Abstract
Bioelectrochemical systems, such as microbial fuel cells and microbial electrosynthesis, are promising technology for energy generation and organic compound production. In the bioelectrochemical systems, extracellular electron transfer is essential in which c-type cytochrome, electrically conductive nanowires, and electron shuttles play key roles. This chapter reviews the underlying molecular mechanisms of the extracellular electron transfer by electrically active microorganisms, such as Geobacter sulfurreducens and Shewanella oneidensis, in the bioelectrochemical systems with recent findings.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
1 Introduction
Electrochemically active microorganisms capable of transferring electrons to/from electrodes play essential roles in bioelectrochemical systems, such as microbial fuel cells and microbial electrosynthesis (MES). Electrochemical and molecular biological studies have demonstrated the detailed mechanisms of extracellular electron transfer (EET) between microorganisms and electrodes. Extensive studies in the last two decades revealed that various microorganisms can transfer electrons to/from electrodes. Detailed mechanisms of electron transfer from microorganisms to electrodes have been intensively studied on two model microorganisms, Geobacter sulfurreducens and Shewanella oneidensis. These microorganisms are also capable of receiving electrons from electrodes, and the mechanisms of electron uptake have been also studied.
2 Extracellular Electron Transfer from Microorganisms to Electrodes
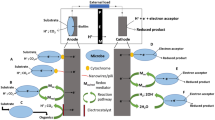
In the bioelectrochemical systems, such as microbial fuel cells, electrons are transferred from electrochemically active microorganisms to the electrodes (Fig. 3.1a). Electrons from cytoplasm are transferred across the cell membranes composed of lipid bilayers via proteins possessing redox-active cofactors, such as c-type cytochromes and iron-sulfur proteins and electrically conductive nanowires (Fig. 3.2). Electron acceptors outside the cells are reduced by primary three different mechanisms, direct contact, electron conductive nanowires, and electron shuttles. The electron transfer mechanisms from the cell surface to the electrode mentioned here are not independently in typical cases but cooperatively, e.g., direct contact and nanowires for G. sulfurreducens and direct contact, nanowires, and electron shuttles for S. oneidensis (Fig. 3.3).
2.1 Direct Contact
In order to transfer electrons from inside the cell to an electrode outside the cell, the electrons must be passed through cell membranes which have insulator property. Electron carrier proteins, c-type cytochrome and/or iron-sulfur protein, localized near the cell membrane play essential roles for electron transfer from the cells to the outside in both well-studied microorganisms, G. sulfurreducens and S. oneidensis (Fig. 3.3). NADH produced in the process of respiration produces quinol by transferring electrons to quinone by NADH dehydrogenase. The electrons of NADH are transferred from inner membrane proteins to the redox-active proteins in periplasm, which subsequently transfer electrons to also redox-active outer membrane proteins.
In G. sulfurreducens, primary c-type cytochromes play essential roles in EET by direct contact (Fig. 3.3a). Inner membrane cytochromes, ImcH and CbcL, play important roles in transferring electrons to periplasm. EET via ImcH and CbcL is proposed to be in a redox potential-dependent manner. CbcL and ImcH are required for electron transfer to low (<−0.1 V [vs. SHE]) and high redox potential (>+0.24 V [vs. SHE]) electrodes, respectively (Levar et al. 2014; Zacharoff et al. 2016).
In the periplasm, triheme c-type cytochromes (most abundant and well-studied PpcA and its homologues PpcB, PpcC, PpcD, and PpcE) are thought to transfer electrons to other redox-active proteins in the outer membrane (Fig. 3.3a) (Lloyd et al. 2003). Among the well-studied outer membrane cytochromes, OmcB is an essential outer membrane cytochrome in the EET from the cells to ferric iron oxide, whereas the deletion of omcB gene showed no significant impact on current production (Leang et al. 2003). Electron conduits composed of ExtABCD (ExtA, a periplasmic c-type cytochrome; ExtB, an outer membrane integral protein with transmembrane domains; ExtC and ExtD, outer membrane lipoprotein c-type cytochromes) are proposed to be involved in electron transfer from periplasm to outer-surface or outer-surface redox-active proteins (Otero et al. 2018). Another essential protein for EET is an octaheme outer membrane cytochrome, OmcZ. OmcZ has a wide redox range (−420 to −60 mV [versus standard hydrogen electrode] for OmcZ) and specifically localized on the surface of the electrode (Inoue et al. 2010; Inoue et al. 2011). It has been also suggested that the wide redox range and multiple hemes contribute to the electron-storage capacity of the biofilms (Malvankar et al. 2012).
In S. oneidensis, also c-type cytochromes play important roles in EET (Fig. 3.3b). Electrons from intracellular quinol are transferred to c-type cytochrome CymA in the intracellular membrane (Myers and Myers 2000). In the periplasm, Fcc3 (flavocytochrome c3) and STC (small tetraheme cytochrome c) are thought to transfer electrons to the outer membrane complex composed of MtrA, MtrB, and MtrC, in the outer membrane (Ross et al. 2007; Fonseca et al. 2012; McMillan et al. 2013). MtrF, a homologue of MtrC and OmcA, is also responsible for electron transfer to the extracellular electron acceptor (Coursolle and Gralnick 2010). These proteins, except for MtrB (integral outer membrane β-barrel protein), are also muti-heme cytochromes. omcA gene-disrupted strain had less power generation capability in microbial fuel cells, and mtrA, mtrB, and mtrC gene-disrupted strains and omcA and mtrC double mutants almost lost power generation capabilities (Coursolle et al. 2010).
EET via direct contact by S. loihica (Newton et al. 2009), Aeromonas hydrophilia (Pham et al. 2003), Rhodoferax ferrireducens (Chaudhuri and Lovley 2003), and Desulfobulbus propionicus (Holmes et al. 2004) has been reported other than G. sulfurreducens and S. oneidensis.
2.2 Electrically Conductive Nanowire
G. sulfurreducens and S. oneidensis are known to produce electrically conductive nanowires. Microscopic and electrochemical analyses of pili produced by G. sulfurreducens using atomic force microscope equipped with a conductive tip revealed that the nanowire was electrically conductive (Reguera et al. 2005). Purified nanowire had temperature-dependent electrical conductivity similar to metals (Malvankar et al. 2011). G. sulfurreducens produces two kinds of electrically conductive nanowires composed of PilA and OmcS.
Deletion mutant of a proposed pilin domain protein, pilA, could not reduce insoluble Fe(III) oxide (Reguera et al. 2005), and, also, the pilA disruption showed severe inhibition of current production (Reguera et al. 2006). A recent biochemical study demonstrated that PilA was stabilized by electrostatic interaction with Spc (short pilin chaperone) encoded by the gene immediately downstream of pilA (Liu et al. 2019). Localization analysis by electron microscopy and immunogold labeling suggests that OmcS is localized along the nanowires (Leang et al. 2010). A recent study using cryoelectron microscopy of purified nanowire extracted from G. sulfurreducens cells revealed that the nanowires were composed of a c-type cytochrome OmcS (Filman et al. 2019; Wang et al. 2019). According to the three-dimensional structure, the nanowire had 46.7–47.5 Å filament repeat, and each subunit contained six hemes corresponding to the heme numbers of OmcS molecule.
S. oneidensis is also thought to produce electrically conductive nanowires (Gorby et al. 2006). The nanowires produced by the OmcA-disrupted mutant and the MtrC-disrupted mutant do not exhibit electrical conductivity, and these c-type cytochromes contribute to the electrical conductivity of the nanowire. A recent study by electron cryotomography revealed that the nanowires were dynamic chains of interconnected outer membrane vesicles (Subramanian et al. 2018). Synechocystis and Pelotomaculum thermopropionicum also produced electrically conductive nanowires other than iron-reducing bacteria (Gorby et al. 2006).
2.3 Electron Shuttle
Electron shuttle is a soluble electron mediator, also called as mediator. The electron shuttles are reduced by receiving electrons from microorganisms and are oxidized by transferring electrons to an extracellular electron acceptor located far from the cells (Watanabe et al. 2009). Oxidized electron shuttles receive electrons again from the microorganism and reduce the electron acceptors. This process is repeated to transfer electrons between the microorganism and the electrode. There are known examples where natural substances, such as humic acid and sulfur, are used for iron reduction (Thygesen et al. 2009; Straub and Schink 2004). Some microorganisms can produce electron shuttles, such as flavin (S. oneidensis [Marsili et al. 2008] and other Shewanella species [Canstein et al. 2008]), riboflavin (Geothrix fermentans [Mehta-Kolte and Bond 2012]), phenazine (Pseudomonas chlororaphis [Hernandez et al. 2004], P. aeruginosa [Rabaey et al. 2005], Pseudomonas sp. [Pham et al. 2008]), quinone (S. putrefaciens [Newman and Kolter 2000], Lactococcus lactis [Freguia et al. 2009]), and melanin (S. algae [Turick et al. 2002]), by themselves. The advantage of electron transfer by the electronic shuttles is that they can transfer electrons to the electrode even if the electrode is physically distant.
3 Extracellular Electron Transfer from Electrodes to Microorganisms
In the microbial electrosynthesis (MES), also called electro-fermentation, electrons are transferred from cathodes to electrochemically active microorganisms (Fig. 3.1b). G. metallireducens and G. sulfurreducens were firstly reported to convert nitrate to nitrite and fumarate to succinate, respectively, by directly accepting electrons from cathodes (Gregory et al. 2004). The “microbial electron uptake” has been observed in various microorganisms, such as Sporomusa ovata (Nevin et al. 2010), Sporomusa sphaeroides, Sporomusa silvacetica, Clostridium ljungdahlii, C. aceticum, Moorella thermoacetica (also known as C. thermoacetica) (Nevin et al. 2011), G. lovleyi (Strycharz et al. 2008), Anaeromyxobacter dehalogenans (Strycharz et al. 2010), Rhodopseudomonas palustris (Bose et al. 2014), Prosthecochloris aestaurii (Ha et al. 2017), Acidithiobacillus ferrooxidans (Nakasono et al. 1997), and Methanobacterium palustre (Cheng et al. 2009), whereas very little is known about the molecular mechanisms of accepting electrons from electrodes in these microorganisms.
The mechanisms of the electron uptake from electrodes in G. sulfurreducens (Gregory et al. 2004; Dumas et al. 2008) and S. oneidensis (Ross et al. 2011) have been studied as well as EET from microorganisms to electrodes. In a G. sulfurreducens cell, PccH (GSU3274), a monoheme c-type cytochrome proposed to be localized at periplasm, plays an important role in electron uptake process (Strycharz et al. 2011). Deletion mutant of pccH did not accept electrons from electrodes, whereas the deletion of c-type cytochromes required for EET, such as OmcZ, OmcS, OmcB, and OmcE, did not show significant impact on electron uptake. Biochemical analysis demonstrated that PccH has unusually low redox potential (−24 mV versus standard hydrogen electrode) (Dantas et al. 2013). In G. sulfurreducens, the predicted electron pathway from electrodes to the cells is different from EET from cells to the electrodes. In contrast, in S. oneidensis, electrons from electrodes were proposed to be transferred via Mtr/CymA pathway by which electrons from cells are transferred to the electrodes (Ross et al. 2011; Okamoto et al. 2014) (Fig. 3.4). In S. oneidensis, MtrDEF was suggested to complement the function of MtrCAB significant partially, and riboflavin could be used as an electron shuttle as well as EET from microorganisms to the electrodes. There are only limited knowledge about the molecular mechanisms of microbial electrosynthesis, and, thus, it requires further investigations for practical applications for producing various compounds.
References
Bose A, Gardel EJ, Vidoudez C, Parra EA, Girguis PR (2014) Electron uptake by iron-oxidizing phototrophic bacteria. Nat Commun 5:339
Canstein H, Ogawa J, Shimizu S, Lloyd JR (2008) Secretion of flavins by Shewanella species and their role in extracellular electron transfer. Appl Environ Microbiol 74:615–623
Chaudhuri SK, Lovley DR (2003) Electricity generation by direct oxidation of glucose in mediatorless microbial fuel cells. Nat Biotechnol 21:1229–1232
Cheng S, Xing D, Call DF, Logan BE (2009) Direct biological conversion of electrical current into methane by electromethanogenesis. Envrion Sci Technol 43:3953–3958
Coursolle D, Gralnick JA (2010) Modularity of the Mtr respiratory pathway of Shewanella oneidensis strain MR-1. Mol Microbiol 77:995–1008
Coursolle D, Baron DB, Bond DR, Gralnick JA (2010) The Mtr respiratory pathway is essential for reducing flavins and electrodes in Shewanella oneidensis. J Bacteriol 192:467–474
Dantas JM, Tomaz DM, Morgado L, Salgueiro CA (2013) Functional characterization of PccH, a key cytochrome for electron transfer from electrodes to the bacterium Geobacter sulfurreducens. FEBS Lett 587:2662–2668
Dumas C, Basseguy R, Bergel A (2008) Microbial electrocatalysis with Geobacter sulfurreducens biofilm on stainless steel cathodes. Electrochim Acta 53:2494–2500
Filman DJ, Marino SF, Ward JE, Yang L, Mester Z, Bullitt E, Lovley DR, Strauss M (2019) Cryo-EM reveals the structural basis of long-range electron transport in a cytochrome-based bacterial nanowire. Commun Biol 2:219
Fonseca MB, Paquete CM, Neto SE, Pacheco I, Soares CM, Louro RO (2012) Mind the gap: cytochrome interactions reveal electron pathways across the periplasm of Shewanella oneidensis MR-1. Biochem J 449:101–108
Freguia S, Masuda M, Tsujimura S, Kano K (2009) Lactococcus lactis catalyses electricity generation at microbial fuel cell anodes via excretion of a soluble quinone. Bioelectrochemistry 76:14–18
Gorby YA, Yanina S, McLean JS, Rosso KM, Moyles D, Dohnalkova A, Beveridge TJ, Chang IS, Kim BH, Kim KS, Culley DE, Reed SB, Romine MF, Saffarini DA, Hill EA, Shi L, Elias DA, Kennedy DW, Pinchuk G, Watanabe K, Ishii S, Logan B, Nealson KH, Fredrickson JK (2006) Electrically conductive bacterial nanowires produced by Shewanella oneidensis strain MR-1 and other microorganisms. Proc Natl Acad Sci U S A 30:11358–11363
Gregory KB, Bond DR, Lovley DR (2004) Graphite electrodes as electron donors for anaerobic respiration. Environ Microbiol 6:596–604
Ha PT, Lindemann SR, Shi L, Dohnalkova AC, Fredrickson JK, Madigan MT, Beyenal H (2017) Syntrophic anaerobic photosynthesis via direct interspecies electron transfer. Nat Commun 8:13924
Hernandez ME, Kappler A, Newman DK (2004) Phenazines and other redox-active antibiotics promote microbial mineral reduction. Appl Environ Microbiol 70:921–928
Holmes DE, Bond DR, Lovley DR (2004) Electron transfer by Desulfobulbus propionicus to Fe(III) and graphite electrodes. Appl Environ Microbiol 70:1234–1237
Inoue K, Qian X, Morgado L, Kim BC, Mester T, Izallalen M, Salgueiro CA, Lovley DR (2010) Purification and characterization of OmcZ, an outer-surface, octaheme c-type cytochrome essential for optimal current production by Geobacter sulfurreducens. Appl Environ Microbiol 76:3999–4007
Inoue K, Leang C, Franks AE, Woodard TL, Nevin KP, Lovley DR (2011) Specific localization of the c-type cytochrome OmcZ at the anode surface in current-producing biofilms of Geobacter sulfurreducens. Environ Microbiol Rep 3:211–217
Leang C, Coppi MV, Lovley DR (2003) OmcB, a c-type polyheme cytochrome, involved in Fe(III) reduction in Geobacter sulfurreducens. J Bacteriol 185:2096–2103
Leang C, Qian X, Mester T, Lovley DR (2010) Alignment of the c-type cytochrome OmcS along pili of Geobacter sulfurreducens. Appl Environ Microbiol 76:4080–4084
Levar CE, Chan CH, Mehta-Kolte MG, Bond DR (2014) An inner membrane cytochrome required only for reduction of high redox potential extracellular electron acceptors. MBio 5:e02034–e02014
Liu X, Zhan J, Jing X, Zhou S, Lovley DR (2019) A pilin chaperon required for the expression of electrically conductive Geobacter sulfurreducens. Environ Microbiol 21:2511–2522
Lloyd JR, Leang C, Myerson ALH, Coppi MV, Cuifo S, Methe B, Sandler SJ, Lovley DR (2003) Biochemical and genetic characterization of PpcA, a periplasmic c-type cytochrome in Geobacter sulfurreducens. Biochem J 369:153–161
Malvankar NS, Vargas M, Nevin KP, Franks AE, Leang C, Kim BC, Inoue K, Mester T, Covalla SF, Johnson JP, Rotello VM, Tuominen MT, Lovley DR (2011) Tunable metallic-like conductivity in microbial nanowire networks. Nat Nanotechnol 6:573–579
Malvankar NS, Mester T, Tuominen MT, Lovley DR (2012) Supercapacitors based on c-type cytochromes using conductive nanostructured networks of living bacteria. ChemPhysChem 13:463–468
Marsili E, Baron DB, Shikhare ID, Coursolle D, Gralnick JA, Bond DR (2008) Shewanella secretes flavins that mediate extracellular electron transfer. Proc Natl Acad Sci U S A 105:3968–3973
McMillan DGG, Marritt SJ, Firer-Sherwood MA, Shi L, Richardson DJ, Evans SD, Elliott SJ, Butt JN, Jeuken LJC (2013) Protein–protein interaction regulates the direction of catalysis and electron transfer in a redox enzyme complex. J Am Chem Soc 135:10550–10556
Mehta-Kolte MG, Bond DR (2012) Geothrix fermentans secretes two different redox-active compounds to utilize electron acceptors across a wide range of redox potentials. Appl Environ Microbiol 78:6987–6995
Myers JM, Myers CR (2000) Role of the tetraheme cytochrome CymA in anaerobic electron transport in cells of Shewanella putrefaciens MR-1 with normal levels of menaquinone. J Bacteriol 182:67–75
Nakasono S, Matsumoto N, Saiki H (1997) Electrochemical cultivation of Thiobacillus ferrooxidans by potential control. Bioelectrochem Bioenerg 43:61–66
Nevin KP, Woodard TL, Franks AE, Summers ZM, Lovley DR (2010) Microbial electrosynthesis: feeding microbes electricity to convert carbon dioxide and water to multicarbon extracellular organic compounds. MBio 1:e00103–e00110
Nevin KP, Hensley SA, Franks AE, Summers ZM, Ou J, Woodard TL, Snoeyebos-West OL, Lovley DR (2011) Electrosynthesis of organic compounds from carbon dioxide is catalyzed by a diversity of acetogenic microorganisms. Appl Envrion Microbiol 77:2882–2826
Newman DK, Kolter R (2000) A role for excreted quinones in extracellular electron transfer. Nature 405:94–97
Newton GJ, Mori S, Nakamura R, Hashimoto K, Watanabe K (2009) Analyses of current-generating mechanisms of Shewanella loihica PV-4 and Shewanella oneidensis MR-1 in microbial fuel cells. Appl Environ Microbiol 75:7674–7681
Okamoto A, Hashimoto K, Nelason KH (2014) Flavin redox bifurcation as a mechanism for controlling the direction of electron flow during extracellular electron transfer. Angew Chem Int Ed 53:10988–10991
Otero FJ, Chan CH, Bond DR (2018) Identification of different putative outer membrane electron conduits necessary for Fe(III) citrate, Fe(III) oxide, Mn(IV) oxide, or electrode reduction by Geobacter sulfurreducens. J Bacteriol 200:e00347–e00318
Pham CA, Jung SJ, Phung NT, Lee J, Chang IS, Kim BH, Yi H, Chun J (2003) A novel electrochemically active and Fe(III)-reducing bacterium phylogenetically related to Aeromonas hydrophila, isolated from a microbial fuel cell. FEMS Microbiol Lett 223:129–134
Pham TH, Boon N, Aelterman P, Clauwaert P, De Schamphelaire L, Vanhaecke L, De Maeyer K, Hofte M, Verstraete W, Rabaey K (2008) Metabolites produced by Pseudomonas sp. enable a Gram-positive bacterium to achieve extracellular electron transfer. Appl Microbiol Biotechnol 77:1119–1129
Rabaey K, Boon N, Hofte M, Verstraete W (2005) Microbial phenazine production enhances electron transfer in biofuel cells. Environ Sci Technol 39:3401–3408
Reguera G, McCarthy KD, Mehta T, Nicoll JS, Tuominen MT, Lovley DR (2005) Extracellular electron transfer via microbial nanowires. Nature 435:1098–1101
Reguera G, Nevin KP, Nicoll JS, Covalla SF, Woodard TL, Lovley DR (2006) Biofilm and nanowire production leads to increased current in Geobacter sulfurreducens fuel cells. Appl Environ Microbiol 72:7345–7348
Ross DE, Ruebush SS, Brantley SL, Hartshorne RS, Clarke TA, Richardson DJ, Tien M (2007) Characterization of protein-protein interactions involved in iron reduction by Shewanella oneidensis MR-1. Appl Environ Microbiol 73:5797–5808
Ross DE, Flynn JM, Baron DB, Gralnick JA, Bond DR (2011) Towards electrosynthesis in Shewanella: energetics of reversing the mtr pathway for reductive metabolism. PLoS One 6:e16649
Straub KL, Schink B (2004) Ferrihydrite-dependent growth of Sulfurospirillum deleyianum through electron transfer via sulfur cycling. Appl Environ Microbiol 70:5744–5749
Strycharz SM, Woodard TL, Johnson JP, Nevin KP, Sanford RA, Löffler FE, Lovley DR (2008) Graphite electrode as a sole electron donor for reductive dechlorination of tetrachlorethene by Geobacter lovleyi. Appl Environ Microbiol 74:5943–5947
Strycharz SM, Gannon SM, Boles AR, Franks AE, Nevin KP, Lovley DR (2010) Reductive dechlorination of 2-chlorophenol by Anaeromyxobacter dehalogenans with an electrode serving as the electron donor. Environ Microbiol Rep 2:289–294
Strycharz SM, Glaven RH, Coppi MV, Gannon SM, Perpetua LA, Liu A, Nevin KP, Lovley DR (2011) Gene expression and deletion analysis of mechanisms for electron transfer from electrodes to Geobacter sulfurreducens. Bioelectrochemistry 80:142–150
Subramanian P, Pirbadianb S, El-Naggar MY, Jensen GJ (2018) Ultrastructure of Shewanella oneidensis MR-1 nanowires revealed by electron cryotomography. Proc Natl Acad Sci U S A 115:E3246–E3255
Thygesen A, Poulsen FW, Min B, Angelidaki I, Thomsen AB (2009) The effect of different substrates and humic acid on power generation in microbial fuel cell operation. Bioresour Technol 100:1186–1191
Turick CE, Tisa LS, Caccavo F (2002) Melanin production and use as a soluble electron shuttle for Fe(III) oxide reduction and as a terminal electron acceptor by Shewanella algae BrY. Appl Environ Microbiol 68:2436–2444
Wang F, Gu Y, O’Brien JP, Yi SM, Yalcin SE, Srikanth V, Shen C, Vu D, Ing NL, Hochbaum AI, Egelman EH, Malvankar NS (2019) Structure of microbial nanowires reveals stacked hemes that transport electrons over micrometers. Cell 177:361–369
Watanabe K, Manefield M, Lee M, Kouzuma A (2009) Electron shuttles in biotechnology. Curr Biotechnol 20:633–641
Zacharoff L, Chan CH, Bond DR (2016) Reduction of low potential electron acceptors requires the CbcL inner membrane cytochrome of Geobacter sulfurreducens. Bioelectrochemistry 107:7–13
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Fujikawa, T., Inoue, K. (2020). Extracellular Electron Transfer in Bioelectrochemically Active Microorganisms. In: Ishii, M., Wakai, S. (eds) Electron-Based Bioscience and Biotechnology . Springer, Singapore. https://doi.org/10.1007/978-981-15-4763-8_3
Download citation
DOI: https://doi.org/10.1007/978-981-15-4763-8_3
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-15-4762-1
Online ISBN: 978-981-15-4763-8
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)