Abstract
This chapter shortly describes key stages of the intrauterine development of the thymus and its postnatal continuation from infancy to old age. The immunohistochemical features and distribution of the major cellular constituents, i.e., thymic epithelial cell subsets, T cells, B cells, macrophages, dendritic cells, and myoid cells are depicted and a short overview on thymic function, thymocyte development, and thymic involution is given.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
The thymus is a primary lympoid organ. It consists of epithelial cells, hematopoietic cells and mesenchymal cells and generates T cells from immature, bone marrow-derived precursors. Through selection processes, the T cells become functional and largely tolerant toward self-antigens and are of key importance for adaptive immune responses. Thymic failure, particularly if congenital, predisposes to life-threatening infections, neoplasia, and autoimmune diseases [1].
1.1 Embryology
The endoderm of the third pharyngeal pouches on both sides of the neck gives rise to “thymic epithelial cells” (TECs). From week 6 of gestation onward, the solid epithelial thymus anlage is present. By week 7, the common thymic/parathyroid primordia are established. The thymic components of the primordia descent along the carotid artery and behind the lower pole of the thyroid to the pre-cardiac region where they fuse [2].
From week 8 onward, the differentiation of cortical and medullary TECs (mTECs, cTECs) begins. By week 16, cortical and medullary compartments are established.
The developmentally indispensable thymic capsule and septae originate from neural crest-derived mesenchymal cells from week 7 onward [3, 4]. The earliest T cell precursors are present at week 8 [2]. T cell maturation and the generation of three-dimensional thymic lobes depend on interactions between NOTCH1 and DLL4 on T cells and TECs, respectively. Early Hassall corpuscles can be recognized by week 12 [5]. Mature T cells leave the thymus between week 14 and 16. The transcription factor FOXN1 is indispensable for the development of the thymus throughout embryonal and adult life [2]. Its defective expression elicits the nude phenotype and immunodeficiency in mice and humans [6, 7].
1.2 Normal and Ectopic Location of the Thymus
The normal position of the thymus is the anterosuperior mediastinum between the upper end of the sternum, the level of the fourth costal cartilage, the upper part of the pericardium and the pre-tracheal fascia, and the dorsal plain of the upper part of the sternum, costal cartilages, and intercostal muscles [8]. The lateral boundaries may extend beyond the phrenic nerves. Ectopic extensions comprise the cervical region up to the base of the skull, the mandibles and salivary glands, the middle and posterior mediastinum, and the intrapericardial and pleural spaces. The frequency of ectopic thoracic and cervical thymic tissue depends on the type of workup. On the microscopic level, frequencies amount to 20–50% [9, 10] but only to 1% in routine autopsies [11].
1.3 Macroscopy
The thymus is composed of two lobes. Their fibrous capsules stick together in the midline. Two upper and two lower “horns” can usually be recognized (Fig. 1.1). In children and adolescents, the cut section of the thymus resembles the cut surface of a lymph node. During the course of involution (see below) it becomes more and more yellow and is barely detectable in the elderly. The average thymus weight is about 15 g at birth (range 5–25 g), reaches a maximum of around 40 g (20–50 g) at 10–15 years of age, and declines thereafter, reaching 10–15 g (range 5–30 g) by age 60 [12].
1.4 Histology
Thymic lobes are composed of many lobules. During childhood, each lobule shows a central medullary compartment that is completely surrounded by an outer cortical layer. From adolescence onward, the architecture gets progressively disturbed, and medullary areas more and more abut on mediastinal fat (Fig. 1.2). In a strict sense, the third thymic compartment, the perivascular space (PVS), is an extrathymic space between the continuous basal membrane of the outermost TECs of thymic lobes and the basal membrane of the vessels that enter and leave the thymus along the septae (Fig. 1.3). Hematopoietic cells that enter or leave the thymus must cross the PVS to egress from or enter into blood vessels, respectively [13]. In the thymic capsule, interlobular septae, and medulla, efferent lymphatic vessels can be found [14].
Histology of thymuses in relation to age. (a) Thymus of a 1-year-old child: distinct lobular architecture with well-developed cortical areas (C) that completely envelope a medullary region (M); many small Hassall corpuscles (HC) and absence of interlobular fat are typical. (b) Thymus of a 30-year-old adult with increase of interlobular fat and medullary areas directly abutting on adipocytes (arrows). (c) Thymus of a 50-year-old adult with further loss of lymphoepithelial parenchyma and more severe distortion of the cortico-medullary architecture. (d) Thymus of a 70-year-old adult with near defect of cortical areas and paucity of lymphocytes within epithelial strands (HE, a–d)
Perivascular spaces (PVS) as highlighted by keratin 19 immunohistochemistry in a normal thymus. (a) Light-staining PVS are epithelial-free spaces reaching from the perithymic fat and along the septae to the cortico-medullary junction (white arrows); the PVS are filled with lymphocytes, the majority of which are mature T cells (C, cortex; M, medulla; HC, Hassall corpuscle). (b) Sharp delineation between an epithelial-free PVS (arrow) and cortex (C) and medulla (M) through a continuous layer of thymic epithelial cells; the disruption of the layer around PVSs is a typical sequela of lymphofollicular hyperplasia in myasthenia gravis. (c) Small capillary vessel (red arrows) within a PVS (immunoperoxidase, Keratin 19, a–c)
Epithelial Cells
Cortical and medullary TECs (cTECs and mTECs) show different histological features: The stellate-shaped cTECs are quite easily detectable due to their large, round nuclei with conspicuous nucleoli, while mTECs are hard to identify among the lymphocytes due to their small, oval nuclei with inconspicuous nucleoli (Fig. 1.4a–d). The distinction of cTECs from mTECs can be achieved by immunohistochemistry, using antibodies, e.g., to the cortex-specific proteasome subunit, Beta5t, and mTEC-restricted proteins such as CD40, Claudin-4, and the tolerance-inducing autoimmune regulator, AIRE [15,16,17] (Table 1.1; Fig. 1.5). AIRE-positive mTECs can develop toward Hassall corpuscles (HC) on downregulation of AIRE. HC are onion-shaped accumulations of concentrically arranged squamoid epithelial cells that can show keratohyalin granules, lose their nuclei toward their cornified centers, and may become calcified or cystic (Fig. 1.6). In contrast to other TECs, HC express cytokeratin 10 and involucrin and fail to express HLA-DR and DP [18]. Since T cell maturation is necessary for HC development, they are lacking in thymuses from patients with T cell developmental defects (historically called “thymic dysplasia”). During aging the number of HC declines [19]. HC have immune tolerogenic functions through shedding of autoantigens and their impact on the development of regulatory T cells [20].
Cytological features of normal cortical and medullary thymic epithelial cells (cTEC, mTECs). (a) cTECs with medium-sized to large vesicular nuclei with conspicuous nucleoli (arrows). (b) Barely visible mTECs (arrows) outside a Hassall corpuscle (HC) with small- to medium-sized nuclei and inconspicuous nucleoli. (c) Nuclei of cTEC highlighted by P40 staining. (d) Significantly smaller nuclei of mTEC outside HC highlighted by P40 immunohistochemistry (HE, a, b; immunoperoxidase, c, d)
Compartment-specific and largely unspecific epithelial markers in the normal thymus. (a) Expression of the thymus-specific proteasome subunit Beta5t exclusively in thymic epithelial cells of the cortex (C). HC, Hassall corpuscle. (b) Nuclear expression of the autoimmune regulator (AIRE) exclusively in a subset of medullary thymic epithelial cells. (c) Expression of keratin 19 in virtually all cortical and medullary epithelial cells and epithelial cells surrounding perivascular spaces (∗). (d) P40 expression in the nuclei of almost all cortical and medullary epithelial cells (immunoperoxidase, a–d)
Hassall corpuscle (HC) (a) HC with regressive changes and apoptotic cells in the medulla; inset, HC composed of vital, epidermoid cells with blue keratohyalin bodies; (b) HC with cystic enlargement containing debris; inset, typical expression of keratin 10 in the outer epithelial layers of a HC (HE, a, b; immunoperoxidase, keratin 10, inset)
T Cells (Thymocytes)
On immunohistochemistry, the cortex appears completely occupied by immature TdT+ thymocytes almost all of which co-express CD1a, CD99, CD3, CD4, CD5, and CD8 and are negative for CD10 and CD34 (so-called CD4/CD8 double-positive (DP) thymocytes) with a Ki67 index >90% (Fig. 1.7a, b). By contrast, the minor, CD34+ subset, CD10+ CD1a− subcapsular subset, and the CD4+CD8−CD3− immature single-positive (iSP) thymocyte subset can only be detected by flow cytometry [21]. In the medulla, almost all cells show a TdT-negative phenotype and a low Ki67 index and belong either to the CD3+CD4+CD8− or CD3+CD4−CD8+ so-called “single-positive” (SP) T cell subsets. Cortical and medullary thymocytes share expression of CD3 and CD5, with particularly strong CD5 expression in the medulla (Fig. 1.7c, d).
T cells in the normal thymus. (a) Restriction of immature, TdT-positive T cells to the cortex (C) with labelling of virtually all lymphoid cells. (b) High Ki67 index (>90%) of cortical thymocytes as compared to few Ki67-positive cells in the medulla (M). (c) CD3 expression on immature and mature T cells in both compartments. (d) Particularly strong expression of CD5 on T cells in the medulla (M) (immunoperoxidase, a–d)
B cells normally occur only in the medulla. The majority is round, while a minority is “asteroid shaped” (i.e., dendritic) if stained for CD20 [22]. The B cell content of medullary areas is highly variable, but B cells are always present. The “asteroid subset” shows a characteristic CD20+CD23+CD21− profile (Fig. 1.8). Thymic B cells can originate through immigration from extrathymic mature B cell sources (e.g., lymph nodes) or through intrathymic development from immature precursors [23, 24]. Thymic B cells are HLA-DR+ and involved in T cell tolerance through negative T cell selection and the induction of regulatory T cells [24, 25].
B cells in the normal thymus. (a) Mainly round CD20+ B cells in the thymic medulla (M) with apparent “spillover” of single B cells into the cortex (arrows). (b) The “asteroid” B cell subset that is generally blurred by the overwhelming majority of round B cells on CD20 immunohistochemistry can be highlighted by CD23 stains; CD23+ B cells must be distinguished from CD23+ follicular dendritic cells that form networks in thymic follicular hyperplasia (immunoperoxidase, a, b)
Macrophages and Dendritic Cells
Macrophages occur in the cortex and medulla, while dendritic cells (DCs) are largely restricted to the medulla with a major focus on the cortico-medullary junction. Macrophages are important for the removal of dying thymocytes during T cell selection [18, 26] and are CD68+ and/or CD163+ (Fig. 1.9a). The small and round subset is strongly HLA-DR+, while the large, stellate-shaped “starry sky macrophages” mainly of the cortex may contain apoptotic thymocytes and are HLA-DRlow [27]. DCs can arise from intrathymic precursors or enter the thymus as mature DCs from outside [28]. They are strongly HLA-DR+ and promote negative T cell selection and the induction of regulatory T cells [20]. Conventional DCs express CD11c (Fig. 1.9b) and may be AIRE+ [29], while the rare plasmacytoid DCs express CD123.
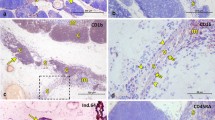
Macrophages and dendritic cells in the normal thymus. (a) Occurrence of CD68-positive macrophages throughout the thymus, including a Hassall corpuscle (HC); greatest frequency in the cortex (C); CD163-positive macrophages show a similar distribution and abundance (not shown). (b) CD11c-positive dendritic cells are largely restricted to the medulla, including the cortico-medullary junction; minor “spillover” CD11c-positive cells to the cortex (C)
Myoid Cells
Thymic myoid cells (TMCs) are fetal-type striated muscle cells of unknown origin in the medulla [30]. They express contractile proteins, including titin [31]. When stained for desmin, they resemble round, immature myoblasts or elongated myotubes (Fig. 1.10). Because they are non-innervated cells, TMCs express fetal and adult skeletal muscle-type nicotinic acetylcholine receptors that likely play a role in the pathogenesis of myasthenia gravis [32, 33]. The normal function of TMCs is unclear, but it has been speculated that they release autoantigens and, thereby, endow DCs with the potential to induce muscle-specific T cell tolerance through negative selection [34].
Thymic myoid cells (TMCs) in the normal thymus: occurrence of desmin-positive TMCs exclusively in the medulla (here abutting on fat cells, right upper part); round, rhabdomyoblast-like TMCs (white arrows) and more elongated, myotube-like TMCs (black arrows) with vague cross-striations are present in the vicinity of a Hassall corpuscle (HC) (immunoperoxidase, desmin)
1.5 Thymic Function
The thymus has three key functions: i) to recruit hematopoietic precursor cells from the blood into the thymus and drive their multistep maturation and expansion (Fig. 1.11) [21, 35], leading to a diverse repertoire of α/βT cells that can recognize millions of antigenic peptides if they are presented by antigen-presenting cells (APCs) on class I and II major histocompatibility (MHC) molecules (“positive selection”); ii) to eliminate from the functional T cells the subset of autoreactive T cells (“negative selection”) through the action of mTECs, DCs, and thymic B cells mainly in the medulla [29, 36, 37]; and iii) to generate immunosuppressive CD4+CD25+ FOXP3+ regulatory T cells (Tregs) that are indispensable for keeping autoreactive T cells in check that inevitably escape from negative selection and reach the peripheral immune system [38]. An essential factor for negative selection is the transcriptional “autoimmune regulator,” AIRE, that drives the expression of thousands of self-antigens in a subset of mTECs and endows them with the capacity to kill T cells if they show high affinity for MHC-presented self-peptides [39]. The thymus is also important for the generation of γ/δ T cells [40] and NKT cells [41].
Maturation of alpha/beta T cells and main levels of operation of positive and negative T cell selection in the human thymus: TSP thymic-seeding precursors (the most immature T cell precursors entering the thymus), DN double-negative cells (in terms of CD4 and CD8 expression, cCD3 denotes CD3 expression in the cytoplasm), iSP immature single-positive cells, DP double-positive cells (constitute more than 90% of the cortical thymocytes; sCD3 denotes CD3 expression on the cell surface), SP single-positive T cells, and pre-emigrants the most mature T cells generated in the thymus that are ready to egress from the thymus at the cortico-medullary junction (CMJ) into perivascular spaces (PVS) and from there into PVS-borne blood vessels and the circulation
1.6 Thymic Involution
Thymic involution denotes the physiological, age-related, and gradual replacement of functional thymic tissue by fat (see above Figs. 1.1–1.3). Morphometry showed that involution starts in the first year of life and continues thereafter, leaving about 5% of thymic parenchyma by the age of 60 [19] . In a broader sense, involution includes thymic atrophy (“accidental involution”) that happens through various “stressors” such as pregnancy, infection/inflammation, malnutrition, and cancer. Factors involved in thymic atrophy are corticosteroids, sex hormones, IFN-α, adipocyte-derived factors (e.g., LIF), TNF-α, IL6, and growth factors [42, 43]. Mechanisms that are operative in relation to age are declining levels of FOXN1, decreasing proliferative activity of TECs with age, exhaustion of TEC progenitor cells, and the declining capacity of cTECs to induce T lineage commitment through NOTCH1 signaling and of mTECs to induce tolerance through expression of self-antigen [44].
These age-related changes lead to a gradual accumulation of senescent T cells and—most likely—to an increased risk of infections and cancer with increasing age [45]. On the other hand, the relative resistance of senescent T cells to regulatory signals and propensity to generate increased amounts of IFN-γ increase the risk for inflammatory tissue reactions and autoimmunity [46].
References
Gupta S, Louis AG. Tolerance and autoimmunity in primary immunodeficiency disease: a comprehensive review. Clin Rev Allergy Immunol. 2013;45(2):162–9.
Farley AM, et al. Dynamics of thymus organogenesis and colonization in early human development. Development. 2013;140(9):2015–26.
Anderson G, et al. MHC class II-positive epithelium and mesenchyme cells are both required for T-cell development in the thymus. Nature. 1993;362(6415):70–3.
Patenaude J, Perreault C. Thymic mesenchymal cells have a distinct transcriptomic profile. J Immunol. 2016;196(11):4760–70.
von Gaudecker B, Muller-Hermelink HK. Ontogeny and organization of the stationary non-lymphoid cells in the human thymus. Cell Tissue Res. 1980;207(2):287–306.
Nehls M, et al. New member of the winged-helix protein family disrupted in mouse and rat nude mutations. Nature. 1994;372(6501):103–7.
Frank J, et al. Exposing the human nude phenotype. Nature. 1999;398(6727):473–4.
Carter BW, et al. ITMIG classification of mediastinal compartments and multidisciplinary approach to mediastinal masses. Radiographics. 2017;37(2):413–36.
Kotani H, et al. Ectopic cervical thymus: a clinicopathological study of consecutive, unselected infant autopsies. Int J Pediatr Otorhinolaryngol. 2014;78(11):1917–22.
Jaretzki A, Steinglass KM, Sonett JR. Thymectomy in the management of myasthenia gravis. Semin Neurol. 2004;24(1):49–62.
Bale PM, Sotelo-Avila C. Maldescent of the thymus: 34 necropsy and 10 surgical cases, including 7 thymuses medial to the mandible. Pediatr Pathol. 1993;13(2):181–90.
Hammar JA. Die Menschenthymus in Gesundheit und Krankheit. Ergebnisse der numerischen Analyse von mehr als tausend menschlichen Thymusdrüsen. Teil I: Das normale Organ. Zugleich eine kritische Beleuchtung der Lehre des “Status thymicus”. Zeitschrift für Mikroskopische Anatomie und Forschung. 1926;6(Suppl):1–570.
Maeda Y, et al. S1P lyase in thymic perivascular spaces promotes egress of mature thymocytes via up-regulation of S1P receptor 1. Int Immunol. 2014;26(5):245–55.
Kato S. Thymic microvascular system. Microsc Res Tech. 1997;38(3):287–99.
Heino M, et al. Autoimmune regulator is expressed in the cells regulating immune tolerance in thymus medulla. Biochem Biophys Res Commun. 1999;257(3):821–5.
Kyewski B, Peterson P. Aire, master of many trades. Cell. 2010;140(1):24–6.
Herzig Y, et al. Transcriptional programs that control expression of the autoimmune regulator gene Aire. Nat Immunol. 2017;18(2):161–72.
Douek DC, Altmann DM. T-cell apoptosis and differential human leucocyte antigen class II expression in human thymus. Immunology. 2000;99(2):249–56.
Strobel P, et al. The ageing and myasthenic thymus: a morphometric study validating a standard procedure in the histological workup of thymic specimens. J Neuroimmunol. 2008;201-202:64–73.
Watanabe N, et al. Hassall corpuscle instruct dendritic cells to induce CD4+CD25+ regulatory T cells in human thymus. Nature. 2005;436(7054):1181–5.
Blom B, Spits H. Development of human lymphoid cells. Annu Rev Immunol. 2006;24:287–320.
Isaacson PG, Norton AJ, Addis BJ. The human thymus contains a novel population of B lymphocytes. Lancet. 1987;2(8574):1488–91.
Akashi K, et al. B lymphopoiesis in the thymus. J Immunol. 2000;164(10):5221–6.
Perera J, et al. Self-antigen-driven thymic B cell class switching promotes T cell central tolerance. Cell Rep. 2016;17(2):387–98.
Lu FT, et al. Thymic B cells promote thymus-derived regulatory T cell development and proliferation. J Autoimmun. 2015;61:62–72.
Surh CD, Sprent J. T-cell apoptosis detected in situ during positive and negative selection in the thymus. Nature. 1994;372(6501):100–3.
Wakimoto T, et al. Identification and characterization of human thymic cortical dendritic macrophages that may act as professional scavengers of apoptotic thymocytes. Immunobiology. 2008;213(9-10):837–47.
Cosway EJ, et al. Formation of the intrathymic dendritic cell pool requires CCL21-mediated recruitment of CCR7(+) progenitors to the thymus. J Immunol. 2018;201(2):516–23.
Fergusson JR, et al. Maturing human CD127+ CCR7+ PDL1+ dendritic cells express AIRE in the absence of tissue restricted antigens. Front Immunol. 2018;9:2902.
Bockman DE. Myoid cells in adult human thymus. Nature. 1968;218(5138):286–7.
Marx A, et al. A striational muscle antigen and myasthenia gravis-associated thymomas share an acetylcholine-receptor epitope. Dev Immunol. 1992;2(2):77–84.
Schluep M, et al. Acetylcholine receptors in human thymic myoid cells in situ: an immunohistological study. Ann Neurol. 1987;22(2):212–22.
Marx A, et al. The different roles of the thymus in the pathogenesis of the various myasthenia gravis subtypes. Autoimmun Rev. 2013;12(9):875–84.
Van de Velde RL, Friedman NB. Thymic myoid cells and myasthenia gravis. Am J Pathol. 1970;59(2):347–68.
Garcia-Leon MJ, et al. Dynamic regulation of NOTCH1 activation and NOTCH ligand expression in human thymus development. Development. 2018;145:16.
Klein L, et al. Positive and negative selection of the T cell repertoire: what thymocytes see (and don’t see). Nat Rev Immunol. 2014;14(6):377–91.
Gies V, et al. B cells differentiate in human thymus and express AIRE. J Allergy Clin Immunol. 2017;139(3):1049–1052.e12.
Bacchetta R, Barzaghi F, Roncarolo MG. From IPEX syndrome to FOXP3 mutation: a lesson on immune dysregulation. Ann N Y Acad Sci. 2018;1417(1):5–22.
Derbinski J, et al. Promiscuous gene expression in medullary thymic epithelial cells mirrors the peripheral self. Nat Immunol. 2001;2(11):1032–9.
Munoz-Ruiz M, et al. Thymic determinants of gammadelta T cell differentiation. Trends Immunol. 2017;38(5):336–44.
Benlagha K, et al. A thymic precursor to the NK T cell lineage. Science. 2002;296(5567):553–5.
Dooley J, Liston A. Molecular control over thymic involution: from cytokines and microRNA to aging and adipose tissue. Eur J Immunol. 2012;42(5):1073–9.
Youm YH, et al. Prolongevity hormone FGF21 protects against immune senescence by delaying age-related thymic involution. Proc Natl Acad Sci U S A. 2016;113(4):1026–31.
Hamazaki Y. Adult thymic epithelial cell (TEC) progenitors and TEC stem cells: models and mechanisms for TEC development and maintenance. Eur J Immunol. 2015;45(11):2985–93.
Palmer S, et al. Thymic involution and rising disease incidence with age. Proc Natl Acad Sci U S A. 2018;115(8):1883–8.
Fessler J, et al. The impact of aging on regulatory T-cells. Front Immunol. 2013;4:231.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Marx, A. (2020). The Normal Thymus. In: Jain, D., Bishop, J.A., Wick, M.R. (eds) Atlas of Thymic Pathology. Springer, Singapore. https://doi.org/10.1007/978-981-15-3164-4_1
Download citation
DOI: https://doi.org/10.1007/978-981-15-3164-4_1
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-15-3163-7
Online ISBN: 978-981-15-3164-4
eBook Packages: MedicineMedicine (R0)