Abstract
Porous molecular crystals (PMCs) with well-defined, self-standing pores have been attracted much attention due to significant functionality provided by selective and reversible inclusion of certain chemical species into the pores. PMCs constructed through preorganized hydrogen bonds (H-bonds) are specifically called as hydrogen-bonded organic frameworks (HOFs). For recent two decades, HOFs have been intensively explored. HOFs are frequently obtained as single crystals, which is convenient to reveal the structure–property relationship. Their regenerable and reusable features are also appealing. However, HOFs are relatively fragile, and their designing strategy needs to be more considered compared with other porous frameworks because of weakness of H-bonds. Regarding this, we have demonstrated that C3-symmetric π-conjugated molecules (C3PIs) possessing o-bis(4-carboxyphenyl)benzene moieties in their periphery give layered frameworks composed of isostructural H-bonded hexagonal networks (H-HexNets) and that the frameworks can effectively provide stable, robust, multifunctional HOFs with permanent porosity. The frameworks also can work as a platform to achieve very unique alignment of functional molecules such as C60. Our strategy for constructing functional HOFs contributes to developing a new field of porous organic materials.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Porous molecular crystal
- Hydrogen-bonded organic framework
- π-conjugated molecule
- Carboxylic acid
- Permanent porosity
1 Introduction: Porous Molecular Crystals
Porous molecular crystals (PMCs) are crystalline materials with permanent porosity constructed from discrete organic molecules through reversible non-covalent intermolecular interactions. A pioneering work, for example, is of tris-o-phenylenedioxycyclotriphosphazene (TPP) [1,2,3]. Although PMCs are closely related to organic inclusion crystals [4], an important feature of PMCs is that they have self-standing pores (i.e., permanent porosity) that can accommodate various and/or specific guest molecules reversibly. PMCs have recently attracted renewed attention from viewpoint of applications such as selective gas storage/separation, catalysis, chemical sensing, drug delivery, and optoelectronics [5,6,7,8].
Specifically, PMCs that are formed via hydrogen bonds (H-bonds) are often called as hydrogen-bonded organic frameworks (HOFs) [9,10,11,12,13], which was introduced by Chen [14]. Although several names and acronyms to describe PMCs constructed through H-bonding can be found in the literature, we call such PMCs as HOFs in this chapter. Typical merits of HOFs are as follows:
-
HOFs are frequently obtained as single crystals via a simple solution process due to the reversible nature of H-bonds, which enables to determine the precise crystal structures by single-crystal X-ray diffraction (SXRD).
-
HOFs do not need metal species for framework construction, allowing lightweight and environmentally friendly porous materials.
-
HOFs have ability to restore its original crystallinity by reannealing.
However, such intrinsic properties of HOFs simultaneously cause the following problems:
-
HOFs tend to collapse when solvent molecules are removed from voids to activate the porous structures.
-
HOFs are difficult to predesign: Even if building block molecules are preorganized thoughtfully, the porous HOFs are not always produced as designed.
-
The H-bonding moieties of building blocks are often trapped by polar solvent molecules used for recrystallization, preventing formation of porous networked frameworks.
Therefore, we need solve these kinds of dilemmas.
An important point to construct stable HOFs with permanent porosity is to combine non-covalent intermolecular interactions such as π/π interactions with H-bonds, because a H-bond alone is too weak to maintain low-density porous materials, compared with a dative or covalent bond. Fortunately, significant progress in the field has resulted in the production of excellent HOFs with permanent porosity, thermal and chemical durability, and functionality [15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41]. Some typical molecular skeletons and supramolecular synthons are listed in Fig. 11.1. For construction of predesigned molecular architectures, the appropriate selection of both a supramolecular synthon (directionality and multiplicity of H-bonding) and molecular skeletons (geometry, size, rigidity, planarity, symmetry, and functionality) to satisfy both H-bonds, and the additional interactions is important. Furthermore, it is remarkable that Cooper, Day, and co-workers recently introduced “energy–structure–function maps” built by combining computational crystal structure prediction with property prediction [42, 43]. The maps describe the possible structures and properties that are available to a candidate molecule and can accelerate development of new functional HOFs.
2 Hydrogen-Bonding Motifs
2.1 Networked Structures Connected by Carboxylic Acid Dimers
A H-bonded dimer of carboxy groups is one of the simplest and the most classical molecular glues to make molecular assemblies [44,45,46]. Meanwhile, the dimer has still been a suitable supramolecular synthon to construct exotic supramolecular architectures, because of the following two features: facile synthesis of derivatives with carboxy groups and its high directional H-bond formation. Particularly, the latter feature enables one to design supramolecular network motifs, combined with geometrically well-defined molecular platforms, as shown in Fig. 11.2.
It is well known that Marsh and Duchamp demonstrated in 1969 that trimesic acid yielded a waved H-bonded honeycomb network, which was then interpenetrated to yield a non-porous crystal [47]. It was in 1987 that layered honeycomb structures of trimesic acid with 1D inclusion channels were constructed by Herbestein and co-workers through template crystallization [48].
Carboxylic acid-based HOFs with permanent porosity have started to be reported intensively since around 2015. Some remarkable examples are described as follows (Fig. 11.3). Rowsell, Zentner, and co-workers demonstrated that 1,3,5-tris(4-carboxyphenyl)benzene (1) yielded porous crystals tcpb with interpenetrated honeycomb sheets [28]. Tris(4-carboxyphenyl)amine (2) gave 3D networked porous framework (HOF-11) with Brunauer–Emmett–Teller surface area (SABET) of 687 m2g−1 [29, 30]. Comotti, Sozzani, and co-workers reported dynamic gas sorption behavior of TCF-1 and-2 composed of 3a and 3b, respectively [38]. Wu, Yuan, and co-workers reported biphenyl derivative 4 gave 3D networked porous framework (HOF-TCBP), which exhibits SABET of 2066 m2g−1 [31]. Liu, Cao, and co-workers applied pylene derivative 5 to construct HOF (PFC-1) with SABET of 2122 m2g−1 and demonstrated proof-of-concept for chemo and photodynamic therapy [34]. Hisaki, Douhal, and co-workers demonstrated construction of highly stable, single-crystalline, isostructural HOFs (CPHAT and CBPHAT) with SABET of 649 m2g−1 and 1288 m2g−1 based on shape-fitted docking strategy with hexaazatriphenylene (HAT) derivatives 6a, b [32, 33, 41]. Stoddart and co-workers reported that triptycene derivative 7 provided interpenetration polymorphs of H-bonded networks [39].
It is remarkable that reported building blocks contain carboxyphenyl groups instead of carboxy groups. This is probably because such building-block molecules can easily synthesized by metal-catalyzed cross-coupling reactions between aryl groups such as Suzuki–Miyaura reaction [49]. Moreover, introduction of carboxyphenyl groups into π-conjugated skeleton improves solubility of the molecule into solvents compared with those directly bonded by carboxy groups [50, 51]. The phenylene group also acts as a spacer to generate pores [52]. However, systematic construction of carboxylic-acid-based HOFs is still challenging.
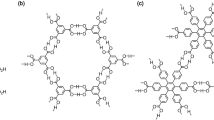
In connection with this, we have proposed that C3-symmetric π-conjugated building blocks (C3PIs) possessing three o-bis(4-carboxyphenyl)benzene moieties in periphery of the core can systematically provide isostructural H-bonded hexagonal networks (H-HexNets) with multiple void spaces, and that the HexNets are stacked without interpenetration to give porous layered assembly of H-HexNets (LA-H-HexNets). This working hypothesis to achieve formation of H-HexNet-based layered HOF is summarized in Fig. 11.4. C3-symmetric, rigid, planar cores with sides of two different lengths are applied for the central part of C3PIs. Such C3PIs enable the formation of a planar 2D H-HexNet motif with multivoid spaces. Moreover, the size and shape of the void can be varied by changing the side length of the C3PIs. The peripheral o-bis(4-carboxyphenyl)benzene moieties can form a H-bonded triangular porous motif, so-called phenylene triangle (PhT) motif to network the C3PI. The PhT motif was first observed in a crystal of hexakis(4-carboxyphenyl)benzene reported by Kobayashi and co-workers in 2000 [53].
2.1.1 Phenylene Triangle (PhT)
As described above, the PhT motif is a key structure for H-HexNet-based layered HOFs. To confirm whether the PhT is a robust H-bonding motif in crystals, o-bis(4-carboxyphenyl)benzene (8a), 4,5-dibromo-1,2-bis(4-carboxyphenyl)benzene (8b), and 1,2,3,4-tetrakis(4-carboxyphenyl)benzene (9) were crystallized from a mixed solution of DMF, 1,2,4-trichlorobenzene (124TCB) and 1,3,5-trichlorobenzene (135TCB) at 50 °C. Pristine 8a yields zigzag-shaped 1D H-bonded chain as in the case of the corresponding meta- and para-substituted derivatives. Brominated derivative 8b, on the other hand, forms the PhT through H-bonding between the carboxy groups (Fig. 11.5a). The PhT of 8b possesses a triangular void with a side of ca. 12.5 Å and diameter of ca. 9.7 Å, in which 124TCB and/or 135TCB molecules are accommodated. Threefold CH/Br intermolecular interactions (H···Br distances: 2.79–2.96 Å, C–H–Br angles: 144–167°) afford formation of the PhT motif, instead of a zigzag chain. Similarly, 9 forms the PhT motif to give a ladder-type porous network structure (Fig. 11.5b). These results indicate that, although the pristine unit does not always form the PhT motif, geometrically or electrostatically well-organized derivatives can form the PhT motif [54].
It is unignorable that the PhT motif exhibits the specific structural feature originated from conformation of the o-terphenyl moiety as described below. The PhT includes conformational frustration. The two peripheral phenylene groups in the ortho-position prefer to incline in the same direction (P or M) to avoid steric repulsion between them (Fig. 11.6a). Therefore, although we have not observed the PhT motif with three frustrated dimers because of its instability (Fig. 11.6b), the motif normally includes at least one conformationally frustrated H-bonded carboxy dimer (Fig. 11.6c, d), except for the case that the all phenylene groups are in the orthogonal conformation as in the case of Kobayashi’s structure [53]. The conformationally frustrated part is sometimes trapped by polar solvent molecule such as DMF through H-bonding to form “truncated catemer”-type dimer to release the distortion (Fig. 11.6e, f) [54].
Geometrical feature of PhT motif. a Cooperative incline of the peripheral phenylene groups. Chemical structures of b PhT with three frustrated dimers, c PhT with one frustrated dimer, and e PhT with one fractured dimer by insertion of DMF molecules. Selected crystal structures of d frustrated and e fractured PhT observed in crystals of T18
3 Construction of HOFs Based on Hexagonal Network
Based on the idea described above, C3PIs possessing three o-bis(4-carboxyphenyl)benzene moiety were designed and synthesized (Fig. 11.7). Recrystallization of the compounds was performed by slow evaporation of a mixed solution of a highly polar solvent such as DMF and a high-boiling aromatic solvent such as 124TCB or methyl benzoate (MeBz) at relatively high temperature (50–120 °C), yielding single crystals suitable for SXRD analysis. It is noteworthy that crystallization at relatively low temperature such as at 30 °C results in formation of solvate crystals, in which some of carboxy groups make H-bonds with solvent molecules, preventing formation of completely networked LA-H-HexNet structures.
In the following part, crystal structures and properties of their LA-H-HexNets are described.
3.1 A Series of Cyclic π-Conjugated Systems with Different Sizes of Macrocycles
C3PIs Tp, T12, T18, and Ex give H-HexNets structures via complemental H-bonding between the carboxy groups (Fig. 11.8a–d) [55]. In the H-HexNet sheet, three kinds of pores are formed. Void I has width of approximately 8 Å is formed within the PhT motif. Void II has a non-regular hexagonal-shaped aperture. Its dimension is varied depending on the size of the core of C3PIs: The longer sides of void II are the same (15.8 Å) in the four systems, while the shorter sides have various lengths ranging from 2.0 to 11.4 Å. Void III is inherent pores located at the center of the cyclic compounds. The H-HexNet sheets stack without interpenetration to give porous LA-H-HexNets (Tp-1, T12-1, T18-1, and Ex-1, respectively). Although the molecules give isostructural H-HexNets sheets, stacking manner of the H-HexNets depends on the molecular structures (Fig. 11.8e–h). The void ratio of Tp-1, T12-1, T18-1, and Ex-1 calculated by PLATON software with probe radius of 1.2 Å is 54%, 41%, 58%, and 59%, respectively. Aromatic solvent molecules used for recrystallization are accommodated in the voids. The neighbored H-HexNet layers are stacked by weak interactions such as face-to-face (π/π), face-to-edge (CH/π), and CH/O interactions. These weak interlayer interactions, as well as rotational flexibility of the peripheral phenylene rings, allow Tp to yield at least four polymorphs of LA-H-HexNets including Tp-1 [56].
To investigate the structural changes upon removal of solvent molecules from the void space, crystalline bulks of Tp-2Ds, T12-1, T18-1, and Ex-1 were subjected to varied temperature (VT)-PXRD measurements as shown in Fig. 11.9, where Tp-2Ds denotes the crystalline bulk of LA-H-HexNet of Tp because it includes either or both of two polymorphic forms Tp-1 and Tp-2. The pattern of the as-formed crystals at first showed almost no recognizable peaks, due to significantly low electron diffraction contrast provided by the low density H-HexNet frameworks composed of no metal but low-weight elements and highly disordered solvent molecules within the frameworks. However, the diffraction peaks became unambiguous upon heating as the solvent molecules were removed. Tp and T18 showed PXRD pattern changes in two or three steps, indicating formation of intermediate phases. After completing removal of the solvents, the materials still exhibit obvious PXRD patterns, which are not in agreement with the original patterns of the solvate crystals, indicating that crystal structure of LA-H-HexNets changes to other crystalline forms upon desolvation. Further increase of the temperature revealed that frameworks of Tp, T12, T18, and Ex decomposed at 323 °C, 360 °C, 242 °C, and 249 °C, respectively.
Changes of PXRD (CuKα) patterns of a Tp-2Ds, b T12-1, c T18-1, and d Ex-1 upon heating from ambient temperature to ca. 215 °C. Temperature was increased at the rate of 1 °C min−1. Patterns were recorded from 3° to 18° of 2θ with the scan rate of 3° min−1. Each scan has a temperature gradient of ca. 5 °C. Since the as-formed crystal of Tp includes either or both of two polymorphic forms, the crystalline bulk was referred as Tp-2Ds
By using PXRD pattern after desolvation, we attempted to identify the crystal structures of activated forms and successfully estimated or solved the reasonable structures for Tp-apo and T12-apo by using the crystal structure prediction technique and powder X-ray analysis including the Rietveld refinement, respectively. The activated structures are shown in Fig. 11.10.
It is noteworthy that Tp-apo crystal retains a layered structure of H-HexNet sheets with permanent porosity. The adjacent Tp cores are stacked with larger overlap compared with Tp-1 and triangular channels with a diameter of ca. 8.5 Å run along the c axis. The void ratio calculated by PLATON software with probe radius of 1.2 Å is 33%. Similarly, T12-apo retains a layered structure of HexNet sheets, although H-bonded moieties are partly deformed. T12 cores are overlapped with an interplanar distance of ca. 3.2 Å through π/π interactions. One-dimensional (1D) channel with a triangular cross section with width of 8.8 Å and branched small voids with a diameter of 2.8 Å are formed. The void ratio of T12-apo is calculated to be 38%.
Activation of LA-H-HexNets was conducted by immersing the as-formed crystalline powders into benzene for overnight at room temperature, followed by lying under vacuum condition (0.2 kPa) for 1 day at 40–100 °C, providing HOFs with permanent porosity (Tp-apo, T12-apo, T18-apo, and Ex-apo). Tp-apo and T12-apo show a type-I N2 sorption isotherm at 77 K with an uptake of 187 cm3g−1 and 85.4 cm3g−1, respectively. Similarly, Tp-apo has a type-I sorption isotherm with an uptake of 194 cm3g−1. SABET was calculated to be 788 m2g−1. The SABET of T12-apo was also calculated based on the CO2 sorption isotherm to be 557 m2g−1. Although T18-apo and Ex-apo also absorbed N2 and CO2, the details are not shown because of their ambiguous structures.
Interestingly, T12-apo shows a two-stepped sorption isotherm with hysteric behavior for CO2 at 195 K. In situ PXRD measurements disclosed that T12-apo experiences up to four kinds of crystalline forms (states 1–4) reversibly during CO2 absorption–desorption process at 195 K (Fig. 11.11) [57]. Although these crystal structures have not been determined precisely, the observed PXRD patterns indicate that the layered structure of the original framework of T12-apo changes by distorting the network, slipping of the H-HexNet layers, and/or increasing in the interlayer distance (states 1–4 shown in Fig. 11.11c). These results indicate that layered organic crystals might be more flexible than those previously considered. The results can aid the construction of soft porous crystalline materials.
Structural changes of T12-apo upon CO2 sorption at 195 K. a CO2 sorption isotherm, where solid and open symbols denote absorption and desorption processes, respectively. b in situ PXRD (λ = 1.000 Å) patterns of T12-apo under a CO2 adsorption–desorption process at 195 K. Patterns a–o are recorded under conditions a–o in the isotherm (a). Pressure (/kPa) that the pattern was recorded at is shown in the parentheses. c Proposed structural changes including slippage, expansion, and deformation of the layered structure
The H-bonded low-density framework is also demonstrated to be capable of applying as a platform to accomplish an isolated arrangement of finite-numbered clusters of C60 molecules. Crystallization of T18 in the presence of C60 gave two types of inclusion crystals T18-C60-1 and -2 (Fig. 11.12). Interestingly, void II in T18-C60-2 accommodates two C60 molecules at its corners, and the resulting dimeric array of C60 is isolated from the adjacent dimers by the H-HexNet framework. The distance between the centroids of the nearest two C60 molecules is 11.2 Å and that between the second nearest two molecules is 15.12 Å. The isolated C60 pair observed in the present system is a unique type of C60 array. Namely, the present system is regarded as the smallest crystalline system of an finite-number-isolated array of C60 and the first example of an isolated C60 dimeric pair within a well-defined, H-bonded, low-density framework. These results imply that the present LA-H-HexNet can be applied as a platform to align functional molecules, aiming to developing such as artificial photosynthetic systems [58].
3.2 Sterically Hindered Hydrogen-Bonding Systems
As described in Sect. 11.2, the peripheral carboxyphenyl groups in the building block molecules are not in coplanar with but twisted against a plane of the central core (Fig. 11.6). This twisted conformation plays a role in stacking ways of the H-HexNets to give layered structures. To explore further effects of twisted conformation of the peripheral groups, triphenylene derivatives (TpMe and TpF) with substituents (Me or F) in the ortho-positions of the carboxy groups were designed and synthesized [59]. The substitution is expected to force the carboxy and phenylene groups to be twisted (Fig. 11.13), and therefore, to change molecular arrangements and properties of the resultant networked crystals.
Although computational calculation revealed that both substituent groups −F and −Me at the ortho-positions had no effects on binding energy of H-bonded dimerization (~15 kcal/mol), the substituents made the both carboxy and phenylene groups twisted, resulting increase of variability of the peripheral conformation. As shown in Fig. 11.14a–c, TpMe gave three polymorphs that exhibit different peripheral conformations and stacking manners of the H-HexNet layers. On the other hand, TpF yielded one crystalline form (Fig. 11.14d–f). However, in the crystals both carboxy and difluorophenylene groups are highly disordered. These observations indicate that introduction of the substituents on the ortho-position results high degree of freedom on the peripheral conformation, which results in generation of polymorphs in the case of larger substituent (i.e. TpMe), while in the case of smaller substituent (i.e. TpF), the single form with highly disordered local structures yields. Thermal stability and permanent porosity are drastically changed by the substituents even the both crystals have quite similar LA-H-HexNet structures. Regarding thermal stability of the LA-H-HexNets, the framework of Tp decomposes at 323 °C, while those of TpF and TpMe at 301 °C and 155 °C, respectively. Especially, the framework of TpF tends to lose its crystallinity as heating. Activation of TpF and TpMe was performed by solvent exchanging with benzene followed by heating up to 100 °C to yield porous materials TpF-1a and TpMe-1a, respectively, although their crystallinity was relatively low. SABET of TpF-1a and TpMe-1a was determined by CO2 sorption isotherms at 195 K to be 561 m2g−1 and 219 m2g−1, respectively. Only TpF-1a shows selective sorption of CO2 over N2.
Crystal structures of three polymorphs of TpMe (Top) and TpF-1 crystal (bottom). a TpMe-1, b TpMe-2, c TpM-3. d Molecular structure of TpF with anisotropic displacement ellipsoids with 50% probability. e Rhombic motif of TpF-1 with local disorder. f Layered framework, which is drawn with one of the disordered structures for clarity reason. Solvent molecules accommodated in the voids are omitted for clarity
3.3 Bowl-Shaped C3PI: A Sumanene Derivative
Former sections describe that planar C3PIs, such as the triphenylene derivative (Tp), form porous H-HexNet structures and that the H-HexNet sheet stacks without interpenetration to give crystalline porous layered frameworks [56]. On the other hand, a periodic 2D framework composed of non-planar π-conjugated molecules is also attractive because such a framework with curved and bumpy surface is expected to show unique electronic, chemical, or physical properties originating from the curved π-system. Furthermore, it also can provide useful information how curved building blocks achieve fully networked layered assemblies. Keeping this in mind, we planned to construct a H-bonded 2D framework by using the C3-symmetric buckybowl, sumanene [60].
CPSM gives three types of crystals, two of which are revealed to have H-bonded HexNet structures (Fig. 11.15). In crystal CPSM-1, all carboxy groups of CPSM form a H-bonded dimer, and the molecules are connected via the PhT motif, giving a waved H-HexNet sheet with a periodicity of 35.3 Å along the b axis due to an alternate alignment of bowl-up and bowl-down buckybowls. Because of geometrically mismatched unfavorable H-bonds, two carboxyphenyl groups bend outward and the bowl becomes shallower: The bowl depth (BD) is 0.985 Å, which is smaller than optimized pristine sumanene (1.11 Å) [61]. Void within and between the waved H-HexNet sheets are filled by solvent molecules, and the void ratio is calculated to be 63% by PLATON software with probe radius of 1.2 Å.
In crystal CPSM-2, the molecule forms a hamburger-like dimer possessing internal void with volume of 140 Å3 by interdigitating peripheral phenylene groups. The dimers are connected through a trefoil knot shaped H-bonding motif to form a 2D HexNet bilayer. The six peripheral carboxy groups of CPSM are capable of forming H-bonds without severe geometrical mismatch: The BD is 1.15 Å. The total void ratio is calculated to be 48%. Regarding network topology, CPSM-1 has a six-connected two-dimensional uninodal hxl net. CPSM-2 also has a six-connected two-dimensional network, while the topology of the network has been hitherto unknown (Fig. 11.15d, h).
Crystal structures composed of CPSM have less contact between the neighboring layers, compared with Tp-1, due to their bumpy surfaces. This inspires us to explore whether the crystals can be deformed by compression. The single crystals of CPSM-1 and CPSM-2 were subjected to X-ray diffraction analysis under high-pressure conditions. Although CPSM-1 lost its crystallinity under high pressure, crystal CPSM-2 kept its single crystallinity and showed significant anisotropic changes of the cell parameters upon addition of isotropic hydrostatic fluid pressure. The crystallographic c axis of CPSM-2 under 970 MPa was shortened by 11.0% compared with that under ambient pressure, while the other parameters showed subtle changes (Fig. 11.16a). These changes were irreversible and the shrunk cell remained after release of the pressure. SXRD analysis at 970 MPa revealed that the bilayered H-HexNet sheets slipped in a deeply offset stacking fashion and that interlayer distance became shorter. As shown in Fig. 11.16b, c, two contacted CPSM molecules (I and II) are slipped along their curved surfaces, resulting in shrinkage of interlayer distance and decrease of overlap between CPSM cores. Such a dynamic behavior between non-planar sheets is hitherto unknown and can provide new insights into 2D-networked architectures based on non-planar π-conjugated systems.
Anisotropic structural changes of CPSM-2 crystal under 970 MPa. a Changes in the unit cell upon increasing pressure. Open symbols refer the cell parameters obtained from SXRD analysis conducted at −120 °C under ambient pressure. Closed symbols refer those obtained from SXRD analysis conducted at ambient temperature with varying pressure. Molecular packing, b under ambient pressure at −120 °C and c under 970 MPa at 20 °C
3.4 Nitrogen-Incorporated C3PI: A Hexaazatrinaphthylene Derivative
Introducing nitrogen atoms into polycyclic aromatic hydrocarbons is capable of altering frontier orbital levels and of interacting and coordinating with cationic species such as metal ions, proton, and other organic cations. Therefore, HOFs composed of such N-hetero-π-conjugated molecules are expected to show multifunctionality. Regarding this, we planned to construct LA-H-HexNets with a hexaazatrinaphthylene derivative CPHATN possessing carboxyphenyl substituents [62].
CPHATN forms the PhT motif via H-bonding of carboxy groups to give a H-HexNet sheet, which then stacks without interpenetration to give a LA-H-HexNet CPHATN-1(124TCB) (Fig. 11.17a, b). It is noteworthy that carboxy groups form no H-bond with the basic pyrazine moieties, which remains non-bonded in the crystal and play a role for acid responsiveness as described later. The framework has 1D channels with width of 6.2 Å, in which 124TCB molecules used for crystallization are accommodated. A void ratio calculated by PLATON software with probe radius of 1.2 Å was 26%.
Crystal structures of as-formed solvate CPHATN-1(124TCB) (a, b) and activated form CPHATN-1a (c–e), solved by SXRD analysis. a, c Stacked three layers of H-HexNet sheets. b, d Relative orientation of the stacked layers, where structural changes upon activation were observed in the conformationally frustrated carboxylic acid dimers in circles. e Visualized surface of the void
TG analysis of CPHATN-1(124TCB) showed weight loss of 22% up to ca. 250 °C, indicating that the framework contains 124TCB with a host–guest ratio of 1:2 (calc. 25%) and solvent molecules were completely removed at around this temperature (Fig. 11.18a). VT-PXRD experiments of as-formed crystalline bulk of CPHATN-1(124TCB) were subsequently carried out as shown in Fig. 11.18b. The initial patterns at around room temperature are not clear as in the case of other systems described in Fig. 11.9. Upon heating, peaks started to appear at 83 °C and slightly shifted to wider angle up to 114 °C, indicating subtle shrinkage of the crystallographic cell. Subsequently, the peak intensity increased as heating, reached plateau at ca. 250 °C, and remained up to 360 °C, indicating that the framework is extremely stable and rigid enough to retain the porous structure at high temperature. SXRD analysis of the activated framework, CPHATN-1a, gave almost the same crystal structure, in which conformationally frustrated dimer was slightly deformed when compared with the structure of CPHATN-1(124TCB) as shown in Fig. 11.17c, d. The void is slightly shrunk from 26 to 20%. SABET was estimated based on N2 sorption isotherm at 77 K to be 379 m2g−1.
Interestingly, CPHATN-1a changes its color from yellow to reddish-brown when exposed to both 37%-HCl aqueous solution and HCl vapor (Fig. 11.19a). Its fluorescence also can be switched OFF or ON in presence or removing of the acid. These changes are reversible. The absorption spectrum exhibits a new band at 500–600 nm upon the exposure (Fig. 11.19b). Simultaneously, the emission band at 539 nm is strongly quenched (Fig. 11.19c). These observations clearly indicate the sensitivity of this HOF to HCl vapors and explain in terms of interactions between the protons and basic nitrogen atoms incorporated in the HATN core. Removal of HCl from the HOF resulted in recover of the original absorption and emission spectra. This is, to our knowledge, the first example of HOFs with external stimuli responsiveness in color and emission. The present results would open a door to develop a new porous materials with stimuli responsiveness.
HCl responsive color changes of CPHATN-1a. a Photographs of the crystalline bulk (i) before experiment, (ii) after 37%-HCl was dropped on, and (iii) after removing HCl by heating at 150 °C for 30 min. b Absorption and c emission spectral changes of the crystalline bulk upon exposure to HCl atmosphere for 40 min and after removing HCl by leaving in air for 48 h
4 Conclusion
It has been regarded that a H-bond is too weak interaction to apply for construction of supramolecular architectures possessing self-standing large pores. Such concernment, however, is about to be gone away. It is getting easy to construct HOFs with permanent porosity by introducing secondary interacting moieties capable of forming well-fitted intermolecular contacts to support the H-bonded networks, in addition to suitable selection of supramolecular synthons and molecular skeleton, namely H-bonding moieties that allow highly directional H-bonds with predictable manners and rigid molecular skeletons with exclusive degree of conformational freedom. As examples of such designed HOFs, we described LA-H-HexNets composed of C3-symmetric π-conjugated molecules (C3PIs) possessing three o-bis(4-carboxyphenyl)benzene moieties in the periphery. Thanks to the triangular H-bonded motif named phenylene triangle (PhT), C3PIs can form isostructural H-HexNet sheets, which further stack via secondary intermolecular interactions such as a π/π interaction to give layered HOFs. Moreover, we revealed that even bowl-shaped C3PI form H-bonded, 2D networked structures, in which all of carboxy groups satisfied through formation of H-bonded dimer. These results can make us realize promising potentials of HOFs. Once the construction ways of HOFs have been established, the properties and functionality of HOFs begin to be investigated. As reported by Cooper and Day, high-throughput exploration for development of functional porous materials is becoming possible by combining with theoretical calculation and robotics [63]. We hope that we would report multifunctional HOFs in the near future.
References
Allcock, H.R., Siegel, L.A.: Phosphonitrilic compounds. III. Molecular inclusion compounds of tris(o-phenylenedioxy)phosphonitrile trimer. J. Am. Chem. Soc. 86, 5140–5144 (1964)
Sozzani, P., Comotti, A., Simonutti, R., Meersmann, T., Logan, J.W., Pines, A.: A porous crystalline molecular solid explored by hyperpolarized xenon. Angew. Chem. Int. Ed. 39, 2695–2698 (2000)
Sozzani, P., Bracco, S., Comotti, A., Ferretti, L., Simonutti, R.: Methane and carbon dioxide storage in a porous van der Waals crystal. Angw. Chem. Int. Ed. 44, 1816–1820 (2005)
Atwood, J.L., Davies, J.E.D., MacNicol, D.D. (eds.): Inclusion Compounds, vol. 1–3. Academic Press, London (1984)
Barbour, L.J.: Crystal porosity and the burden of proof. Chem. Comm. 1163–1168 (2006)
Tian, J., Thallapally, P.K., McGrail, B.P.: Porous organic molecular materials. CrystEngComm 14, 1909–1919 (2012)
Mastalerz, M.: Permanent Porous materials from discrete organic molecules—towards ultra-high surface areas. Chem. Eur. J. 18, 10082–10091 (2012)
Atwood, J.L., Barbour, L.J., Jerga, A.: Storage of methane and freon by interstitial van der Waals confinement. Science 296, 2367–2369 (2002)
Adach, T., Ward, M.D.: Versatile and resilient hydrogen-bonded host frameworks. Acc. Chem. Res. 49, 2669–2679 (2016)
Lu, J., Cao, R.: Porous organic molecular frameworks with extrinsic porosity: a platform for carbon storage and separation. Angew. Chem. Int. Ed. 55, 9474–9480 (2016)
Luo, J., Wang, J.-W., Zhang, J.-H., Lai, S., Zhong, D.-C.: Hydrogen-bonded organic frameworks: design, structures and potential applications. Zhong, CrystEngComm 20, 5884–5898 (2018)
Lin, R.-B., He, Y., Li, P., Wang, H., Zhou, W., Chen, B.: Multifunctional porous hydrogen-bonded organic framework materials. Chen. Chem. Soc. Rev. 48, 1362–1389 (2019)
Hisaki, I., Chen, X., Takahashi, K., Nakamura, T.: Designing hydrogen-bonded organic frameworks (HOFs) with permanent porosity. Angew. Chem. Int. Ed. 58, 11160–11170 (2019)
He, Y., Xiang, S., Chen, B.: A microporous hydrogen-bonded organic framework for highly selective C2H2/C2H4 separation at ambient temperature. J. Am. Chem. Soc. 133, 14570–14573 (2011)
Maly, K.E., Gagnon, E., Maris, T., Wuest, J.D.: Engineering hydrogen-bonded molecular crystals built from derivatives of hexaphenylbenzene and related compounds. J. Am. Chem. Soc. 129, 4306–4322 (2007)
Yang, J., Dewal, M.B., Profeta, S., Smith Jr., M.D., Li, Y., Shimizu, L.S.: Origins of selectivity for the [2 + 2] cycloaddition of α, β-unsaturated ketones within a porous self-assembled organic Framework. J. Am. Chem. Soc. 130, 612–621 (2008)
Comotti, A., Bracco, S., Distefano, G., Sozzani, P.: Methane, carbon dioxide and hydrogen storage in nanoporous dipeptide-based materials. Chem. Commun. 284–286 (2009)
Yang, W., Greenaway, A., Lin, X., Matsuda, R., Blake, A.J., Wilson, C., Lewis, W., Hubberstey, P., Kitagawa, S., Champness, N.R., Schröder, M.: Exceptional thermal stability in a supramolecular organic framework: porosity and gas storage. J. Am. Chem. Soc. 132, 14457–14469 (2010)
Mastalerz, M., Oppel, I.: Rational construction of an extrinsic porous molecular crystal with an extraordinary high specific surface area. Angew. Chem. Int. Ed. 51, 5252–5255 (2012)
Luo, X.-Z., Jia, X.-J., Deng, J.-H., Zhong, J.-L., Liu, H.-J., Wang, K.-J., Zhong, D.-C.: A microporous hydrogen-bonded organic framework: exceptional stability and highly selective adsorption of gas and liquid. J. Am. Chem. Soc. 135, 11684–11687 (2013)
Lü, J., Perez-Krap, C., Suyetin, M., Alsmail, N.H., Yan, Y., Yang, S., Lewis, W., Bichoutskaia, E., Tang, C.C., Blake, A.J., Cao, R., Schröder, M.: A robust binary supramolecular organic framework (SOF) with high CO2 adsorption and selectivity. J. Am. Chem. Soc. 136, 12828–12831 (2014)
Comotti, A., Bracco, S., Yamamoto, A., Beretta, M., Hirukawa, T., Tohnai, N., Miyata, M., Sozzani, P.: Engineering switchable rotors in molecular crystals with open porosity. J. Am. Chem. Soc. 136, 618–621 (2014)
Chen, T.-H., Popov, I., Kaveevivitchai, W., Chuang, Y.-C., Chen, Y.-S., Daugulis, O., Jacobson, A.J., Miljanić, O.Š.: Thermally robust and porous noncovalent organic framework with high affinity for fluorocarbons and CFCs. Nat. Commun. 5, 5131 (2014)
Tian, J., Zhou, T.-Y., Zhang, S.-C., Aloni, S., Altoe, M.V., Xie, S.-H., Wang, H., Zhang, D.-W., Zhao, X., Liu, Y., Li, Z.-T.: Three-dimensional periodic supramolecular organic framework ion sponge in water and microcrystals. Nat. Commun. 5, 5574 (2014)
Li, P., He, Y., Zhao, Y., Weng, L., Wang, H., Krishna, R., Wu, H., Zhou, W., O’Keeffe, M., Han, Y., Chen, B.: A rod-packing microporous hydrogen-bonded organic framework for highly selective separation of C2H2/CO2 at room temperature. Angew. Chem. Int. Ed. 54, 574–577 (2015)
Wang, H., Li, B., Wu, H., Hu, T.-L., Yao, Z., Zhou, W., Xiang, S., Chen, B.: A flexible microporous hydrogen-bonded organic framework for gas sorption and separation. J. Am. Chem. Soc. 137, 9963–9970 (2015)
Yadav, V.N., Comotti, A., Sozzani, P., Bracco, S., Bonge-Hansen, T., Hennum, M., Görbitz, C.H.: Microporous molecular materials from dipeptides containing non-proteinogenic sesidues. Angew. Chem. Int. Ed. 54, 15684–15688 (2015)
Zentner, C.A., Lai, H.W.H., Greenfield, J.T., Wiscons, R.A., Zeller, M., Campana, C.F., Talu, O., FitzGerald, S.A., Rowsell, J.L.C.: High surface area and Z′ in a thermally stable 8-fold polycatenated hydrogen-bonded framework. Chem. Commun. 51, 11642–11645 (2015)
Nandi, S., Chakraborty, D., Vaidhyanathan, R.: A permanently porous single molecule H-bonded organic framework for selective CO2 capture. Chem. Commun. 52, 7249–7252 (2016)
Yang, W., Wang, J., Wang, H., Bao, Z., Zhao, J.C.-G., Chen, B.: Highly interpenetrated robust microporous hydrogen-bonded organic framework for gas separation. Cryst. Growth Des. 17, 6132–6137 (2017)
Hu, F., Liu, C., Wu, M., Pang, J., Jiang, F., Yuan, D., Hong, M.: An ultrastable and easily regenerated hydrogen-bonded organic molecular framework with permanent porosity. Angew. Chem. Int. Ed. 56, 2101–2104 (2017)
Hisaki, I., Ikenaka, N., Gomez, E., Cohen, B., Tohnai, N., Douhal, A.: Hexaazatriphenylene-based hydrogen-bonded organic framework with permanent porosity and single-crystallinity. Chem. Eur. J. 23, 11611–11619 (2017)
Hisaki, I., Suzuki, Y., Gomez, E., Cohen, B., Tohnai, N., Douhal, A.: Docking strategy to construct thermostable, single-crystalline, hydrogen-bonded organic framework with high surface area. Angew. Chem. Int. Ed. 57, 12650–12655 (2018)
Yin, Q., Zhao, P., Sa, R.-J., Chen, G.-C., Lü, J., Liu, T.-F., Cao, R.: An ultra-robust and crystalline redeemable hydrogen-bonded organic framework for synergistic chemo-photodynamic therapy. Angew. Chem. Int. Ed. 57, 7691–7696 (2018)
Yamagishi, H., Sato, H., Hori, A., Sato, Y., Matsuda, R., Kato, K., Aida, T.: Self-assembly of lattices with high structural complexity from a geometrically simple molecule. Science 361, 1242–1246 (2018)
Hashim, M.I., Le, H.T.M., Chen, T.-H., Chen, Y.-S., Daugulis, O., Hsu, C.-W., Jacobson, A.J., Kaveevivitchai, W., Liang, X., Makarenko, T., Miljanić, O.Š., Popovs, I., Tran, H.V., Wang, X., Wu, C.-H., Wu, J.I.: Dissecting porosity in molecular crystals: influence of geometry, hydrogen bonding, and [π···π] stacking on the solid-state packing of fluorinated aromatics. J. Am. Chem. Soc. 140, 6014–6026 (2018)
Gomez, E., Gutierrez, M., Cohen, B., Hisaki, I., Douhal, A.: Single crystal fluorescence behavior of a new HOF material: potential candidate for a new LED. J. Mater. Chem. C 6, 6929–6939 (2018)
Bassanetti, I., Bracco, S., Comotti, A., Negroni, M., Bezuidenhout, C., Canossa, S., Mazzeo, P.P., Marchil, L., Sozzani, P.: Flexible porous molecular materials responsive to CO2, CH4 and Xe stimuli. J. Mater. Chem. A 6, 14231–14239 (2018)
Li, P., Li, P., Ryder, M.R., Liu, Z., Stern, C.L., Farha, O.L., Stoddart, J.F.: Interpenetration isomerism in triptycene-based hydrogen-bonded organic frameworks. Angew. Chem. Int. Ed. 58, 1664–1669 (2019)
Han, B., Wang, H., Wang, C., Wu, H., Zhou, W., Chen, B., Jiang, J.: Postsynthetic metalation of a robust hydrogen-bonded organic framework for heterogeneous catalysis. J. Am. Chem. Soc. 141, 8737–8740 (2019)
Gomez, E., Suzuki, Y., Hisaki, I., Moreno, M., Douhal, A.: Spectroscopy and dynamics of a HOF and its molecular units: remarkable vapor acid sensing. J. Mater. Chem. C 7, 10818–10832 (2019)
Pulido, A., Chen, L., Kaczorowski, T., Holden, D., Little, M.A., Chong, S.Y., Slater, B., McMahon, D.P., Bonillo, B., Stackhouse, C.J., Stephenson, A., Kane, C.M., Clowes, R., Hasell, T., Cooper, A.I., Day, G.M.: Functional materials discovery using energy-structure-function maps. Nature 543, 657–666 (2017)
Day, G.M., Cooper, A.I.: Energy–structure–function maps: cartography for materials discovery. Adv. Mater. 1704944 (2017)
Desiraju, G.R.: Supramolecular synthons in crystal engineering—a new organic synthesis. Angew. Chem. Int. Ed. Engl. 34, 2311–2327 (1995)
Ivasenko, O., Perepichka, D.F.: Mastering fundamentals of supramolecular design with carboxylic acids common lessons from X-ray crystallography and scanning tunneling microscopy. Chem. Soc. Rev. 40, 191–206 (2011)
Moulton, B., Zawarotko, M.J.: From molecules to crystal engineering: supramolecular isomerism and polymorphism in network solids. Chem. Rev. 101, 1629–1658 (2001)
Duchamp, D.J., Marsh, R.E.: The crystal structure of trimesic acid (benzene-1,3,5-tricarboxylic acid). Acta Crystallogr. B 25, 5–19 (1969)
Herbstein, F.H., Kapon, M., Reisner, G.M.: Catenated and non-catenated inclusion complexes of trimesic acid. J. Inclusion Phenom. 5, 211–214 (1987)
Miyaura, N., Suzuki, A.: Palladium-catalyzed cross-coupling reactions of organoboron compounds. Chem. Rev. 95, 2457–2483 (1995)
Hisaki, I., Sakamoto, Y., Shigemitsu, H., Tohnai, N., Miyata, M., Seki, S., Saeki, A., Tagawa, S.: Superstructure-dependent optical and electrical properties of an unusual face-to-face, π-stacked, one-dimensional assembly of dehydrobenzo[12]annulene in the crystalline state. Chem. Eur. J. 14, 4178–4187 (2008)
Hisaki, I., Senga, H., Sakamoto, Y., Tuzuki, S., Tohnai, N., Miyata, M.: Specific interaction between chloroform and the pockets of triangular annulene derivatives providing symmetry carry-over crystallization. Chem. Eur. J. 15, 13336–13340 (2009)
Hisaki, I., Senga, H., Shigemitsu, H., Tohnai, N., Miyata, M.: Construction of 1D π-stacked superstructures with inclusion channels through symmetry-decreasing crystallization of discotic molecules of C3 symmetry. Chem. Eur. J. 17, 14348–14353 (2011)
Kobayashi, K., Shirasaka, T., Horn, E., Furukawa, N.: Two-dimensional hexagonal hydrogen-bonded network with triangle-like large cavities: hexakis(4-carboxyphenyl)benzene. Tetrahedron Lett. 41, 89–93 (2000)
Hisaki, I., Nakagawa, S., Tohnai, N., Miyata, M.: A C3-symmetric macrocycle-based, hydrogen-bonded, multiporous hexagonal network as a motif of porous molecular crystals. Angew. Chem. Int. Ed. 54, 3008–3012 (2015)
Hisaki, I., Nakagawa, S., Ikenaka, N., Imamura, Y., Katouda, M., Tashiro, M., Tsuchida, H., Ogoshi, T., Sato, H., Tohnai, N., Miyata, M.: A series of layered assemblies of hydrogen-bonded, hexagonal networks of C3-symmetric π-conjugated molecules: a potential motif of porous organic materials. J. Am. Chem. Soc. 138, 6617–6628 (2016)
Hisaki, I., Ikenaka, N., Tohnai, N., Miyata, M.: Polymorphs of layered assemblies of hydrogen-bonded hexagonal networks caused by conformational frustration. Chem. Commun. 52, 300–303 (2016)
Hisaki, I., Nakagawa, S., Suzuki, Y., Tohnai, N.: CO2 sorption of layered hydrogen-bonded organic framework causes reversible structural changes involving four different crystalline states under ambient pressure. Chem. Lett. 47, 1143–1146 (2018)
Hisaki, I., Nakagawa, S., Sato, H., Tohnai, N.: Alignment of paired molecules of C60 within a hexagonal platform net-worked through hydrogen bonds. Chem. Commun. 52, 9781–9784 (2016)
Hisaki, I., Ikenaka, N., Tsuzuki, S., Tohnai, N.: Sterically crowded hydrogen-bonded hexagonal network frameworks. Mater. Chem. Front. 2, 338–346 (2018)
Hisaki, I., Toda, H., Sato, H., Tohnai, N., Sakurai, H.: A hydrogen-bonded hexagonal buckybowl framework. Angew. Chem. Int. Ed. 56, 15294–15298 (2017)
Sakurai, H., Daiko, T., Sakane, H., Amaya, T., Hirao, T.: Structural elucidation of sumanene and generation of Its benzylic anions. J. Am. Chem. Soc. 127, 11580–11581 (2005)
Hisaki, I., Suzuki, Y., Gomez, E., Ji, Q., Tohnai, N., Nakamura, T., Douhal, A.: Acid responsive hydrogen-bonded organic frameworks. J. Am. Chem. Soc. 141, 2111–2121 (2019)
Greenaway, R.L., Santolini, V., Bennison, M.J., Alston, B.M., Pugh, C.J., Little, M.A., Miklitz, M., Eden-Rump, E.G.B., Clowes, R., Shakil, A., Cuthbertson, H.J., Armstrong, H., Briggs, M.E., Jelfs, K.E., Cooper, A.I.: High-throughput discovery of organic cages and catenanes using computational screening fused with robotic synthesis. Nat. Commun. 9, 2849 (2018)
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Hisaki, I., Ji, Q., Takahashi, K., Nakamura, T. (2020). Layered Hydrogen-Bonded Organic Frameworks as Highly Crystalline Porous Materials. In: Sakamoto, M., Uekusa, H. (eds) Advances in Organic Crystal Chemistry. Springer, Singapore. https://doi.org/10.1007/978-981-15-5085-0_11
Download citation
DOI: https://doi.org/10.1007/978-981-15-5085-0_11
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-15-5084-3
Online ISBN: 978-981-15-5085-0
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)