Abstract
Xylan, the most abundant hemicellulose and major component in lignocellulosic biomass requires depolymerisation in order for it to be used as a precursor for the production of value added products (VAPs). As global interest in using lignocellulosic feedstocks for VAPs production increases, an accompanying knowledge on how to efficiently depolymerise these feedstocks is required. Thermochemical depolymerisation of xylan in lignocellulose is non-specific and environmentally unfriendly, and as a result, enzymatic means of xylan depolymerisation is favoured. Due to the complex nature of xylans, a consortium of xylanolytic enzymes is required for the efficient degradation of xylans. Interesting applications of xylanolytic enzymes include uses in plant-based industries such as use as feed additives, in waste treatment, biofuel production, bread making, probiotics and fruit juice production, and pulp and paper bleaching. Although many xylanolytic enzymes exhibit certain desirable characteristics for industry, no individual enzyme is capable of meeting the needs of all xylanolytic enzyme requiring industries. In addition, as industry requires cheaper enzyme processing costs, efficient catalysis by xylanolytic enzymes is required. This review examines recent advances in the use of xylanolytic enzymes and their synergistic associations with each other and with other classes of enzymes which can improve their industrial applications.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
Introduction
Hemicelluloses are the second most abundant polysaccharide in nature, after cellulose, and represent about 20–35% of the dry weight of the lignocellulosic biomass (Saha 2003). Generally, hemicelluloses are named according to the major monosaccharide residues found in their structural backbones and most of these are linked together by β-1,4-glycosidic bonds (Saha 2003; Van Zyl et al. 2010). The classes of hetero-polysaccharides that hemicellulose represents or denotes to includes plant cell wall polysaccharides such as xylans, mannans, β-glucans and xyloglucans (Peng and She 2014).
The distribution of hemicellulose in plants varies greatly—hardwoods and grasses contain xylans as the major hemicellulosic component, whereas softwoods and some specialized structures, such as plant seeds and fruits, are comprised of mannans as a more prominent type of hemicellulose (Moreira and Filho 2008; Saha 2003). Among hemicelluloses, xylan is the major carbohydrate, and is composed of a backbone of β-1,4-d-xylose residues substituted with acetyl, α-l-arabinofuranosyl, α-galactosyl, α-glucuronyl, and 4-O-methylglucuronyl groups.
Xylan-containing lignocellulosic biomass is a renewable and carbon-neutral energy source that has considerable potential as a feedstock for the large scale production of liquid fuels, prebiotics, artificial sweeteners and other value added products (VAPs), with the production of most of these VAPs requiring the xylan portion of lignocellulose to be hydrolysed into its monomers, xylose, or at least into xylo-oligosaccharides (XOS) in some instances (Charoensiddhi et al. 2017; Saha 2003; York and O’Neill 2008). Currently, thermochemical pre-treatment of the xylan-containing lignocellulosic biomass is the predominantly used method for hydrolysing xylan. During this procedure, the lignocellulosic biomass is generally treated with a diluted acid such as 2% (w/v) sulfuric acid by steam-heating to 120 °C or higher for 2 h (Alvira et al. 2010). The solid to liquid ratio in this process is typically around 1:7 to 1:15, respectively (Wang et al. 2017). Technical drawbacks in xylan hydrolysis via thermochemical means include: energy consumption (accounts for 40% of total energy consumption), corrosion of equipment, production of waste water containing acids and salts which accounts for 30% of the total water consumption and can cause heavy environment pollution and production of undesirable by-products resulting from dehydration of xylose and other monosaccharides, which increase the difficulty of subsequent purification of target products (Alvira et al. 2010; Hendriks and Zeeman 2009; Wang et al. 2017).
Recent studies have demonstrated synergistic interactions between various xylanolytic enzymes and other enzyme classes and, as a result, improve xylan and xylan-containing biomass industrial utilization (Malgas et al. 2017a). Here, we summarize recent studies that report on advances in industrial utilization of xylanolytic enzymes for value added products derived from lignocellulosic biomass.
Xylans
Xylans are classified into three subfamilies: glucuronoxylan, arabinoxylan and arabinoglucuronoxylan or glucuronoarabinoxylan. Structural properties and occurrence of these various xylan subfamilies are briefly explained in this review (Fig. 1).
General structure of hetero-xylans. (a) A typical arabinoxylan structure, (b) a typical glucuronoxylan structure, and (c) a typical arabinoglucuronoxylan or glucuronoarabinoxylan structure. Grey hexagons represent xylose while black hexagons represent reducing end xylose, pentagons represent arabinose, circles represent (methyl)-glucuronic acid residues and lines represent glycosidic bonds between sugar moieties
Arabinoxylans
Arabinoxylans (AXs) consist of a main chain of 1,4-linked β-d-xylopyranosyl residues to which mono- or di-α-l-arabinofuranosyl substituents are attached through O-2 and/or O-3 positions of xylose (Revanappa et al. 2015; Schendel et al. 2015). The arabinofuranosyl residues in AXs can be esterified with hydroxycinnamic acid derivatives, such as ferulic and p-coumaric acid (Lagaert et al. 2014). AX solubility and viscosity can vary depending on their molecular weight, arabinose: xylose ratio and degree of cross-linking with other polymers in the plant cell wall (Schendel et al. 2015). Arabinoxylans are the main non-starch hemicelluloses found in the cell walls of endosperms of Gramineae such as wheat, bamboo, rice, sorghum, sugarcane and ryegrass (Peng and She 2014; Revanappa et al. 2015).
Glucuronoxylans
Glucuronoxylans (GXs) consist of a main chain of 1,4-linked β-d-xylopyranosyl residues to which α-1,2-d-linked glucupyranosyluronic acid residues are attached to O-2 of each tenth xylose residue (Togashi et al. 2009). Some hardwood species may have some or all of their glucuronic acid substituents methylated at O-4 position; however, in Eucalyptus globulus wood, about one third of the 4-O-methyl-α-d-glucuronopyranosyl residues are substituted at O-2 by d-galactopyranosyl and d-glucopyranosyl units (Pinto et al. 2005). Therefore, in hardwood species, generally, the majority of hemicelluloses are O-acetyl-4-O-methylglucurono-β-d-xylans, their content varying between 15 and 30% (wood weight basis) (Pinto et al. 2005). The degree of polymerization of these hemicelluloses in wood is approximately 150–200 (Singh et al. 2003).
Arabinoglucuronoxylan and Glucuronoarabinoxylan
According to Gatenholm and Tenkanen (2003), and Huisman and co-workers (Huisman et al. 2000), arabinoglucuronoxylans (AGX) and glucuronoarabinoxylans (GAXs) consist of a β-d-(1,4)- linked xylopyranoside backbone and can be substituted with α-l-arabinofuranose on C2 and/or C3, α-d-glucopyranosyluronic acid, or its 4-O-methyl derivative on C2, acetyl on C2 or C3 of some xylose residues. These are considered the most complex types of xylans. AGXs are the dominant hemicelluloses in cereals and grasses, and are also present in coniferous species (Ebringerová 2006; Gatenholm and Tenkanen 2003).
Xylan Degradation
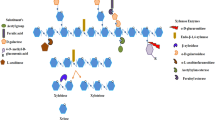
Complete degradation of xylans requires the synergistic action of a consortium of glycoside hydrolase (GH) enzymes, including β-xylanase, β-xylosidase, α-glucuronidase and α-arabinofuranosidase, and accessory enzymes, such as acetyl xylan esterase, ferulic acid esterase and p-coumaric acid esterase (Fig. 2). Here we give a brief summary of the properties of each xylanolytic glycoside hydrolase enzyme required during xylan degradation—in-depth information on these enzymes has been reviewed elsewhere (Collins et al. 2005; Biely et al. 2016; Juturu and Wu 2012).
An illustration of the enzyme sites of the enzymes required to completely degrade hetero-xylans. Grey hexagons represent xylose while black hexagons represent reducing end xylose, pentagons represent arabinose, circles represent (methyl)-glucuronic acid residues, lines represent glycosidic bonds between sugar moieties and arrows represent the enzyme required for cleaving a glycosidic bond between specific sugar moieties
Xylanases
Endo-β-1,4-xylanases (also called 1,4-β-d-xylan xylanohydrolases, EC 3.2.1.8) are enzymes which randomly cleave the β-(1,4)-linkages between two d-xylopyranosyl residues in xylan backbones (Mendis and Simsek 2015). Xylanases have been classified into glycoside hydrolase families GH5, 8, 10, 11, 30, 43, 62 and 98 in the CAZy database, with GH10 and 11 being the best characterized families. GH10 fungal and bacterial xylanases often contain a catalytic domain linked to one or more non-catalytic modules (such as CBM22). CBM22 possesses the ability to bind four to five xylosyl residues in insoluble xylans via hydrophobic stacking interactions (Motta et al. 2013; Simpson et al. 2003). According to Paës et al., CBMs probably help the xylanase to indirectly localize the xylan substrate, since it is in close association with cellulose in lignocellulosic biomass (Paës et al. 2012). GH11 xylanases can only hydrolyse xylosidic bonds where the two corresponding xylose moieties in subsites (−1) and (+1) are not branched, while GH10 xylanases attack the glycosidic linkage next to a single or double substituted xylose toward the non-reducing end and require two unsubstituted xylose residues between branched residues (Mendis and Simsek 2015; Paës et al. 2012).
Arabinofuranosidases
Arabinofuranosidases (also called Abfs, EC 3.2.1.55) are enzymes involved in the hydrolysis of terminal non-reducing α-l-arabinofuranoside (l-Araf) residues in α-l-arabinans containing α-(1,2)- or α-(1,3)- and/or α-(1,5)-linkages, arabinoxylans, arabinoxylo-oligosaccharides and arabinogalactans (Lagaert et al. 2014). Abfs belong to the glycoside families GH2, 3, 10, 43, 51, 54 and 62 in the CAZy database. All Abf families perform glycosidic bond hydrolysis with retention of the anomeric configuration, except for GH62 which still has an unknown mechanism (www.cazy.org). Abfs that release l-Araf units from mono-substituted main-chain d-xylopyranosyl (d-Xylp) motifs are termed AXH-m, while those that release single l-Araf residues from double-substituted main-chain d-Xylp motifs are termed AXH-d. Only GH43 and 51 Abfs have been demonstrated to readily de-branch l-arabinose groups on both mono- and di-substituted d-xylopyranosides (Lagaert et al. 2014).
Glucuronosidases and Glucuronidases
Xylan α-1,2-glucuronosidases and α-glucuronidases (EC 3.2.1.131 and EC 3.2.1.139, respectively) are enzymes that hydrolyse α-(1,2)-d-(4-O-methyl)-glucuronosyl links in the main chains of glucuronoxylans and glucuronoarabinoxylans. According to Nurizzo et al. (2002), some GH67 glucuronidases prefer short glucuronic acid substituted xylo-oligomers and are generally intracellular or membrane associated, while others have specificity for polymeric glucuronic acid-containing polymeric xylans, these being consequently extracellularly produced enzymes. The reaction mechanism of GH67 glucuronidases has been elucidated as net inversion (Shallom and Shoham 2003). Generally, GH115 glucuronidases are more active on polymeric glucuronic acid-containing polymeric xylans (McKee et al. 2016; Tenkanen and Siika-Aho 2000) compared to GH67 glucuronidases (Golan et al. 2004; Nagy et al. 2002).
Xylosidases
According to the CAZy database, xylosidases are presently found in glycoside hydrolase families GH3, 30, 39, 43, 52, 54, 116 and 120. Some GH39 xylosidases exist as tetramers in solution and have also been reported to exhibit glycosylation activity on xylo-oligomers (Lagaert et al. 2014). Xylosidases catalyse the hydrolysis of xylobiose and also attack the non-reducing ends of short xylo-oligosaccharides, but do not hydrolyse xylan, which is a polysaccharide (Huy et al. 2015; Knob et al. 2010; Yan et al. 2008).
Exo-xylanases
Exo-xylanases (also called oligosaccharide reducing-end xylanase (Rex), EC. 3.2.1.156) show a different mode of action when compared to the β-xylanases and β-xylosidases, and are strictly found in GH8 according to the CAZy database. Exo-xylanases hydrolyse the xylan back-bone from the reducing ends producing xylo-oligomers. Interestingly, these enzymes can also produce xylose from short xylo-oligomers with a degree of polymerization of 2 or 3 (Juturu and Wu 2012; Malgas and Pletschke 2019; Lagaert et al. 2014). Rex’s are said to recognize the xylose unit of the reducing end in a very strict manner, even discriminating the β-anomeric hydroxyl configuration from the α-anomer or 1-deoxyxylose (Fushinobu et al. 2005).
Since numerous comprehensive reviews on the characteristics of xylanolytic enzymes have been published (Juturu and Wu 2013; Lagaert et al. 2014; Paës et al. 2012), this review will focus on the role of xylanolytic enzymes and their synergism with other enzymes in industrial applications.
Synergistic Actions of Xylanolytic Enzymes on Xylans
As previously mentioned, an array of xylan specific hemicellulolytic enzymes synergistically acts on xylans, leading to their complete hydrolysis into monomeric sugars. According to Moreira and Filho (2008), two types of synergies have been identified between mannanolytic enzymes, namely homeosynergy and heterosynergy. Based on the complexity of xylan, which includes the presence of substituents on its backbone, we propose that xylanolytic enzymes can also exhibit these two types of synergies during its hydrolysis. Homeosynergy can be defined as the cooperation between two main-chain-cleaving enzymes (e.g. β-xylanase and β-xylosidase) or two side-chain-cleaving enzymes (e.g. α-arabinofuranosidase and α-glucuronidase), while heterosynergy can be defined as the synergistic association between a side-chain-cleaving and a main-chain-cleaving enzyme (e.g. β-xylanase and α-arabinofuranosidase).
Sources of Xylanolytic Enzymes
Xylanolytic enzymes are produced by a diverse variety of microorganisms including archaea, bacteria, fungi, yeast etc. Different niches such as insect gut, manure, waste water, hot environmental samples and chicken cecum have been reported as sources which harbour xylanolytic enzyme producing micro-organisms (Chadha et al. 2019). Some bacterial and fungal strains which produce aggressive xylanolytic enzymes include Aspergillus sp., Bacillus sp., Thermoascus auranticus, Talaromyces emersonii, Thermomyces lanuginosus, Melanocarpus albomyces, Sporotrichum thermophile, Geobacillus stearothermophilus, Caldoceum saccharolyticum, Clostridium sp., Trichoderma sp. and Thermomonospora sp. (Chadha et al. 2019; Collins et al. 2005). Table 1 lists bacterial and fungal sources of xylanolytic machinery and the characterised xylanolytic enzyme gene products the micro-organisms produce as reported in the CAZy database (http://www.cazy.org). In addition to the production of a variety of hydrolytic enzymes, many microorganisms can produce multiple isozymes of the same enzyme. A classic example of this is Aspergillus niger which is reported to encode a multiple of up to fifteen extracellular xylanases (Malgas et al. 2017b).
Xylanase Market
It is reported that the global market for industrial enzymes was around $6.3 billion in 2017 and will continue to grow at around 6.8% compound annual rate of growth (CAGR) up to 2024, with xylanases expected to be worth $35 million in the same year (http://www.alliedmarketresearch.com). According to Collins and co-workers, presently the technical industries, dominated by the detergent, starch, textile and fuel alcohol industries, account for the majority of the total enzymes market, with the feed and food enzymes together totalling only about 35% (Collins et al. 2005). The biofuels segment is expected to be the fastest-growing application area for industrial enzymes during the forecast period, with a CAGR of about 7.3% through to 2024. The United States Patent and Trademark Office lists 3975 patents to date with reference to xylanases (http://www.uspto.gov/).
The major global manufacturers and suppliers of industrial enzymes include: AB Enzymes, Adisseo, ABF Group, BioZyme, Danisco, Dyadic, Genencor, DSM, Elanco, Iogen, Roche, Alltech, Basf, Takabio and Novozymes. The top three companies holding the biggest enzyme market share (approximately 75%) are Novozymes, AB Enzymes and Genencor at 47, 21 and 20%, respectively (Li et al. 2012). These companies are focusing on improving their production capacities and upgrading their solutions to differentiate their services from their competitors.
Industrial Applications of Xylanases
Xylanases and other xylanolytic enzymes are reported to be useful in numerous industrial applications beyond their use as feed additives, waste treatment, biofuel production, bread making, probiotics and fruit juice production, and pulp and paper bleaching. Table 2 lists possible industrial applications of xylanolytic enzymes and the specific roles they play in those certain processes.
Application of Xylanases in Prebiotics Production
According to Yasmin and co-workers, prebiotics are simply defined as non-digestible food components that selectively stimulate the growth or activity of specific indigenous bacteria (prebiotics) in the digestive tract in a manner claimed to be beneficial for the host (Yasmin et al. 2015). Well known probiotics include bacteria such as lactobacilli and Bifidobacterium and non-pathogenic yeast, Saccharomyces boulardii (Yasmin et al. 2015; Vandenplas 2016). Prebiotics are also said to decrease the toxin-producing bacteria like Streptococcus pneumonia, proteolytic Clostridia and Escherichia coli (Charoensiddhi et al. 2017). Prebiotics are primarily carbohydrates (oligosaccharides and polysaccharides) in nature. Oligosaccharides included in this category are fructooligosaccharides (FOS), xylooligosaccharides (XOS), galactooligosaccharides (GOS), mannooligosaccharides (MOS) and pectin oligosaccharides (POS) as well as some sugar alcohols (Hutkins et al. 2016; Moreno et al. 2017).
It is reported that prebiotic oligosaccharides can be found naturally in foods or, alternatively, they can be produced by enzymatic or chemical synthesis from disaccharides or other substrates, as well as by hydrolysis of polysaccharides (Moreno et al. 2017). Hydrolysis of polysaccharides is normally the most reliable choice for oligosaccharide production on a large scale, due to its reproducibility and high yield. Lyases such as pectate lyases and glycoside hydrolases (GHs) including cellulases, xylanases, mannanases and amylases are the enzymes required to degrade lignocellulose derived polysaccharides into oligosaccharides which can subsequently be used as prebiotics (Juturu and Wu 2013; Pedrolli et al. 2009). In this section, we shall examine the synergistic use of xylanolytic enzymes for the production of prebiotic XOS.
During prebiotic XOS production, it is noteworthy to mention that homeosynergistic interactions between backbone cleaving enzymes such as xylanases may be required. However, enzymes with xylosidase activity are not desirable as XOS with a degree of polymerisation (DP) of 2 and higher are reported to elicit a prebiotic effect, therefore xylose production is undesirable (Samanta et al. 2015). Synergism between GH10 and 11 xylanases during the degradation of sugarcane bagasse xylan has been reported previously by Gonçalves and co-workers in a protein mass ratio of 50:50%; however, the synergistic effect was enzyme source specific (Goncalves et al. 2015). Malgas and co-workers also recently demonstrated that XT6 and Xyn2A in a mass ratio of 25:75% could synergise and efficiently depolymerize beechwood glucuronoxylan and wheat-flour arabinoxylan into XOS compared to when the enzymes were used alone (Malgas and Pletschke 2019). On the contrary, another study reported that the simultaneous use of XYL10 and XYL11 xylanases from Thermobacillus xylanilyticus does not result in a synergistic action on insoluble wheat bran arabinoxylan degradation (Beaugrand et al. 2004).
Malgas and co-workers have postulated that reasons for the differences with respect to both the enzyme ratios and synergism trends (synergy vs anti-synergy/competition) reported in literature could be due to both enzyme and substrate specific properties (Malgas and Pletschke 2019). They further postulated that differences such as the enzyme source and presence or absence of CBMs in enzymes, and solubility or insolubility and/or degree of substitution of substrates have been reported to have an effect on the trend of synergy observed (Malgas and Pletschke 2019; Van Dyk and Pletschke 2012). In summary, it appears as if the synergistic application of xylanases from different GH families holds promise for improving the production of high yields of XOS from xylan substrates to be used as prebiotics.
Application of Xylanases in Xylose and Xylitol Production
Xylitol, a five carbon sugar alcohol used as an artificial sweetener, can be produced from the second most abundant polysaccharide, xylan, which upon hydrolysis produces xylose (Rao et al. 2016). Xylitol is widely used as a sugar substitute in the food industry because of its properties, such as similarity in sweetness to sucrose, no requirement for insulin, inhibition of dental caries and low calories (Kamat et al. 2013; Li et al. 2015). Xylitol can also serve as a valuable synthetic building block for various chemical compounds such as xylaric acid, succinic acid and glycols (Guo et al. 2013). In this section we describe the role of xylanolytic enzymes in producing xylose from the xylan fraction of lignocellulosic biomass for use as a precursor for xylitol production (Li et al. 2013).
A recent study showed that a sugar-rich effluent from pre-hydrolysis of eucalyptus wood (pre-hydrolysate) composed of XOS is an abundant by-product of paper and pulp industries which could be completely hydrolysed to xylose with concentrations reaching 50.41 g/L after 24 h using a xylanolytic cocktail composed of xylanase (1333.33 U/g), β-xylosidase (60 U/g) and acetyl-xylan-esterase (27 U/g) (Wang et al. 2017). Another study demonstrated that Aspergillus terreus derived xylanolytic enzymes, xylanase (722 U/g) and β-xylosidase (196 U/g), could be used to hydrolyse corncob hemicellulose for xylitol synthesis with sugars of 18.03 g/L xylose and 4.87 g/L glucose being obtained after 8 h of hydrolysis (Li et al. 2013). An integrated xylitol production pathway, directly using xylan as the substrate, was constructed in Candida tropicalis BIT-Xol-1 which could efficiently convert xylose into xylitol using a xylanase gene (atn) and a xylosidase gene (atl) were cloned from Aspergillus terreus which were constructed onto the episomal plasmid pAUR123 of Candida tropicalis (Guo et al. 2013).
These studies all showed that a combination of xylanolytic enzymes was more efficient at xylose production from xylan (for use as a precursor for xylitol production) compared to when only xylanases were used for xylose production.
Application of Xylanases in Biofuels
Bioethanol is a renewable liquid fuel that can be produced from lignocellulosic biomass via a combination of physical or chemical pre-treatment, and enzymatic hydrolysis to produce fermentable sugars (e.g. glucose and xylose) from the biomass and their fermentation by microorganisms. The major components of lignocellulose are carbohydrates such as cellulose and hemicellulose (approximately 75% dry weight), and lignin. For maximum utilization of lignocellulosic biomass, efficient hydrolysis of hemicellulose is required. This is mainly based on the following two considerations: (1) the utilization of hemicellulose can increase the theoretical ethanol yield and improve the economics of lignocellulose conversion and (2) degradation of hemicellulose will further facilitate the access of cellulases to cellulose, leading to more efficient utilization of cellulose, since cellulose is embedded in a matrix of hemicellulose (Van Dyk and Pletschke 2012).
Traditional glucose fermenting organisms such as Saccharomyces cerevisiae and Zymomonas mobilis cannot ferment other sugars such as xylose to bioethanol efficiently. As a result, research has focused on exploring other yeasts such as Pachysolen tannophilus, Pichia stipitis, and Candida shehate which have the capability to ferment xylose to ethanol. However, commercial exploitation of these yeasts for bioethanol production from xylose is still not viable due to their low ethanol tolerance, slow rates of fermentation, difficulty in controlling the rate of oxygen supply at the optimal level, in addition to sensitivity to inhibitors generated during pretreatment and hydrolysis of lignocellulosic substrates. Due to this, other efforts have focused on converting xylose to xylulose using the enzyme xylose isomerase and this (xylulose) can be fermented to ethanol by traditional yeasts.
Cellulase to endo-xylanase synergism is so well studied, to such an extent that it is known that glycoside hydrolase (GH) family 10 xylanases show better synergistic cooperation with canonical hydrolytic cellulases than GH11 xylanases on various pre-treated lignocellulosic substrates such as sugarcane bagasse (Hu and Saddler 2018) and wheat straw (Zhang et al. 2011).
From these numerous studies, it appears that an intimate network between cellulose and hemicellulose exists, and as xylanolytic enzymes synergistically interacted with cellulases during the degradation of lignocellulosic biomass both glucose and xylose yields were improved. In this regard, a previous review of ours showed that as the xylan content increases for a particular lignocellulosic substrate, the presence of a xylanase becomes necessary in the enzyme cocktail and the degree of synergy between cellulase and xylanase also increases with respect to both glucose and xylose release (Malgas et al. 2017a, b). Finally, it is clear that the addition of xylanolytic enzymes in cellulolytic cocktails leads to improvement in the specific cellulase performance or activity and results in the reduction of grams of enzyme required for achieving equivalent hydrolysis by the xylanase free cocktail (Malgas et al. 2017a; Pletschke et al. 2016).
Application of Xylanases in Animal Feeds
Xylanases have been documented for their use as feed additives for monogastric animals (particularly poultry and pigs) for more than 20 years (Paloheimo et al. 2012). Monogastric animals are classified as non-ruminant animals which includes pigs, poultry such as turkeys, ducks and chickens (both broilers and layers); fish and crustaceans (e.g. shrimp and prawns). Xylanases have also been reported to be effective feed additives for ruminant animals such as sheep, goats and cattle (e.g. beef cattle and dairy cows). In this review, we will focus on reported advances in the application of xylanases as well as their synergistic associations with non GH enzymes as feed additives for both poultry and pigs.
Cereal grains are the important and major component of animal feeds. In cereal grains, starch is the major nutritional component, however, cereal grains also contain non-starch polysaccharides (NSPs) which cannot be digested by the digestive system of many monogastric animals such as poultry and pigs (Fischer and Petterson 2014; Paloheimo et al. 2012). NSPs in cereal-based feeds include polysaccharides such as xylan (particularly AX), mannan, beta-glucans and cellulose. Grains such as wheat, triticale and maize contain AX as a major anti-nutritional factor which makes up 50% (w/w-dry matter) of the NSPs, whereas for barley, sorghum, rye and rice the AX makes up approximately 25–45% of the NSPs. In grains, AX exists in two forms, water soluble and water insoluble fractions. Insoluble-AX is known for encapsulating nutrients such as starch and protein. The soluble AX, on the other hand, has been shown to lead to an increase in the viscosity of digesta. This encapsulation and high viscosity digesta leads to valuable nutrients bypassing the digestive process in the gut of monogastric animals. Therefore, xylanases are used to improve the feed digestibility of livestock and in cutting expenses with profit gain. Discussed below are recent studies detailing the use of xylanolytic enzymes—either alone or in concert with other classes of enzymes as additives in animal feeds.
The use of xylanases and xylan-debranching enzymes has been reported to solubilize insoluble-AX, and as a result, expose the nutrients which are encapsulated, and also fragments the soluble AX, leading to lowered digesta viscosity (Fischer and Petterson 2014; Lei et al. 2016). Latorre et al. demonstrated that wheat, barley, rye or oat diet increased proliferation of the Clostridium perfringens in the guts of the broilers (Latorre et al. 2015). The inclusion of Bacillus subtilis (AM1002) and Bacillus amyloliquefaciens (AM0938 and JD17), which produced the xylanase in these diets, reduced the viscosity of the feed in the intestinal tract of poultry and significantly reduced C. perfringens proliferation. Bacillus sp. were incorporated into feed diet through a direct fed microbial method.
In addition, AX is reported to cause gut health problems for broilers, which are fed a wheat-based diet (Fischer and Petterson 2014). AX generates viscous chyme in the guts of broilers, which lead to a proliferation of pathogenic bacteria, intestinal inflammation, impaired intestinal barrier function, and severe intestine lesions (Fischer and Petterson 2014). Lei et al. (2016) demonstrated that xylanase and xylan debranching enzymes Abf and feruloyl esterase (FE) produce XOS which were important for the gut health of the broilers. On the other hand, a GH11 endo-xylanase from Thermomyces lanuginosus demonstrated high activity on wheat, rye and barley AX (Ravn et al. 2016). The main products of the xylanase hydrolysate were short oligosaccharides with a degree of polymerization below 100. Microscopy studies revealed that the xylanase led to a complete removal of AX from the cell walls of the grains. These results suggest that this enzyme have potential use in a wide range of monogastric animal feeds.
Nguyen and co-workers used a commercial enzyme cocktail produced by DSM Nutritional Products, Ltd., which consisted of xylanase and proteases, and demonstrated that the addition of these enzymes in corn and soybean diets improved the body weight gain of broilers (Nguyen et al. 2017). The growth performance and body weight gain were attributed to the feed conversion rates and nutrient absorption by broilers. The xylanase in the cocktail degraded the soluble and insoluble AX, which both reduced the viscosity of the feed and improved feed conversion rates (FCR). A commercial endo-xylanase from Neocallimastix patriciarum supplied by Asiapac (Dongguan, China) was added to the wheat diet of the broiler chicken (Lei et al. 2016). The finding showed that the broilers’ average daily growth (ADG) increased from day 1 up to 31 days in the study. However, Lei et al. argued that the ADG was even better when xylanase was used in concert with an arabinofuranosidase produced by Bacillus pumilus and a feruloyl esterase (EC 3.1.1.73) produced by Clostridium thermocellum purchased from Megazyme (Dublin, Ireland). The benefits of this enzyme synergism included (1) significantly decreased digesta viscosity, (2) higher FCR of broilers from day 1 to 36, and (3) reduced intestinal lesions induced by an exclusive wheat-based diet (particularly reduction of duodenum, jejunum, and ileum lesion) (Lei et al. 2016).
From these studies, it is clear that the use of xylanases as feed additives exhibited greater benefit to livestock when the xylanases were used in concert with other xylanolytic enzymes.
Conclusions and Future Perspectives
The diverse industrial uses of xylanolytic enzymes has led to the conduct of several studies to design synergistic tailored xylanolytic enzyme cocktails to improve the hydrolytic potential of enzymes, which, in turn, would lead to the reduction of enzyme loads during industrial applications and, as a result, lower enzyme costs. The two main types of xylanolytic enzyme synergism discussed here were: (1) between xylanolytic enzymes during xylan degradation, and (2) between xylanolytic enzymes and other classes of enzymes during the bioconversion of xylan and other polymers associated with it in lignocellulosic biomass. In both cases, an improvement in the bioconversion of lignocellulosic biomass into VAPs (or precursors thereof) was most critical consideration.
The use of microbial xylanolytic enzymes for biomass hydrolysis is still cost-intensive compared to the use of acids. As a result, more effort is required in producing genetically modified xylanolytic enzymes with improved properties for their large scale production and applications in industry. Biely and co-workers also alluded to another challenge for protein engineers, which is to construct chimeric proteins that would be composed of several catalytic domains which act in synergy, for example xylanases and arabinofuranosidases or xylanases and acetyl-xylan esterases (Biely et al. 2016). They further suggested an alternative approach, which would consist of a combination of catalytic domain(s) with various carbohydrate binding module(s). Empirical elucidation of more synergistic interactions between various GH family xylanolytic enzymes and with other enzyme classes still remains an area of exploration, as enzymes with a similar classification may show a relaxed substrate specificity due to differences in their structure, including active sites, the presence of multifunctional domains and varied carbohydrate-binding domains. Embarking on these future prospects in the study of xylanolytic enzymes will, without a doubt, further extend the current repertoire, understanding and applications of these enzymes in industry.
References
Alvira P et al (2010) Pretreatment technologies for an efficient bioethanol production process based on enzymatic hydrolysis: a review. Bioresour Technol 101(13):4851–4861
Beaugrand J et al (2004) Impact and efficiency of GH10 and GH11 thermostable endoxylanases on wheat bran and alkali-extractable arabinoxylans. Carbohydr Res 339(15):2529–2540
Biely P, Singh S, Puchart V (2016) Towards enzymatic breakdown of complex plant xylan structures: state of the art. Biotechnol Adv 34(7):1260–1274. Available at http://linkinghub.elsevier.com/retrieve/pii/S0734975016301070
Chadha BS et al (2019) Thermostable xylanases from thermophilic fungi and bacteria : Current perspective. Bioresour Technol 277:195–203
Charoensiddhi S et al (2017) The development of seaweed-derived bioactive compounds for use as prebiotics and nutraceuticals using enzyme technologies. Trends Food Sci Technol 70:20–33. Available at http://linkinghub.elsevier.com/retrieve/pii/S0924224417302947
Collins T, Gerday C, Feller G (2005) Xylanases, xylanase families and extremophilic xylanases. FEMS Microbiol Rev 29(1):3–23
Ebringerová A (2006) Structural diversity and application potential of hemicelluloses. Macromol Symp 232(333):1–12
Fischer M, Petterson D (2014) Xylanase for animal feed. WO 2008/037757Al
Fushinobu S et al (2005) Structural basis for the specificity of the reducing end xylose-releasing exo-oligoxylanase from Bacillus halodurans C-125. J Biol Chem 280(17):17180–17186
Gatenholm P, Tenkanen M (2003) Hemicelluloses: science and technology. In: Gatenholm P, Tenkanen M (eds) ACS symposium series. American Chemical Society, Washington, pp 2–22. Available at http://pubs.acs.org/isbn/9780841238428
Golan G et al (2004) Crystal structures of Geobacillus stearothermophilus alpha-glucuronidase complexed with its substrate and products: mechanistic implications. J Biol Chem 279(4):3014–3024
Goncalves GAL et al (2015) Synergistic effect and application of xylanases as accessory enzymes to enhance the hydrolysis of pretreated bagasse. Enzym Microb Technol 72:16–24
Guo X et al (2013) A novel pathway construction in Candida tropicalis for direct xylitol conversion from corncob xylan. Bioresour Technol 128:547–552
Hendriks a TWM, Zeeman G (2009) Pretreatments to enhance the digestibility of lignocellulosic biomass. Bioresour Technol 100(1):10–18
Hu J, Saddler JN (2018) Biomass and bioenergy: why does GH10 xylanase have better performance than GH11 xylanase for the deconstruction of pretreated biomass? Biomass Bioenergy 110:13–16
Huisman MMH, Schols HA, Voragen AGJ (2000) Glucuronoarabinoxylans from maize kernel cell walls are more complex than those from sorghum kernel cell walls. Carbohydr Polym 43(3):269–279
Hutkins RW et al (2016) Prebiotics: why definitions matter. Curr Opin Biotechnol 37:1–7
Huy ND et al (2015) Putative endoglucanase PcGH5 from Phanerochaete chrysosporium is a β-xylosidase that cleaves xylans in synergistic action with endo-xylanase. J Biosci Bioeng 119(4):416–420
Juturu V, Wu JC (2012) Microbial xylanases: engineering, production and industrial applications. Biotechnol Adv 30(6):1219–1227
Juturu V, Wu JC (2013) Insight into microbial hemicellulases other than xylanases: a review. J Chem Technol Biotechnol 88(3):353–363
Kamat S et al (2013) Coupled production of single cell oil as biodiesel feedstock, xylitol and xylanase from sugarcane bagasse in a biorefinery concept using fungi from the tropical mangrove wetlands. Bioresour Technol 135:246–253
Knob A, Terrasan CRF, Carmona EC (2010) β-xylosidases from filamentous fungi: an overview. World J Microbiol Biotechnol 26(3):389–407
Kumar V, Dangi AK, Shuklar P (2018) Engineering thermostable xylanases toward its industrial applications. Mol Biotechnol 60:226–235
Lagaert S et al (2014) β-Xylosidases and α-L-arabinofuranosidases: Accessory enzymes for arabinoxylan degradation. Biotechnol Adv 32(2):316–332. Available at. https://doi.org/10.1016/j.biotechadv.2013.11.005
Latorre JD et al (2015) Selection of Bacillus spp. for cellulase and xylanase production as direct-fed microbials to reduce digesta viscosity and Clostridium perfringens proliferation using an in vitro digestive model in different. Front Verter Sci 2:1–8
Lei Z et al (2016) Combination of xylanase and debranching enzymes specific to wheat arabinoxylan improve the growth performance and gut health of broilers. J Agric Food Chem 64(24):4932–4942
Li S et al (2012) Technology prospecting on enzymes: application, marketing and engineering. Comput Struct Biotechnol J 2(3):e201209017
Li Z et al (2013) Direct and efficient xylitol production from xylan by Saccharomyces cerevisiae through transcriptional level and fermentation processing optimizations. Bioresour Technol 149:413–419
Li Z et al (2015) An environment friendly and efficient process for xylitol bioconversion from enzymatic corncob hydrolysate by adapted Candida tropicalis. Chem Eng J 263:249–256
Malgas S, Pletschke BI (2019) The effect of an oligosaccharide reducing-end xylanase, Bh Rex8A, on the synergistic degradation of xylan backbones by an optimised xylanolytic enzyme cocktail. Enzym Microb Technol 122:74–81
Malgas S et al (2017a) Formulation of an optimized synergistic enzyme cocktail, HoloMix, for effective degradation of various pre-treated hardwoods. Bioresour Technol 245:52–65
Malgas S et al (2017b) Time dependence of enzyme synergism during the degradation of model and natural lignocellulosic substrates. Enzym Microb Technol 103:1–11
McKee LS et al (2016) A GH115 α-glucuronidase from Schizophyllum commune contributes to the synergistic enzymatic deconstruction of softwood glucuronoarabinoxylan. Biotechnol Biofuels 9(1):2
Mendis M, Simsek S (2015) Production of structurally diverse wheat arabinoxylan hydrolyzates using combinations of xylanase and arabinofuranosidase. Carbohydr Polym 132:452–459
Moreira LRS, Filho EXF (2008) An overview of mannan structure and mannan-degrading enzyme systems. Appl Microbiol Biotechnol 79(2):165–178
Moreno FJ et al (2017) Current state and latest advances in the concept, production and functionality of prebiotic oligosaccharides. Curr Opin Food Sci 13:50–55
Motta FL, Andrade CC, Santana MH (2013) In: Chandel A, da Silva SS (eds) A review of xylanase production by the fermentation of xylan: classification, characterization and applications. InTech, London
Nagy T et al (2002) The membrane-bound α -glucuronidase from Pseudomonas cellulosa hydrolyzes 4-O-methyl-D-glucuronoxylooligosaccharides but not 4-O- methyl-D-glucuronoxylan. J Biotechnol 184(17):4925–4929
Nguyen D et al (2017) Influence of a cocktail of protease and xylanase in different energy densities of corn- and soybean-meal-based diet on growth performance, nutrient digestibility, carcass quality. Can J Anim Sci 98(2):271–278
Nurizzo D et al (2002) The structural basis for catalysis and specificity of the Pseudomonas cellulosa alpha-glucuronidase, GlcA67A. Structure 10(4):547–556
Paës G, Berrin JG, Beaugrand J (2012) GH11 xylanases: structure/function/properties relationships and applications. Biotechnol Adv 30(3):564–592
Paloheimo M, Piironen J, Vehmaanpera J (2012) In: Bedford MR, Partridge GG (eds) Enzymes in farm animal nutrition. CAB International, Wallingford
Pedrolli DB et al (2009) Pectin and pectinases: production, characterization and industrial application of microbial pectinolytic enzymes. Open Biotechnol J 3(1):9–18
Peng P, She D (2014) Isolation, structural characterization, and potential applications of hemicelluloses from bamboo: a review. Carbohydr Polym 112:701–720
Pinto PC, Evtuguin DV, Neto CP (2005) Structure of hardwood glucuronoxylans: modifications and impact on pulp retention during wood kraft pulping. Carbohydr Polym 60(4):489–497
Pletschke BI et al (2016) Enzyme synergism: a powerful tool for decreasing enzyme loading for efficient biomass conversion. In: 24th European biomass conference and exhibition, 6–9 June 2016, Amsterdam, The Netherlands, pp 68–82
Rao LV et al (2016) Bioconversion of lignocellulosic biomass to xylitol: an overview. Bioresour Technol 213:299–310
Ravn JL et al (2016) A commercial GH 11 xylanase mediates xylan solubilisation and degradation in wheat, rye and barley as demonstrated by microscopy techniques and wet chemistry methods. Anim Feed Sci Technol 219:216–225
Revanappa SB, Nandini CD, Salimath PV (2015) Structural variations of arabinoxylans extracted from different wheat (Triticum aestivum) cultivars in relation to chapati-quality. Food Hydrocoll 43:736–742
Saha BC (2003) Hemicellulose bioconversion. J Ind Microbiol Biotechnol 30(5):279–291
Samanta AK et al (2015) Xylooligosaccharides as prebiotics from agricultural by-products: production and applications. Bioact Carbohydr Diet Fibre 5:62–71
Schendel RR et al (2015) Isolation and characterization of feruloylated arabinoxylan oligosaccharides from the perennial cereal grain intermediate wheat grass (Thinopyrum intermedium). Carbohydr Res 407:16–25
Shallom D, Shoham Y (2003) Microbial hemicellulases. Curr Opin Microbiol 6(3):219–228
Simpson DJ et al (2003) Structure and function of cereal and related higher plant (1 -> 4)-beta-xylan endohydrolases. J Cereal Sci 37(2):111–127
Singh S, Madlala AM, Prior BA (2003) Thermomyces lanuginosus: properties of strains and their hemicellulases. FEMS Microbiol Rev 27(1):3–16
Tenkanen M, Siika-Aho M (2000) An α-glucuronidase of Schizophyllum commune acting on polymeric xylan. J Biotechnol 78(2):149–161
Togashi H, Kato A, Shimizu K (2009) Enzymatically derived aldouronic acids from Eucalyptus globulus glucuronoxylan. Carbohydr Polym 78(2):247–252
Van Dyk JS, Pletschke BI (2012) A review of lignocellulose bioconversion using enzymatic hydrolysis and synergistic cooperation between enzymes-factors affecting enzymes, conversion and synergy. Biotechnol Adv 30(6):1458–1480
Van Zyl WH et al (2010) Fungal β-mannanases: Mannan hydrolysis, heterologous production and biotechnological applications. Process Biochem 45(8):1203–1213
Vandenplas Y (2016) Use of probiotics and prebiotics in infant feeding. Best Pract Res Clin Gastroenterol 30(1):39–48. https://doi.org/10.1016/j.bpg.2016.01.001
Wang Y et al (2017) Post-hydrolysis of the prehydrolysate from eucalyptus pulping with xylanase. J Clean Prod 142:2865–2871
Yan QJ et al (2008) A xylose-tolerant β-xylosidase from Paecilomyces thermophila: characterization and its co-action with the endogenous xylanase. Bioresour Technol 99(13):5402–5410
Yasmin A et al (2015) Prebiotics, gut microbiota and metabolic risks: unveiling the relationship. J Funct Foods 17:189–201
York WS, O’Neill MA (2008) Biochemical control of xylan biosynthesis—which end is up? Curr Opin Plant Biol 11(3):258–265
Zhang J et al (2011) Comparison of the synergistic action of two thermostable xylanases from GH families 10 and 11 with thermostable cellulases in lignocellulose hydrolysis. Bioresour Technol 102(19):9090–9095
Acknowledgements
S. M. is currently funded by Senior Chair of Energy Research (CoER) in Biofuels. M. S. M is currently funded by the National Research Foundation (NRF) of South Africa. B. I. P is an academic representative from Rhodes University. Any opinion, findings and conclusions or recommendations expressed in this material are those of the author(s) and therefore the NRF does not accept any liability in regard thereto.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Ethics declarations
The authors report no further conflicts of interest. The authors are responsible for the content and writing of this article.
Rights and permissions
Copyright information
© 2020 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Malgas, S., Mafa, M.S., Pletschke, B.I. (2020). The Effects of Xylanase Synergistic Interactions During Lignocellulose Degradation and Their Significance for Industry. In: Shrivastava, S. (eds) Industrial Applications of Glycoside Hydrolases . Springer, Singapore. https://doi.org/10.1007/978-981-15-4767-6_9
Download citation
DOI: https://doi.org/10.1007/978-981-15-4767-6_9
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-15-4766-9
Online ISBN: 978-981-15-4767-6
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)