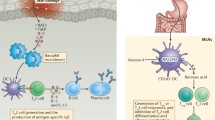
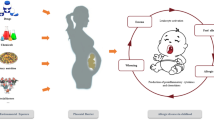
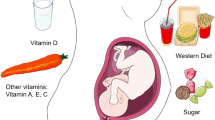
Abstract
Allergic diseases, including asthma, food allergies, atopic dermatitis (eczema), allergic rhinitis or hay fever, and anaphylaxis, have gradually been increasing in prevalence. Allergies have both a genetic and an environmental component. Several harmful environmental factors during early life may interrupt the function of helper T cells (Th1/Th2), cytokines, and immunoglobulin levels and lead to allergic reactions. In this chapter, we specifically focused on the influence of prenatal and postnatal environmental chemical exposures, such as persistent organic pollutants (POPs) and short half-life compounds, on early childhood allergic diseases. We also investigate the possible pathophysiological mechanisms involved based on experimental data. We emphasized the importance of further investigation of the effects of these environmental chemicals, as well as alternative chemicals, on asthma and other allergies.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
An allergy is a chronic condition involving an abnormal reaction to an ordinarily harmless substance (i.e., an allergen). The resultant response can lead to conditions such as asthma, food allergies, atopic dermatitis (eczema), allergic rhinitis or hay fever, and anaphylaxis. Allergens can include aeroallergens such as dust mites, mold, and plants pollen, as well as food allergens such as milk, eggs, wheat, nuts, or fish proteins. Responses to allergens result in allergic symptoms, including increased levels of immunoglobulin-E (IgE). Allergies first present in early childhood. Interestingly, the severity of symptoms of allergic asthma in childhood predicts disease persistence into adulthood. Type 2 immune responses are the basis of an allergic response. However, increasing allergies in early childhood cannot be simply explained by type 2 immune responses only. It is clear that the genetic component certainly contributes to allergy susceptibility. In fact, various gene variants associated with Th2 cell differentiation are noted as risk factors for allergy [15]. For example, loss-of-function mutation(s) in the filaggrin gene is a risk factor of atopic dermatitis. However, filaggrin gene mutations can explain only 40% of the cases of atopic dermatitis [67], and the remaining 60% are thought to occur due to environmental risk factors. Furthermore, epigenetic modifications play a key role in the differentiation of allergy-related T-cell lineages as well as influence the balance between distinct Th cell populations such as Th1, Th2, and regulatory T cells. Epigenetic regulation also mediates the effect of various environmental exposures resulting in both allergy protection and conferring susceptibility to allergic diseases [65]. Many environmental factors have been identified and investigated as “environmental influences of allergic diseases.” These include various toxic and harmful physical, chemical, biological, and sociopsychological factors. Such factors are introduced into people’s lives through their environment (e.g., via air), water source, soil, indoor public spaces, food, etc. In regard to chemical factors, the prevalence of asthma has increased since the 1970s, which coincides with an increased use of numerous new chemicals in manufacturing processes, with increased exposure of the general population during the same period. Asthma and allergy have not only a genetic but also an environmental component, and, because of the increases in incidence, prevalence cannot be due solely to genetics. These newly produced synthetic chemicals have shown endocrine-disrupting effects on humans, which interfere in some way with hormone action and can alter endocrine function such as immune, reproductive, endocrine, cardiopulmonary, and brain systems [84]. In this section, we provide up-to-date evidence based on epidemiological findings, specifically focused on the influence of prenatal and postnatal environmental chemical exposures, such as persistent organic pollutants (POPs) and short half-life compounds, on early childhood allergic diseases outcomes. We also investigate the possible pathophysiological mechanisms involved based on experimental data.
2 Persistent Organic Pollutants (POPs)
2.1 Perfluoroalkyl Substances (PFAS)
Perfluoroalkyl substances (PFAS) are persistent bioaccumulative chemicals which are widely used in industry including textiles, non-stick housewares, food packaging, furnishings, fire-fighting formulations, and more. The main exposure routes of PFAS for people are through the intake of contaminated food and water [28], although inhalation and ingestion of indoor dust are also contributing factors [40]. Since perfluorooctane sulfonate (PFOS) and perfluorooctanoate (PFOA) were included in the Stockholm Convention on Persistent Organic Pollutants, both substances have been replaced by shorter or longer carbon-chain PFAS. In human biomonitoring, results have shown that the exposure levels of PFOS and PFOA have decreased in recent years, while the levels of shorter and longer carbon-chain have increased [31, 61, 78]. Despite the introduction of the shorter or longer carbon-chain PFAS, epidemiological studies have reported that these substances are immunotoxic and immunomodulating (Table 1). Data from a birth cohort from the “Hokkaido Study” showed that prenatal exposure to PFOS was linked to decreased cord blood IgE levels among female infants [63] and reduced risk of eczema among 2-year-old girls [62]. In the same population, prenatal exposure to longer chain PFAS, such as perfluorohexane sulfonate (PFHxS), perfluorododecanoic acid (PFDoDA), and perfluorotridecanoic acid (PFTrDA), was linked to reduced risk of eczema at 4 years old [34]. Similar results have been reported from other birth cohorts. The Norwegian Mother and Child (MoBA) cohort has reported that prenatal exposure to longer carbon-chain PFAS and perfluoroundecanoic acid (PFUnDA) was inversely linked to a risk of atopic eczema [44]. Furthermore, a study conducted in Greenland and Ukraine showed that a principal component of PFAS in maternal blood, dominated by PFOS, was inversely linked to wheeze at 5–9 years old [68]. On the other hand, some studies are incongruent with those results. A study from Taiwanese asthmatic case-control study reported that positive dose-response associations were found between PFAS concentrations and risk of asthma at 15 years old [25]. National Health and Nutrition Examination Survey, a cross-sectional study, reported that the levels of PFOS were linked to higher odds of diagnosed asthma at 12–15 years old [43].
Data from the same population as Goudarzi et al. showed that prenatal exposure to PFHxS, PFDoDA, and PFTrDA was linked to an increasing risk of infectious diseases at 4 years old [33]. The Denmark Odense Child Cohort has reported that exposure to PFOS and PFOA during the prenatal period increases the prevalence of fever at 1–4 years of age [22]. This inverse association of allergic symptoms and infectious diseases is thought to be plausible due to the immunosuppressive effects of PFAS. Grandjean et al. first reported the possibilities of the immunosuppressive effects of PFAS when they showed that a twofold increase in PFAS levels was linked to a decline in diphtheria antibody levels [35]. Similar associations have been found between prenatal or postnatal exposure to PFAS and childhood vaccination antibodies, such as mumps and rubella [36, 71]. Moreover, the Guangzhou Birth Cohort Study has reported that cord blood PFAS was linked to a significant increase in the risk of hand-food-mouth-disease antibody concentration below the level of clinical protection among 3-month-old infants [86]. These findings are in line with animal studies. It has been demonstrated that in PFOS-exposed mice, IgM, which was suppressed, responses to T-cell-dependent and T-cell-independent antigens and natural killer (NK)-cell function [24, 37]. However, the experimental conditions employed used higher doses than the general exposure levels seen in humans. Evidence from experimental studies cannot be directly related to human health outcomes. Moreover, epidemiological findings are not fully consistent; this may be due to study designs, the age to which the health outcomes are related, confounding factors, and varied definitions of outcomes. Evidence regarding the immunosuppressive effects of PFAS, especially longer carbon-chain of PFAS in early childhood, is still under consideration.
2.2 PCBs/PCDDs/PCDFs
Polychlorinated biphenyls (PCBs), polychlorinated dibenzo-p-dioxins, and polychlorinated dibezofurans (PCDDs/PCDFs) have a long half-life and are categorized as POPs. PCBs, PCDDs, and PCDFs exist persistently not only in the food chain but also in the environment. Primary sources of exposure to these compounds are food items such as fish, seafood, meat, and eggs. PCBs, PCDDs. For many years, it has been shown that PCDFs accumulate mainly in adipose tissue due to the high lipophilicity and resistance to biodegradation. In bodies, they traverse the placenta and are transferred to fetal tissues and cord blood. The most toxic congener of PCDDs/PCDFs is 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD). The offspring of maternal rats treated with TCDD during pregnancy are more sensitive to immune toxicity caused by TCDD compared to adult rats and the negative influence that occurs at crucial windows of maturation persists later in life. Besides, the aforementioned toxic susceptibility appears to be sex-dependent. The offspring of male rats is more sensitive to TCDD-mediated inhibition of T cell activity than that of female rats [83].
There have been reports of exposure of high levels of PCBs/PCDDs/PCDFs, accidental poisoning, or contaminated food intake of PCBs/PCDDs/PCDFs in humans (Table 2). In one such incident in Taiwan Yucheng, pregnant women were exposed to high levels of PCDFs, and their children were more frequent to have bronchitis and reduced serum levels of immunoglobulins at 6 months of age and more likely to develop diseases of influenza and otitis media at 6 years of age. In Japan, pregnant women exposed to contaminated food were exposed to high levels of PCB. The children that these women bore were more likely to catch colds and gastrointestinal symptoms [30]. In Inuit infants born to mothers who were exposed to contaminated marine mammals, higher levels of prenatal PCB caused an apparent increased incidence of infections (e.g., acute otitis) and respiratory problems [20, 21]. On the Faroe Islands, PCB levels in maternal serum were inversely linked to an antibody response to diphtheria toxoid in 18-month-old children and tetanus toxoid in 7-year-old children [41]. According to a study in Eastern Slovakia, higher PCB levels in maternal serum were linked to newborns with a smaller thymus, the organ associated with lymphocyte maturation [64].
The associations between prenatal exposure to PCBs and dioxins at environmental levels and allergies or infectious diseases among general population have been also reported (Table 3). In the Rotterdam study, children’s otitis media and chicken pox were more prevalent due to maternal exposure to PCBs during pregnancy. Moreover, these implications were associated with a decrease in measles, mumps, and rubella reactivity after primary vaccination and a growing number of T lymphocytes in 42-month-old children [79, 80]. In contrast, in the Amsterdam study, children’s allergies were found to have decreased at 8 years of age due to increased maternal dioxin levels [73]. PCB levels in cord blood have no associations with the prevalence of asthma in 4-year-old children in Spain [72]. In Japan, PCBs/PCDDs/PCDF levels in breast milk were notably linked to an increase of lymphocyte subset ratio in the peripheral blood of breast-fed infants at 10 months of age [60]; however, there was no observation in another cohort of Japanese infants at 12 months of age [47]. A systematic review published by Gascon et al. included several studies demonstrating a higher risk of respiratory infections, including acute otitis media, among infants [16, 20, 32, 58] with regard to in utero exposure to PCBs and/or dioxins. The majority of the current knowledge surrounding in utero exposure to PCBs/PCDDs/PCDFs in the context of downregulation of immune response and higher incidences of infectious symptoms in early infancy has been relatively consistent.
Regarding long-term effects, in a Danish cohort, the number of children with asthma was higher during a follow-up period lasting for 20 years according to higher maternal PCBs during pregnancy. Airway obstruction in children but not allergic sensitization, at age 20, was associated with maternal PCB levels during pregnancy in that cohort [39]. The Hokkaido Study in Japan reported that exposure to PCBs/PCDDs/PCDFs in utero may modify immune responses in offspring, resulting in a growing risk of allergy among school-age children. Besides, male infants may be more susceptible to maternal exposure to PCBs/PCDDs/PCDFs [57]. In summary, excluding accidental poisoning and contaminated food intake, the general population is continually exposed to PCBs/PCDDs/PCDF at relatively low levels. Even at low-level exposure, prenatal PCB/PCDD/PCDF exposure may modify the immune responses of the offspring of exposed individuals, resulting in an increased risk of allergy and infection during childhood and adolescence. There is insufficient evidence detailing the long-term effects of exposure to PCBs/PCDDs/PCDFs. Therefore, further studies targeted at the general population are vital to elucidate the long-term influence of such exposure.
2.3 Brominated Flame Retardants (BFRs)
Brominated flame retardants (BFRs) consist of different groups of chemicals with a range of physiochemical properties. BFRs are widely used in industrial applications such as production of polybrominated diphenyl ethers (PBDEs), hexabromocyclododecane (HBCD), tetrabromobisphenol A (TBBPA), as well as other brominated compounds. PBDEs contain up to 10 bromine atoms attached to 2 aromatic rings, which results in 209 different congeners which are structurally similar to PCBs. Exposure to BFRs from indoor environments is of particular concern because of their widespread use within household interiors, building materials, electrical appliances, as well as rubber products. More specifically, house dust is thought to be a significant means of exposure to PBDEs for toddlers, children, teenagers, and adults, accounting for more than 50% of the total PBDE intake [45]. Widespread contamination of PBDEs in the environment and their detection in people and wildlife have raised concerns regarding adverse health effects [55]. Several reviews have suggested that BFRs, especially PBDEs, have potentially adverse effects on endocrine functions, the central nervous system, and reproductive systems in experimental studies [19, 54]. PBDEs and HBCDD have been listed as POPs in the Stockholm Convention because of their potentially negative influence on human health, their persistence in the environment, as well as bioaccumulation potential. In epidemiological studies, adverse effects on neurodevelopment [77], birth outcomes [17], changes in the levels of thyroid hormones [18, 77], and reproductive hormone levels [27] have been reported. As for immunological effects, however, experimental and epidemiological data is lacking. An in vitro study suggested that BFRs can induce or enhance immune or allergic responses by increasing antigen presentation-related molecule expression and IL-4 production [49]. A case-control study reported that, serum levels of BDE-209, which is the most widely used PBDE in China and was dominantly detected in the study population, was linked to an increased risk of asthma among children. However, levels of BDE-209 or other PBDEs in house dust were not associated with any allergic symptoms [14, 56].
3 Short Half-Lived Chemicals
3.1 Phthalates
Phthalates are plastic additives (plasticizers), which are added to plastics and perfumes and are widely contained in consumer products and personal care products (PCPs). Phthalates are nonpersistent, not bioaccumulative, and therefore short half-life compounds which are excreted in urine within hours to 2 days. Due to their endocrine-disrupting effects, some phthalates, such as di-2-ethylhexyl phthalate (DEHP) and di-n-butyl phthalate (DnBP), have been partly regulated for their use in food containers for fatty foods, as well as in children’s toys and mouthing products. However, their use in other consumer products such as PCPs, furniture, and indoor materials is not regulated. We are exposed to phthalates from various consumer products on a daily basis. It is, therefore, important to investigate the possible associations between exposure to phthalates and allergic diseases. This is especially critical since the hand-to-mouth behavior of infants and toddlers is thought to be the main exposure route of phthalate exposure among young children. Moreover, phthalates are known to have adjuvant effects on allergies, which are consistent with experimental studies [48, 53]. In experimental studies, adjuvants induced production and differentiation of Th2 cells in mice, which in turn promoted IgE and IgG1 production. In a human experimental study, healthy human participants showed no inflammatory responses to short-term inhalation of house dust which contains DEHP. Nevertheless, nasal exposure to dust mite in allergic participants showed elevated IL-5, IL-6, granulocyte-colony stimulating factor, and eosinophil cationic protein levels even after short-term exposure to low DEHP doses [23].
Epidemiological studies are mainly designed to use the levels of phthalate metabolites in urine or of phthalates in house dust as exposure assessments (Table 4). In human biological samples, both prenatal and postnatal exposure levels are considered; however, in dust samples, only postnatal time periods are investigated. Currently, evidence regarding the relations between prenatal exposure to phthalates in house dust and allergies in early childhood is lacking. Data generated using phthalates in house dust as a proxy for postnatal exposure assessment shows quite accordant results, in which higher house dust levels of butyl benzyl phthalate (BBzP), DEHP, and DnBP were linked to an increase in the risk for allergies among school-age children [1, 6, 8, 42, 51]. Some of these studies investigated the relations with urinary phthalate metabolite levels; however, no associations were found between urinary phthalate metabolites and allergies. In prenatal exposure, metabolites of diisononyl phthalate (DiNP) were associated with an increase in the risk for childhood asthma [7]. In this study, two spot urine samples were collected from pregnant women during the first and second trimester of pregnancy. Additionally, the prenatal exposure to diisodecyl phthalate (DiDP) was linked to wheeze at 5 years old [76]. Data seems to suggest that high molecular weight phthalates, such as DiNP and DIDP which are used as replacements for DEHP, are associated with asthma and respiratory symptoms. However, evidence for the effects of postnatal exposure to phthalates on childhood allergies is still very limited. Since phthalates are nonpersistent and short half-life compounds, the inconsistency of postnatal exposure to phthalates may be due to this and compounded by the fact that only one spot urine collection was used for assessing the effects of phthalate exposure. Intra- and inter-day variances of phthalate metabolites have been reported, and urine sampling in several time points is recommended. Therefore, only one spot urine collection is one of the limitations of exposure assessment of phthalates. On the other hand, levels of phthalates in house dust might not change dramatically on a daily basis, as well as intra-day basis. It is also quite challenging to collect urine samples from children at several time points. Nonetheless, further studies are needed to challenge this and to allow for robust analysis of postnatal phthalate exposure.
3.2 Phosphorus Flame Retardants and Plasticizers (PFRs)
Phosphorus flame retardants and plasticizers (PFRs) are mostly applied in polyurethane foam (PUF), thermoplastics, resins, polyvinyl chloride, synthetic rubbers, and textiles [75]. Tris(2-butoxyethyl) phosphate (TBOEP), which is used in waxes, floor polishes, and plasticizers in floor coverings, takes up the highest fraction of PFR concentrations in indoor dust [46]. PFR levels in house dust have been reported in several studies; however, human biomonitoring data is still rather limited. Studies investigating associations between PFRs and allergies are also very limited for both epidemiological and experimental studies (Table 5). Tris(1,3-dichloroisopropyl) phosphate (TDCIPP) found in dust was linked to noticeable rises in ORs of atopic dermatitis/eczema among elementary school children. Furthermore, in the same population, urinary TDCIPP, tris(2-chloroisopropyl) phosphate (TCIPP), and TBOEP metabolites were linked to eczema and/or rhinoconjunctivitis among elementary school children [4]. Metabolites of triphenyl phosphate (TPHP) and 2-ethylhexyl phenyl phosphate (EHPHP) and bis(2-butoxyethyl) phosphate (BBOEP) were linked to increased levels of oxidative stress biomarkers [2]. The allergy-inducing mechanisms of PFRs remain unclear. TBOEP, TCIPP, and TDCIPP cause mild to moderate skin irritation in rabbits [81, 82], while TCIPP and TDCIPP cause skin and eye irritation in rats [75, p. 1213]. A transcriptome study illustrated hepatic pro-inflammatory influence of TBOEP on the immune responses as well as on the lipid and steroid hormone metabolism [52]. Under in vitro conditions, TDCIPP and TPHP were found to be immunocytotoxic and induced oxidative stress [13]. Moreover, TDCIPP had antagonistic effects on androgen receptors, glucocorticoid receptors, and pregnane X receptors (PXRs). TBOEP and TCIPP also exhibited antagonistic activity against PXR [50]. These effects suggest that certain PFRs are likely to be endocrine disruptors. Nevertheless, the mechanisms underlying the immunological effects of PFRs in humans remain unknown. Further experimental and epidemiological researches are urgently required to elucidate these mechanisms.
3.3 Bisphenols (BPs)
Bisphenol A (BPA), a man-made chemical that is widely used for polycarbonate plastics and epoxy resins, can be found in certain food and beverage packaging, dental sealants, and receipts. With rising concerns regarding endocrine-disrupting effects on humans, the use of BPA in products has been phasing down, and other analogues, i.e., bisphenol F (BPF) and bisphenol S (BPS), are being used instead. Due to its widespread use, BPA is detected in more than 90% of human urine samples in Canadians [12], US children [59], and Asian populations (i.e., Chinese, Vietnamese, Malaysians, Indian, Japanese, and Korean people) [87]. Recent reviews have suggested that early-life exposure to BPA is linked to altered neurological developments leading to behavioral problems, low fetal growth, low birth weight, and thyroid dysfunction [9, 10, 66]. In regard to asthma, allergies, and immunological effects on humans, several epidemiological studies have been conducted. In a birth cohort of the Health Outcomes and Measures of the Environment (HOME) study, urine samples were collected from pregnant women twice during pregnancy (at 16 weeks and 26 weeks of gestation), and BPA levels were measured in each sample [69, 70]. Parent-reported child wheeze was assessed every 6 months until they were 5 years-old. Lung function was also assessed when the children were 4 and 5 years old. Maternal mean urinary BPA concentrations were not linked to wheeze under 3 years old; however, they were marginally linked to increased risk of wheezing at 5 years old. Moreover, maternal mean urinary BPA concentrations were linked to decreased lung function at 4 years old, but no relation was found at 5 years old. Urinary BPA concentrations in samples collected at 16 weeks, but not 26 weeks, were linked to increased risk of wheeze. Child urinary BPA concentrations were not linked to lung function or wheeze. Gascon et al. reported that prenatal exposure to BPA was linked to increased risk of wheeze as well as bronchitis under 7 years old [29]. Another birth cohort study in the USA reported that prenatal exposure to BPA was inversely linked to a risk of wheeze among 5-year-old children [26]. In the same study, postnatal exposure to BPA was also investigated. The data show that urinary BPA concentrations in 3-year-old children were linked with wheeze at 5 and 6 years of age. These epidemiological studies investigated both prenatal and postnatal exposure to BPA; however, the findings are inconsistent in each study. This may be due to BPA has a short half-life, resulting in variability in urinary BPA concentrations within and between days [10, 11, 74]. An additional confounding factor is that the timings of urine collections from pregnant women in these studies are different. Spanier et al. and Gascon et al. collected spot urine samples during the first and third trimester of pregnancy, and they used average concentrations of BPA as exposure assessments, while Donohue et al. collected samples during the third trimester.
Several experimental studies haves been suggested some direct mechanistic links between prenatal exposure to BPA and the influence on immune system. For example, pre- and postnatal developmental exposure to 50 μg/kgBW/day enhanced lung inflammation in the mucosal sensitization model [5]. Yang et al. demonstrated that prenatal exposure to BPA influences asthma risk through upregulation of Th2 pathways and possibly reduces regulatory T-cell counts [85]. Another possible mechanism suggests that BPA may bind to estrogen receptors to disrupt estrogen function and the response of immune cells [3]. However, regarding the other bisphenol analogues (e.g., BPF and BPS), no clear mechanism of action has been defined because of the limited research in this area. Although exposure levels of BPF and BPS are lower than BPA, it has been suggested that BPS and BPF could have equivalent or even greater toxicity than BPA [66]. In addition, the production and usage of BPA are reducing; however, BPF and BPS are increasing. Further epidemiological and experimental studies including other bisphenol analogues are needed to investigate both in detail.
4 Conclusions
In this section, the current epidemiological knowledge regarding the possible influence of environmental chemicals on childhood allergies were summarized. In spite of reported inconsistencies of the immunosuppressive effects of longer carbon-chain PFAS, PFOS, and PFOA, they showed consistent immunosuppressive effects on humans, which decreased risk of allergies and increased the risk of infectious diseases. Prenatal exposure to PCBs/PCDDs/PCDFs may modify the immune responses of children born to exposed mothers. Moreover, these children are at an increased risk of allergy and infection in their childhood and adolescence. Currently, there is insufficient evidence for the long-term effects of PCBs/PCDDs/PCDFs. Some BFRs have been banned due to widespread contaminations and concerns regarding the adverse health effects (e.g., neurodevelopment and reproductive) of BFRs in people. Despite a number of experimental and epidemiological studies, conclusive evidence on immune functions and allergies is very much lacking. Precise exposure assessment of short half-life compounds such as phthalate, PFRs, and bisphenols using human urine samples is difficult which may have led to inconsistent results within each epidemiological study. Nevertheless, even with the limited amount of available data, epidemiological and experimental studies have displayed that PFR exposure from house dust increases the risk of asthma and allergies in childhood. The influence of BPA on the developmental immune system is consistent within experimental studies; however, other bisphenols such as BPF and BPS have not yet been evaluated. Further investigation of the effects of these environmental chemicals, as well as alternative chemicals, on asthma and allergies are essential.
References
Ait Bamai Y, Araki A, Kawai T, Tsuboi T, Saito I, Yoshioka E, Cong S, Kishi R (2016) Exposure to phthalates in house dust and associated allergies in children aged 6–12years. Environ Int 96:16–23
Ait Bamai Y, Bastiaensen M, Araki A, Goudarzi H, Konno S, Ito S, Miyashita C, Yao Y, Covaci A, Kishi R (2019) Multiple exposures to organophosphate flame retardants alter urinary oxidative stress biomarkers among children: the Hokkaido Study. Environ Int 131:105003
Alonso-Magdalena P, Laribi O, Ropero AB, Fuentes E, Ripoll C, Soria B, Nadal A (2005) Low doses of bisphenol A and diethylstilbestrol impair Ca2+ signals in pancreatic alpha-cells through a nonclassical membrane estrogen receptor within intact islets of Langerhans. Environ Health Perspect 113:969–977
Araki A, Bastiaensen M, Ait Bamai Y, Van den Eede N, Kawai T, Tsuboi T, Ketema RM, Covaci A, Kishi R (2018) Associations between allergic symptoms and phosphate flame retardants in dust and their urinary metabolites among school children. Environ Int 119:438–446
Bauer SM, Roy A, Emo J, Chapman TJ, Georas SN, Lawrence BP (2012) The effects of maternal exposure to bisphenol A on allergic lung inflammation into adulthood. Toxicol Sci 130:82–93
Beko G, Callesen M, Weschler CJ, Toftum J, Langer S, Sigsgaard T, Host A, Kold Jensen T, Clausen G (2015) Phthalate exposure through different pathways and allergic sensitization in preschool children with asthma, allergic rhinoconjunctivitis and atopic dermatitis. Environ Res 137:432–439
Berger K, Eskenazi B, Balmes J, Kogut K, Holland N, Calafat AM, Harley KG (2019) Prenatal high molecular weight phthalates and bisphenol A, and childhood respiratory and allergic outcomes. Pediatr Allergy Immunol 30:36–46
Bornehag CG, Sundell J, Weschler CJ, Sigsgaard T, Lundgren B, Hasselgren M, Hagerhed-Engman L (2004) The association between asthma and allergic symptoms in children and phthalates in house dust: a nested case-control study. Environ Health Perspect 112:1393–1397
Braun JM (2017) Early-life exposure to EDCs: role in childhood obesity and neurodevelopment. Nat Rev Endocrinol 13:161–173
Braun JM, Hauser R (2011) Bisphenol A and children’s health. Curr Opin Pediatr 23:233–239
Braun JM, Kalkbrenner AE, Calafat AM, Bernert JT, Ye X, Silva MJ, Barr DB, Sathyanarayana S, Lanphear BP (2011) Variability and predictors of urinary bisphenol A concentrations during pregnancy. Environ Health Perspect 119:131–137
Bushnik T, Haines D, Levallois P, Levesque J, Van Oostdam J, Viau C (2010) Lead and bisphenol A concentrations in the Canadian population. Health Rep 21:7–18
Canbaz D, Logiantara A, van Ree R, van Rijt LS (2017) Immunotoxicity of organophosphate flame retardants TPHP and TDCIPP on murine dendritic cells in vitro. Chemosphere 177:56–64
Canbaz D, van Velzen MJ, Hallner E, Zwinderman AH, Wickman M, Leonards PE, van Ree R, van Rijt LS (2016) Exposure to organophosphate and polybrominated diphenyl ether flame retardants via indoor dust and childhood asthma. Indoor Air 26:403–413
Candi E, Schmidt R, Melino G (2005) The cornified envelope: a model of cell death in the skin. Nat Rev Mol Cell Biol 6:328–340
Chao WY, Hsu CC, Guo YL (1997) Middle-ear disease in children exposed prenatally to polychlorinated biphenyls and polychlorinated dibenzofurans. Arch Environ Health 52:257–262
Chen L, Wang C, Cui C, Ding G, Zhou Y, Jin J, Gao Y, Tian Y (2015) Prenatal exposure to polybrominated diphenyl ethers and birth outcomes. Environ Pollut (Barking, Essex: 1987) 206:32–37
Chevrier J, Harley KG, Bradman A, Sjödin A, Eskenazi B (2011) Prenatal exposure to polybrominated diphenyl ether flame retardants and neonatal thyroid-stimulating hormone levels in the CHAMACOS study. Am J Epidemiol 174:1166–1174
Czerska M, Zieliński M, Kamińska J, Ligocka D (2013) Effects of polybrominated diphenyl ethers on thyroid hormone, neurodevelopment and fertility in rodents and humans. Int J Occup Med Environ Health 26:498–510
Dallaire F, Dewailly E, Muckle G, Vezina C, Jacobson SW, Jacobson JL, Ayotte P (2004) Acute infections and environmental exposure to organochlorines in Inuit infants from Nunavik. Environ Health Perspect 112:1359–1365
Dallaire F, Dewailly E, Vezina C, Muckle G, Weber JP, Bruneau S, Ayotte P (2006) Effect of prenatal exposure to polychlorinated biphenyls on incidence of acute respiratory infections in preschool Inuit children. Environ Health Perspect 114:1301–1305
Dalsager L, Christensen N, Husby S, Kyhl H, Nielsen F, Host A, Grandjean P, Jensen TK (2016) Association between prenatal exposure to perfluorinated compounds and symptoms of infections at age 1–4years among 359 children in the Odense Child Cohort. Environ Int 96:58–64
Deutschle T, Reiter R, Butte W, Heinzow B, Keck T, Riechelmann H (2008) A controlled challenge study on Di(2-ethylhexyl) phthalate (DEHP) in house dust and the immune response in human nasal mucosa of allergic subjects. Environ Health Perspect 116:1487–1493
Dong G-H, Zhang Y-H, Zheng L, Liu W, Jin Y-H, He Q-C (2009) Chronic effects of perfluorooctanesulfonate exposure on immunotoxicity in adult male C57BL/6 mice. Arch Toxicol 83:805–815
Dong GH, Tung KY, Tsai CH, Liu MM, Wang D, Liu W, Jin YH, Hsieh WS, Lee YL, Chen PC (2013) Serum polyfluoroalkyl concentrations, asthma outcomes, and immunological markers in a case-control study of Taiwanese children. Environ Health Perspect 121:507–513
Donohue KM, Miller RL, Perzanowski MS, Just AC, Hoepner LA, Arunajadai S, Canfield S, Resnick D, Calafat AM, Perera FP, Whyatt RM (2013) Prenatal and postnatal bisphenol A exposure and asthma development among inner-city children. J Allergy Clin Immunol 131:736–742
Eskenazi B, Rauch SA, Tenerelli R, Huen K, Holland NT, Lustig RH, Kogut K, Bradman A, Sjödin A, Harley KG (2017) In utero and childhood DDT, DDE, PBDE and PCBs exposure and sex hormones in adolescent boys: the CHAMACOS study. Int J Hyg Environ Health 220:364–372
Fromme H, Tittlemier SA, Volkel W, Wilhelm M, Twardella D (2009) Perfluorinated compounds – exposure assessment for the general population in Western countries. Int J Hyg Environ Health 212:239–270
Gascon M, Casas M, Morales E, Valvi D, Ballesteros-Gomez A, Luque N, Rubio S, Monfort N, Ventura R, Martinez D, Sunyer J, Vrijheid M (2015) Prenatal exposure to bisphenol A and phthalates and childhood respiratory tract infections and allergy. J Allergy Clin Immunol 135:370–378
Gascon M, Morales E, Sunyer J, Vrijheid M (2013) Effects of persistent organic pollutants on the developing respiratory and immune systems: a systematic review. Environ Int 52:51–65
Glynn A, Berger U, Bignert A, Ullah S, Aune M, Lignell S, Darnerud PO (2012) Perfluorinated alkyl acids in blood serum from primiparous women in Sweden: serial sampling during pregnancy and nursing, and temporal trends 1996-2010. Environ Sci Technol 46:9071–9079
Glynn A, Thuvander A, Aune M, Johannisson A, Darnerud PO, Ronquist G, Cnattingius S (2008) Immune cell counts and risks of respiratory infections among infants exposed pre- and postnatally to organochlorine compounds: a prospective study. Environ Health Glob 7
Goudarzi H, Miyashita C, Okada E, Kashino I, Chen CJ, Ito S, Araki A, Kobayashi S, Matsuura H, Kishi R (2017) Prenatal exposure to perfluoroalkyl acids and prevalence of infectious diseases up to 4 years of age. Environ Int 104:132
Goudarzi H, Miyashita C, Okada E, Kashino I, Kobayashi S, Chen CJ, Ito S, Araki A, Matsuura H, Ito YM, Kishi R (2016) Effects of prenatal exposure to perfluoroalkyl acids on prevalence of allergic diseases among 4-year-old children. Environ Int 94:124–132
Grandjean P, Andersen EW, Budtz-Jorgensen E, Nielsen F, Molbak K, Weihe P, Heilmann C (2012) Serum vaccine antibody concentrations in children exposed to perfluorinated compounds. JAMA 307:391–397
Granum B, Haug LS, Namork E, Stolevik SB, Thomsen C, Aaberge IS, van Loveren H, Lovik M, Nygaard UC (2013) Pre-natal exposure to perfluoroalkyl substances may be associated with altered vaccine antibody levels and immune-related health outcomes in early childhood. J Immunotoxicol 10:373–379
Guruge KS, Hikono H, Shimada N, Murakami K, Hasegawa J, Yeung LWY, Yamanaka N, Yamashita N (2009) Effect of perfluorooctane sulfonate (PFOS) on influenza A virus-induced mortality in female B6C3F1 mice. J Toxicol Sci 34:687–691
Hansen S, Strom M, Olsen SF, Dahl R, Hoffmann HJ, Granstrom C, Rytter D, Bech BH, Linneberg A, Maslova E, Kiviranta H, Rantakokko P, Halldorsson TI (2016) Prenatal exposure to persistent organic pollutants and offspring allergic sensitization and lung function at 20 years of age. Clin Exp Allergy: J Br Soc Allergy Clin Immunol 46:329–336
Hansen S, Strom M, Olsen SF, Maslova E, Rantakokko P, Kiviranta H, Rytter D, Bech BH, Hansen LV, Halldorsson TI (2014) Maternal concentrations of persistent organochlorine pollutants and the risk of asthma in offspring: results from a prospective cohort with 20 years of follow-up. Environ Health Perspect 122:93–99
Haug LS, Huber S, Schlabach M, Becher G, Thomsen C (2011) Investigation on per- and polyfluorinated compounds in paired samples of house dust and indoor air from Norwegian homes. Environ Sci Technol 45:7991–7998
Heilmann C, Grandjean P, Weihe P, Nielsen F, Budtz-Jorgensen E (2006) Reduced antibody responses to vaccinations in children exposed to polychlorinated biphenyls. PLoS Med 3:e311
Hsu NY, Lee CC, Wang JY, Li YC, Chang HW, Chen CY, Bornehag CG, Wu PC, Sundell J, Su HJ (2012) Predicted risk of childhood allergy, asthma, and reported symptoms using measured phthalate exposure in dust and urine. Indoor Air 22:186–199
Humblet O, Diaz-Ramirez LG, Balmes JR, Pinney SM, Hiatt RA (2014) Perfluoroalkyl chemicals and asthma among children 12-19 years of age: NHANES (1999–2008). Environ Health Perspect 122:1129–1133
Impinen A, Longnecker MP, Nygaard UC, London SJ, Ferguson KK, Haug LS, Granum B (2019) Maternal levels of perfluoroalkyl substances (PFASs) during pregnancy and childhood allergy and asthma related outcomes and infections in the Norwegian Mother and Child (MoBa) cohort. Environ Int 124:462–472
Johnson-Restrepo B, Kannan K (2009) An assessment of sources and pathways of human exposure to polybrominated diphenyl ethers in the United States. Chemosphere 76:542–548
Kanazawa A, Saito I, Araki A, Takeda M, Ma M, Saijo Y, Kishi R (2010) Association between indoor exposure to semi-volatile organic compounds and building-related symptoms among the occupants of residential dwellings. Indoor Air 20:72–84
Kaneko H, Matsui E, Shinoda S, Kawamoto N, Nakamura Y, Uehara R, Matsuura N, Morita M, Tada H, Kondo N (2006) Effects of dioxins on the quantitative levels of immune components in infants. Toxicol Ind Health 22:131–136
Koike E, Yanagisawa R, Sadakane K, Inoue K, Ichinose T, Takano H (2010) Effects of diisononyl phthalate on atopic dermatitis in vivo and immunologic responses in vitro. Environ Health Perspect 118:472–478
Koike E, Yanagisawa R, Takigami H, Takano H (2013) Brominated flame retardants stimulate mouse immune cells in vitro. J Appl Toxicol 33:1451–1459
Kojima H, Takeuchi S, Itoh T, Iida M, Kobayashi S, Yoshida T (2013) In vitro endocrine disruption potential of organophosphate flame retardants via human nuclear receptors. Toxicology 314:76–83
Kolarik B, Naydenov K, Larsson M, Bornehag CG, Sundell J (2008) The association between phthalates in dust and allergic diseases among Bulgarian children. Environ Health Perspect 116:98–103
Krivoshiev BV, Beemster GTS, Sprangers K, Cuypers B, Laukens K, Blust R, Husson SJ (2018) Toxicogenomics of the flame retardant tris (2-butoxyethyl) phosphate in HepG2 cells using RNA-seq. Toxicol in Vitro 46:178–188
Larsen ST, Lund RM, Nielsen GD, Thygesen P, Poulsen OM (2002) Adjuvant effect of di-n-butyl-, di-n-octyl-, di-iso-nonyl- and di-iso-decyl phthalate in a subcutaneous injection model using BALB/c mice. Pharmacol Toxicol 91:264–272
Linares V, Bellés M, Domingo JL (2015) Human exposure to PBDE and critical evaluation of health hazards. Arch Toxicol 89:335–356
Lyche JL, Rosseland C, Berge G, Polder A (2015) Human health risk associated with brominated flame-retardants (BFRs). Environ Int 74:170–180
Meng G, Nie Z, Feng Y, Wu X, Yin Y, Wang Y (2016) Typical halogenated persistent organic pollutants in indoor dust and the associations with childhood asthma in Shanghai, China. Environ Pollut 211:389–398
Miyashita C, Bamai YA, Araki A, Itoh S, Minatoya M, Kobayashi A, Kajiwara J, Hori T, Kishi R (2018) Prenatal exposure to dioxin-like compounds is associated with decreased cord blood IgE and increased risk of wheezing in children aged up to 7 years: The Hokkaido study. Sci Total Environ 610:191–199
Miyashita C, Sasaki S, Saijo Y, Washino N, Okada E, Kobayashi S, Konishi K, Kajiwara J, Todaka T, Kishi R (2011) Effects of prenatal exposure to dioxin-like compounds on allergies and infections during infancy. Environ Res 111:551–558
Morgan MK, Jones PA, Calafat AM, Ye X, Croghan CW, Chuang JC, Wilson NK, Clifton MS, Figueroa Z, Sheldon LS (2011) Assessing the quantitative relationships between preschool children’s exposures to bisphenol A by route and urinary biomonitoring. Environ Sci Technol 45:5309–5316
Nagayama J, Tsuji H, Iida T, Nakagawa R, Matsueda T, Hirakawa H, Yanagawa T, Fukushige J, Watanabe T (2007) Immunologic effects of perinatal exposure to dioxins, PCBs and organochlorine pesticides in Japanese infants. Chemosphere 67:S393–S398
Okada E, Kashino I, Matsuura H, Sasaki S, Miyashita C, Yamamoto J, Ikeno T, Ito YM, Matsumura T, Tamakoshi A, Kishi R (2013) Temporal trends of perfluoroalkyl acids in plasma samples of pregnant women in Hokkaido, Japan, 2003–2011. Environ Int 60:89–96
Okada E, Sasaki S, Kashino I, Matsuura H, Miyashita C, Kobayashi S, Itoh K, Ikeno T, Tamakoshi A, Kishi R (2014) Prenatal exposure to perfluoroalkyl acids and allergic diseases in early childhood. Environ Int 65:127–134
Okada E, Sasaki S, Saijo Y, Washino N, Miyashita C, Kobayashi S, Konishi K, Ito YM, Ito R, Nakata A, Iwasaki Y, Saito K, Nakazawa H, Kishi R (2012) Prenatal exposure to perfluorinated chemicals and relationship with allergies and infectious diseases in infants. Environ Res 112:118–125
Park HY, Hertz-Picciotto I, Petrik J, Palkovicova L, Kocan A, Trnovec T (2008) Prenatal PCB exposure and thymus size at birth in neonates in Eastern Slovakia. Environ Health Perspect 116:104–109
Potaczek DP, Harb H, Michel S, Alhamwe BA, Renz H, Tost J (2017) Epigenetics and allergy: from basic mechanisms to clinical applications. Epigenomics 9:539–571
Rochester JR (2013) Bisphenol A and human health: A review of the literature. Reprod Toxicol 42:132–155
Sandilands A, Terron-Kwiatkowski A, Hull PR, O’Regan GM, Clayton TH, Watson RM, Carrick T, Evans AT, Liao H, Zhao Y, Campbell LE, Schmuth M, Gruber R, Janecke AR, Elias PM, van Steensel MA, Nagtzaam I, van Geel M, Steijlen PM, Munro CS, Bradley DG, Palmer CN, Smith FJ, McLean WH, Irvine AD (2007) Comprehensive analysis of the gene encoding filaggrin uncovers prevalent and rare mutations in ichthyosis vulgaris and atopic eczema. Nat Genet 39:650–654
Smit LA, Lenters V, Hoyer BB, Lindh CH, Pedersen HS, Liermontova I, Jonsson BA, Piersma AH, Bonde JP, Toft G, Vermeulen R, Heederik D (2015) Prenatal exposure to environmental chemical contaminants and asthma and eczema in school-age children. Allergy 70:653–660
Spanier AJ, Kahn RS, Kunselman AR, Hornung R, Xu Y, Calafat AM, Lanphear BP (2012) Prenatal exposure to bisphenol A and child wheeze from birth to 3 years of age. Environ Health Perspect 120:916–920
Spanier AJ, Kahn RS, Kunselman AR, Schaefer EW, Hornung R, Xu Y, Calafat AM, Lanphear BP (2014) Bisphenol a exposure and the development of wheeze and lung function in children through age 5 years. JAMA Pediatr 168:1131–1137
Stein CR, McGovern KJ, Pajak AM, Maglione PJ, Wolff MS (2016) Perfluoroalkyl and polyfluoroalkyl substances and indicators of immune function in children aged 12–19 y: National Health and Nutrition Examination Survey. Pediatr Res 79:348–357
Sunyer J, Torrent M, Munoz-Ortiz L, Ribas-Fito N, Carrizo D, Grimalt J, Anto JM, Cullinan P (2005) Prenatal dichlorodiphenyldichloroethylene (DDE) and asthma in children. Environ Health Perspect 113:1787–1790
ten Tusscher GW, Steerenberg PA, van Loveren H, Vos JG, von dem Borne AE, Westra M, van der Slikke JW, Olie K, Pluim HJ, Koppe JG (2003) Persistent hematologic and immunologic disturbances in 8-year-old Dutch children associated with perinatal dioxin exposure. Environ Health Perspect 111:1519–1523
Townsend MK, Franke AA, Li X, Hu FB, Eliassen AH (2013) Within-person reproducibility of urinary bisphenol A and phthalate metabolites over a 1 to 3 year period among women in the Nurses’ Health Studies: a prospective cohort study. Environ Health 12:80
van der Veen I, de Boer J (2012) Phosphorus flame retardants: properties, production, environmental occurrence, toxicity and analysis. Chemosphere 88:1119–1153
Vernet C, Pin I, Giorgis-Allemand L, Philippat C, Benmerad M, Quentin J, Calafat AM, Ye X, Annesi-Maesano I, Siroux V, Slama R, Group, E.M.C.C.S (2017) In utero exposure to select phenols and phthalates and respiratory health in five-year-old boys: a prospective study. Environ Health Perspect 125:097006
Vuong AM, Braun JM, Webster GM, Thomas Zoeller R, Hoofnagle AN, Sjödin A, Yolton K, Lanphear BP, Chen A (2018a) Polybrominated diphenyl ether (PBDE) exposures and thyroid hormones in children at age 3 years. Environ Int 117:339–347
Wang M, Park JS, Petreas M (2011) Temporal changes in the levels of perfluorinated compounds in California women’s serum over the past 50 years. Environ Sci Technol 45:7510–7516
Weisglas-Kuperus N, Patandin S, Berbers GA, Sas TC, Mulder PG, Sauer PJ, Hooijkaas H (2000) Immunologic effects of background exposure to polychlorinated biphenyls and dioxins in Dutch preschool children. Environ Health Perspect 108:1203–1207
Weisglas-Kuperus N, Vreugdenhil HJ, Mulder PG (2004) Immunological effects of environmental exposure to polychlorinated biphenyls and dioxins in Dutch school children. Toxicol Lett 149:281–285
WHO (1998) Frame Retardants: Tris(chloropropyl)phosphate and Tris(2-chloroethyl) Phosphate. World Health Organization, Geneva
WHO (2000) Flame Retardants: Tris (2-butoxyethyl) phosphate, Tris (2-ethylhexyl)phosphate and Tetrakis (hydroxymethyl)phosphonium salt. World Health Organization, Geneva
WHO (2012) Endocrine disruptoers and child health: possible developmental early effects of endocrine disrupters on child health. World Health Organization, Geneva
WHO/UNEP. State of the science of endocrine disrupting chemicals – 2012. 2013
Yan H, Takamoto M, Sugane K (2008) Exposure to Bisphenol A prenatally or in adulthood promotes T(H)2 cytokine production associated with reduction of CD4CD25 regulatory T cells. Environ Health Perspect 116:514–519
Zeng XW, Bloom MS, Dharmage SC, Lodge CJ, Chen D, Li S, Guo Y, Roponen M, Jalava P, Hirvonen MR, Ma H, Hao YT, Chen W, Yang M, Chu C, Li QQ, Hu LW, Liu KK, Yang BY, Liu S, Fu C, Dong GH (2019) Prenatal exposure to perfluoroalkyl substances is associated with lower hand, foot and mouth disease viruses antibody response in infancy: findings from the Guangzhou Birth Cohort Study. Sci Total Environ 663:60–67
Zhang Z, Alomirah H, Cho HS, Li YF, Liao C, Minh TB, Mohd MA, Nakata H, Ren N, Kannan K (2011) Urinary bisphenol A concentrations and their implications for human exposure in several Asian countries. Environ Sci Technol 45:7044–7050
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Ait Bamai, Y., Miyashita, C., Araki, A., Kishi, R. (2020). Early-Life Environmental Influences on Allergic Diseases. In: Xia, Y. (eds) Early-life Environmental Exposure and Disease. Springer, Singapore. https://doi.org/10.1007/978-981-15-3797-4_9
Download citation
DOI: https://doi.org/10.1007/978-981-15-3797-4_9
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-15-3796-7
Online ISBN: 978-981-15-3797-4
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)