Abstract
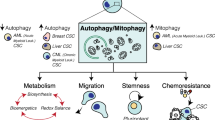
Autophagy is critical for the survival and stemness maintenance of cancer stem cells (CSCs) and is an enhancer of CSC tumorigenesis. At the same time, autophagy contributes to conditions optimal for facilitating the invasion and metastasis of CSCs. Moreover, autophagy induces the dormant state of CSCs to help them resist the cytotoxic effects of chemotherapy and radiotherapy, thereby improving the likelihood of their survival. The combination of autophagy inhibitors with specific drugs targeting specific CSC subpopulations is expected to act specifically on CSCs and produce fewer toxic side effects on normal tissues. This in-depth study is very timely and important for further identifying the potential role of autophagy in different states of CSCs and places a particular emphasis on exploring molecular mechanisms in the regulation of autophagy via advanced techniques based on molecular biology.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
There is a rare type of cancer cell in tumour tissue that has the potential for self-renewal, proliferation and differentiation, and it plays an important role in the processes of tumour occurrence, development, recurrence and metastasis. Due to their many properties that are similar to those of stem cells, they are called cancer stem cells (CSCs). First discovered in the haematopoietic system, cancer stem cells have been identified in a variety of tumours through the detection of various markers such as CD44, CD24, Epcam and CD133 (Clevers 2011; Meng et al. 2012; Wang et al. 2015). They divide asymmetrically, separating into cancer stem cells with an identical nature and into non-tumorigenic cancer cells that make up the bulk of tumours. In the process of tumour development, a tumour microenvironment characterized by hypoxia, reduced pH and nutrient deficiency is formed. However, in such an environment, which is harsh for normal cells, cancer stem cells can grow well. What is the mechanism that helps cancer stem cells cope with the harsh environment in tumour tissues? Autophagy is believed to enable the body to survive under severe living conditions by ‘degrading itself to provide materials and energy and remove harmful substances’. At present, a large number of studies have confirmed that autophagy plays an important role in cell differentiation, development and adaptation to environmental stress and contributes to the occurrence of a variety of diseases. However, a large number of studies have also found that during the emergence and development of tumours, autophagy plays a dual role. In normal tissues, autophagy-mediated injury relief can effectively inhibit tumorigenesis, while in tissues with tumours, the macromolecular recycling induced by autophagy can alleviate only the energy demands of cancer stem cells to maintain their survival. In this case, inhibition of autophagy is beneficial for antitumour therapy. However, both the inhibition and activation of autophagy inhibit tumour growth by removing cancer stem cells. We all know that the most important feature of stem cells is their ability to self-renew and differentiate. Currently, increasing evidence has suggested that autophagy is involved in the resting, self-renewal and differentiation of stem cells during physical and other kinds of stress conditions. Is the function of autophagy in CSC self-renewal and differentiation similar to that of other stem cells? Or do cancer stem cells have other special regulatory functions not present in other stem cells? Recent studies have suggested that in addition to its role in normal embryonic development and adult stem cells, autophagy also plays an important role in the origin, maintenance and migration of cancer stem cells. The role of autophagy in maintaining the survival and function of cancer stem cells is described below.
1 Autophagy Is Involved in the Maintenance of the Stemness of Cancer Stem Cells
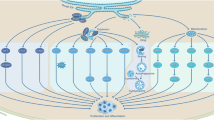
Tumour stem cells comprise a small subgroup of cells within the tumour that are closely related to the growth, drug resistance, recurrence and metastasis of the tumour. Tumour stem cells have the potential for self-renewal and differentiation into a variety of somatic cells and can maintain their undifferentiated state, thus leading to the heterogeneity of tumour tissues. Transplantation of a small number of tumour stem cells resulted in tumour formation in immunodeficient mice (Sharif et al. 2017). Numerous reports have shown that many factors are related to the maintenance of the stemness of tumour stem cells, and among them, the POU domain transcription factor (POU5F1) plays an important role during embryonic development and pluripotency maintenance of tumour stem cells (Ng and Surani 2011). In addition, cytokines secreted by tumour cells, such as interleukin-6 (IL-6) and transforming growth factor-β (TGF-β), can stimulate the transformation of some tumour cells into tumour stem cells. In addition, it has been reported that the activation level of autophagy increases in a variety of tumour stem cells, such as breast cancer stem cells, pancreatic cancer stem cells and liver cancer stem cells. The high infiltration and rapid growth of tumour cells lead to insufficient blood supply inside the tumour, which results in hypoxia. Simulation of the intermittent hypoxic environment in vitro could reprogramme pancreatic cancer cells such that they obtain a stem-like phenotype and promote the transformation of pancreatic cancer cells into CD133+ tumour stem cells, which are mainly dependent on autophagy activation induced by hypoxia-inducible factor-1 (HIF-1α). In a breast cancer study, researchers found that both genetic and pharmacological inhibition of cell autophagy activity can make breast cancer stem cells more inclined to show an epithelioid phenotype while inhibiting their ability to show mesenchymal phenotypes, such as the CD44+ CD24low immune phenotype, which is often considered a marker of breast cancer stem cells. The same phenomenon was found in ovarian cancer. In ovarian cancer stem cells, whether the autophagy level is downregulated by CQ or by interference from ATG5, the pellet-forming ability of ovarian cancer stem cells and the expression of dry genes are inhibited, and FOXA2 is involved in this downregulation. Regarding the process whereby cirrhosis of the liver leads to the development of liver cancer, researchers found that autophagy can drive Axin2+ cells to become Axin2+ CD90+ cells, that CD90 is the marker of liver cancer stem cells and that high expression of the other stem cell markers OCT4 and SOX2 promotes colony formation in vitro and tumour formation in nude mice, in which HGF signalling pathways are involved (Peng et al. 2017). The results described above suggested that autophagy can promote the acquisition of tumour stem cells and the maintenance of their stemness (Peng et al. 2017). With ongoing research, different opinions have emerged. A report in 2017 made a unique point; that is, in a study of teratoma cells, researchers used inhibited or promoted autophagy levels in different ways and obtained the same result: stemness markers of cancer stem cells were reduced, and differentiation markers were increased; hence, they noted that maintaining the balance of the foundational autophagy level is vital to maintaining the stemness of cancer stem cells (Sharif et al. 2017).
2 The Role of Autophagy in the Adaptation of Tumour Stem Cells to Harsh Environments
In the process of tumour development, the tumour also forms a tumour microenvironment characterized by tissue hypoxia, decreased pH, nutritional deficiency and tumour angiogenesis. The ‘seed and soil’ theory is used to explain these phenomena: tumour stem cells are seeds, while the tumour microenvironment is the soil on which the tumour stem cells live. However, the soil is not warm and fertile but relatively barren. Local hypoxia and a relative lack of nutrients caused by insufficient blood supply are common in solid tumours and are important characteristics of the tumour microenvironment. At the start of tumorigenesis and metastasis, tumour stem cells must face and overcome this unfavourable tumour microenvironment. However, autophagy is actually a survival mechanism cells use in response to adverse environments, and hypoxia and hyponutrition are typical autophagy-inducing factors. In the environment of nutrient deficiency, cells use autophagy and lysosomes to degrade the damaged organelles and macromolecules and thus maintain the balance of protein metabolism, stabilize the intracellular environment and provide the minimum energy required for cell survival.
Studies have confirmed that hypoxia can induce autophagy in tumour cells by hypoxia-induced factor-1α (HIF-1α), while hypoxia-induced autophagy can promote tumour cell survival and even promote the proliferation of tumour stem cells. Studies have found that CD133+ hepatocellular carcinoma stem cells have higher levels of basal autophagy and ischaemia deficiency-induced autophagy than CD133− cells in vitro, whereas inhibition of autophagy reduced their viability, suggesting that cancer stem cells with higher levels of autophagy may respond more rapidly to the anoxic microenvironment. In addition to hypoxia, an important feature of the tumour microenvironment is ischaemia, which refers to a lack of nutrients. In addition to escaping biophysical limitations, starving cancer cells must be able to utilize renewable resources by activating catabolic processes, i.e. recycling intracellular components to maintain the balance of intracellular metabolism and cell viability. The function and activity of cancer stem cells depend on their ability to ensure adequate bioenergy supplementation in a timely manner. An increasing number of studies have found a close relationship between cancer stem cells and autophagy and have also suggested that the protein metabolism function of autophagy has a protective effect when tumour stem cells face starvation. The expression level of autophagy-related genes was significantly greater in cells with globular proteins that could be isolated from ductal carcinoma lesions. Treatment with the autophagy inhibitor chloroquine induced apoptosis in genetically abnormal spheroid cells, which were eventually cleared. Allograft tumours cannot be formed by reducing the expression of autophagy-related genes. The above evidence suggested that autophagy is critical for the survival of tumour stem cell-like precursor cells in pre-malignant lesions. Of course, in addition to helping cancer stem cells cope with harsh conditions in the tumour microenvironment, autophagy can also help the survival of cancer stem cells that are treated with chemotherapeutic drugs. Curcumin is considered as a chemotherapeutic drug that can induce tumour cell apoptosis through a variety of mechanisms. Researchers have found that while curcumin induces the apoptosis of colon cancer stem cells, autophagy is activated, thereby promoting colon cancer stem cells survival and enhancing their tolerance to the chemotherapeutic drug. However, unfortunately, there is still no literature that explains how autophagy can increase the tolerance of cancer stem cells to stress environments while maintaining their function and even promoting their expansion.
3 Autophagy and Tumour Stem Cell Apoptosis
Apoptosis refers to the self-ordered death pattern of cells controlled by genes to maintain homeostasis. Apoptosis is a fundamental biological phenomenon of cells that plays a necessary role in the removal of unwanted or abnormal cells by multicellular organisms. Tumour cells, especially cancer stem cells, have a prominent feature in which their proliferative capacity is abnormal and apoptosis is inhibited. Therefore, the treatment of tumours should not be limited to killing tumour cells and inhibiting tumour cell division, and studying the induction of the apoptosis of cancer stem cells will be an important direction for antitumour research for a long time. In recent years, it has been found that in tumour stem cells, autophagy can not only promote cell survival but also regulate the apoptosis of cancer stem cells under certain conditions. At present, the study of the regulation of autophagy on apoptosis has created different perspectives. In the process of using a higher concentration of a plant-derived chemotherapeutic drug, rottlerin, to induce apoptosis in human prostate cancer stem cells, researchers have also found autophagy in tumour stem cells (cytoplasmic vacuoles and autophagosomes were observed under an electron microscope, and autophagy-related gene expression levels were significantly increased), and treatment of cells with the autophagy inhibitor 3-methyladenine could inhibit the apoptosis of cancer stem cells. This research suggested that the activation of autophagy in cancer stem cells may induce apoptosis. On the other hand, studies have shown that autophagy can inhibit apoptosis of cancer stem cells. In a study of breast cancer, the use of the autophagy inhibitor chloroquine could induce apoptosis in both breast cancer cells and breast cancer stem cells. The combination of chloroquine and chemotherapeutic drugs could enhance chemosensitivity. Quinacrine, another autophagy inhibitor, could also induce the apoptosis of breast cancer stem cells while inhibiting lysosomal acidification (Han et al. 2018). The mechanism by which autophagy regulates apoptosis is not clear but has been studied in tumour cells. In a leukaemia study, the researchers found that the autophagy inhibitor spautin-1 could promote cell apoptosis while inhibiting autophagy. This pro-apoptotic phenomenon was associated with activation of the key effector molecule GSK3β downstream of PI3K/Akt and downregulation of the apoptosis-related proteins Mcl-1 and Bcl-2. Therefore, the inhibitor has potential application in the treatment of tumours (Liu et al. 2011; Shao et al. 2014). A related study on adipose tissue-derived stem cells indicated that inhibition of autophagy promotes glucose-induced apoptosis and that ROS/JNK signalling pathways are critical in the induction of autophagy (Li et al. 2018).
4 Bidirectional Regulation of Autophagy on Glucose Catabolism of Tumour Stem Cells
In normal mammalian cells, glycolysis is inhibited under aerobic conditions. However, German biochemist Warburg found that under oxygen-rich conditions, malignant cells are also active in glycolysis. This aerobic glycolysis is a metabolic characteristic called the Warburg effect. It is characterized by a high glucose uptake rate, active glycolysis, and high lactic acid content of metabolites. Glycolysis not only produces ATP efficiently but also avoids the generation of unwanted endogenous reactive oxygen species (ROS). In addition, glycolysis can support the defence efficacy of antioxidants by inducing the production of NADPH, which is required for the formation of key antioxidant enzymes, and reducing glutathione. Studies have suggested that autophagy may have a certain promoting effect on the level of glycolysis during stemness acquisition and malignant transformation of tumour stem cells. Loss of autophagy reduces glucose uptake, resulting in insufficient levels of glycolytic activity in cancer stem cells, which ultimately reduces the ability of mammalian stem cells to proliferate. Autophagy levels were found to be continuously activated in Kras-mutated pancreatic epithelial tumours and advanced pancreatic ductal carcinomas but not in normal pancreatic epithelium or early pancreatic epithelial tumours. Inhibition of autophagy can slow the proliferation and tumorigenesis of pancreatic cancer cells by reducing the oxidative phosphorylation of cancer stem cells. This finding suggests that autophagy promotes the mitochondrial oxidative phosphorylation activity of tumour cells. Urinary epithelial tumour stem cells showed a high level of autophagy, and the expression of glycolytic-related genes was also high. Inhibition of autophagy reduced the expression of glycolytic genes in cancer stem cells. The results suggested that autophagy promoted cell survival by buffering the bioenergy requirements necessary to meet the high levels of glycolytic reactions in cancer stem cells (Ojha et al. 2016). On the other hand, studies have suggested that autophagy may also act as an antagonist of the Warburg effect in specific cases. A study on acute leukaemia indicated that rapamycin induced autophagy but inhibited glycolysis and promoted cell proliferation (Watson et al. 2015).
5 Autophagy Required for the Migration and Tumorigenicity of Tumour Stem Cells
It is well known that tumour metastasis is one of the important biological characteristics of malignant tumours and one of the main factors for poor prognosis in patients with malignant tumours. Although 90% of tumour cells can ‘escape’ early from the primary tumour and eventually reach the pre-metastasis stage, fewer than 2% of these cells can form micro-metastatic deposits, and only 0.02% or fewer of these cells eventually develop into metastatic tumours, which means that tumour cells with certain intrinsic properties can maintain self-renewal and proliferation in the microenvironment throughout the complete metastatic process. Tumour stem cells showing aggressive invasiveness and tumorigenicity are the best candidates for being such cells. In recent years, studies have found that autophagy is essential for the survival of cancer stem cells. One study found that SDCBP/MDA-9/Syntenin signalling-mediated protective autophagy plays an important role in anoikis-resistant glioma stem cells (Talukdar et al. 2018). Autophagy inhibitors not only increase the death of chronic myeloid leukaemia tumour stem cells but also combine with tyrosine kinase inhibitors to significantly suppress their stem cell-like properties and functions. On the other hand, the epithelial to mesenchymal transition (EMT) plays an important role in tumour invasion and metastasis. An increasing number of studies have found that cancer stem cells have an EMT phenotype that can confer migratory and invasive properties to tumour cells, giving them the characteristics of stem cells. Autophagy also plays an important role in the maintenance of the interstitial properties of tumour stem cells, including their migratory and invasive characteristics. Autophagy can promote tumour invasion and metastasis through the renewal of local adhesion molecules and upregulation of metastatic cytokine secretion. Studies have shown that autophagy promotes proto-oncogene RAS-mediated invasion by secreting the pro-metastatic cytokine IL-6. Autophagy can also decompose focal adhesions to promote the dissociation of locally adhered molecules and the migration of metastatic tumour cells through the combination of the autophagy molecule LC3 and paxillin regulated by the proto-oncogene SRC (Sharifi et al. 2016). Moreover, the autophagy-related genes for BECN1 and ATG5 gene silencing can inhibit autophagy in highly metastatic hepatoma cells and significantly suppress pulmonary metastasis of HCC in mice by impairing the anoikis resistance and colonization of the HCC cells. Autophagy inhibition may promote EMT through the ROS/HO-1 pathway in ovarian cancer cells (Zhao et al. 2016). In addition, the autophagy-related molecules DRAM1 and p62 regulate the migration and invasion of malignant glioma tumour stem cells. Increasing data from clinical studies have confirmed that autophagy corresponds to targeted cancer therapy. The autophagy core protein ATG4B is a potential biomarker and therapeutic target for chronic myeloid leukaemia CD34+ haematopoietic stem/progenitor cells (Rothe et al. 2014). Autophagy-related factors are highly expressed in highly invasive tumour cells. The Thr300Ala variant of ATG16L1 is associated with a decreased risk of brain metastasis in patients with non-small-cell lung cancer (Li et al. 2017b). These studies have demonstrated that autophagy maintains the stemness and survival of tumour cells and enhances their invasive and migratory abilities. These studies also have provided new therapeutic strategies and targets for clinical intervention.
In addition to high metastatic ability, cancer stem cells also show high levels of tumorigenicity and demonstrate other biological characteristics. A very small number of cancer stem cells can be cultured in vitro to generate tumour cell colonies and can also form tumours when injected into experimental animals; however, in these cases, a large number of tumour cells are needed to form tumours. The highly tumorigenic characteristic of cancer stem cells has always been an important obstacle for the cure rate of tumours. Therefore, understanding the mechanism for inducing high tumorigenicity in cancer stem cells is conducive to improving the prognosis of patients. In recent years, studies have found that autophagy is required not only for the invasion and migration capacity of cancer stem cells but also for its role in tumorigenicity. Researchers isolated a subset of cells with stem cell characteristics from the bladder cancer cell lines T24 and UM-UC-3, and high levels of autophagy were observed in these side populations without any autophagy-inducing factors. Compared with other cells, the mortality of the side population cell groups was significantly increased after the inhibition of autophagy, and the colony formation ability was reduced for those with weak tumorigenicity. Similar studies have demonstrated that hypoxia-inducible factor-1 α-induced autophagy played an important role in the conversion of non-stem pancreatic cancer cells into CD133+ pancreatic cancer stem-like cells (Zhu et al. 2013). Beclin 1 and autophagy were required for the tumorigenesis of breast cancer stem cells. In the pathologic microenvironment of cirrhosis, autophagy-dependent generation of Axin2+ cancer stem-like cells promoted the development of hepatocellular carcinoma (Li et al. 2017a). Recent observations have suggested that autophagy promotes tumour-like stem cell niche occupancy (Zhao et al. 2018). Autophagy can also maintain stemness by preventing senescence (Garcia-Prat et al. 2016). In this regard, there are also different research perspectives, and studies have shown that autophagy suppresses the self-renewal and tumorigenicity of glioma-initiating cells by promoting the degradation of Notch1 (Tao et al. 2018). The studies described above confirm that autophagy contributes to the survival and stemness maintenance of cancer stem cells and enhances the tumorigenicity of cancer stem cells. At the same time, autophagy also contributes to conditions suitable for the invasion and metastasis of cancer stem cells that have high metastaticity. Traditional cancer treatments combined with autophagy inhibitors enhance the therapeutic antitumour effect (Fig. 21.1).
6 Autophagy and Cancer Stem Cells with Resistance to Chemotherapy and Radiotherapy
In addition to surgical treatment, radiotherapy and chemotherapy have become two of the most important treatments for cancer. Radiotherapy and chemotherapy can act quickly on tumour lesions and reduce tumour burden. However, the largest challenge encountered in current chemoradiotherapy treatments is the resistance of tumour cells to chemotherapy and radiotherapy, which leads to tumour recurrence. A large number of recent studies have shown that tumour resistance to radiotherapy and chemotherapy is mainly achieved by the cancer stem cells, while radiotherapy and chemotherapy are used for selectively destroying dividing cells. However, current studies have found that autophagy is critical for the dormancy and quiescence of haematopoietic stem cells and muscle stem cells. The stem cells can switch from dormancy into metabolically active cells and can be prevented from irreversible ageing. Therefore, autophagy confers a dormancy phenotype in tumour stem cells to help them escape the cytotoxic effects of chemotherapeutic treatment and continue to survive. How to reduce the chemosensitivity of cancer stem cells has become a hot topic in the study of tumour therapy. At the same time, research on the mechanism of action for cancer stem cell resistance to radiotherapy and chemotherapy has become more urgent.
On the one hand, a large body of evidence indicates that autophagy plays an important role in the radiotherapy resistance of cancer stem cells. In gliomas, the CD133+ cell population increased after radiation, and the survival rate of CD133+ cells was significantly higher than that of CD133− cells. Further studies found that radiation can induce autophagy in glioma stem cells, and the level of autophagy in CD133+ cells was significantly higher than that of CD133− cells, and the sensitivity of CD133+ cells to radiation was enhanced after inhibition of autophagy. The survival rate of tumour stem cells and the colony-forming ability in vitro decreased significantly after irradiation, and while CD133− was inhibited after autophagy, the sensitivity of these cells to radiation was only slightly enhanced, suggesting that cancer stem cells play a leading role in resistance to radiotherapy. This study suggested that the induction of autophagy contributes to the response of glioma stem cells to the toxic effects of radiation and increases their rate of survival. In addition, it was found that the radiotherapy protocol induced the upregulation of autophagy-related genes in tumour cells, upregulated the expression of beclin 1, atg3, atg4b, atg4c, atg5 and atg12 and induced the aggregation of phagosomes. Inhibition of autophagy-related genes enhanced the sensitivity of tumour cells to radiotherapy, but researchers also found that the basal clonogenicity of untreated resistant cells may even be enhanced by the inhibition of autophagy. The results suggested that different tumour types and different tumour states, phenotypic characteristics of tumour cells and different individual inflammatory microenvironments in patients with tumours need to be considered for tumour therapy when autophagy is inhibited to optimize the options for cancer treatment.
Autophagy has also been found to be involved in the chemoresistance characteristics of cancer stem cells. Chronic myelogenous leukaemia (CML) stem cells were the first cancer stem cells to be isolated. Imatinib mesylate (IM) is the standard therapeutic drug for treating CML, but CML stem cells instinctively produce resistance to IM and accelerate the progression of CML. IM-induced autophagy can counteract tumour cell death, and autophagy inhibitors help restore the sensitivity of CML stem cells to IM treatment. In addition, the toxic effects of fluorouracil on colon cancer CD133+ cells were significantly enhanced through autophagy inhibition. Another study found that the chemotherapy drug 5-FU and cisplatin induced the death of oesophageal cancer cells. It was found that chemotherapy-sensitive oesophageal cancer cell lines showed increased apoptosis, while chemotherapy-resistant oesophageal cancer cell lines showed increased autophagy. The study found that autophagy in response to chemotherapy is the mechanism that promotes cell recovery and enhances chemotherapy resistance. The results suggested that selective inhibition of autophagy regulators could increase the effect of chemotherapy. In addition, the study found that the expression of the autophagic molecule LC3 in pancreatic cancer tumour tissues was closely related to the expression of the tumour stem cell markers aldehyde dehydrogenase 1 (ALDH1), CD133 and CD44. In pancreatic cancer cell lines, higher LC3-II expression was observed in the sphere-forming cells. The elevated expression levels of LC3-II suggested elevated levels of autophagy, and the researchers found that the chemotherapy drug gemcitabine combined with autophagy inhibitors had a significant effect on pancreatic cancer (Yang et al. 2015). In addition, studies have found that the level of autophagy in ovarian cancer stem cells was significantly higher than it was in non-stem cells, and the combination of the chemotherapy drug carboplatin and autophagy inhibitors improved the therapeutic effect on ovarian cancer (Pagotto et al. 2017). The increased autophagy level of tumour stem cells in the face of chemoradiotherapy is also a spontaneous protective behaviour of cells in harsh environments. Autophagy for cancer stem cells can provide a new target for clinical tumour therapy.
Current studies have indicated that autophagy acts as a ‘double-edged sword’ in cancer stem cells. In antitumour therapy, autophagic death can be induced in cells, and a variety of chemotherapeutic drugs achieve therapeutic effects through this route. In drug-resistant cancer stem cells, autophagy also appears to be a protective mechanism to reduce the killing effect of drugs on cells. The combination of autophagy inhibitors with traditional cancer treatment can induce the death of drug-resistant cancer stem cells to achieve better chemotherapeutic effects. An in-depth study on the regulatory mechanisms of autophagy in cancer stem cells may reveal a more valuable reference and therapeutic target for tumour therapy.
7 Antitumour Strategy for Targeting Autophagy in Cancer Stem Cells
Although there have been many reports on the research and clinical application of antitumour therapy and autophagy, some core problems remain controversial. Autophagy has a role in the promotion and inhibition of tumour development and metastasis processes. On the one hand, the tumour has not yet formed in the presence of damage, and autophagy is thought to prevent genomic instability or cell death and inflammation by inhibiting protein aggregation and eliminating damaged organelles and chromosome damage. It is theoretically feasible to prevent the occurrence of these injuries and ultimately inhibit the occurrence of tumours. Of course, it has been confirmed that autophagy is necessary for drugs to exert their therapeutic effects in the treatment of cancer. For example, mammalian target of rapamycin (mTOR) kinase inhibitors are used to treat renal cell carcinoma and metastatic breast cancer, and inhibition of the mTOR kinase function produces an inhibitory effect on cell proliferation. As a central regulator of autophagy, mTOR kinase can inhibit the activation of autophagy. In addition, when the EGFR inhibitor drug erlotinib is used to treat lung cancer, autophagy is required to maximize its inhibitory effect. On the other hand, autophagy is a protective mechanism for cells in the face of harsh environments and has a certain role in promoting tumour development and metastasis. The rapid proliferative capacity of tumour cells requires more energy expenditure, but the efficiency of glycolytic metabolism is not high. Therefore, it is necessary to activate autophagy to produce more energy. Under this type of metabolic stress, autophagy inhibitors can induce tumour cell death. Imatinib has been studied as a means to block the fusion of autophagosomes and lysosomes in malignant glioma cells, increase mitochondrial damage, induce apoptosis and inhibit malignant glioma. The combination of chemotherapy drugs and autophagy inhibitors can enhance the effectiveness of tumour treatment. It has been found that the addition of the autophagy inhibitor 3-methyladenine (3-MA) or chloroquine (CQ) can enhance the apoptotic activity of human cervical cancer cells treated with cisplatin to improve the chemotherapeutic effect of cisplatin, and the use of chloroquine in bladder cancer cells can inhibit autophagy and activate apoptosis to enhance the sensitivity of tumour cells to radiotherapy (Wang et al. 2018). It has also been found that chloroquine potentiates the radiosensitivity of glioma-initiating cells by inhibiting autophagy and activating apoptosis (Ye et al. 2016). However, due to the lack of specific cell targets during autophagy therapy, autophagy also affects the function of normal cells and produces certain side effects. According to the theory of the origin of cancer stem cells, it is believed that cancer stem cells are critical for the malignant transformation, invasion and metastasis of tumours. Therefore, the combination of autophagy inhibitors and specific drugs that target cancer stem cells is expected to become more effective and produce fewer side effects than antitumour treatments.
8 Conclusions
Tumour stem cells are the key factors for tumorigenesis, growth, metastasis and chemoradiotherapy resistance. Cancer stem cells maintain the vitality of tumour cell populations through self-renewal and immortalization, and the migratory and invasive ability of cancer stem cells promotes the metastatic spread of the tumour. Cancer stem cells can be dormant for a long time and are not sensitive to external physical and chemical factors because of their drug resistance ability. Therefore, cancer stem cells are considered to be the keys to a breakthrough in cancer therapy. How to remove cancer stem cells or enhance their sensitivity to various treatments without affecting normal stem cells is a difficulty for tumour treatment in the future. It is especially important to understand the key mechanisms of tumour stem cell survival, migration and chemoradiotherapy resistance. Existing studies have shown that autophagy plays an important role in tumour stem cell survival and invasion and metastasis, and autophagy has different effects on tumour stem cells at different stages of tumorigenesis and development. In the early stage of carcinogenesis and prior to the induction of malignant tumours, autophagy can inhibit the formation of tumour stem cells by eliminating damaged organelles and proteins and maintaining genomic stability. In the late stage of tumour development, autophagy can protect cancer stem cells in the harsh environment, such as that produced by ischaemia and hypoxia, to promote their survival and inhibit tumour stem cell apoptosis. However, does autophagy maintain the same status in different types of tumours? Is the heterogeneity of cancer still relevant? Autophagy can remove excess organelles, misfolded proteins and other waste materials in the cytoplasm. Autophagy can also provide the energy and substances cells need by degrading their own macromolecules. What is the regulatory role of autophagy in cancer stem cells? What are the similarities and differences between these various roles? These answers are still needed and require further study.
Although some existing results have shown a close relationship between autophagy and cancer stem cells, the importance of autophagy in cancer stem cells has started to emerge as a component of tumour prevention and treatment. However, most of the current related studies have been based on the observation of gene knockout animal models. Some autophagy-related genes are likely to have functions other than those related to autophagy. Therefore, when autophagy-related gene mutation analysis is used to study autophagy, phenotypic changes and other non-autophagy factors should be considered. The analysis of experimental results should also be considered to reach a conclusion. In addition, the current research on the relationship between autophagy and tumour stem cell characteristics mostly focuses on the observable phenomena, while the specific mechanism of autophagy regulation remains unclear. Further exploration of the role of autophagy during different life stages of stem cells is still urgent, and study of the molecular mechanism of autophagy regulation using modern biomedical research techniques is still necessary. These are important directions for research if autophagy is to serve as a powerful target for therapeutic applications in the future.
References
Clevers H (2011) The cancer stem cell: premises, promises and challenges. Nat Med 17:313–319
Garcia-Prat L, Martinez-Vicente M, Perdiguero E et al (2016) Autophagy maintains stemness by preventing senescence. Nature 529:37–42
Han Y, Fan S, Qin T et al (2018) Role of autophagy in breast cancer and breast cancer stem cells (Review). Int J Oncol 52:1057–1070
Li J, Hu SB, Wang LY et al (2017a) Autophagy-dependent generation of Axin2+ cancer stem-like cells promotes hepatocarcinogenesis in liver cirrhosis. Oncogene 36:6725–6737
Li QX, Zhou X, Huang TT et al (2017b) The Thr300Ala variant of ATG16L1 is associated with decreased risk of brain metastasis in patients with non-small cell lung cancer. Autophagy 13:1053–1063
Li Q, Yin Y, Zheng Y et al (2018) Inhibition of autophagy promoted high glucose/ROS-mediated apoptosis in ADSCs. Stem Cell Res Ther 9:289
Liu J, Xia H, Kim M et al (2011) Beclin1 controls the levels of p53 by regulating the deubiquitination activity of USP10 and USP13. Cell 147:223–234
Meng E, Long B, Sullivan P et al (2012) CD44+/CD24− ovarian cancer cells demonstrate cancer stem cell properties and correlate to survival. Clin Exp Metastasis 29:939–948
Ng HH, Surani MA (2011) The transcriptional and signalling networks of pluripotency. Nat Cell Biol 13:490–496
Ojha R, Jha V, Singh SK (2016) Gemcitabine and mitomycin induced autophagy regulates cancer stem cell pool in urothelial carcinoma cells. Biochim Biophys Acta 1863:347–359
Pagotto A, Pilotto G, Mazzoldi EL et al (2017) Autophagy inhibition reduces chemoresistance and tumorigenic potential of human ovarian cancer stem cells. Cell Death Dis 8:e2943
Peng Q, Qin J, Zhang Y et al (2017) Autophagy maintains the stemness of ovarian cancer stem cells by FOXA2. J Exp Clin Cancer Res 36:171
Rothe K, Lin H, Lin KB et al (2014) The core autophagy protein ATG4B is a potential biomarker and therapeutic target in CML stem/progenitor cells. Blood 123:3622–3634
Shao S, Li S, Qin Y et al (2014) Spautin-1, a novel autophagy inhibitor, enhances imatinib-induced apoptosis in chronic myeloid leukemia. Int J Oncol 44:1661–1668
Sharif T, Martell E, Dai C et al (2017) Autophagic homeostasis is required for the pluripotency of cancer stem cells. Autophagy 13:264–284
Sharifi MN, Mowers EE, Drake LE et al (2016) Autophagy promotes focal adhesion disassembly and cell motility of metastatic tumor cells through the direct interaction of paxillin with LC3. Cell Rep 15:1660–1672
Talukdar S, Pradhan AK, Bhoopathi P et al (2018) Regulation of protective autophagy in anoikis-resistant glioma stem cells by SDCBP/MDA-9/Syntenin. Autophagy 14:1845–1846
Tao Z, Li T, Ma H et al (2018) Autophagy suppresses self-renewal ability and tumorigenicity of glioma-initiating cells and promotes Notch1 degradation. Cell Death Dis 9:1063
Wang J, Chen D, He X et al (2015) Downregulated lincRNA HOTAIR expression in ovarian cancer stem cells decreases its tumorgeniesis and metastasis by inhibiting epithelial-mesenchymal transition. Cancer Cell Int 15:24
Wang F, Tang J, Li P et al (2018) Chloroquine enhances the radiosensitivity of bladder cancer cells by inhibiting autophagy and activating apoptosis. Cell Physiol Biochem 45:54–66
Watson AS, Riffelmacher T, Stranks A et al (2015) Autophagy limits proliferation and glycolytic metabolism in acute myeloid leukemia. Cell Death Discov 1:15008
Yang MC, Wang HC, Hou YC et al (2015) Blockade of autophagy reduces pancreatic cancer stem cell activity and potentiates the tumoricidal effect of gemcitabine. Mol Cancer 14:179
Ye H, Chen M, Cao F et al (2016) Chloroquine, an autophagy inhibitor, potentiates the radiosensitivity of glioma initiating cells by inhibiting autophagy and activating apoptosis. BMC Neurol 16:178
Zhao Z, Zhao J, Xue J et al (2016) Autophagy inhibition promotes epithelial-mesenchymal transition through ROS/HO-1 pathway in ovarian cancer cells. Am J Cancer Res 6:2162–2177
Zhao S, Fortier TM, Baehrecke EH (2018) Autophagy promotes tumor-like stem cell niche occupancy. Curr Biol 28(3056–3064):e3
Zhu H, Wang D, Liu Y et al (2013) Role of the Hypoxia-inducible factor-1 alpha induced autophagy in the conversion of non-stem pancreatic cancer cells into CD133+ pancreatic cancer stem-like cells. Cancer Cell Int 13:119
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Science Press and Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Yang, X., Ye, F., Jing, Y., Wei, L. (2020). Autophagy and Tumour Stem Cells. In: Le, W. (eds) Autophagy: Biology and Diseases. Advances in Experimental Medicine and Biology, vol 1207. Springer, Singapore. https://doi.org/10.1007/978-981-15-4272-5_21
Download citation
DOI: https://doi.org/10.1007/978-981-15-4272-5_21
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-15-4271-8
Online ISBN: 978-981-15-4272-5
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)