. A mitochondrial sensor (mTOR) is aberrantly overactivated in the brain during the pathogenesis of PD. Studies have shown that the autophagy and mTOR are crucial in the pathophysiology of PD [40]. MSDC-0160 is believed to reduce mTOR activity by regulation of mitochondrial pyruvate carrier (MPC), which is activated by mTOR. MSDC-0160 has shown to decrease the neuronal toxicity due to α-syn protein in Caenorhabditis elegans model and further investigations in humans are yet to be documented [41]. Another target of interest is c-Abl (ABL1, Abelson tyrosine kinase), which is effective in α-syn protein aggregation and neuronal degeneration. Inhibitors of c-Abl act via mitochondrial functions and also through posttranslational modifications of α-syn protein. As a result, the autophagic pathways get activated [41]. The clinical trials of c-Abl inhibitor, nilotinib, are still under examination [42].
The aggregation of α-syn protein is shown to be reduced or inhibited in the presence of intrabodies, which are small fragments of antibodies tailored to bind to the protein and prevent oligomerization. A small molecule, NPT200-11 (developed by Neuropore Therapies in connection with UCB Pharma), is reported to intervene with the interaction of membranes with α-syn and hence hinder its oligomerization [41]. A Phase I clinical investigation has been completed but no data from the investigation has been furnished [43]. Another protein, NPT-088 (developed by Proclara Biosciences), is reported to bind to amyloid protein. When tested in mouse models with overexpressed levels of α-syn, NPT-088 was found to decrease the levels of the protein α-syn [41]. A multiple-dose safety study was completed in patients with Alzheimer’s disease and the results are yet to be furnished [44]. The study in patients with PS needs to be initiated.
7 Cell-Based Therapy
Replacement of dopaminergic neurons and reestablishment of the neural circuit using cell-based approaches are an attractive field for meeting the clinical challenges in PD. Many reports and investigations are currently in the pipeline on cell-based therapy. Some of the different stem cell types explored include embryonic stem cells (ESCs), fetal ventral mesencephalic (FVM) tissues, neural stem (NS cells), induced pluripotent stem cells (iPSCs), and mesenchymal stem cells (MSCs) [22].
ESCs are highly proliferative cells that are derived from inner mass of blastoclasts and these are able to retain the pluripotent property for long durations. Large-scale production and capability to differentiate into dopaminergic neurons have thus considered ESCs as a tool for PD-based therapy. Researchers have studied and demonstrated the ability of ESC-derived dopaminergic neurons to survive and reinnervate the striatum and consequent improvement in motor symptoms in animal models [22]. Despite the promising results, ethical issues and practical side of its availability have hindered its clinical application and thus limited the clinical use [45]. Furthermore, instability of grafts may risk out to form tumors.
Fetal ventral mesencephalic (FVM) tissues, derived from the midbrain of fetuses, have precursors for dopaminergic neurons [46], and have been transplanted into the striatum of patients with PD [46]. The results were good in many patients. Similar to the problems linked to ESCs, FVM dopaminergic cells also have major ethical issues and technical issues such as limited in vivo differentiation and inability to yield consistent dopaminergic neurons [22, 45]. A clinical investigation for grafting fetal tissue into the brain of patients with PD is ongoing with results expected to be published by 2021 [47].
Neural stem (NS cells) or precursor cells are isolated from an adult central nervous system and later cultured as neurospheres (freely floating) with the aid of supporting growth factor(s) like endothelium growth factor and fibroblast growth factor. These cells are able to differentiate in various cellular types (namely astrocytes, neurons, and oligodendrons) of the central nervous system. Thus, the replacement of the lost/degenerated neurons follows to reestablish the dopamine release and transmission [22]. Several reports have revealed poor survival and inability/mild ability of the cells to improve the progress of treatment on PD-induced animal models. This is due to poor differentiation into dopaminergic neurons. Other reports have stressed on the importance of NSCs that were isolated from the midbrain alone, as only these cells would be capable of differentiating into dopaminergic neurons. Later, another study proposed an alternative to use NSCs developed by genetic engineering and further cultured with the necessary developmental factors or signals. These cells would induce the differentiation of dopaminergic neurons and also enhance the yield of dopamine [48]. Nevertheless, the survival and stability of these grafts still remain to be proven, based on which transplantation effects could be investigated in humans.
Induced pluripotent stem cells (iPSCs) are reprogrammed skin or blood cells to pluripotent cells in its embryonic state that facilitates the development of any type of human cell as per the need for therapy. These cells have shown better genomic stability, viability, and transcription ability as that of ESCs [22]. Research on iPSC grafting into the striatum of PD animal models has demonstrated the ability to survive, differentiate, and ameliorate the symptoms in PD animals. Despite the promising results, tumor formation can follow if differentiation is not complete. These type of cells are patient specific and thus will have no limitation. However, the differentiation protocols would be tedious till standardization and hence expensive [49]. The first human trial for iPSC transplantation for PD has marked off in 2018 [50]. Due to the nonhomogeneous differentiated tissues of iPSCs obtained, a new type of cell was sorted to be known as induced neural cells. These were developed through viral vectors from adult somatic cells that would circumvent the intermediate phase of stem cell. Human induced neurons have demonstrated survival and stability on rodents [39]. But, similar to that of iPSCs, the protocols still lack consistency and efficiency to differentiate as dopaminergic neurons.
Another possible treatment intervention under cell-based therapy is mesenchymal stem cells (MSCs). These cells are adult stem cells that are self-renewable and are capable to differentiate. These cells have surface markers and are devoid of hemopoietic surface markers. Initial studies have shown the ability of naïve and predifferentiated MSCs to stabilize and redevelop degenerated neuronal sites partially with no/minimal tumorigenicity and immunogenicity. The mechanism of differentiation and therapeutic effect was to be deduced [51, 52]. In the later years, secretomes were related with MSCs as the presence of immunomodulatory cytokines and neurotrophic factors in secretomes would reduce neuroinflammation and provide favorable environment for differentiation of MSCs into new dopaminergic neurons and also for survival of the remaining dopaminergic neurons. Based on the tissue from which they are extracted, MSCs are denoted as adipose tissue type (aMSCs), bone marrow type (bMSCs), umbilical cord type (uMSCs), and others. After isolation, the cells may be transplanted either in its raw (undifferentiated) or in the cultured (differentiated) form [53]. Due to the promising results obtained from the treatment of different types of MSCs in numerous experimental PD models, the approach was opened for testing in humans [54]. Currently clinical trials using MSCs for PD therapy [53] are under study from which relevant information for therapeutic reality, route of administration, immunological responses, and other related ones are to be known. Such clinical data will be contributory to the use of MSCs for treatment of PD.
8 Future Prospects
The therapeutic strategy for PD extends from the conventional pharmacological and non-pharmacological approaches (based on the symptoms) to the latest interventions, namely gene-based, cellular based, and immune-based therapy. Many of these lines are effective either as single or as combination therapy. The symptomatic therapy is managed by maintaining and reinstating the levels of dopamine through drugs such as levodopa, dopaminergic agonists, COMT, and MAO-B inhibitors. Apart from these, several new drugs are administered as add-on drugs to the existing regimen. Recently, literature has revealed the newer methods and efforts that are focused on disease modifications instead of relying only on symptom-based therapy. Despite the effort, there has been very limited success in modifying the disease condition in PD. Hence intense research is still ongoing for a better therapeutic approach. Preclinical data are known in some biological pathways. A myeloperoxidase (MPO) inhibitor, AZD5904, reduces oxidative stress occurring during neuroinflammation, which is involved in the pathophysiology of PD [55]. LRRK2 inhibition is another target that is extensively under study. DNL151 and DNL201 are currently under clinical study for safety and tolerability [56, 57]. Similarly, G-protein-coupled receptor 6 (GPR6) is an indirect modulator of dopamine pathway for treating PD [58]. Glucocerebrosidase (GCase) is also found to be a target of interest in PD therapy as decreased activity of GCase is found to show increased levels of α-syn aggregates and other proteins associated with neurodegenerative disorders [59]. Furthermore, the concept of drug repurposing/repositioning has also been applied and analyzed by clinical trials for the disease-modifying potential in clinical trials. Drugs such as isradipine (calcium channel blocker), exenatide (glucagon-like peptide-1/GLP-1 agonist), nilotinib (c-Abl (Abelson) tyrosine kinase inhibitor), and simvastatin (3-hydroxy-3-methylglutaryl-coenzyme A/HMG-CoA inhibitor) are some of the drugs that have been extensively studied for their effect on PD. Mitochondrial based treatments have also been implicated as an emerging alternative for PD [60]. Table 1 summarizes the different potential targets/approaches that may be proven beneficial for the treatment of PD. These information, though not exhaustive, show an insight into different targets or approaches intended for clinical use in the future.
9 Conclusion
PD is a neurodegenerative disorder and is second in order after Alzheimer’s disease. Numerous biological systems/targets/approaches are presently addressed to meet the therapeutic needs of PD. Apart from the newer drugs, clinically and preclinically under study, the advent of newer strategies like cell transplantation therapy and gene engineering has been extensively explored for meeting the disease challenges. Despite some crucial realizations, several areas still remain to be managed. This has to be predominantly tackled through appropriate knowledge and experience in upcoming technologies, especially when iPSCs, secretome-based MSCs, and gene engineering are concerned. Ongoing research with better understanding of various targets and approaches should no doubt assist in a multidimensional therapeutic regimen for PD.
References
Ellis JM, Fell MJ (2017) Current approaches to the treatment of Parkinson’s disease. Bioorg Med Chem Lett 27:4247–4255
Stopping Parkinson’s Disease before it Starts (2019). https://medicalxpress.com/news/2019-06-parkinson-disease.html. Accessed 14 Aug 2019
Dorszewska J, Kozubski W (2016) Genetic and biochemical factors in Parkinson’s disease. In: Dorszewska J, Kozubski W (eds) Challenges in Parkinson’s disease. IntechOpen, London
Dorszewska J, Prendecki M, Lianeri M, Kozubski W (2014) Molecular effects of L-dopa therapy in Parkinson’s disease. Curr Genomics 15:11–17
Parkinson’s Disease (2019). https://www.mayoclinic.org/diseases-conditions/parkinsons-disease/diagnosis-treatment/drc-20376062. Accessed 16 Aug 2019
Perez-Lloret S, Barrantes FJ (2016) Deficits in cholinergic neurotransmission and their clinical correlates in Parkinson’s disease. NPJ Park Dis 2:16001
What are Anticholinergics? (2019). https://parkinsonsdisease.net/medications/anticholinergics/. Accessed 16 Aug 2019
Medications Used To Treat Parkinson’s (2019). https://parkinsonsdisease.net/medications/. Accessed 16 Aug 2019
Yadav A, Agarwal S, Tiwari SK, Chaturvedi RK (2014) Mitochondria: prospective targets for neuroprotection in Parkinson’s disease. Curr Pharm Des 20:5558–5573
Freitas ME, Fox SH (2016) Nondopaminergic treatments for Parkinson’s disease: current and future prospects. Neurodegenerative Dis Manag 6:249–268
FDA (2019) FDA approves new add-on drug to treat off episodes in adults with Parkinson’s disease. https://www.fda.gov/news-events/press-announcements/fda-approves-new-add-drug-treat-episodes-adults-parkinsons-disease. Accessed 23 Aug 2019
Parkinson’s Tozadenant Trial Discontinued (2019). https://www.michaeljfox.org/news/parkinsons-tozadenant-trial-discontinued. Accessed 23 Aug 2019
Yuan G, Jankins TC, Patrick CG Jr, Philbrook P, Sears O, Hatfield S, Sitkovsky M, Vasdev N, Liang SH, Ondrechen MJ, Pollastri MP, Jones GB (2017) Fluorinated adenosine A2A receptor antagonists inspired by preladenant as potential cancer immunotherapeutics. Int J Med Chem 2017:1–8. https://www.hindawi.com/journals/ijmc/2017/4852537. Accessed 23 Aug 2019
Isaacson SH, Skettini J (2014) Neurogenic orthostatic hypotension in Parkinson’s disease: evaluation, management, and emerging role of droxidopa. Vasc Health Risk Manag 10:169–176
Sánchez-ferro Á, Benito-león J, Gómez-esteban JC (2013) The management of orthostatic hypotension in Parkinson’s disease. Front Neurol 4:1–11
Treatment of Orthostatic Intolerance in Patients With Parkinson’s Disease using Midodrine (2019). https://clinicaltrials.gov/ct2/show/NCT02365012. Accessed 23 Aug 2019
Buddhala C, Loftin SK, Kuley BM, Cairns NJ, Campbell MC, Perlmutter JS, Kotzbauer PT (2015) Dopaminergic, serotonergic, and noradrenergic deficits in Parkinson disease. Ann Clin Transl Neurol 2:949–959
Trenkwalder C et al (2015) Prolonged-release oxycodone—naloxone for treatment of severe pain in patients with Parkinson’s disease (PANDA): a double-blind, randomised, placebo-controlled trial. Lancet Neurol 14:1161–1170
Blair HA, Dhillon S (2017) Safinamide: a review in Parkinson’s disease. CNS Drugs 31:169–176
FDA Accepts New Drug Application for Opicapone as Add-on Therapy (2019). https://parkinsonsnewstoday.com/2019/07/15/fda-accepts-new-drug-application-opicapone-add-on-therapy-parkinsons-neurocrine-biosciences/. Accessed 24 Aug 2019
Monti DA, Zabrecky G, Kremens D, Liang TW, Wintering NA, Cai J, Wei X, Bazzan AJ, Zhong L, Bowen B, Intenzo CM, Iacovitti L, Newberg AB (2016) N-acetyl cysteine may support dopamine neurons in Parkinson’s disease: preliminary clinical and cell line data. PLoS One 11:1–15
Pires AO, Teixeira FG, Sousa N (2017) Old and new challenges in Parkinson’s disease therapeutics. Prog Neurobiol 156:69–89
Pandey S (2012) Parkinson’s disease: recent advances. J Assoc Physicians India 60:30–32
Shukla AW, Okun MS (2013) Surgical treatment of Parkinson’s disease: patients, targets, devices, and approaches. Neurotherapeutics 11:47–59
A Responsive Closed-Loop Approach to Treat Freezing of Gait in Parkinson’s Disease (2019). https://clinicaltrials.gov/ct2/show/NCT02318927. Accessed 25 Aug 2019
Combined Deep Brain Stimulation for Parkinson’s Disease (2019). https://clinicaltrials.gov/ct2/show/NCT01485276. Accessed 25 Aug 2019
Randomised Crossover Trial of Deep Brain Stimulation of Differential Posterior Subthalamic Area Regions in Parkinson’s Disease and Tremor (2019). https://clinicaltrials.gov/ct2/show/NCT01945567. Accessed 25 Aug 2019
Mandler M, Valera E, Rockenstein E, Mante M, Weninger H, Patrick C, Adame A, Schmidhuber S, Santic R, Schneeberger A, Schmidt W, Mattner F, Masliah E (2015) Active immunization against alpha-synuclein ameliorates the degenerative pathology and prevents demyelination in a model of multiple system atrophy. Mol Neurodegener 10:1–15
George S, Brundin P (2015) Immunotherapy in Parkinson’s disease: micromanaging alpha-synuclein aggregation. J Park Dis 5:413–424
Jankovic J, Goodman I, Safirstein B, Marmon TK, Schenk DB, Koller M, Zago W, Ness DK, Griffith SG, Grundman M, Soto J, Ostrowitzki S, Boess FG, Martin-Facklam M, Quinn JF, Isaacson SH, Omidvar O, Ellenbogen A, Kinney GG (2018) Safety and tolerability of multiple ascending doses of PRX002/RG7935, an anti-alpha-synuclein monoclonal antibody, in patients with Parkinson disease: a randomized clinical trial. JAMA Neurol 75:1206–1214
Brys M, Fanning L, Hung S, Ellenbogen A, Penner N, Yang M, Welch M, Koenig E, David E, Fox T, Makh S, Aldred J, Goodman I, Pepinsky B, Liu Y, Graham D, Weihofen A, Cedarbaum JM (2019) Randomized phase I clinical trial of anti-alpha-synuclein antibody BIIB054. Mov Disord 34:1154–1163
Single-Ascending Dose Study of BIIB054 in Healthy Participants and Early Parkinson’s Disease (2019). https://clinicaltrials.gov/ct2/show/NCT02459886. Accessed 30 Aug 2019
Zella SMA, Metzdorf J, Ciftci E, Ostendorf F, Muhlack S, Gold R, Tönges L (2019) Emerging immunotherapies for Parkinson disease. Neurol Ther 8:29–44
AFFITOPE®PD03A (2019) Affiris announces top line results of first-in-human clinical study using AFFITOPE®PD03A, Confirming immunogenicity and safety profile In Parkinson’s disease patients. https://affiris.com/news/affiris-announces-top-line-results-of-first-in-human-clinical-study-using-affitope/. Accessed 30 Aug 2019
Axelsen TM, Woldbye DPD (2018) Gene therapy for Parkinson’s disease, an update. J Park Dis 8:195–215
Sanders TH, Jaeger D (2016) Optogenetic stimulation of cortico-subthalamic projections is sufficient to ameliorate bradykinesia in 6-OHDA lesioned mice. Neurobiol Dis 95:225–237
Uehara T, Choong CJ, Nakamori M, Hayakawa H, Nishiyama K, Kasahara Y, Baba K, Nagata T, Yokota T, Tsuda H, Obika S, Mochizuki H (2019) Amido-bridged nucleic acid (AmNA)-modified antisense oligonucleotides targeting α-synuclein as a novel therapy for Parkinson’s disease. Sci Rep 9:1–13
Nakamori M, Junn E, Mochizuki H, Mouradian MM (2019) Nucleic acid-based therapeutics for Parkinson’s disease. Neurotherapeutics 16:287–298
de Ynigo-Mojado L, Martin-Ruiz I, Sutherland JD (2011) Efficient allele-specific targeting of LRRK2 R1441 mutations mediated by RNAi. PLoS One 6:1–10
Zhu Z, Yang C, Iyaswamy A, Krishnamoorthi S, Sreenivasmurthy SG, Liu J, Wang Z, Tong BC, Song J, Lu J, Cheung KH, Li M (2019) Balancing mTOR signaling and autophagy in the treatment of Parkinson’s disease. Int J Mol Sci 20:1–15
Sardi SP, Cedarbaum JM, Brundin P (2018) Targeted therapies for Parkinson’s disease: from genetics to the clinic. Mov Disord 33:684–696
Nilotinib in Parkinson’s Disease (NILO-PD) (2019). https://clinicaltrials.gov/ct2/show/NCT03205488. Accessed 2 Sept 2019
Phase 1 Study of NPT200-11 in Healthy Subjects (2019). https://clinicaltrials.gov/ct2/show/NCT02606682. Accessed 2 Sept 2019
Multiple Dose Safety Study of NPT088 in Patients With Mild to Moderate Probable Alzheimer’s Disease (2019). https://clinicaltrials.gov/ct2/show/NCT03008161. Accessed 3 Sept 2019
Gonzalez C, Bonilla S, Flores AI, Cano E, Liste I (2016) An update on human stem cell-based therapy in Parkinson’s disease. Curr Stem Cell Res Ther 11:561–568
Barker RA, Drouin-Ouellet J, Parmar M (2015) Cell-based therapies for Parkinson disease-past insights and future potential. Nat Rev Neurol 11:492–503
TRANSEURO (2019) TRANSEURO Open Label Transplant Study in Parkinson’s Disease. https://clinicaltrials.gov/ct2/show/NCT01898390. Accessed 4 Sept 2019
Parmar M, Torper O, Drouin-Ouellet J (2019) Cell-based therapy for Parkinson’s disease: a journey through decades toward the light side of the force. Eur J Neurosci 49:463–471
Stoker TB, Blair NF, Barker RA (2017) Neural grafting for Parkinson’s disease: challenges and prospects. Neural Regen Res 12:389–392
Medicine P, Pluripotent I (2019) Treatment of Parkinson’s disease through personalized medicine and induced pluripotent stem cells. Cell 8:2–15
Hayashi T, Wakao S, Kitada M, Ose T, Watabe H, Kuroda Y, Mitsunaga K, Matsuse D, Shigemoto T, Ito A, Ikeda H, Fukuyama H, Onoe H, Tabata Y, Dezawa M (2013) Autologous mesenchymal stem cell-derived dopaminergic neurons function in parkinsonian macaques. J Clin Invest 123:272–284
Khoo MLM, Tao H, Meedeniya ACB, Mackay-Sim A, Ma DDF (2011) Transplantation of neuronal-primed human bone marrow mesenchymal stem cells in hemiparkinsonian rodents. PLoS One 6:1–16
Filho DM, Ribeiro PDC, Oliveira LF, de Paula DRM, Capuano V, de Assunção TSF, da Silva VJD (2018) Therapy with mesenchymal stem cells in Parkinson disease history and perspectives. Neurologist 23:141–147
Inden M, Yanagisawa D, Hijioka M (2016) Annals of neurodegenerative disorders therapeutic effects of mesenchymal stem cells for Parkinson’s disease. Ann Neurodegener Disord 1002:1–8
AZD5904 Mechanism of action: Myeloperoxidase (MPO) inhibitor (2019). https://openinnovation.astrazeneca.com/azd5904.html. Accessed 10 Sept 2019
First Parkinson’s Patient Dosed in Early Trial of DNL151, Potential LRRK2 Inhibitor (2019). https://parkinsonsnewstoday.com/2019/09/10/1st-parkinsons-patient-dosed-in-phase-1b-trial-of-dnl151-potential-lrrk2-inhibitor/. Accessed 10 Sept 2019
Study to Evaluate DNL201 in Subjects With Parkinson’s Disease (2019). https://clinicaltrials.gov/ct2/show/NCT03710707. Accessed 10 Sept 2019
Lemos A, Melo R, Preto AJ, Almeida JG, Moreira IS, Dias Soeiro Cordeiro MN (2018) In silico studies targeting G-protein coupled receptors for drug research against Parkinson’s disease. Curr Neuropharmacol 16:786–848
Balestrino R, Schapira AHV (2018) Glucocerebrosidase and Parkinson disease: molecular, clinical, and therapeutic implications. Neuroscientist 24:540–559
Elkouzi A, Vedam-Mai V, Eisinger RS, Okun MS (2019) Emerging therapies in Parkinson disease—repurposed drugs and new approaches. Nat Rev Neurol 15:204–233
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Mohammed, M., Alenezi, S.K. (2020). Advancement and Challenges in Parkinson’s Disease: A Recent Outlook. In: Mathew, B., Thomas Parambi, D.G. (eds) Principles of Neurochemistry. Springer, Singapore. https://doi.org/10.1007/978-981-15-5167-3_8
Download citation
DOI: https://doi.org/10.1007/978-981-15-5167-3_8
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-15-5166-6
Online ISBN: 978-981-15-5167-3
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)