Abstract
This chapter describes the evidence of early-life environmental influences on growth and development. The most environmental influences discussed include the exposure to pesticides, endocrine-disrupting chemicals (EDCs), metals, and persistent organic pollutants (POPs). The exposure outcome focused includes obesity, asthma, allergies, neurodevelopmental disorders, during gestation, infancy and childhood, and/or reproductive development later in life. EDCs have been reported to act as an agonist or antagonists of the estrogen receptor (ER), androgen receptor (AR), and aryl hydrocarbon receptor (AhR). Other mechanisms such as oxidative stress and DNA alterations and inflammation also play important roles in effect of the early exposure to environmental contaminants on growth and development. In this chapter, the combined effect of all the compounds present in the human body was also emphasized to be considered for risk assessment.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Pesticides
- Endocrine-disrupting chemicals (EDCs)
- Metals
- Persistent organic pollutants (POPs)
- Health effects and early life
1 Introduction
Environmental chemical exposures are ubiquitous; however, they are largely invisible. Human beings are worldwide exposed to thousands of chemicals daily [1, 2], including many known toxicants, as well as numerous potentially hazardous chemicals with more or less well-characterized risks. These chemicals are commonly detected in blood and urine [3,4,5,6]. The categorization of these chemicals is based on their uses in commerce (e.g., pesticides), routes of exposure (e.g., inhalation, drinking water, and food), toxicological effects (e.g., neurotoxicity and immune toxicity), or their persistence being hardly metabolized in biological tissues or the environment (e.g., long half-lives).
Exposure to some chemicals may increase the risk of health effects such as obesity, asthma, allergies, neurodevelopmental disorders, and/or reproductive development [7, 8]. Pesticides (e.g., pyrethroids), naturally occurring metals (e.g., lead, mercury), endocrine-disrupting chemicals (EDCs) (e.g., bisphenol A (BPA), and persistent organic pollutants (POPs) are listed chemicals to have health effects to humans.
Endocrine-disrupting chemicals (EDCs) are a class of chemicals which have the potential to alter the homeostasis or action of endogenous hormones or other signaling compounds of the endocrine system and consequently interfere with fetal growth and development, therefore increasing the risk of disease across the lifespan [9]. By altering the production, release, transport, metabolism, binding, action, or elimination of endogenous hormones important for programming and/or maintaining normal growth and development, EDCs might increase the risk of diseases. There is a concern that the fetus, infant, or child may have higher exposure to some EDCs or be more vulnerable compared to adults [7].
Epidemiological studies are important to quantify how the risk of diseases in childhood and adult life is influenced by early-life environmental chemical exposures. There are challenges in identifying the signals of associations between chemical exposure and childhood health including accurately estimating chemical exposure, confounding from causes of both exposure and disease, identifying periods of heightened vulnerability to chemical exposures, and determining the effects of chemical mixtures.
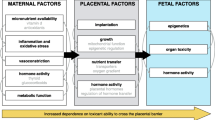
Exposure to chemicals during fetal, infant, and child development might be higher than adult due to time-dependent and synchronized nature of rapidly developing organ systems, which makes them to be more sensitive to environmental inputs that disrupt growth and development. Moreover, because of the different in pharmacokinetics compared to adults, a higher chemical body burdens for a given dose of exposure to the fetus, infant, and child may alter the absorption and distribution of chemicals and decrease their capacity to metabolize and excrete chemicals.
To understand the mechanisms behind the exposure and health effects, experimental in vitro and ex vivo studies might help to elucidate which cellular and molecular mechanisms are involved.
Chemical exposures do not occur to single compound alone, and individuals are exposed to multiple chemicals daily during life. A “one chemical at a time” approach that treats exposures as if they occur in isolation from each other has been used by most epidemiological studies to examine these exposures. However, this does not reflect the nature of exposure to a multitude of chemicals that can have cumulative or interactive effects on human health. Thus, identifying signals from chemical mixtures may improve our understanding of risk factors for fetal and childhood diseases.
2 Epidemiological Data on Environmental Exposure and Fetal Growth and Development
2.1 Pyrethroid Pesticides
Pyrethroids are commonly used to control insects around the home and in agricultural production; it is likely for people to come into contact with one or more pyrethroid insecticides. There were dominant metabolites of pyrethroids detected in urine samples collected from the general population. It was suggested that children and adults are widely exposed to one or more pyrethroids. Exposure to pyrethroids in non-occupational (e.g., general population) mainly occurs through ingestion of food, ingestion after household use, or skin contact with contaminated house dust or particles attached to the surface. Although clinical features of acute accidental exposure to pyrethroids are well documented (e.g., respiratory, eye, skin irritation, and paranesthesia), there is limited information on the chronic effects at low concentrations. Recent epidemiological studies address their potential adverse effects on pregnancy outcomes, reproductive hormones, sperm DNA, sperm quality, and early neurobehavioral development [10]. Most epidemiology studies about pyrethroids relied upon a single urinary sample of pyrethroid metabolites, using a poor exposure metric, which could result in misclassification of past exposures. Additionally, cross-sectional studies prevented an evaluation of the exposure–disease association. Moreover, toxicological effects found in the epidemiological studies were inconsistent with the observed effects in parathyroid animal experiments. To provide more reliable evidence on the underlying health effects of low-dose pyrethroid exposure, future epidemiological studies are needed to characterize adverse outcomes, quantify exposure over time, and verify components’ exposure [11].
2.2 Bisphenol A
Bisphenol A (BPA) has estrogenic properties that can enter the food and water supply. Researches on exposure-associated health outcomes in humans have revealed the endocrine-disrupting properties of BPA to have the potential effect on children development. An individual may be predisposed to diseases at doses below the prescribed oral reference dose (RfD) by the environmental protection agencies.
A random effects meta-analysis on prenatal exposure to BPA in 3 human studies and 29 rodent studies showed increases in hyperactivity in male rodent, and there is an association between early BPA exposure and hyperactivity in both boys and girls [12]. Several epidemiological studies observed the associations between BPA exposure and altered thyroid function of pregnant women, neonates, or adolescent [7]. Studies found that BPA exposure during prenatal and postnatal periods was related to behavior problems in children, but there were inconsistencies with the greatest vulnerable period to exposure (prenatal vs. infancy vs. childhood) and sex-specific effects. The reason for this heterogeneity could be the variation of urinary BPA concentrations that might result in BPA exposure misclassification [7]. The obesogenic effects of BPA exposure during early life still remain unclear. Both decreases and increases in adiposity with higher early-life BPA exposure were observed [7, 12].
Searhrist et al. have reviewed in vivo literature for the carcinogenic potential of BPA and confirmed from the rodent studies that BPA exposure during early life below the RfD could lead to ascending susceptibility to prostate and mammary cancer [13]. Studies also proposed that BPA may act as a carcinogen in the breast or prostate because of its tumor-promoting properties [13].
2.3 Phthalates
Phthalates, which act as a kind of EDCs, are used in consumer products, including medications, personal care products, and plastics. Biomonitoring studies worldwide indicate that infants, children, and pregnant women are exposed to phthalate universally. Exposure occurs through food, inhalation, or dermal absorption. Moreover, phthalates could pass through the placenta and expose the fetus [7].
Phthalates may disturb the metabolism or action of thyroid hormones, androgens, and glucocorticoids. Potential mechanisms of phthalates are anti-androgenic and reduced testosterone production in testicular and decreases of the expression of genes involved in steroidogenesis and steroid trafficking [7]. Both human and animal studies showed that several phthalates may reduce triiodothyronine concentration and thyroxine in pregnant women and children, antagonize T3 binding to thyroid receptor-β, and reduce cellular T3 uptake, affecting transcription of the sodium-iodine transporter. Phthalates could inhibit 11-β-hydroxysteroid dehydrogenase-2 as well, which deactivates cortisol. Additionally, phthalate exposure may affect offspring health by causing oxidative stress [14] or via epigenetic reprogramming of the fetus and placenta.
There is concern about the health effects of phthalate mixtures since humans are exposed to multiple phthalates simultaneously and rodent studies demonstrated that phthalates have concentration additive effects on fetal androgen production. Thus, the aggregate of individual phthalate exposures may have an additive impact on human health since individual phthalates share a common mechanism of action.
Epidemiological studies suggested that there may be associations between prenatal exposure and child behavioral problems and cognitive decrements. However, the conclusions about the association between early-life phthalate exposure and obesity or adiposity risk of child are inconsistent. The reason for this inconsistency across the studies might be due to the different time windows of exposure, misclassification, for example, using urine samples to assess exposure, child age, and/or neurodevelopment assessment [7].
2.4 Persistent Organic Pollutants
Persistent organic pollutants (POPs) are carbon-based chemicals found ubiquitously in the environment, originating from industrial processes. POPs are transported along sea currents and atmospheric movement and are accumulated in the food chain, especially the arctic marine food chain [15,16,17]. Humans are exposed to POPs due to their persistence through the diet, including meat, fish, and dairy products [18,19,20,21,22]. Several studies have found that the body burden of lipophilic POPs in the Arctic populations, for example, Greenlandic Inuit, is much higher than that of the people living close to major emission points [3, 23,24,25].
The POPs include the amphiphilic perfluoroalkylated substances (PFASs), the lipophilic POPs (e.g., polychlorinated biphenyls (PCBs)), and the organochlorine pesticides (OCPs). Due to their resistance to degradation, they biomagnify and bioaccumulate via the marine food chain [26,27,28].
Previous studies have shown that POPs, including PFASs, are negatively associated with cognitive development of children [29,30,31], immune disruption [32,33,34,35], cancer, reduced reproductive ability, and metabolic alternations [29, 36, 37]. It has been confirmed by several studies that the endocrine system could be disrupted by POPs and body’s defense against oxidative stress could be decreased [3, 29].
Since the 1930s, many organochlorine compounds including PCBs started to be produced. They have been used in an array of products. However, it was prohibited to be used in electrical devices since the late 1970s in most industrialized countries [38,39,40]. As alternatives to PCBs, polybrominated diphenyl ethers (PBDEs) and polybrominated biphenyls (PBBs) were then introduced as flame retardants. The mixture of PBBs and two types of PBDE (i.e., pentaBDEs and octaBDEs) has been weeded out around the world, and a third type (i.e., decaBDEs) is being regulated [41, 42]. Several OCPs including dichlorodiphenyltrichloroethane (DDT) and hexachlorobenzene (HCB) are banned from use [39, 43]. In the Stockholm Convention, several PCBs, PBDEs, and OCPs are registered as persistent organic pollutants (POPs) [44].
The amphiphilic PFAAs have been used as surfactants since the 1940s [45]. The long-chain PFAAs (six or more carbon atoms) are extremely bioaccumulative and persistent with average serum half-life of 4–8 years [46]. Food including fish, meat, and cereals, being among the largest contributors, is the major source of exposure to PFAAs [47,48,49]. Drinking water, dust, and air could also be other sources [49]. Humans are also under the threat of being exposed to PFAAs and their precursors from consumer products such as food packaging [50, 51], nonstick cookware, dental floss, cosmetics, leather sofa, etc. [52].
2.5 Prenatal Exposure and Fetal Growth
Family exposure was expected to be similar because food intake and environmental exposure. A study on PFASs that was carried out in matched parental and cord serum in 369 families from a birth cohort in Shandong, one of the regions seriously polluted by PFASs in China, reported that PFAS levels were positively correlated among family members. Moreover, PFAAs, PFOA levels in particular, were extremely high and had a positive correlation between parents and in cord blood, suggesting that there was a common source of exposure for the family and heavy pollution in this region [53].
Another study on biosamples and data from a prospective birth cohort study in the Shandong province, China, investigated the efficiency of maternal–fetal transfer (369 pairs) of perfluoroalkyl and polyfluoroalkyl substances [54]. The ten PFASs analyzed were nearly detected in all samples including both umbilical cord and maternal serum. There was a close correlation between maternal and cord levels for most PFASs. The transplacental transfer efficiency (TTE) was significantly affected by chain length and functional group carbon. Perfluoroalkylsulfonates (PFSAs) had a lower ratio of maternal to fetal transfer when compared to perfluoroalkyl-carboxylates (PFCAs). A U-shaped relationship between carbon chain length and TTE was observed for PFCAs, while a monotonic descending trend was identified between TTE and the increasing carbon chain length for PFSAs. Thus, PFAAs could easily pass through the placenta. Both carbon chain length and functional group are crucial determinants of the TTE of PFASs [54].
Studies have investigated whether prenatal exposure to POPs is associated with fetal growth. Results of meta-analyses including 12 European birth cohorts showed that low-level exposure to PCB (or correlated exposures) had hazardous effects on fetal growth. Evidence from this study confirmed that low-level exposure to PCBs has a negative association with fetal growth [55]. Another European study on pooled analysis of seven birth cohorts showed that prenatal exposure to p,p’-DDE has an increased association with infant growth, and postnatal PCB-153 with decreased growth at European exposure levels [56].
In the Greenlandic birth cohort ACCEPT, POPs had a negative effect on fetal growth. For all lipophilic POPs, an overall trend of negative associations to fetal growth indices was observed. Whereas PFOA affects birth weight and head circumference negatively, a positive association with gestational age (GA) was observed [57].
Similar associations between PFOA and birth weight from previous studies have been observed [58,59,60], but it is inconsistent with other studies [61, 62]. The reason for this inconsistency could be due to numerous factors not included in the studies which may have impact on the fetal growth. The surprising observation of positive association between PFOA and GA contrasts most observed negative associations of POP exposure and GA [57]. The underlying hypothesis could be that the mother’s body is trying to prolong the gestational age to compensate for the lower birth weight caused by PFOA exposure.
For the first time, it was demonstrated that the combined PFAA-induced xenoestrogenic activity in serum of Danish pregnant women was associated with an inverse effect on fetal growth [63]. The actual PFAA mixture was extracted from the serum of 702 pregnant women in Denmark. The PFAA-induced estrogenic receptor transactivation was determined by use of the human stable transfected MVLN cell line [63]. There was an association between higher serum PFAA-induced xenoestrogenic activities with lower birth weight and birth length of the offspring, which suggested that the actual serum PFAA mixtures could have an effect on fetal growth through disruption of the ER function [63].
This study highlights the importance of mixture studies with respect to fetal development and health risk. In the same Danish Birth Cohort studying the “one chemical at a time” approach for 16 PFAAs, PFAAs were not found to be associated with birth weight or other indices of fetal growth consistently [64].
2.6 Prenatal Exposure and Fetal Growth (Metals)
Metals and other elements exist naturally in the Earth’s crust. However, there are numerous metal exposures in environment with growing applications of heavy metals in industry, agriculture, technology, and medicine, as well as from domestic settings and workplaces [65,66,67].
According to the World Health Organization (WHO), mercury (Hg), lead (Pb), arsenic (As), cadmium (Cd), and chromium (Cr) rank among chemicals which are of major public health concerns due to relatively high toxicity [68,69,70]. Exposure to heavy metals could result in increased risk of diseases, ranging from cancer and allergies to neurological diseases, decreased cognition, and endocrine disorders [24, 71,72,73,74]. The exposure may affect the fetal development because of pregnant women being particularly vulnerable [24, 38, 72,73,74,75]. Some contaminants are transferred from the mother to fetus through the placental barrier, as well to the newborn through breastfeeding [3, 4, 65, 67, 76,77,78].
The Greenlandic Birth Cohort ACCEPT study found negative associations between Pb, Cd, and Cu levels and birth outcomes. For females, Pb and Ni were important risk factors for low birth weight, but Cr correlated positively with birth weight. In addition, female head circumference and gestational age were negatively associated with Ni, while not for males [79].
Previous studies also report that lead exposure affects birth outcome with increased prevalence of low birth weight and small for gestation age among term infants [73, 80].
In the ACCEPT study, a higher risk of low birth weight upon exposure to Cd was observed to be similar to the previous findings [73]. Moreover, Bank-Nielsen et al. [79] also found the indication of increased levels of Ni might increase the risk of low birth weight similar to the previous studies, though rarely investigation was documented; the known toxic effects of nickel includes hemolysis, genetic alterations, and oxidative stress [81,82,83].
2.7 Environmental Exposure and Child Health and Developmental Effects
The timing of the exposure may determine the toxicity of environmental chemicals to some degree. Thalidomide, as one of the most infamous teratogens, was used to treat nausea in pregnant women in the 1950s and 1960s, leading to limb defects in thousands of children [84]. Notably, exposure between 21 and 36 days after conception was necessary to cause these birth defects, demonstrating the presence of limb defects depended on the timing of thalidomide use. Another example was diethylstilbestrol (DES), a pharmaceutical given to women from the 1940s–1970s to prevent spontaneous abortion. The offspring of women who were prescribed DES in the first half of their pregnancy had increased risk of developing vaginal or cervical clear cell adenocarcinoma [85], as well as reproductive problems and some cancers [86, 87]. The question is how could these compounds be available on the market, and the answer might be that the compounds’ risk was assessed in rodents but did not respond clearly with adverse outcome upon exposure to thalidomide [84], whereas later studies demonstrated for DES in both rodents and humans [85].
2.8 Immunological Effects (Autism, Vaccination, Asthma, and Allergy)
2.8.1 Autism
During pregnancy and after birth, infections played a potential role in the pathophysiology of autism [88]. Children developing autism were more likely to have decreased levels of both T helper-1(Th-1)-like cytokines (i.e., IFN-γ) and Th-2like cytokines (i.e., IL-4, IL-10), suggesting a depressed or hypoactive immune cell activity during neonatal period in autism [89]. The analysis of neonatal inflammatory chemokine levels in amniotic fluid of children diagnosed later in life with autism and controls cautiously suggested an altered cell-mediated immunity during the early neonatal period in autism [90].
2.8.2 Vaccination Response
Prenatal exposure to POPs can influence vaccination response and the immunological protection against infections. Heilmann et al. 2010 [34] reported prenatal exposure to PCBs affect the serum concentrations of antibodies against diphtheria and tetanus vaccines. Examination at 5 and/or 7 years of age showed that the immune system development in early life appears to be particularly vulnerable when exposed to PCBs, whereas Jusko et al. 2016 [91] found little evidence for specific antibody responses at 6 months of age related to maternal or early postnatal PCB exposure.
Another study examined whether PFAA exposure was associated with antibody response to childhood vaccinations similar to PCBs. Higher exposures to PFAAs were associated with reduced humoral immune response to diphtheria and tetanus vaccines in routine childhood immunizations in children aged 5 and 7 years [33]. These findings were consistent with other studies [92,93,94], though not all [95] experimental studies in rodents, in which adverse effects of PFOS on humoral immune function were observed at serum concentrations similar to those reported in the present study and at levels prevalent in the United States [96].
In a birth cohort study of maternal and infant serum PCB-153 and DDE concentrations on responses to infant tuberculosis vaccination, the results indicated that higher 6-month infant concentrations of PCB-153 and DDE were strongly associated with lower 6-month Bacille Calmette–Guérin (BCG) vaccine-specific antibody levels [91].
These studies link prenatal and child exposure to POP with deficits in immune system functions that might cause less protection against infectious diseases.
2.8.3 Asthma
Exposure to air pollutants during pregnancy and early life is associated with developmental and functional alterations in lung and other negative respiratory conditions in childhood (asthma, wheezing) that may last into adulthood. Plausible mechanisms include changes in maternal physiology such as hypoxia, oxidative stress and maternal systemic inflammation, and DNA methylation in the fetus [97].
POPs existing in maternal serum concentrations have been reported to be linked with increased risk of asthma in the offspring [98], and researches showed that breastfeeding can modify offspring risk of asthma [99].
2.8.4 Allergy
Greenlandic pregnant women have a high frequency of smoking—and it has been related to higher accumulation of serum POPs [[100]]. A preliminary study on the Greenlandic ACCEPT Birth Cohort children at 3–5 years of age observed that the risk of getting allergy among the offspring was higher to maternal smoking exposure and the child being breastfeed <12 months. Furthermore, we found that children with eczema and breastfed >12 months were predisposed to having asthma and allergy [101].
2.8.5 Neurological Effects
Follow-up studies in neurological effects showed conflicting results, indicating associations between neurodevelopmental outcome in children and prenatal exposure to POPs, while other studies observed no associations [102].
Prenatal exposure to PCBs was found to be linked with an increase in attention-deficit/hyperactivity disorder (ADHD)-like behaviors and lower intelligence levels in children and less optimal long-term memory in adolescents [103,104,105,106,107], whereas other findings showed no associations between prenatal exposure to PCBs and attention in adolescents, learning in 12- to 15-year-old adolescents [108,109,110], and memory in children at school age [111].
Also prenatal exposure to PBDEs was reported to be associated with reduced motor speed and lower intelligence levels [112, 113]. As for PCB exposures, prenatal exposure to DDE was shown to be associated with ADHD-like behaviors in 7- to 11-year-old children [114], while several other studies found no association with intelligence even at a higher level of exposure to DDE [108, 115].
Berghuis et al. 2018 [116] reported for a follow-up of two Dutch birth cohorts on prenatal exposure to POPs that several OH-PCBs were linked to more optimal sustained attention and balance, whereas hexabroomcyclododecane, with lower performance intelligence, and PCB-183 were linked to lower total intelligence. PCBs, PBDEs, and OH-PCBs were negatively linked to verbal memory.
Numerous studies have indicated that PFASs may interfere with thyroid hormone homeostasis in pregnant women. A recent systematic review reported that three PFASs were negatively correlated with free thyroxine and positively correlated with thyroid-stimulating hormone [117,118,119,120]. Thyroid hormones transferred from the mother to the embryo and fetus might be essential for normal brain development of the offspring [121]. Subclinical maternal hypothyroxinemia has been associated with adverse neurodevelopmental outcomes. Deficiency of severe thyroid hormone during gestation might cause cretinism and cognitive and/or mental disorders [122,123,124,125,126]. Research has also observed that even a slight reduction of circulating free thyroid hormone in mothers might cause a loss of 4–7 IQ points in children [127].
We conducted a review on “Exposure to perfluoroalkyl acids and fetal and maternal thyroid status.” Including 13 studies, the results indicated a mainly positive relationship between maternal PFAA exposure and TSH levels, and a suggestion of an inverse association with T4 and/or T3 levels. Associations of infant TH upon PFAA exposure were less consistent (Boesen et al. submitted).
A study on the Danish National Birth Cohort showed gestational-week-specific associations between high exposure to several PFAAs and TSH level in early gestations [128]. Further researches within the biology and the adverse clinical outcome regarding thyroid hormones disruptions in early pregnancy are needed.
Few data have been reported on PFAA exposure and child IQ. A study on the Danish National Birth Cohort found no strong associations between a natural-log unit increase in each of the seven PFASs and child IQ scores, while a few positive and negative associations were found in the sex-stratified PFAS quartile analyses, but the patterns were inconsistent [129].
3 Possible Molecular Mechanisms
In vitro and ex vivo cell systems have been introduced for the assessment of EDCs such as the integrated level of xenobiotic cellular effects in human beings. In this section, the in vitro and ex vivo studies of EDCs mainly using cell-based reporter gene bioassays are described. In addition, some in vivo animal studies about the development effect are also elaborated.
3.1 In Vitro (and In Vivo)
3.1.1 Pesticides
Previous in vitro studies showed that pesticides (methiocarb, endosulfan, dieldrin, and fenarimol) acted as both estrogen receptor (ER) agonists and androgen receptor (AR) antagonists. Prochloraz reacted as both an estrogen and an androgen antagonist. Furthermore, fenarimol and prochloraz were potent aromatase inhibitors, while endosulfan was a weak inhibitor. Hence, there are at least three different ways in which pesticides potentially disturb sex hormone actions. Chlorpyrifos, deltamethrin, tolclofos-methyl, and tribenuron-methyl induced weak estrogenic responses, while endosulfan and pirimicarb, propamocarb, and daminozide potentiated the estrogenic response of natural ER ligand 17-estradiol. Methomyl, pirimicarb, propamocarb, and iprodione weakly stimulated activity of aromatase, an enzyme converting testosterone to estrogen. Although the potencies of the pesticides were low compared to the natural ligands, the integrated response in the organism might be amplified by the ability of the pesticides to act via several mechanisms and the frequent simultaneous exposure to several pesticides [43].
Furthermore, studies showed that the pesticides endosulfan, prochloraz, tolclofos-methyl, and propamocarb elicited the estrogenic potential in both stable and transient transfected cell lines [130] and demonstrated organochlorine and organophosphorus pesticides such as prochloraz, fenarimol, and chlorpyrifos possess the ability to interfere with the ERα and ERβ mRNA steady-state levels [131, 132].
Different receptor conformations induce diverse effects of different natural and synthetic ER ligands, allowing differential interactions with other transcription factors. It was reported that the peptide recognition pattern induced by a group of chlorinated pesticides including 2, 4-dichlorodiphenyl-dichloroethylene (DDE) is different from the classical ER ligand [133].
A recent in vitro study showed a weakly induced ER transactivity by the pesticides propiconazole, terbuthylazine, cypermethrin, prothioconazole, and malathion [134], while bitertanol, propiconazole, and mancozeb antagonized the AR activity in a concentration-dependent manner. The mixture consisting of five pesticides (bitertanol, terbuthylazine, cypermethrin, malathion, propiconazole) induced the aromatase activity and the ER transactivity while additively antagonized the AR transactivity [134].
A study on the fungicide fenarimol indicated estrogenic effects both in vitro and in vivo and a dual effect being estrogenic at higher concentrations and aromatase inhibitor at low concentrations [135].
Studies have reported that prochloraz, an imidazole fungicide, elicits multiple mechanisms of action in vitro, antagonizing the androgen and the estrogen receptor and inhibiting activity of aromatase [43] while agonizing the aryl hydrocarbon receptor (AhR) [136]. The in vivo Hershberger assay using castrate-immature male rats showed that prochloraz as an antiandrogen might reduce weights of reproductive organs, affects androgen-regulated gene expressions in the prostate, and increases luteinizing hormone (LH) levels [137]. A study investigating the developmental effects of prochloraz, upon exposure of pregnant Wistar rat dams, observed that plasma and testicular testosterone levels in gestational day 21 male fetuses were significantly reduced, whereas testicular progesterone was increased by prochloraz, indicating that the male offspring might be feminized by perinatal prochloraz exposure and these effects are due to diminished fetal steroidogenesis. Thus prochloraz acts as an antiandrogen eliciting dual mechanisms of action both by blocking the androgen receptor and by inhibiting fetal steroidogenesis [138].
The potential endocrine activity was studied on pesticides bitertanol, propiconazole, cypermethrin, malathion, and terbuthylazine alone and as mixtures in vitro and in vivo. It was shown that the pesticides alone and as mixtures affected steroidogenesis in adrenal cells in vitro and caused the increases in progesterone and decreases in testosterone. The mixture of five pesticides elicited an increase in estradiol, indicating increased aromatase activity [139]. Furthermore, the in vivo animal study showed a decreased estradiol and reduced placental testosterone for dams exposed to pesticide mixture and a significant increase in aromatase mRNA-levels in female adrenal glands [139]. This study indicated the potential aromatase induction of the mixture of the five pesticides both in vitro and in vivo. However, the hormonal responses in vitro were only partly reflected in vivo, probably due to some toxicokinetic issues, since the amount of compound mixtures affect pesticide levels negatively in the amniotic fluid [139].
The aryl hydrocarbon receptor (AhR) acts as a ligand-activated transcription factor mediating many of the biologic and toxicological effects of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) and related compounds. The cross-talks between AhR and ER, AR have been reported [140]. AhR is involved in syntheses of steroids and metabolism of steroids and xenobiotic compounds [141]. Studies showed that the pesticides iprodione, chlorpyrifos, prochloraz, terbuthylazine, propiconazole, mancozeb, cypermethrin, and tau-fluvalinate elicited dose-dependent AhR agonistic effects [136, 142]. The mixture of bitertanol, propiconazole, cypermethrin, malathion, and terbuthylazine induced as well AhR transactivity [142].
The actions of thyroid hormones (THs) are mediated by the thyroid hormone receptor (TR). Agonistic effects on cell growth (cell proliferation) may be caused by interaction of compounds with the TRs, whereas interference with the triiodothyronine (T3)-TR association or binding of antagonists to the TRs may result in inhibiting effects on T3-mediated cell growth. Studies showed that the pesticides prochloraz, chlorpyrifos, prothioconazole, malathion, tau-fluvalinate, cypermethrin, terbuthylazine, and mancozeb significantly stimulated rat pituitary GH3 cell proliferation and bitertanol and propiconazole slightly reduced the GH3 cell proliferation [142, 143]. In the presence of triiodothyronine (T3), prochloraz, iprodione, prothioconazole, tau-fluvalinate, propiconazole, cypermethrin, and bitertanol significantly antagonized the T3-induced GH3 cell proliferation [142, 143]. Moreover, an additive combined effect on TH-dependent cell proliferation of pesticide mixture composed of terbuthylazine, propiconazole, cypermethrin, and malathion was observed [142].
3.1.2 Plasticizers/Phthalates
In vitro studies showed that widely used phenols and plasticizers including bisphenol A (BPA) and bisphenol A dimethacrylate (BPA-DM), 4-n-nonylphenol (nNP), and 4-n-octylphenol (nOP) have endocrine-disrupting potentials and the effects can be mediated via several cellular pathways, including the two sex steroid hormone receptors (ER and AR), aromatase activity, and AhR [144]. Moreover, BPA and BPA-DM elicited an inhibitory effect on thyroid hormone (TH)-dependent cell growth [143]. In addition, benzyl butyl phthalate (BBP), 4-chloro-3-methylphenol (CMP), 2,4-dichlorophenol (2,4-DCP), and resorcinol affected AR and AhR transactivity, whereas bis(2-ethylhexyl) phthalate (DEHP), diisodecyl phthalate (DIDP), and dibutyl phthalate (DBP) affected only the AhR and 4-tert-octylphenol (tOP), and 2-phenylphenol (2-PP) antagonized the AR activity in vitro [145].
The mixture composed of six plasticizers, of which one weakly induced the AhR but all others antagonized the AR-elicited additive effects for both AR and AhR. This in vitro data indicate that when assessing the risk to human health, the effect of a mixture depends on the character, potency, concentration, and composition of the single mixture compounds and also the combined effects of the compounds should be taken into account [145].
Furthermore, in vitro study showed that BBP, DBP, dioctyl phthalate (DOP), DIDP, diisononyl phthalate (DINP), DEHP, bis(2-ethylhexyl) adipate (DEHA), tOP, CMP, 2,4-DCP, and resorcinol significantly affected the thyroid hormone-dependent GH3 cell proliferation: tOP, BBP, and DBP activated ER transactivity, whereas DEHP antagonized the 17-estradiol-induced ER function. The mixture of six plasticizers significantly induced ER transactivity in an additive manner, whereas elicited antagonized effect for the thyroid hormone-dependent GH3 cell proliferation [146].
3.1.3 Persistent Organic Pollutants
3.1.3.1 Lipophilic POPs
There are many interactions in the bloodstream of mammals connected with the transport of lipophilic xenobiotic compounds. POPs including DDT, and especially its metabolite DDE would cause a range of deleterious health effects by interacting with nuclear hormone receptors and resulting in malfunction. A study of lipoprotein receptors in mouse embryonic fibroblast cells in conjunction with uptake of DDT–lipoprotein compounds from supplemented media in vitro showed that DDT uptake decreased with increased low-density lipoprotein (LDL) concentration. However, there was no strong evidence for a receptor-mediated uptake of the DDT–lipoprotein complex, suggesting DDT might be transported as a DDT–lipoprotein complex without receptors to cross cell membranes, since passive diffusion constitutes a major passageway [147].
Among the lipophilic POPs, PCBs are ubiquitous environmental POPs giving rise to potential health hazard. Dioxin-like PCBs such as PCB 105, PCB 118, PCB126, PCB 156, exert dioxin-like activities mediated through AhR. The di-ortho, multiple-chloro-substituted biphenyls (PCB138, PCB153, and PCB180) were shown to have pleiotropic effects on the estrogen receptor (ER) and androgen receptor (AR). They can compete with the binding of the natural ligand to ER and AR and thus possess the ability to interfere with sexual hormone-regulated processes [148].
The PCB metabolites (OH-PCB 106, OH-PCB 121, OH-PCB 69) and brominated flame retardants (tetrabromobisphenol A) were shown to interfere with the TH-dependent GH3 cell proliferation alone or upon co-treatment with T3 [143].
Several rodent researches reported behavioral alterations after developmental, neonatal, or adult exposure to PBDEs, among which subtle structural and functional changes in brains were observed. In the brain, functional effects have been found on synaptic plasticity and the glutamate–nitric oxide–cyclic guanosine monophosphate pathway. Furthermore, some studies reported expression changes of genes and proteins involved in synapse and axon formation, neuronal morphology, cell migration, synaptic plasticity, ion channels, and vesicular neurotransmitter release. Cellular and molecular mechanisms include effects on neuronal differentiation and migration, neuronal viability (via apoptosis and oxidative stress), neurotransmitter release/uptake, neurotransmitter receptors and ion channels, calcium (Ca2+) homeostasis, and intracellular signaling pathways [149,150,151].
3.1.3.2 Amphiphilic POPs (PFAAs)
An in vitro study analyzed seven PFAA congeners [perfluorooctanoate (PFOA), perfluorooctane sulfonate (PFOS), perfluorodecanoate (PFDA), perfluorononanoate (PFNA), perfluorohexane sulfonate (PFHxS), perfluorododecanoate (PFDoA), and perfluoroundecanoate (PFUnA)] for their potential to affect estrogen receptor (ER) and androgen receptor (AR) transactivity as well as aromatase enzyme activity in individual and in an equimolar mixture. The results showed that PFHxS, PFOS, and PFOA and a mixture of the seven PFAAS significantly induced the ER transactivity [152], while PFHxS, PFOS, PFOA, PFNA, and PFDA significantly antagonized the AR activity in a concentration-dependent manner. A synergistic mixture effect on AR function was observed for the equimolar mixture of the seven PFAAs. Moreover, PFDA weakly decreased the aromatase activity at a high test concentration [152].
Furthermore, the above seven PFAAs were shown to elicit a dose-dependent inhibition of GH3 cell growth and PFOS, PFHxS, PFNA, and PFUnA antagonized the T3-induced GH3 cell proliferation [153], while only PFDoA and PFDA elicited an activating effect on the AhR [153].
PFAAs were shown to affect oxidative stress biomarkers in vitro. Wielsøe M et al. [154] reported that PFHxS, PFOA, PFOS, and PFNA showed a dose-dependent increase in DNA damage; except for PFDoA, all the other PFAAs increased the generation of reactive oxygen species (ROS) significantly with PFHxS and PFUnA dose-dependently. PFOA decreased the total antioxidant capacity (TAC) level in human liver cells [154].
3.1.4 Phytoestrogens
Phytoestrogens (PEs) can mimic or modulate the production or action of endogenous hormones resulting in biologic responses in vertebrates. PEs are produced in a large range of plants as naturally occurring plant components.
An in vitro study examined the mixtures of 12 foods relevant PEs including coumestrol, isoflavonoids and lignans for the effects on estrogenic and aromatase activity, steroid hormone production, and for the interaction with the androgen receptor. The results indicated an induced aromatase activity by the mixture of all tested PEs causing increased estradiol production and decreased testosterone production of adrenal corticocarcinoma cells in human. Furthermore, various mixtures of PEs significantly stimulated human breast adenocarcinoma cell growth and induced aromatase activity in human choriocarcinoma cells. It was reported that isoflavonoid may cause reduction of testosterone, and isoflavonoid mixture and coumestrol might decrease ERα expression while increase progesterone receptor protein expression [155].
Among the 12 PEs mentioned above, most PEs and their mixtures showed effects on both the TH and AhR cell system. Single isoflavonoid metabolites and their mixture and coumestrol induced T3-dependent GH3 cell growth and AhR transactivity dose-dependently. Furthermore, isoflavonoid metabolites may also affect T3-dependent GH3 cell growth and cause a synergistic effect on the AhR transactivity [156]. Therefore, nutrition-relevant PEs, alone and in mixture, may possess endocrine-disrupting potential through various modes of actions.
3.1.5 Other Compounds
Based on previous studies, polyhalogenated carbazoles (PHCZs) are a general class of contaminants characterized by persistence and bioaccumulation property. It was shown that nine PHCZs significantly activated AhR in a concentration-dependent manner, with 1000–100,000-fold less potential than that of the most potent AhR ligand TCDD. The AhR activation was partly consistent with the induction of AhR-mediated CYP1A1 expression. In in silico analysis, the nine PHCZs could be docked into the same pocket as TCDD, owing to their high structural similarity. However, the shrunk size of the heterocyclic moieties in PHCZs compared with that in TCDD dramatically contributed to a lower compound stability provided by inter-molecular interactions. Moreover, two distinguished docking poses adopted by the nine PHCZs were found. Thus PHCZ may possess the toxicity via interaction with AhR [157].
Dehydroepiandrosterone sulfate (DHEAS) and estrone sulfate (E1S) are two of the most abundant steroids existing in the human circulation. The enzyme steroid sulfatase (STS) cracks the sulfate group of DHEAS and E1S resulting in biosynthesis of endogenous hormones such as testosterone and estrone. In vitro study showed both E1S and DHEAS dose-dependently transactivated the ER and the AR in dose-dependent manners [158].
3.2 Ex Vivo Studies (as Exposure Biomarker)
The toxicological assessment of EDCs in human is complicated. The adverse health effects of environment contaminants are probably due to disruption of various hormonal systems, e.g., ER, AR, TR, and/or AhR. Toxicological studies have shown that the individual POPs possess very different biological effects and potentials; many of the bioaccumulated POPs are estrogenic while others are antiestrogenic, as well as anti-androgenic, and some have dioxin-like potentials. Additive enhancement of hormone actions has been reported in vitro and in vivo. Therefore, it is of great significance to evaluate the integrated biological effect of the actual chemical mixture in human body. Extractions followed by cell culture system analyses have recently been introduced to assess the ex vivo integrated effect on hormone receptor activities of xenobiotic compounds such as POPs in human adipose tissue and in human serum [29].
Extracting the compounds from human tissues, such as human serum, is one way to study the effects of the actual mixture of compounds related to the human body [29]. A method of extracting lipophilic POPs from human serum, including PCBs and organochlorine pesticides (OCPs) but free of endogenous hormones, was established and validated, making the extraction method as a valuable tool to assess the combined effects such as additive/synergistic and agonistic/antagonistic effects of ER and AR of serum lipophilic POPs and may give an overall estimate of exposure and bioactivity [159]. Recently, a method of extracting in parallel the lipophilic POPs and the amphiphilic PFAAs, free of endogenous hormones, separated from the same serum sample, was developed and validated [160]. This method can be used for studying the effects of the actual serum lipophilic POPs mixture as well as PFAA mixture on both steroid hormones and other hormonal systems, e.g., thyroid hormone function, and possibly elucidate the relationship between exposure to lipophilic POPs and PFAAs and related biological effects and health outcomes [161].
The lipophilic POPs were extracted from Greenland Inuit serum, and the extract elicited in cell culture ex vivo xenoestrogenic and xenoandrogenic transactivities and AhR transactivity which were related to age, marine food intake, and smoking years and negatively correlated to the serum levels of lipophilic POPs. These data indicate that the POP mixture induced xenohormone and AhR transactivities can serve as a comprehensive biomarker of POP exposure and lifestyle characteristics. The data clearly showed the hormone-disruptive potentials of serum POPs [162,163,164].
A study measured the xenoestrogenic activity in human serum extracts consisting of mixtures of PFAAs of 397 Danish nulliparous pregnant women. Fifty-two percent of the PFAA serum extracts agonized the ER transactivation, and 46% further enhanced the 17β estradiol-induced ER transactivation. The serum PFAA extracts induced the ER in a non-monotonic concentration-dependent manner. The serum extract containing the actual mixtures of PFAAs induced the estrogenic activity corresponding to the effect of 0.5 pg 17β estradiol per milliliter serum. Thus, nearly all of the serum extracts containing the PFAA mixtures from pregnant women’s serum elicited estrogenic potentials, agonizing the ER, and further promoted the E2-induced effects in non-monotonic concentration-dependent manners [165].
The lipophilic POPs induced AhR transactivity were analyzed in serum samples from Danish schoolchildren and their mothers living in urban and rural areas. The serum lipophilic POP-induced AhR transactivities were dramatically higher in schoolchildren living in the urban area compared with the rural. A high correlation between the AhR transactivity of mothers and children was observed. The results also showed that AhR transactivity can be measured as a biomarker of exposure and effects in blood samples from children and women and indicated that people living in urban areas may be exposed to higher concentrations of PCBs, dioxins, and dioxin-like chemicals, which may result in a greater risk of adverse effects for urban populations [166].
3.2.1 POP-Induced Receptor Transactivity and Health Outcome (as Effect Biomarker)
3.2.1.1 Fetal Growth
A newly published ex vivo study showed that the maternal serum extract of PFAAs in cell culture-induced xenoestrogenic receptor transactivation (XER) was related to a decrease in birth weight and birth length [63]. The associations of maternal serum dioxin-like compounds measured as ex vivo AhR transactivity and birth outcomes are inconsistent [167, 168]. An international study showed the cord blood AhR transactivity was inversely associated with gestational age but found a tendency of a positive, non-significant association between maternal serum AhR transactivity and birth weight and gestational age [169].
3.2.1.2 Autism (Molecular Mechanisms)
Several studies support the hypothesis that the pathogenesis of autism is most likely to be polygenic [170], and environmental factors may interact with genetic factors to increase the risk of autism [171, 172]. Although the genetic risk factors remain difficult to identify, several chromosomal disorders and single gene disorders are associated with an increased risk for autism. Furthermore, evidence has indicated that some non-inherited factors such as exposure to environmental pollutants bear upon autism [173]. The epigenetic mechanisms play an important role in the gene–environment interactions in autism [174]. In addition, prenatal stress and maternal immune dysregulation are also associated with autism [175].
A Danish case-control study measured the amniotic fluid (AF) levels of EDCs and metals as well as the receptor transactivity induced by AF and also investigated the possible connection between prenatal exposure to EDCs and heavy metals and risk of autism [176]. The biomarkers of effect such as ER-, AR-, AhR-, and TH-like transactivity were determined in the AF samples, which indicated the presence of EDCs in amniotic fluid. This study suggested that EDCs might alter the risk of autism by affecting the hormone receptor function. The observed inverse correlation between PFAAs and autism risk might be associated with the weak estrogenic activities and anti-androgenic activities of PFAAs. As autism is a typical male trait, regardless of whether EDCs together with endogenous hormones play a role in the development of autism, the observed tendency of positive association between the ratio of combined androgenic effect to the combined estrogenic effect and autism risk needs further studies to explore [176].
4 Summary
Humans are exposed to complicated mixtures of chemicals, which have individually significant differences in biological potentials and effects. Endocrine disruptors (EDCs) can mimic or block endogenous hormones, thereby disrupting normal hormone homeostasis. EDCs include compounds with long half-life such as POPs and non-accumulating compounds such as certain pesticides, BPA, and phthalates.
In particular, prenatal and children’s exposure to chemicals in surrounding environment are of great concern that may have the potential to influence the risk of diseases in childhood and adult life.
Epidemiological researches play a critical part in quantifying how environmental chemical exposures prenatally and postnatally contribute to diseases in childhood and adult life. Although there are some challenges for estimating accurately exposure and the period of exposure sensitivity that needs further exploration.
In epidemiological studies, pesticides such as pyrethroids effects were reported for acute accidental exposure to pyrethroids such as paranesthesia; respiratory, eye, and skin irritation; and so on, whereas information on their chronic effects at low concentrations is not only limited but also controversial. The effects observed in the epidemiological literature were inconsistent with toxicological effects observed in extensive testing of pyrethroids in animals.
Considerable research on BPA exposure-associated health risks in humans has elucidated BPA’s endocrine-disrupting properties, suggesting BPA’s potential impact on development during early life. This may make individuals more susceptible to diseases, including neurological and carcinogenic potential, below the oral reference dose (RfD) determined by the environmental protection agencies.
Epidemiological studies on exposure to phthalates during pregnancy suggest behavioral and cognitive effects in children as well as child obesity.
POPs, including lipophilic POPs and amphiphilic PFASs and some metals, were relevant to negative influence on development of fetal, child cognitive, immune, and reproductive disruption. POPs can disrupt the endocrine system and decrease the defense against oxidative stress. A new method to study the actual serum mixture of POPs has suggested that the mixtures exposure approach reflect more precisely the real daily exposure to multiple chemicals instead of “one chemical at a time” approach.
POP exposures were also associated with decreased vaccination response and immunological protection to tetanus, diphtheria, and tuberculosis.
Prenatal environmental exposures were also associated with the risk of autism/ADHD, asthma, and allergy.
5 Mechanisms
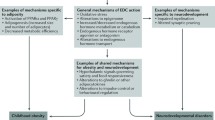
EDCs such as pesticides, PCBs, PFAAs, plasticizers/phthalates, phenols, and phytoestrogens are shown to work as an estrogen receptor (ER) agonist/antagonist, androgen receptor (AR), and aryl hydrocarbon receptor (AhR), to interfere with thyroid hormone (TH) function and to interfere with the steroid enzymes such as aromatase enzyme that convert testosterone to E2 (17b-estradiol) and can work through a variety of mechanisms. In addition, due to the cross interaction of the AhR with the ER, AR, and TH, the effects of EDCs on AhR can help to elucidate the cellular mechanisms behind hormone disorders. The final response may be determined by the interaction of all pathways implicated. Moreover, the mixture analyses showed that the combined effect of all the compounds present in the human body must be taken into consideration for risk assessment.
Ex vivo studies have shown comprehensive biomarker effect of complex mixture extracted from human serum to reflect the regional differences in serum levels of bioaccumulated POPs, e.g., in the Arctic region. Xenobiotic receptor activity can be used as an in vitro biomarker of POP exposure and effects. The potential impact of the comprehensive effect of EDCs on human health has been supported by epidemiological and in vitro/ex vivo studies.
Other mechanisms such as oxidative stress and DNA alterations and inflammation also play important roles in effects of the early exposure to environmental contaminants on growth and development (Fig. 1).
References
Agency, E.C. Available from: https://echa.europa.eu/information-on-chemicals
EP, A (2017) How to access the TSCA inventory 2017 [cited 2017 June 22]. Available from: https://www.epa.gov/tsca-inventory/how-access-tsca-inventory#download
AMAP (2015) AMAP assessment 2015: human health in the Arctic. Arctic Monitoring and Assessment Programme (AMAP), Oslo, vii + 165 pp. https://www.amap.no/documents/download/2594
AMAP (2017) AMAP assessment 2015 chemicals of emerging arctic concern. 2015, Arctic Monitoring and Assessment Programme (AMAP): Oslo, Norway
CDC CfDCaP, U.T Fourth national report on human exposure to environmental chemicals, Updated Tables 2012. Available from: http://www.cdc.gov/exposurereport/pdf/FourthReport_UpdatedTables_Feb2012.pdf
OECD (2019) Environmental performance reviews: Denmark 2019, Chapter 5, Chemical management. Available from: https://books.google.dk/books?id=bT6DwAAQBAJ&pg=PA202&lpg=PA202&dq=biomonitorering+in+Denmark&source=bl&ots=tUaRkEStZ&sig=ACfU3U19a2IHP7IGnaYy7SxkaNFAvz_soQ&hl=da&sa=X&ved=2ahUKEwiPm5L6_PmAhVO_qQKHVFhBKIQ6AEwBXoECAoQAQ#v=onepage&q=biomonitorering%20in%20Denmark&f=false
Braun JM (2017) Early-life exposure to EDCs: role in childhood obesity and neurodevelopment. Nat Rev Endocrinol 13(3):161–173
Grandjean P, Landrigan PJ (2014) Neurobehavioural effects of developmental toxicity. Lancet Neurol 13(3):330–338
Zoeller RT et al (2012) Endocrine-disrupting chemicals and public health protection: a statement of principles from The Endocrine Society. Endocrinology 153(9):4097–4110
Saillenfait AM, Ndiaye D, Sabate JP (2015) Pyrethroids: exposure and health effects – an update. Int J Hyg Environ Health 218(3):281–292
Burns CJ, Pastoor TP (2018) Pyrethroid epidemiology: a quality-based review. Crit Rev Toxicol 48(4):297–311
Rochester JR, Bolden AL, Kwiatkowski CF (2018) Prenatal exposure to bisphenol A and hyperactivity in children: a systematic review and meta-analysis. Environ Int 114:343–356
Seachrist DD et al (2016) A review of the carcinogenic potential of bisphenol A. Reprod Toxicol 59:167–182
Ferguson KK et al (2015) Urinary phthalate metabolites and biomarkers of oxidative stress in pregnant women: a repeated measures analysis. Environ Health Perspect 123(3):210–216
Barrie LA et al (1992) Arctic contaminants: sources, occurrence and pathways. Sci Total Environ 122(1–2):1–74
Burkow IC, Kallenborn R (2000) Sources and transport of persistent pollutants to the Arctic. Toxicol Lett 112–113:87–92
Hung H et al (2016) Temporal trends of Persistent Organic Pollutants (POPs) in arctic air: 20 years of monitoring under the Arctic Monitoring and Assessment Programme (AMAP). Environ Pollut 217:52–61
Diamanti-Kandarakis E et al (2009) Endocrine-disrupting chemicals: an Endocrine Society scientific statement. Endocr Rev 30(4):293–342
Thomsen C, Liane VH, Becher G (2007) Automated solid-phase extraction for the determination of polybrominated diphenyl ethers and polychlorinated biphenyls in serum – application on archived Norwegian samples from 1977 to 2003. J Chromatogr B Anal Technol Biomed Life Sci 846(1–2):252–263
Brauner EV et al (2011) Predictors of polychlorinated biphenyl concentrations in adipose tissue in a general Danish population. Environ Sci Technol 45(2):679–685
Brauner EV et al (2012) Predictors of adipose tissue concentrations of organochlorine pesticides in a general Danish population. J Expo Sci Environ Epidemiol 22(1):52–59
Halldorsson TI et al (2008) Linking exposure to polychlorinated biphenyls with fatty fish consumption and reduced fetal growth among Danish pregnant women: a cause for concern? Am J Epidemiol 168(8):958–965
Bonefeld-Jorgensen EC (2010) Biomonitoring in Greenland: human biomarkers of exposure and effects – a short review. Rural Remote Health 10(2):1362
Long M et al (2015) Food intake and serum persistent organic pollutants in the Greenlandic pregnant women: the ACCEPT sub-study. Sci Total Environ 529:198–212
Frank W, Mackay D (1993) Global fractionation and cold condensation of low volatility organochlorine compounds in polar regions. Ambio 22(1):10–18
Borga K et al (2004) Biological and chemical factors of importance in the bioaccumulation and trophic transfer of persistent organochlorine contaminants in Arctic marine food webs. Environ Toxicol Chem 23(10):2367–2385
Corsolini S, Sara G (2017) The trophic transfer of persistent pollutants (HCB, DDTs, PCBs) within polar marine food webs. Chemosphere 177:189–199
Krafft MP, Riess JG (2015) Per- and polyfluorinated substances (PFASs): Environmental challenges. Curr Opin Colloid Interface Sci 20(3):192–212
Bonefeld-Jorgensen EC et al (2014) Biomonitoring and hormone-disrupting effect biomarkers of persistent organic pollutants in vitro and ex vivo. Basic Clin Pharmacol Toxicol 115(1):118–128
Boucher O et al (2012) Response inhibition and error monitoring during a visual go/no-go task in Inuit children exposed to lead, polychlorinated biphenyls, and methylmercury. Environ Health Perspect 120(4):608–615
Lam J et al (2017) Developmental PBDE exposure and IQ/ADHD in childhood: a systematic review and meta-analysis. Environ Health Perspect 125(8):086001
Dallaire F et al (2006) Effect of prenatal exposure to polychlorinated biphenyls on incidence of acute respiratory infections in preschool Inuit children. Environ Health Perspect 114(8):1301–1305
Grandjean P et al (2012) Serum vaccine antibody concentrations in children exposed to perfluorinated compounds. JAMA 307(4):391–397
Heilmann C et al (2010) Serum concentrations of antibodies against vaccine toxoids in children exposed perinatally to immunotoxicants. Environ Health Perspect 118(10):1434–1438
Schaebel LK et al (2017) The influence of persistent organic pollutants in the traditional Inuit diet on markers of inflammation. PLoS One 12(5):e0177781
Ghisari M et al (2014) Polymorphisms in phase I and phase II genes and breast cancer risk and relations to persistent organic pollutant exposure: a case-control study in Inuit women. Environ Health 13(1):19
Weihe P et al (2016) Health effects associated with measured levels of contaminants in the Arctic. Int J Circumpolar Health 75:33805
Takeuchi S et al (2011) Characterization of steroid hormone receptor activities in 100 hydroxylated polychlorinated biphenyls, including congeners identified in humans. Toxicology 289(2–3):112–121
Noren K, Meironyte D (2000) Certain organochlorine and organobromine contaminants in Swedish human milk in perspective of past 20–30 years. Chemosphere 40(9–11):1111–1123
Medehouenou TC et al (2011) Polychlorinated biphenyls and organochlorine pesticides in plasma of older Canadians. Environ Res 111(8):1313–1320
Wu JP et al (2011) Several current-use, non-PBDE brominated flame retardants are highly bioaccumulative: Evidence from field determined bioaccumulation factors. Environ Int 37(1):210–215
Sjodin A et al (2004) Retrospective time-trend study of polybrominated diphenyl ether and polybrominated and polychlorinated biphenyl levels in human serum from the United States. Environ Health Perspect 112(6):654–658
Andersen HR et al (2002) Effects of currently used pesticides in assays for estrogenicity, androgenicity, and aromatase activity in vitro. Toxicol Appl Pharmacol 179(1):1–12
Stockholm_Convention (2009) Listing of POPs in Stockholm convention [cited 2014 July 24th]. Available from: http://chm.pops.int/Convention/ThePOPs/ListingofPOPs/tabid/2509/Default.aspx
Schultz MM, Barofsky DF, Field JA (2003) Fluorinated alkyl surfactants. Environ Eng Sci 20(5):487–501
Olsen GW et al (2007) Half-life of serum elimination of perfluorooctanesulfonate, perfluorohexanesulfonate, and perfluorooctanoate in retired fluorochemical production workers. Environ Health Perspect 115(9):1298–1305
Haug LS et al (2011) Characterisation of human exposure pathways to perfluorinated compounds – comparing exposure estimates with biomarkers of exposure. Environ Int 37(4):687–693
Haug LS et al (2010) Diet and particularly seafood are major sources of perfluorinated compounds in humans. Environ Int 36(7):772–778
Trudel D et al (2008) Estimating consumer exposure to PFOS and PFOA. Risk Anal 28(2):251–269
D’Eon JC et al (2009) Observation of a commercial fluorinated material, the polyfluoroalkyl phosphoric acid diesters, in human sera, wastewater treatment plant sludge, and paper fibers. Environ Sci Technol 43(12):4589–4594
Trier X, Granby K, Christensen JH (2011) Polyfluorinated surfactants (PFS) in paper and board coatings for food packaging. Environ Sci Pollut Res Int 18(7):1108–1120
Ramli MR et al (2020) Level and determinants of serum perfluoroalkyl acids (PFAAs) in a population in Klang Valley, Malaysia. Int J Hyg Environ Health 223(1):179–186
Han W et al (2018) Perfluoroalkyl and polyfluoroalkyl substances in matched parental and cord serum in Shandong, China. Environ Int 116:206–213
Wang Y et al (2019) Efficiency of maternal-fetal transfer of perfluoroalkyl and polyfluoroalkyl substances. Environ Sci Pollut Res Int 26(3):2691–2698
Govarts E et al (2012) Birth weight and prenatal exposure to polychlorinated biphenyls (PCBs) and dichlorodiphenyldichloroethylene (DDE): a meta-analysis within 12 European Birth Cohorts. Environ Health Perspect 120(2):162–170
Iszatt N et al (2015) Prenatal and postnatal exposure to persistent organic pollutants and infant growth: a pooled analysis of seven European birth cohorts. Environ Health Perspect 123(7):730–736
Hjermitslev MH et al (2020) Persistent organic pollutants in Greenlandic pregnant women and indices of foetal growth: The ACCEPT study. Sci Total Environ 698:134118
Apelberg BJ et al (2007) Cord serum concentrations of perfluorooctane sulfonate (PFOS) and perfluorooctanoate (PFOA) in relation to weight and size at birth. Environ Health Perspect 115(11):1670–1676
Chen MH et al (2012) Perfluorinated compounds in umbilical cord blood and adverse birth outcomes. PLoS One 7(8):e42474
Cao W et al (2018) Perfluoroalkyl substances in umbilical cord serum and gestational and postnatal growth in a Chinese birth cohort. Environ Int 116:197–205
Bach CC et al (2016) Perfluoroalkyl acids in maternal serum and indices of fetal growth: the Aarhus Birth Cohort. Environ Health Perspect 124(6):848–854
Darrow LA, Stein CR, Steenland K (2013) Serum perfluorooctanoic acid and perfluorooctane sulfonate concentrations in relation to birth outcomes in the Mid-Ohio Valley, 2005–2010. Environ Health Perspect 121(10):1207–1213
Bjerregaard-Olesen C et al (2019) Associations of fetal growth outcomes with measures of the combined xenoestrogenic activity of maternal serum perfluorinated alkyl acids in Danish pregnant women. Environ Health Perspect 127(1):17006
Bach CC et al (2015) Perfluoroalkyl acids and time to pregnancy revisited: An update from the Danish National Birth Cohort. Environ Health 14:59
Adal A, Wiener S, Louden M. Heavy metal toxicity: back ground, pathophysiology, epidemiology [cited 2018 February 14]. Available from: https://emedicine.medscape.com/article/814960-overview#a5
Jaishankar M et al (2014) Toxicity, mechanism and health effects of some heavy metals. Interdiscip Toxicol 7(2):60–72
Tchounwou PB et al (2012) Heavy metal toxicity and the environment. Exp Suppl 101:133–164
Gavidia T et al (2011) Children’s environmental health – from knowledge to action. Lancet 377(9772):1134–1136
WHO (2010) World Health Organization: Action is needed on chemicals of major public health concern. World Health Organization, Geneva. http://www.who.int/ipcs/features/10chemicals_en.pdf
WHO (2011) World Health Organization: adverse health effects of heavy metals in children. Children’s Health and the Environment WHO Training Package for the Health Sector World Health Organization, pp 1–77. https://www.who.int/ce
Boucher O et al (2014) Domain-specific effects of prenatal exposure to PCBs, mercury, and lead on infant cognition: results from the Environmental Contaminants and Child Development Study in Nunavik. Environ Health Perspect 122(3):310–316
Knudsen AS et al (2018) Persistent organic pollutants and haematological markers in Greenlandic pregnant women: the ACCEPT sub-study. Int J Circumpolar Health 77(1):1456303
Luo Y et al (2017) Maternal blood cadmium, lead and arsenic levels, nutrient combinations, and offspring birthweight. BMC Public Health 17(1):354
Vrijheid M et al (2016) Environmental pollutants and child health-A review of recent concerns. Int J Hyg Environ Health 219(4–5):331–342
Veyhe AS et al (2015) The Northern Norway Mother-and-Child Contaminant Cohort (MISA) Study: PCA analyses of environmental contaminants in maternal sera and dietary intake in early pregnancy. Int J Hyg Environ Health 218(2):254–264
Caserta D et al (2013) Heavy metals and placental fetal-maternal barrier: a mini-review on the major concerns. Eur Rev Med Pharmacol Sci 17(16):2198–2206
Odland JO, Nieboer E (2012) Human biomonitoring in the Arctic. Special challenges in a sparsely populated area. Int J Hyg Environ Health 215(2):159–167
Zietz BP et al (2008) Long-term biomonitoring of polychlorinated biphenyls and organochlorine pesticides in human milk from mothers living in northern Germany. Int J Hyg Environ Health 211(5–6):624–638
Bank-Nielsen PI, Long M, Bonefeld-Jorgensen EC (2019) Pregnant Inuit women’s exposure to metals and association with fetal growth outcomes: ACCEPT 2010–2015. Int J Environ Res Public Health 16(7):1171
Berkowitz Z et al (2006) Lead exposure and birth outcomes in five communities in Shoshone County, Idaho. Int J Hyg Environ Health 209(2):123–132
Brocato J, Costa M (2015) 10th NTES conference: Nickel and arsenic compounds Alter the epigenome of peripheral blood mononuclear cells. J Trace Elem Med Biol 31:209–213
McDermott S et al (2015) Systematic review of chromium and nickel exposure during pregnancy and impact on child outcomes. J Toxicol Environ Health A 78(21–22):1348–1368
Zhang Y et al (2015) Toxicity of nickel ions and comprehensive analysis of nickel ion-associated gene expression profiles in THP-1 cells. Mol Med Rep 12(3):3273–3278
Kim JH, Scialli AR (2011) Thalidomide: the tragedy of birth defects and the effective treatment of disease. Toxicol Sci 122(1):1–6
Swan SH (2000) Intrauterine exposure to diethylstilbestrol: long-term effects in humans. APMIS 108(12):793–804
Hoover RN et al (2011) Adverse health outcomes in women exposed in utero to diethylstilbestrol. N Engl J Med 365(14):1304–1314
Braun JM, Gray K (2017) Challenges to studying the health effects of early life environmental chemical exposures on children’s health. PLoS Biol 15(12):e2002800
Abdallah MW et al (2012) Infections during pregnancy and after birth, and the risk of autism spectrum disorders: a register-based study utilizing a Danish historic birth cohort. Turk Psikiyatri Derg 23(4):229–235
Abdallah MW et al (2012) Neonatal levels of cytokines and risk of autism spectrum disorders: an exploratory register-based historic birth cohort study utilizing the Danish Newborn Screening Biobank. J Neuroimmunol 252(1–2):75–82
Abdallah MW et al (2013) Neonatal chemokine levels and risk of autism spectrum disorders: findings from a Danish historic birth cohort follow-up study. Cytokine 61(2):370–376
Jusko TA et al (2016) A birth cohort study of maternal and infant serum PCB-153 and DDE concentrations and responses to infant tuberculosis vaccination. Environ Health Perspect 124(6):813–821
Fair PA et al (2011) Effects of environmentally-relevant levels of perfluorooctane sulfonate on clinical parameters and immunological functions in B6C3F1 mice. J Immunotoxicol 8(1):17–29
Keil DE et al (2008) Gestational exposure to perfluorooctane sulfonate suppresses immune function in B6C3F1 mice. Toxicol Sci 103(1):77–85
Peden-Adams MM et al (2008) Suppression of humoral immunity in mice following exposure to perfluorooctane sulfonate. Toxicol Sci 104(1):144–154
Qazi MR et al (2010) Dietary exposure to perfluorooctanoate or perfluorooctane sulfonate induces hypertrophy in centrilobular hepatocytes and alters the hepatic immune status in mice. Int Immunopharmacol 10(11):1420–1427
Kato K et al (2009) Polyfluoroalkyl compounds in pooled sera from children participating in the National Health and Nutrition Examination Survey 2001-2002. Environ Sci Technol 43(7):2641–2647
Veras MM et al (2017) Before the first breath: prenatal exposures to air pollution and lung development. Cell Tissue Res 367(3):445–455
Hansen S et al (2013) Maternal concentrations of persistent organochlorine pollutants and the risk of asthma in offspring: results from a prospective cohort with 20 years of follow-up. Environ Health Perspect 122(1):93–99
Silvers KM et al (2012) Breastfeeding protects against current asthma up to 6 years of age. J Pediatr 160(6):991–996. e1
Deutch B et al (2003) Smoking as a determinant of high organochlorine levels in Greenland. Arch Environ Health 58(1):30–36
Haugaard Rasmussen IM, Bonefeld-Jorgensen EC, Long M (2019) Greenlandic women s lifestyle and diet during pregnancy and child risk for asthma, eczema and allergy: an ACCEPT-substudy. Int J Circumpolar Health 78(1):1682421
Berghuis SA et al (2015) Developmental neurotoxicity of persistent organic pollutants: an update on childhood outcome. Arch Toxicol 89(5):687–709
Neugebauer J et al (2015) The influence of low level pre- and perinatal exposure to PCDD/Fs, PCBs, and lead on attention performance and attention-related behavior among German school-aged children: results from the Duisburg Birth Cohort Study. Int J Hyg Environ Health 218(1):153–162
Polanska K, Jurewicz J, Hanke W (2013) Review of current evidence on the impact of pesticides, polychlorinated biphenyls and selected metals on attention deficit/hyperactivity disorder in children. Int J Occup Med Environ Health 26(1):16–38
Newman J et al (2009) Analysis of PCB congeners related to cognitive functioning in adolescents. Neurotoxicology 30(4):686–696
Chen YC, Guo YL, Hsu CC (1992) Cognitive development of children prenatally exposed to polychlorinated biphenyls (Yu-Cheng children) and their siblings. J Formos Med Assoc 91(7):704–707
Lai TJ et al (2002) A cohort study of behavioral problems and intelligence in children with high prenatal polychlorinated biphenyl exposure. Arch Gen Psychiatry 59(11):1061–1066
Lee DH, Jacobs DR, Porta M (2007) Association of serum concentrations of persistent organic pollutants with the prevalence of learning disability and attention deficit disorder. J Epidemiol Community Health 61(7):591–596
Newman J et al (2014) PCBs and ADHD in Mohawk adolescents. Neurotoxicol Teratol 42:25–34
Strom M et al (2014) Persistent organic pollutants measured in maternal serum and offspring neurodevelopmental outcomes – a prospective study with long-term follow-up. Environ Int 68:41–48
Orenstein ST et al (2014) Prenatal organochlorine and methylmercury exposure and memory and learning in school-age children in communities near the New Bedford Harbor Superfund site, Massachusetts. Environ Health Perspect 122(11):1253–1259
Zhang H et al (2017) Prenatal PBDE and PCB exposures and reading, cognition, and externalizing behavior in children. Environ Health Perspect 125(4):746–752
Kicinski M et al (2012) Neurobehavioral function and low-level exposure to brominated flame retardants in adolescents: a cross-sectional study. Environ Health 11:86
Sagiv SK et al (2010) Prenatal organochlorine exposure and behaviors associated with attention deficit hyperactivity disorder in school-aged children. Am J Epidemiol 171(5):593–601
Gaspar FW et al (2015) Prenatal DDT and DDE exposure and child IQ in the CHAMACOS cohort. Environ Int 85:206–212
Berghuis SA et al (2018) Prenatal exposure to persistent organic pollutants and cognition and motor performance in adolescence. Environ Int 121(Pt 1):13–22
Wang Y et al (2014) Association between maternal serum perfluoroalkyl substances during pregnancy and maternal and cord thyroid hormones: Taiwan maternal and infant cohort study. Environ Health Perspect 122(5):529–534
Wang Y et al (2013) Association between perfluoroalkyl substances and thyroid stimulating hormone among pregnant women: a cross-sectional study. Environ Health 12(1):76
Webster GM et al (2014) Associations between perfluoroalkyl acids (PFASs) and maternal thyroid hormones in early pregnancy: a population-based cohort study. Environ Res 133:338–347
Ballesteros V et al (2017) Exposure to perfluoroalkyl substances and thyroid function in pregnant women and children: a systematic review of epidemiologic studies. Environ Int 99:15–28
Lazarus JH (1999) Thyroid hormone and intellectual development: a clinician’s view. Thyroid 9(7):659–660
Hong T, Paneth N (2008) Maternal and infant thyroid disorders and cerebral palsy. Semin Perinatol 32(6):438–445
Modesto T et al (2015) Maternal mild thyroid hormone insufficiency in early pregnancy and attention-deficit/hyperactivity disorder symptoms in children. JAMA Pediatr 169(9):838–845
Oppenheimer JH, Schwartz HL (1997) Molecular basis of thyroid hormone-dependent brain development. Endocr Rev 18(4):462–475
Andersen SL et al (2014) Attention deficit hyperactivity disorder and autism spectrum disorder in children born to mothers with thyroid dysfunction: a Danish nationwide cohort study. BJOG 121(11):1365–1374
Andersen SL et al (2018) Maternal thyroid function in early pregnancy and neuropsychological performance of the child at 5 years of age. J Clin Endocrinol Metab 103(2):660–670
Haddow JE et al (1999) Maternal thyroid deficiency during pregnancy and subsequent neuropsychological development of the child. N Engl J Med 341(8):549–555
Inoue K et al (2019) Perfluoroalkyl substances and maternal thyroid hormones in early pregnancy; findings in the Danish national birth cohort. Environ Health Perspect 127(11):117002
Liew Z et al (2018) Prenatal exposure to perfluoroalkyl substances and IQ scores at age 5; A Study in the Danish National Birth Cohort. Environ Health Perspect 126(6):067004
Bonefeld-Jorgensen EC, Grunfeld HT, Gjermandsen IM (2005) Effect of pesticides on estrogen receptor transactivation in vitro: a comparison of stable transfected MVLN and transient transfected MCF-7 cells. Mol Cell Endocrinol 244(1–2):20–30
Grunfeld HT, Bonefeld-Jorgensen EC (2004) Effect of in vitro estrogenic pesticides on human oestrogen receptor alpha and beta mRNA levels. Toxicol Lett 151(3):467–480
Hofmeister MV, Bonefeld-Jorgensen EC (2004) Effects of the pesticides prochloraz and methiocarb on human estrogen receptor alpha and beta mRNA levels analyzed by on-line RT-PCR. Toxicol in Vitro 18(4):427–433
Sumbayev VV et al (2005) A novel pesticide-induced conformational state of the oestrogen receptor ligand-binding domain, detected by conformation-specific peptide binding. FEBS Lett 579(2):541–548
Kjeldsen LS, Ghisari M, Bonefeld-Jorgensen EC (2013) Currently used pesticides and their mixtures affect the function of sex hormone receptors and aromatase enzyme activity. Toxicol Appl Pharmacol 272:453
Andersen HR et al (2006) Estrogenic effects in vitro and in vivo of the fungicide fenarimol. Toxicol Lett 163(2):142–152
Long M et al (2003) Effects of currently used pesticides in the AhR-CALUX assay: comparison between the human TV101L and the rat H4IIE cell line. Toxicology 194(1–2):77–93
Vinggaard AM et al (2002) Antiandrogenic effects in vitro and in vivo of the fungicide prochloraz. Toxicol Sci 69(2):344–353
Vinggaard AM et al (2006) Prochloraz: an imidazole fungicide with multiple mechanisms of action. Int J Androl 29(1):186–192
Taxvig C et al (2013) In vitro-in vivo correlations for endocrine activity of a mixture of currently used pesticides. Toxicol Appl Pharmacol 272(3):757–766
Pocar P et al (2005) Molecular interactions of the aryl hydrocarbon receptor and its biological and toxicological relevance for reproduction. Reproduction 129(4):379–389
Baba T et al (2005) Intrinsic function of the aryl hydrocarbon (dioxin) receptor as a key factor in female reproduction. Mol Cell Biol 25(22):10040–10051
Ghisari M et al (2015) Effects of currently used pesticides and their mixtures on the function of thyroid hormone and aryl hydrocarbon receptor in cell culture. Toxicol Appl Pharmacol 284(3):292–303
Ghisari M, Bonefeld-Jorgensen EC (2005) Impact of environmental chemicals on the thyroid hormone function in pituitary rat GH3 cells. Mol Cell Endocrinol 244(1–2):31–41
Bonefeld-Jorgensen EC et al (2007) Endocrine-disrupting potential of bisphenol A, bisphenol A dimethacrylate, 4-n-nonylphenol, and 4-n-octylphenol in vitro: new data and a brief review. Environ Health Perspect 115(Suppl 1):69–76
Kruger T, Long M, Bonefeld-Jorgensen EC (2008) Plastic components affect the activation of the aryl hydrocarbon and the androgen receptor. Toxicology 246(2–3):112–123
Ghisari M, Bonefeld-Jorgensen EC (2009) Effects of plasticizers and their mixtures on estrogen receptor and thyroid hormone functions. Toxicol Lett 189(1):67–77
Hjelmborg PS, Andreassen TK, Bonefeld-Jorgensen EC (2008) Cellular uptake of lipoproteins and persistent organic compounds – an update and new data. Environ Res 108(2):192–198
Bonefeld-Jorgensen EC et al (2001) Effect of highly bioaccumulated polychlorinated biphenyl congeners on estrogen and androgen receptor activity. Toxicology 158(3):141–153
Dingemans MM et al (2007) Neonatal exposure to brominated flame retardant BDE-47 reduces long-term potentiation and postsynaptic protein levels in mouse hippocampus. Environ Health Perspect 115(6):865–870
Llansola M et al (2007) Prenatal exposure to polybrominated diphenylether 99 enhances the function of the glutamate-nitric oxide-cGMP pathway in brain in vivo and in cultured neurons. Eur J Neurosci 25(2):373–379
Dingemans MM, van den Berg M, Westerink RH (2011) Neurotoxicity of brominated flame retardants: (in)direct effects of parent and hydroxylated polybrominated diphenyl ethers on the (developing) nervous system. Environ Health Perspect 119(7):900–907
Kjeldsen LS, Bonefeld-Jorgensen EC (2013) Perfluorinated compounds affect the function of sex hormone receptors. Environ Sci Pollut Res Int 20:8031
Long M, Ghisari M, Bonefeld-Jorgensen EC (2013) Effects of perfluoroalkyl acids on the function of the thyroid hormone and the aryl hydrocarbon receptor. Environ Sci Pollut Res Int 20:8045
Wielsoe M et al (2015) Perfluoroalkylated substances (PFAS) affect oxidative stress biomarkers in vitro. Chemosphere 129:239–245
Taxvig C et al (2010) Effects of nutrition relevant mixtures of phytoestrogens on steroidogenesis, aromatase, estrogen, and androgen activity. Nutr Cancer 62(1):122–131
Long M et al (2012) Effects of selected phytoestrogens and their mixtures on the function of the thyroid hormone and the aryl hydrocarbon receptor. Nutr Cancer 64(7):1008–1019
Ma D et al (2019) Aryl hydrocarbon receptor activity of polyhalogenated carbazoles and the molecular mechanism. Sci Total Environ 687:516–526
Bjerregaard-Olesen C et al (2016) Estrone sulfate and dehydroepiandrosterone sulfate: transactivation of the estrogen and androgen receptor. Steroids 105:50–58
Hjelmborg PS, Ghisari M, Bonefeld-Jorgensen EC (2006) SPE-HPLC purification of endocrine-disrupting compounds from human serum for assessment of xenoestrogenic activity. Anal Bioanal Chem 385(5):875–887
Bjerregaard-Olesen C et al (2015) Extraction of perfluorinated alkyl acids from human serum for determination of the combined xenoestrogenic transactivity: a method development. Chemosphere 129:232–238
Bjerregaard-Olesen C, Bonefeld-Jørgensen E (2015) Receptor research on xenohormone effects of human serum extracts containing the actual mixture of perfluorinated alkyl acids: a short review. Receptor Clin Invest 2:e702
Kruger T et al (2008) Xenohormone transactivities are inversely associated to serum POPs in Inuit. Environ Health 7:38
Long M, Deutch B, Bonefeld-Jorgensen EC (2007) AhR transcriptional activity in serum of Inuits across Greenlandic districts. Environ Health 6:32
Kruger T et al (2012) The combined effect of persistent organic pollutants in the serum POP mixture in Greenlandic Inuit: xenoestrogenic, xenoandrogenic and dioxin-like transactivities. Biomarkers 17(8):692–705
Bjerregaard-Olesen C, Ghisari M, Bonefeld-Jorgensen EC (2016) Activation of the estrogen receptor by human serum extracts containing mixtures of perfluorinated alkyl acids from pregnant women. Environ Res 151:71–79
Morck TA et al (2014) PCB concentrations and dioxin-like activity in blood samples from Danish school children and their mothers living in urban and rural areas. Basic Clin Pharmacol Toxicol 115(1):134–144
Halldorsson TI et al (2009) Dioxin-like activity in plasma among Danish pregnant women: dietary predictors, birth weight and infant development. Environ Res 109(1):22–28
Konishi K et al (2009) Prenatal exposure to PCDDs/PCDFs and dioxin-like PCBs in relation to birth weight. Environ Res 109(7):906–913
Vafeiadi M et al (2014) In utero exposure to compounds with dioxin-like activity and birth outcomes. Epidemiology 25(2):215–224
Bill BR, Geschwind DH (2009) Genetic advances in autism: heterogeneity and convergence on shared pathways. Curr Opin Genet Dev 19(3):271–278
Gardener H, Spiegelman D, Buka SL (2011) Perinatal and neonatal risk factors for autism: a comprehensive meta-analysis. Pediatrics 128(2):344–355
Hallmayer J et al (2011) Genetic heritability and shared environmental factors among twin pairs with autism. Arch Gen Psychiatry 68(11):1095–1102
Windham GC et al (2006) Autism spectrum disorders in relation to distribution of hazardous air pollutants in the San Francisco bay area. Environ Health Perspect 114(9):1438–1444
Tordjman S et al (2014) Gene x environment interactions in autism spectrum disorders: role of epigenetic mechanisms. Front Psychiatry 5:53
Beversdorf DQ, Stevens HE, Jones KL (2018) Prenatal stress, maternal immune dysregulation, and their association with autism spectrum disorders. Curr Psychiatry Rep 20(9):76
Long M et al (2019) Autism spectrum disorders, endocrine disrupting compounds, and heavy metals in amniotic fluid: a case-control study. Mol Autism 10:1
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Bonefeld-Jorgensen, E.C., Long, M. (2020). Early-Life Environmental Influences on Growth. In: Xia, Y. (eds) Early-life Environmental Exposure and Disease. Springer, Singapore. https://doi.org/10.1007/978-981-15-3797-4_7
Download citation
DOI: https://doi.org/10.1007/978-981-15-3797-4_7
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-15-3796-7
Online ISBN: 978-981-15-3797-4
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)