Abstract
For the last few decades, with the emergence of nanoscience and nanotechnology, nanoparticles gained enormous attention due to their high surface/volume ratio and other novel, unique, and remarkable properties. Traditionally, nanoparticles are fabricated by either chemical or physical approaches which not only utilize toxic chemicals but also are energy-intensive and consequently costly. The microbe-based synthesis of nanoparticles is biocompatible, economical, eco-friendly, and energy-intensive. Metallic and nonmetallic, metal oxide, and sulfide nanoparticles are synthesized by bacteria, virus, fungi, and algae. These nanoparticles act as an adsorbent for the remediation of water and wastewater pollutants clearly due to their physicochemical properties, nano size, controlled growth, and surface modification. The carbohydrates, proteins, and enzymes present in such microbes act as surfactant and capping agents which reduce the use of harmful chemical surfactants. Nanoparticles find application in the remediation of dyes, heavy metals, microbial contaminants, and pesticides, in the area of wastewater treatment. The widely used adsorbents are iron oxide nanoparticle, zinc oxide, alumina, and titanium dioxide. The present chapter highlights the synthesis of nanoparticles from bacteria, algae, fungi, and viruses and their application for wastewater treatment.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
7.1 Introduction
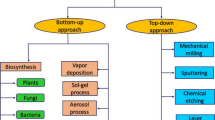
Increasing population and advanced lifestyle have resulted in mounting pressure on environmental resources, deteriorating the process feasibilities of multiple natural environmental conservation pathways. The effects seem to be convincingly regulated, after the reports of environmental pollution assessment agencies, reflecting inescapable approaching threats. Though several efforts and resolving inroads are rapidly emerging to tackle this issue, nothing serious seems to shake the generating source, owing to which there is an urgent need to counteract the probable risk factors with powerful and stronger prediction technologies. For example, on a daily basis, more than half of the natural processes require conversion of harmful and complex materials into simpler and self-decomposable entities, through interventions that are increasingly energy sensitive, whereby stress levels and contaminants are bound to affect the environmental quality. So better and more powerful solutions, consuming little and effecting much higher problem domain, are urgently needed. In this context, the unique physical and chemical properties of nanoscale materials have been the focus of sheer interest ever since their inception. Nanotechnology and nanoparticles have drawn the attention of the scientific community towards them (Seqqat et al. 2019). Mostly nanoparticles due to their size tunable and fascinating properties find applications in the field of electronics, medicine, research, catalysis, and environmental cleanup (Khan et al. 2019), due to which their demand has increased drastically, and the load on the industries for their synthesis has increased manyfold. The commercial synthesis of nanoparticles is generally carried out by various chemical and physical routes. Among the chemical approaches, the most common methods are sol-gel, coprecipitation, hydrothermal, solvothermal (Ganachari et al. 2017), etc. On the other hand, the physical synthesis method includes laser sputtering, chemical vapor deposition (CVD), and ball milling. In addition to their advantages, these chemical and physical methods have several disadvantages as well. Chemical methods utilize numerous hazardous chemicals as surfactant reducing agents, or reactants, which are toxic to the environment. While the physical methods require sophisticated machinery for their synthesis, they are highly energy-intensive, which makes them expensive. So there is a need for another alternative source which should overcome the drawbacks of the abovementioned methods. A generalized method of nanoparticle synthesis is depicted in Fig. 7.1.
The biological synthesis of nanoparticles is the best alternative method which will overcome the drawback of the abovementioned problems. Among the biological methods, microbial-based synthesis of nanoparticles is of utmost importance due to its cost-effectiveness, ease of synthesis, and eco-friendly nature (Singh et al. 2016a; Prasad et al. 2016). Since the source of preparation is economically viable and renewable, interest in their scientific evaluation has attracted unconditional support from different parts of the globe. Besides this, the biosynthesized nanoparticles are also biocompatible which can be used directly in the field of medicine and drug delivery. Microorganisms are the organisms whose sizes fall in the range of microns. Generally, they can be classified into two classes: prokaryotic and eukaryotic. Prokaryotic involves bacteria and Actinomycetes, whereas eukaryotes involve algae, fungi, and yeast. There were several reports of using viruses as the templates for the synthesis of several nanoparticles. Microorganisms have numerous biomolecules such as enzymes, organic acids, polysaccharides, etc. known to play a key role in the synthesis process. Moreover, such biomolecules encapsulate the nanoparticle which further prevents the aggregation by acting as a capping agent. Microorganisms can synthesize all types of nanoparticles by reduction, for instance, single metallic nanoparticles (like gold, silver, copper, Se, Fe, or NZVI) (Das et al. 2017) or metal oxides (titanium oxide, zinc oxide, iron oxide, silica, and aluminum oxide) or metal sulfides (like PbS, CdS, PbSe, CdSe, ZnS). Metallic nanoparticles have almost replaced conventional catalysts in different biochemical and even industrial activities. The generalized flowchart for extracellular and intracellular biosynthesis is shown in Fig. 7.2.
The biologically synthesized biocompatible and eco-friendly nanoparticles have potential for wastewater treatment like remediation of water pollutants such as dyes, heavy metals, pesticides, and other organic/inorganic pollutants (Das et al. 2017). The nanoparticles being small in size have a high surface/volume ratio (SVR) and comparatively high surface energies have a special role in wastewater treatment (Shah et al. 2015a). Moreover, their microbial origin provides them special advantage like capping by biomolecules, which not only prevents their aggregation but also provides different functional active sites. These active sites help in the adsorption of pollutants from the wastewater.
The contamination of wastewater streams and its associated risks of environmental degradation is the source of polluting water bodies owing to which aquatic organisms are at risk (Bhateria and Jain 2016). The risk factors are enhanced significantly due to the heterogeneous nature of contaminants, requiring differential treatment measure for each specific kind of pollutant. The NPs with their high surface areas (SA) are able to initiate multiple interactions with chemically distinct pollutants at the same instant of time, owing to which in the same time interval, pollutant load is reduced to a greater extent as compared to conventional technologies (Lin et al. 2014). Keeping such characteristics of metal/metal oxide NPs in observance, the present chapter focuses on the advances in the field of microbial synthesis of single metals and metal oxide nanoparticles and their potential applications in the field of remediation of various organic and inorganic pollutants from the water and wastewater.
7.2 Advantages of Bacteria-Mediated Synthesis of Nanoparticles
Biological systems especially bacteria, due to their high growth rate, act as a unique candidate to achieve nonhazardous nanoparticle synthesis. In chemical methods, the growth of NPs is usually expensive and ends with toxic end products released into the environment. In biological systems, an individual bacterium can be a center and a matrix for the controlled growth on NPs.
Studies on bacterially synthesized NPs reveal interesting variations, with extracellular as well as intracellular formation sites, variability in the shapes and sizes, and numerous others. A key concern in all the cutting-edge applications of NPs (ranging from industrial, chemical, biological, and many others) is the extent for which these entities retain their low sizes (Nath and Banerjee 2013). The diverse chemical environments of application sites often lead to the aggregation of these moieties, so it is very important that these nanomaterials retain their native sizes for sufficiently longer durations. That is why physically prepared NPs are often ineffective in most applications and the problem of aggregation is frequently encountered. With biological sources, there is an advantage that an aggregation preventing agent does not need to be added separately, and the polysaccharides, protein fractions, and fatty acid conjugates of microbes serve as aggregation stabilizing agents (Siddiqi et al. 2018). Apart from this, culturing bacteria is relatively easy (since it is the largest active microbe among all varieties) and relatively less costly as compared to reducing agents of the chemical method. Furthermore, an incentive with the bacterial synthesis is that even if we have one bacterial strain, culturing it in different chemical environments (characterized by pH and temperature variation) could form different NPs at the same instant of time. For example, if the salt precursor for Fe is added in the culture medium of magnetotactic bacterium, we will get Fe-O NPs, while if a cobalt salt is added, the likely product would be Co (as such) or in some combined form as NPs. The reason is that both Co and Fe are magnetically sensitive and have unpaired electrons in their molecular orbitals. Similarly, the NPs of two metals belonging to the same group of the periodic table could be formed in a simplistic manner as they would have similar chemical characteristics. The only distinction is the presence of salt (for each metal type) in the culture medium of the chosen bacterial strain. So, low cost lesser synthesis time, variability, and better stability of the product (here NPs) are some of the reasons to choose a bacterial route for NP synthesis (Shah et al. 2015a).
The exact mechanism of microbial-based nanoparticle formation is not known and most of the studies project consensus for the metabolism of toxic compounds by bacteria after which a whole range of defensive genes is activated to combat the resultant toxicities. The activation of these genes is accompanied by the sequential modulations in oxidation and reduction chemical balances which ultimately result in the prevalence of a peculiar form that neutralizes the toxicity to a maximum extent. In this reference, some authentic claims are put forward by Ghashghaei et al. in their 2015 compilation, describing distinctive three-step nanoparticle formation by bacteria (Gahlawat and Choudhury 2019). The first discusses the bacterial self-assembled nanostructures such as pilus, flagellum, S-layer bipolyester, cyanophycin inclusions, phage, rhodopsin, and alginates. The second possibility projects the metal and metal oxide nanoparticle formation as bacterial metabolism by-products. In this mechanism, there seems to be an implicit role of bacterial enzymes. The third step of nanoparticle formation by bacteria relies on bacterial polymers (using polysaccharides and polysaccharide derivatives) which can be thereafter processed to NPs. Derivatives of plant products are highly reliable sources of preventing aggregation owing to their natural mode of existence, least energy requirements for operation, and much lower toxicity than chemical agents accomplishing the same. Discussing the diversity of mechanisms explaining nanoparticle formation, they concluded one common aspect in all approaches, involving the trapping of metal ions either on the surface of or inside microbial cells. Subsequently, these are reduced to NPs through enzymatic or nonenzymatic methods. Holding detoxification pathways as prime sources responsible for bacterial (and even other) nanoparticle formation, they illustrate a nonspecific uptake of toxic metal ions via the cationic membrane transport systems, which regulate the transport of metabolically important cations. Now since these uptaken metal ions are toxic, they are not tolerated by bacteria beyond a threshold concentration which is accomplished either via mutagenesis of metal-resistant bacterial strains or by complementation to identify the resistance restoring genes. The identification of genes responsible for causing toxic responses paves the way for their enzymatic inactivation of toxic ionic forms of the metal into nontoxic metal salts, modulation in metal ion efflux system activities, and diminishing membrane permeability. Figure 7.3 discusses the concentration-dependent enzymatic induction controls in a bacterium to counter the toxicity threats to the bacterium itself.
The higher the toxicity (caused by an increasing population of metal ions), the more pronounced is the activation of enzymes restoring the normal pH and chemical balance. Diversity of the product manifested by changes in the process parameters, such as pH, temperature, duration of culture, type of strain, culture conditions, and their mutual optimization, is a key factor affecting the nature of NPs formed. Interesting aspects within these predictors are the differences in the product’s nature attained through differential regulation of comprising factors. For example, maintaining the temperature at 45 °C for half an hour is likely to confer different features compared to assuring the same for an hour or more. Similarly, the concentration of a characteristic protein source in the culture medium could be responsible for a peculiar nature of the product which may vary with the change in the protein source. The obvious reason is that proteins are composed of amino acids that play decisive roles in regulating 1:1 dispersion. Constituent amino acids may exhibit an altogether distinct response if they are dextro-rotatory instead if leave or vice versa. Likewise, composition of dispersion medium in the culture plays a critical role where if the water is the major source, polar activities are likely to be aggressive contrary to aqueous ethanol or some other additive (organic) source. On a similar note, if we change the pH, the dispersion trend is likely to be varied that is reflected in the ζ-potential trend. It may be possible that the shape and size of NPs being prepared are more regulatory and optimized at acidic pH which the alkaline pH is unlikely to support. The discussed factors present preliminary aspects, which, if replicated on a molecular scale, can have quite significant consequences. For instance, acidic pH may be supportive inactivating a particular bacterial gene/enzyme in a specific manner that the basic pH is unable to do. At the same point, it is imperious to elucidate the consequence of a peculiar pH on the expression of neighborhood genes and enzyme secretion, so that growth remains unaffected. An accurate generalization of the bacterial activities in different growth phases is the key to the choice of a bacterial species for a certain kind of nanoparticle synthesis. Generally, in the log phase, the most active nutrients are synthesized and the stationary phase is the domain where the functional activities could be stimulated.
Thus, exploiting the bacterial genetic makeup is the groundwork required for thoughtful and optimized regulation of nanoparticle formation. The mechanistic insights probably offer incentives that, with the variations in culture ingredients, even hybrid or core-shell NPs could also be prepared. Similar robustness is not attainable with fungal cultures since fungi require adequate growth of mycelia to exhibit the desired metabolic performance. Apart from this, the protein content of fungi is very much different from that of a bacterium, owing to which growth conditions remain less controlled with the former. Nevertheless, the logical estimation of obtaining still better results with consortium cultures suits well for the kind of NPs being formed.
7.3 Bacteria-Mediated Synthesis of Metallic Nanoparticles
Microbes used for preparing metal and metal oxide NPs comprise bacteria, fungi, virus, and algae, thanks to the robust chemical actions of microbial enzymes which remain operational even at extreme temperature and pH conditions. An interesting aspect of microbial biochemical activities is that the expression of enzymes could be modulated by changing the chemical compositions of their culture media. Optimization of culture parameters such as pH, light exposure, temperature, medium salt content, and mixing speed is a pivotal factor which can significantly modulate the enzyme activity (Sarker et al. 2013; Kirthi et al. 2011a).
Bacteria can significantly reduce heavy metal ions to produce nanoparticles; many researchers proved that bacteria facilitated interactive pathways accountable for the reduction of metal ions and their ability to precipitate metals to the nanometer scale (Kirthi et al. 2011a). One of the major benefits of bacteria-mediated nanoparticle synthesis is their large-scale production with negligible use of hazardous chemicals; however, there are firm boundaries like laborious bacterial culturing procedures and less control over their shape, size, and distribution.
So keeping track of media composition-dependent enzyme expressions easily serves as the lead for facilitating the requisite extent of chemical reduction. For example, the most common bacteria, E. coli, acts as a probiotic within the physiological environment, hinting that changing the pH and the chemical composition of the surrounding environment could activate another set of E. coli enzymes whereby functions other than the probiotic activities could be exercised (Fijan 2014). Another important feature responsible for regulating nanoparticle formation by bacteria is their ability to interact with their nearby environment arising due to the polarity of their lipidic membranes that catalyzes diverse oxidation-reduction processes (most of the nanoparticle preparation is enacted through a chemical reduction of metallic salts) (Singh et al. 2018). The distinction in cell wall polarities of gram-positive (GP) and gram-negative (GN) stains (dissimilar peptidoglycan extents) is, in itself, quite a significant prospect of bacterial structural diversity, which is missing in all other microbes. Another critical factor affecting NP formation by bacteria is the choice of a particular species (Singh et al. 2018; Wang et al. 2017). This is because all bacteria do not have a similar genetic makeup and that is why culturing two different species in the same media is likely to result in differential functional expressions of similar redox enzymes. To meet such challenges, a consortium of the bacterial population, comprising two or more species, generally performs better than a single bacterium. Furthermore, studies on the microbial synthesis of NPs have notified that only those biological moieties that have potential to accumulate the metallic precursors in the form of oxides, hydroxides, sulphates, etc. have the best chance of forming metal NPs (Zargar et al. 2011).
Synthesis of NPs using biological moieties (enzymes, sugar, and proteins secreted by a bacterial strain) is a bottom-up approach, producing less waste material and enabling the formation process to be stopped as per the requirement. This is particularly exciting, as applications of NPs are substantially expressed through their shape and size variations, which acutely affect their interaction abilities. Another advantage with bacterially synthesized NPs is that the enzymes responsible for initiating biochemical reduction of corresponding metallic precursors could be amplified in their expression through knowledge of their regulatory genes and pathways. Similarly, the introduction of these enzymes from the outside could facilitate an extracellular synthesis of both, i.e., pure metallic and their oxides NPs, the absence of which drives the synthesis at intracellular locations (Patra and Baek 2014; Malik et al. 2014).
7.4 Inception and Progression of Bacterially Formed NPs
7.4.1 Bacterial Synthesis of Metallic Nanoparticles
Bacteria bear exceptional abilities for reducing metallic ions into their zerovalent forms (the nanoparticle state) and are perhaps the most befitting candidates for nanoparticle synthesis attributed to their ease of handling and robust culture medium requirement (Ruttkay-Nedecky et al. 2017a). In comparison to other microbes, it is very easy to mold and manipulate genes in bacteria for retrieval of metal ions. Generally, bacteria are exposed to harsh and increased heavy metal ion concentration in their surroundings. To combat these stressful conditions, bacteria have developed numerous defense processes, for instance, intracellular sequestration, efflux pumps, fluctuation in concentration ions of metals, and extracellular precipitation. These defense mechanisms form the basis of shape- and size-specific nanoparticle synthesis by bacteria. Table 7.1 comprises the various bacterial strains used for the synthesis of NPs along with their specific applications. The first incidence of bacterial synthesis of Au NPs was reported by Beveridge and Murray, who noted the extracellular deposition of Au NPs on Bacillus subtilis cell wall when AuCl4 solution was exposed to its colonies (Beveridge and Murray 1980). Almost at the same time, Klaus-Joerger intracellularly synthesized AgNPs < 200 nm using an NADH-dependent reductase as an electron source and, in course, itself getting oxidized to NAD+. It was noted that e− transfer from NADH results in a biological reduction of Ag + ions to their zerovalent form (the nanoparticle form) (You et al. 2013). The emergence of bacteria-mediated nanoparticle synthesis acquired attention in 2012 when Srivastava et al. intracellularly synthesized Pd, Ag, Rh, Ni, Fe, Co, Pt, and Li NPs (Srivastava and Constanti 2012). Interestingly, these synthesis procedures do not require an external stabilizing agent and electron donors and also did not need any pH modification in the course of biomineralization of different metal ions. Various metallic nanoparticles synthesized using various microbes are summarized in Table 7.1.
Some of the bacteria have shown exceptional phenomenon of nanoparticle synthesis, like synthesis at extremely high concentration of metal ions, acidic pH, and higher temperature. Ps. stutzeri and Ps. aeruginosa are bacteria that can grow at higher metallic concentration (Lu et al. 2016).
Besides this, there are a few iron-reducing bacteria that can reduce ferric to ferrous form when grown on elemental sulfur, i.e., Thiobacillus ferrooxidans, T. thiooxidans, and Sulfolobus acidocaldarius. This was reported by Brock and Gustafson, and the detailed process is given in IONP synthesis by non-magnetiotactic bacteria (Brock and Gustafson 1976). Other biomineralization phenomena, such as the formation of tellurium (Te) in Escherichia coli K12 (Iravani 2014), the direct enzymatic reduction of Tc (VII) by resting cells of Shewanella putrefaciens and Geobacter metallireducens, and the reduction of selenite to selenium by Enterobacter cloacae, Desulfovibrio desulfuricans, and Rhodospirillum rubrum (Kessi et al. 1999), have been reported as well.
Mullen et al. reported the leaching capability of Ag+, Cd2+, and Cu2+ from solution by Bacillus cereus, P. aeruginosa, B. subtilis, and E. coli. Some of them can bind to large metallic ions, while some can form magnetic nanoparticles (Kessi et al. 1999) which are shown below in Table 7.1.
Multiple studies of the past 5 years have reported the quick and fast synthesis of NPs possessing varying size and shape from several bacterial strains, such as E. coli, B. subtilis, B. cereus, B. megaterium, Ps. aeruginosa, Klebsiella pneumoniae, Alteromonas, and Ochrobactrum. The idea of bacterial enzymatic functions can be well judged from the simplistic procedures, involving minimum time periods without any sophisticated pH and temperature optimization. For example, Das et al. reported that B. cereus could synthesize AgNPs extracellularly at ambient temperature within 24 h (Lakshmi Das et al. 2014). Kumari et al. reported enhanced antifungal activity of AgNPs prepared using biological route (fungus, Trichoderma viride) contrary to that of chemical method, although, in both modes, the sizes and shapes of produced NPs were similar. It was observed that biologically prepared particles effected (20 and 48.8)% higher decrements (compared to those prepared chemically) in the dry weight of fungal pathogens, namely, Fusarium oxysporum and Alternaria brassicicola. Staining assays using nitro blue tetrazolium and propidium iodide dyes revealed an enhanced generation of superoxide radicals, as the probable cause of death for the biologically prepared NPs. Scanning electron microscopy (SEM) analysis revealed altered osmotic balance and membrane disintegrity as the primary source of cell death (Kumari et al. 2019). This study, therefore, concluded that compared to the chemically produced NPs, the bacteria synthesized NPs effected comparatively higher ROS generation, antioxidant pathway downregulation, and disruption of osmotic balance and cellular integrity. From this research, the observations infer a higher toxic expression of biologically prepared NPs, which confers them a preference compared to the chemically prepared NPs in the treatment of wastewater streams. The hidden prospect of this study is the size of the synthesized NPs, which have an intricate effect on their functional properties. It is well known that smaller size at the nanoscale argues for stronger quantum confinement which has a direct correlation with the particle energies. Since this study observed higher toxicity for biologically synthesized NPs, it seems certain that biological sources could have produced a lower size as lower size could have increased the kinetic stability owing to which interactive potential of the formed NPs increases. Such size controls are attainable with chemical methods where the stabilizing or aggregation preventing agent needs to be added separately to prevent the size growth beyond a stage.
Further, AgNPs was also synthesized extracellularly by Kulkarni et al. using Deinococcus radiodurans. D. radiodurans are an extremely robust bacterium species which can survive cold, dehydration, vacuum, radiation, and acid exposures (Kulkarni et al. 2015). The NPs were synthesized by the reduction of AgCl solution, following which these were screened for their antibacterial and antibiotic responses towards GN and GP bacteria. The radiation resistance properties of Deinococcus radiodurans enabled its activity even in extreme environments, owing to which good results were obtained with regard to anticancer studies. Results of this study have encouraged the scientific community to optimize Deinococcus radiodurans growth kinetics (via changes in the culture medium composition) so that palladium, platinum, and several other NPs could also be produced with similar control and ease. An incentive with such microbial species is that they can be as such added to wastewater streams where they can feed and regulate their metabolism through processed consumption of organic contents, such as discarded proteins, carbohydrates, fats, nitrogen sources, and several others. The temperature-resistant functioning of Deinococcus radiodurans offers special benefits as it is likely to cause minimal structural changes of synthesized NPs owing to which the NPs retain an unaltered application potential to a longer extent. Digging for the mechanism of bacterial NP formation, Sneha et al. replicated the attempt of Liu et al. (synthesis of AuNP Bacillus megaterium dried cells) using Corynebacterium species to obtain NPs and proposed a nonenzymatic reduction mechanism to be the source of nanoparticle formation (Liu et al. 2000). They noted that the formation of NPs by bacteria depended on two major requirements, where the first was the presence of organic functional groups in the bacterial cell wall, while the second factor was the maintenance of optimum temperature and pH in the surrounding environment. Studies by He et al. provide the most significant justification of this fact where working on Rhodopseudomonas capsulata bacterium growth optimization, they noted that the peculiar morphology of the formed particle can be governed by concentration of metallic salts and pH of the medium. There was formation of spherical AuNPs within 10–20 nm at pH 6 using diluted AuCl4 solutions. However, when the salt concentration was increased, the reaction formed Au nanowires (He et al. 2008). Interestingly, reduction of pH to 4 (enhancing the acidity), diluting salt concentrations, formed both spheres and triangular nanometer-scale plates. This study clearly established the role of pH and precursor concentration in the formation efficacy, size, and shape regime of NPs. So the same bacterium being fed on a similar metallic precursor could produce differently shaped NPs with the variation in medium pH. Similar attempts with Lactobacillus sp. A09 and B. megaterium D01 produced AgNPs through distinct reduction of silver ions (Gowramma et al. 2015). For details on the bacterial potential to synthesize NPs that are not discussed above (palladium, platinum, and some other metals), readers can refer to more specific literature sources (Shah et al. 2015b).
7.5 Bacterial Synthesis of Gold (Au) and Silver (Ag) Nanoparticles
Both Ag and AuNPs have drawn huge attention towards them due to their applications in medicine, electronics, cosmetics, coatings, packaging, and biotechnology. The production of nanoparticles emerges as an environment-friendly and exciting approach due to the use of microorganisms. Kushwaha et al. (2015) synthesized silver nanoparticles using E. coli and assessed their antibacterial potential against bacteria Bacillus subtilis and Klebsiella pneumoniae after confirming by UV-Vis and TEM (Sabri et al. 2016).
7.5.1 Gold (Au) Nanoparticles
In the biotechnology and biomedical field, the gold and silver NPs are considered very essential and interesting for several applications. Gold and silver NPs mainly find applications in the biomedical field such as diagnosis and therapy. Gold nanoparticle synthesis gained a lot of interest from many researchers. Rhodopseudomonas capsulate, Shewanella oneidensis, Escherichia coli, Yarrowia lipolytica, and Plectonema boryanum have been applied for the formation of gold nanoparticles (Sehgal et al. 2018). Gold nanoparticles (AuNPs) are mainly applied in the field of drug delivery for cancer, diabetes mellitus, and heart-related diseases. It also finds application in medicine, mainly diagnosis and therapy of cancer and as anti-arthritic and malarial agents (Prasad et al. 2018). AuNPs are synthesized by Klebsiella pneumoniae, which is pathogenic bacteria, and their antimicrobial activity is assessed against pathogenic bacteria, i.e., E. coli, Staphylococcus epidermidis, S. aureus, Pseudomonas aeruginosa, and Bacillus subtilis (Prema et al. 2016). AuNPs of various shapes i.e. rectangular, square, cubic, and triangular shape with an average diameter of 60 nm were synthesized using Rhodomonas capsulata, which was later on applied for the treatment of human colon, lung, prostate, heart, and breast cancer (Menon et al. 2017). Pourrali et al. (2017) synthesized gold nanoparticles (AuNPs) using bacteria Bacillus cereus and fungus Fusarium oxysporum in vitro and their confirmation was done by UV-Vis, TEM, and XRD. Further, AuNPs were also used for the treatment of human fibroblast cell line CIRC-HLF (Pourali et al. 2017). Srinath et al. (2017) reported the synthesis of biocompatible gold nanoparticles using Brevibacillus formosus and assesed their antibacterial activity against Staphylococcus aureus and Escherichia coli (Srinath et al. 2017).
AuNPs were synthesized by using bacterium Ps. fluorescens 417, and properties confirmed by UV-Vis, FTIR, and TEM and finally showed their antimicrobial potential against Ps. aeroginosa, Bacillus subtilis, E. coli, S. aureus, and Klebsiella pneumoniae (Syed et al. 2016). Nangia et al. (2009) synthesized AuNPs using bacterium Stenotrophomonas maltophilia (AuRed02) and characterized them by electrophoresis, zeta potential, and FTIR (Nangia et al. 2009). Sharma et al. (2012) biosynthesized AuNPs using bacterium Marinobacter pelagius and analyzed them with TEM, UV-Vis, and dynamic light scattering (Sharma et al. 2012).
Besides bacteria, there are few reports in literature for the biosynthesis of AuNPs and AgNPs using microalgae (Agarwal et al. 2019). These microalgae are rich in proteins, flavonoids, and several other enzymes which are absent or present in very less content than the bacteria. Moreover, microalgae being a eukaryotic, have different modes of synthesis of both metallic and metal oxide nanoparticles. Sintubin et al. synthesized both Au and AgNPs using dried powders of Spirogyra spp. (Sintubin et al. 2011).
7.5.2 Silver (Ag) Nanoparticles
As we have already discussed above, AgNPs have various applications, especially in the field of medicine and drug delivery. They exhibit almost all applications shown by AuNPs. AgNPs are applied in food pacakaging in order to prevent their spoilage by foodborne pathogens. They are also used as antimicrobial agents for plant crop protection. All these applications of AgNPs are possible due to their economical and biocompatible nature (Hoseinnejad et al. 2017) (Siddiqi et al. 2018). In pharmaceutical products, AgNPs could be used as antimicrobials, agents for targeted drug delivery, and anti-biofilm agents. While in agriculture, it could be used for plant disease management. Further, it could also be used for environmental cleanup like water electrolysis, treatment of wastewater, degradation of dyes and pesticides, detection of pathogens and contaminants as a biosensor, etc. Silver nanoparticles showed antimicrobial potential against B. cereus, B. subtilis, S. aureus, Corynebacterium rubrum, E. coli, Vibrio parahemolyticus, K. pneumoniae, Salmonella typhi, Ps. aeruginosa, Citobacter koseri, and Salmonella typhimurium (Thomas et al. 2015). Gahlawat and Choudhury (2019) have already reported earlier the biosynthesis of AgNPs from various pathogenic and nonpathogenic bacteria (Gahlawat and Choudhury 2019).
In the field of research, Kumar and Ghosh (2016) studied that, in history, silver has been used as an antibiotic in human health care (Kumar and Ghosh 2016). For many centuries, the antimicrobial property of silver has been well known to cultures around the world. Nalenthiran et al. (2009) reported the synthesis of AgNPs from Bacillus sp. (Brevibacillus borstelensis_MTCC10642) by exposing them with AgNO3 solution and further characterizing them with advanced instruments. The size of AgNPs was 5–15 nm, formed intracellularly in the periplasmic space of the bacteria (Nalenthiran et al. 2009). Suman et al. (2014) reported the synthesis of AgNPs from Agrococcus sp. and characterized them with TEM, UV-Vis, and FTIR. Silver nanoparticles were prepared using the bacterium Escherichia coli in liquid broth medium (Suman et al. 2014). Silver nanoparticles showed antimicrobial potential against Lactobacillus fermentum, Streptomyces sp., Bacillus cereus, Brevibacterium casei, Staphylococcus aureus, Bacillus licheniromis, Enterobacteria, and Ureibacillus thermosphaerius (Chintamani et al. 2018). Fang et al. (2019) synthesized AgNPs using bacteria Bacillus amyloliquefaciens, Bacillus subtillis, and Stenotrophomonas sp. BHU-S7, whereas gold nanoparticles were prepared using bacterium Deinococcus radiodurans (Fang et al. 2019).
Karthika et al. (2015) synthesized AgNPs from bacterium Serratia marcescens, whose characterization was done by sophisticated instruments. Here, the AgNPs exhibited antibacterial capability against E. coli, Staphylococcus sp., and Pseudomonas sp. (Karthika et al. 2015). El-Saadony et al. (2019) synthesized AgNPs from Bacillus pseudomycoides MT32 and showed antifungal properties against a group of Aspergillus spp. (Asp. flavus, Asp niger, and Asp. tereus), Penicillum notatum, Rhizoctonias olina, Fusarium solani, Fusarium oxysporum, Trichoderma viride, Verticillium dahlia, and Pythium spinosum. Characterization and analysis of silver nanoparticles were done using TEM, XRD, EDS, DLS, zeta potential, and UV-Vis (El-Saadony et al. 2019). Further, AgNPs synthesized from bacterium Haemophilus influenzae showed antimicrobial potential against various pathogenic bacteria like Ps. aeruginosa, Klebsiella spp., Streptococcus spp., Serratia spp., S. aureus, E. coli, and yeast (Candida albicans) using the agar well diffusion method. Characterization of AgNPs was confirmed using atomic force microscopy (Gahlawat and Choudhury 2019). Yadav and Fulekar (2018) reported the synthesis of AgNPs from bacterium Pseudomonas sp. ARS-22 and characterized them using various microscopic and spectroscopic instruments (Archana et al. 2015).
Further, Kumar and Ghosh (2016) reported the synthesis of AgNPs from bacterium Brevibacillus centrosporus DSM8445T, B. levickii LKG22481, B. invocatus NCIMB13772T, B. panacihumi DCY35, and B. choshinensis DSM8552T, and the confirmation of AgNPs was done by sophisticated instruments (Kumar and Ghosh 2016). The schematic representation of the biosynthesis of AgNPs and AuNPs is shown in Fig. 7.4.
7.6 Algal-Mediated Synthesis of Metallic Nanoparticles
Algae like fungi are eukaryotic organisms, which are widely utilized for the formation of both metal and metal oxide NPs, though their mode of synthesis of nanoparticles is more or less similar to prokaryotes. Several approaches have been made which are available in literature for the microalga-mediated formation of metal and metal oxide nanoparticles from their respective aqueous salt solutions (Kuppusamy et al. 2016). But the synthesis of nanoparticles by algae has numerous drawbacks as it is highly time taking, require more space for harvesting and tedious task (Agarwal et al. 2019). Simultaneously, there are several advantages also like photosynthetic property, ability to convert solar energy and CO2 into biomass, and accumulation of nutrients in the form of C, N, and P. All these properties have direct impact on the morphological properties of nanocrystals. Microalgae could synthesize NPs in four different ways:
-
1.
Direct utilization of biomolecules extracted after the breakage of the algae
-
2.
Using supernatant obtained from microalgae culture media
-
3.
Directly exposing the salts with the microalgae cells
-
4.
Using living cells of microalgae
Merin et al. and several others have synthesized AgNPs from microalgae from the family of Haptophyta, Chlorophyta, and Ochrophyta (Mohseniazar et al. 2011). Mahdieha et al. reported synthesis of AgNPs by using living biomass of Spirulina platensis (Mahdieh et al. 2012), while Luangpipat et al. incubated gold chloride with Chlorella vulgaris under optimal conditions and reported the synthesis AuNPs, which was confirmed by TEM (Luangpipat et al. 2011). Microalgae due to their richness in various biomolecules like sulfated polysaccharides and proteins and pigments were used for the synthesis of Ag, Pd, Cd, and NPs, by either whole cells, supernatant, or dried biomass. Microalgae, mainly exploited so far for the above NPs, are cyanobacteria, Chlorella spp., Lyngbya majuscula, Spirulina platensis, and other Chlorophyta spp. (Patel et al. 2014).
7.7 Microbial-Mediated Synthesis of Metal Oxide Nanoparticles (MONPs)
7.7.1 Bacteria-Mediated Synthesis of Metal Oxide Nanoparticle
Metal oxide NPs have changed the face of industries due to its application in research, medicine, ceramics, space, defense, wastewater treatment, and composite preparation (Patel et al. 2014). Some of the most commonly used metal oxide nanoparticles are iron oxide nanoparticles, silica nanoparticles, and alumina nanoparticles. These nanoparticles are mostly synthesized from a commercial precursor using chemical and physical approaches. The most common chemical approaches employ sol-gel, coprecipitation, hydrothermal and solvothermal decomposition (Bilton et al. 2012), while chemical vapor deposition and laser ablation are common examples of the physical method. Because it is highly energy-intensive, both of these approaches are not only expensive, but also hazardous due to applications of various chemicals in the synthesis. So the biosynthesis of metal oxide NPs is an economical, biocompatible, and green method (Jiang et al. 2018). Among the biological approaches, various photoproducts and microbes have also been used. Plant products like leaf, flower, fruit, bark, and stem have various proteins, phytochemicals, flavonoids, terpenoids, and enzymes that act as a reducing agent, while microbes like bacteria, fungi, yeast, and algae have numerous biomolecules (proteins and enzymes and organic acids) that act as both reducing and capping agent in addition to transforming the precursor into a biocompatible product (Shah et al. 2015a).
The mechanism of synthesis of metal oxides from the precursor material by microorganisms is similar to single metals. Generally, microorganisms can synthesize metal oxide NPs either intra- or extracellularly. In the previous process, metal precursors are taken up from the medium by the microbes and transform them into respective metal oxides using their machinery, whereas in the extracellular process, the metal precursors are present in the medium, where the microbes secrete their microbial products. The secreted microbial products interact with the metal precursors and get transformed into metal oxide nanoparticles (Mohd Yusof et al. 2019). Out of these two processes, extracellular mechanisms are considered advantageous, as the metal oxide nanoparticles form outside, so the recovery or downstream processing will be easier and economical in comparison to the intracellular process. Moreover, in the extracellular process, there are fewer chances of contamination.
Metal oxide nanoparticles are very effective in inhibiting the growth of various GP and GN bacteria and they have emerged as the most promising candidate as antimicrobial agents. The most common metal oxides include ZnO, TiO2, CuO, Fe2O3, and MgO. The biological material used for the synthesis of NPs like bacteria, fungi, yeast, and plant extract lies on the principles of green chemistry and is compatible with the use of microorganisms (Mohd Yusof et al. 2019). The biologically synthesized NPs have various applications such as drug delivery, biolabeling, coating of medical products, and treatment of cancer (Yadav and Fulekar 2018). Opposite to physicochemical methods, biosynthesized NPs are nontoxic, making them suitable for biomedical applications. Moreover, the oxidized form of synthesized NPs is even more useful due to their physicochemical properties. Like metal NPs, bacteria could also synthesize metal oxide NPs by means of intracellular or extracellular process. Based on several works, it was found that among bacteria, NADH-dependent nitrate reductase (NDNR) enzyme activity plays a key role in the conversion of metallic ions to nanoparticles. The general synthesis methodology for the biosynthesis of nanoparticles using bacteria is shown in Fig. 7.5.
7.7.2 Iron Oxide Nanoparticles (IONPs): A Special Class
The unique physicochemical properties of FeO nanoparticles have fueled their demand not only from research and industrial viewpoints, but perhaps these are one of the most preferred nanomaterials in biotechnology and microbiological domains (Gu et al. 2006; Tamer et al. 2009; Chang et al. 2008; Dong et al. 2011; Meng et al. 2011). The common link with hemoglobin present in the blood has recently fascinated research towards them in modified forms, whereby these entities could be prepared using facile approaches. On the basis of microbial stain and respective nanoparticle concentration, NPs of FeO could either stimulate or suppress microbial growth. The superparamagnetic attributes of these particles make them befitting heat coupling sources, forming the basis of their suitability in targeted drug delivery to injurious or damaged cell or organ systems and improving the efficacy of modified drug delivery through hypothermic coupling (Khanna et al. 2018; Xue et al. 2018). The heat coupling efficacy of FeO NPs is already in robust consideration towards improving the antimicrobial efficacy of AgNPs (Senthil and Ramesh 2012). Similarly, the magnetically responsive ability of FeO NPs has rapidly increased their implication in biosensing (separation of pathogenic and nonpathogenic microbial strains), immobilization of select microbial population(s), and process intensification of biotechnological processes. Till date, FeO NPs are produced by several Fe-reducing bacterial species such as Thiobacillus thiooxidans, T. ferrooxidans, Gallionella, Shewanella, and magnetotactic bacterium, with existence in several phases, such as magnetite (γ-Fe3O4), hematite (β-Fe2O3), and maghemite (α-Fe2O3) (Ali et al. 2016). Two broad mechanisms for synthesizing FeO NPs include either intervention of magnetotactic bacteria or through exposure of Fe precursors to non-magnetotactic bacteria. The two approaches are discussed ahead with their working distinctions.
7.7.2.1 Magnetotactic Bacteria: Magnetite Nanoparticles
Magnetotactic bacteria comprise a peculiar category of bacteria, owing to their capability of intracellular generation of magnetite NPs in specialized organelles, called magnetosomes. These structures encode specialized globular protein, ferrin, which, in turn, comprises 24 distinctive subunits (Sakaguchi et al. 1993; Philipse and Maas 2002). This encapsulation aids in the storage of Fe in a nontoxic and soluble form, inside the magnetosomes, which could be extracted from bacteria through mechanical or chemical means (extracted magnetosomes could be ruptured to harvest magnetite NPs). The magnetite NPs are arranged in a crystalline manner within the magnetotactic bacteria with the entire population having equal size and shape. A characteristic/unique aspect of these magnetite NPs inside the magnetotactic bacteria is their ability to align them along the earth’s magnetic field (Blakemore 1975). As harvested magnetic NPs are being used in diverse fields of cutting-edge, substantiating drug delivery, life sciences, MRI, CT scan, electronically equipped memory storage devices, ink printing, and several others. The most common bacterial species studied for the generation of magnetite are Magnetospirillum magnetotacticum, generating two distinct kinds of NPs, the first being magnetic (Fe3O4) NPs (having chain like structure), while the second being greigite (Fe3S4) NPs (Xie et al. 2009). Rarely, synthesis of both nanostructures is also reported from the same microbial species (but definitely under different culturing conditions). Popular species, among other bacteria well known for intracellular formation of magnetite (Fe3O4) NPs, include Aquaspirillum magnetotacticum, Magnetospirillum candidatus, Magnetoglobus multicellularis, and magnetotactic bacterium MV-1.
Magnetosomes are capable of forming robust crystalline as well as noncrystalline nanocrystal morphologies, having diverse shapes ranging from hexagonal to octahedral and faceted cuboctahedral shape and octahedral symmetry with either tied down or collected morphology within the phospholipid bilayers. It is pertinent to discuss some of the cahracteristic formations of magnetic NPs by bacterial stains, with the first being Desulfovibrio magneticus (RS-1), an anaerobic bacteria capable of reducing sulfur and intracellular accumulation of magnetite NPs, with sizes of most accumulated magnetite crystals remaining in the order of 30 nm (all particles being superparamagnetic in nature). In an interesting study, Klaus-Joerger demonstrated the possible replacement of Fe in biosynthesized magnetite NPs by Co, Cr, and Ni (all having an unpaired electron that contributes to spin-based characteristic magnetic sensitivity) in Thermoanaerobacter ethanolicus, a heat-enduring Fe-reducing bacterium. These substitutions produced octahedral magnetite NPs of <12-nm sizes in significant quantities, coexisting with poorly crystalline magnetite phase near the cell surface. Elblbesy and coworkers documented the formation of magnetic NPs from the Magnetospirillum strain AMB-1 to produce 47-nm-sized magnetite NPs, further working on the investigation of magnetic behavior as a function of varying incubation temperatures. Almost working on the same principle, Philipse and Maas reported the synthesis of magnetite crystals in single-domain, folded-chain, and flux-closure ring morphologies in Magnetospirillum magnetotacticum through regulation of bacterium locomotion via varying the externally applied magnetic field (Philipse and Maas 2002). Organisms living in Fe-rich surroundings harness their energy through a reduction of mFe+3, with the partial reduction resulting in magnetosome having a peculiar protein-constituted phospholipid bilayer as its membrane. Several studies have screened the ability of diverse bacterial species isolated from Fe ore mining sites for their magnetic property conferring ability at laboratory conditions. In one such attempt, the Thiobacillus thioparus strain was identified through rybotyping, with microbial growth and magnetite production being optimized at varying pH, temperature, and substrate concentrations (Katayama and Kuraishi 1978). Moreover, magnetic NPs were purified via growth and lysis of bacteria and the magnetic properties were screened using empirical observations under the influence of magnetic field. With an interest to pursue their specific biological impact, the harvested NPs were monitored for their suitability in SDS-polyacrylamide gel electrophoresis (PAGE) in conjunction to those of coprecipitated magnetic NPs as well as particles coated with bacterial protein. The observations revealed that purified particles were synthesized using isolated bacterial strains having a protein coating, visualized on the stained polyacrylamide gel. The fluorescence property of the solution under magnetic field and aggregation of the particle along the edge of the wells in the absence of protein coating displayed the presence of monodispersive magnetic NPs in the preparation. Another attempt focused on the utilization of magnetically insensitive anaerobic bacterium GS-15, to reduce nonmagnetic brown disordered ferric oxide into a magnetic black solid particle, with the synthesized crystals residing outside the cell in a nonaligned hierarchy (Elcey et al. 2014).
7.7.2.2 IONP Synthesis by Non-Magnetotactic Bacteria
Several studies have reported the synthesis of FeO NPs using non-magnetotactic bacteria, through culturing in peculiar environments at specific pH and temperature. One such attempt reports the biological synthesis of 73.30-nm-sized FeO NPs through Lactobacillus rhamnosus strain, followed by characterization using atomic force microscopy (AFM) and FTIR spectroscopy (Mohammed et al. 2016). Another attempt reports the utility of P. islandicum strain in getting rid of heavy metal manifested as pollution through a transformation of Fe, Cu, Cr, and U to their oxides at high temperatures (Tchounwou et al. 2012). Study results like these provide highly crucial information about the optimization of microbial species to attain optimum biological degradation as the genes coding for concerned biochemical conversions could be selected and engineered for a higher expression to maximize the detoxification efficacy. Many investigations report better efficiency of biodegradation using symbiotic cultures compared to single microbes, inferring a probable synergistic mode of functioning by microorganisms. These possibilities could be further optimized for enhancing the performance as requirement of distinctive nutrients from culture media would allow better growth of a bacterial and fungal consortium compared to bacteria or fungi alone.
7.7.2.2.1 Maghemite Nanoparticles (γ-Fe2O3)
Microbial species like Actinobacter (an aerobic bacterium) have been optimized to produce superparamagnetic Fe2O3 NPs at extracellular locations, using FeCl3 and FeSO4 under ambient culture conditions. Exploring the untapped potential of biologically abundant and environmentally safer technologies to utilize inorganic metals and their varying stoichiometry compounds has been on the rise over the past few years. The justified reason for this is the renewable nature of energy input needed to catalyze the bioconversions and catalysis processes. It seems quite overwhelming when the microbes residing in marine and terrestrial habitats are cultured for development into biologically altered forms that could selectively act on harmful synthetic dyes and poisonous heavy metals and their compounds, recalcitrants. These technologies reflect the untapped potential of natural biomaterials or bioresources to clean the environment and regulate the net balance of several useful metals and nonmetals, via precipitation, decomposition, and degradation. For instance, Bacillus species encode a significant population of enzymes and proteins aiding in the synthesis of metal oxide NPs, such as that of hexagonal and protein-capped crystalline Fe2O3 NPs by Bacillus cereus using FeCl2 as a precursor. Capping with microbial proteins during synthesis ensures a thorough stability of as synthesized NPs using the same material as reducing and capping agents (Fang et al. 2019). So no separate capping agent needs to be added and NPs or nanomaterials made in this manner find astounding significance in drug delivery applications (where cytotoxicity has to be controlled).
The use of a thermophilic bacterium, P. islandicum, is immensely significant in this regard, through which amorphous Fe (III) oxyhydroxide is reduced to magnetically receptive iron oxides at 65 °C. Similarly, the enzyme extract of Lactobacillus casei, upon incubation with FeSO4 solution (1 mM) at 5.6 pH, 37 °C, and in 5% CO2-containing environment for 3 weeks, provided spherical NPs of 15 nm (evaluated using transmission electron microscopy) size. Preliminary formation of NPs was noted by the transparent to black color changes of the culture medium, after which confirmatory screening was made using electron microscopy and X-ray diffraction. Approaches like these offer benign methodology for FeO NP synthesis, with simple, efficient, and economic methodology, owing to which the as-harvested product could be readily utilized for drug delivery and pharmaceutical applications (Torabian et al. 2018). Similarly, Bacillus subtilis strains (isolated from rhizosphere-rich soil) are reported for the extracellular synthesis of Fe3O4 NPs, using a supernatant fraction of their culture medium (to write details). It would be interesting to note here that same yield of NPs is not possible with supernatant and pellet as the population of biological catalysts differs, thereby affecting the NP formation activity (Sundaram et al. 2012).
A peculiar aspect in FeO NP formation by magnetotactic bacteria involves the movement of Fe particles, leading to the formation of siderophores, which are the magnetosome vesicles facilitating Fe reduction from the +3 (ferric) to +2 (ferrous) state. After this biological reduction, controlled biomineralization of magnetite (in the last stage) happens. An important consideration here is the formation of siderophores, characteristic magnetic vesicles formed in magnetotactic bacteria, typically low-molecular-weight (0.5–1.5-kDa) Fe+3-chelating molecules synthesized by most bacteria under Fe limiting conditions (Arakaki et al. 2008). In general, these siderophores are natively synthesized by magnetotactic bacteria.
Table 7.2 presents an account of different metal oxide NPs synthesized using bacterial species, with the characteristic shape, size, and species. Yet again, it is interesting to note the NPs of similar size, shape, and extent, which consequently form the basis of their distinct applications (owing to size- and shape-dependent NP functional responses).
7.7.2.3 Fungus-Mediated Synthesis of FeO NPs
Recognized as eukaryotic organisms for their decomposition activities, fungi are ubiquitous in several ordinary lodgings and are one of the most well-known decomposer organisms. Biological diversity and robustness of living requirements for the fungi could be well judged from the supposedly 1.5 million species (reported on earth), even though only 70,000 of these are distinctly known. Digestion of extracellular food sources through secretion of specified enzymes to hydrolyze complex chemical compounds into simpler fractions that are finally absorbed and utilized as energy resources is a characteristic feature of these microorganisms. Latest data enabled through high-throughput screening methods estimate a prevalence of nearly 5.1 million fungal species (Wu et al. 2019). Higher tolerance of culture environment adversities (such as temperature and pH diversity) along with greater metal bioaccumulation abilities by the fungi renders them highly suitable compared to bacterial species (Archana et al. 2015). Apart from the higher culture fluctuation tolerance of fungi, the fascinating features for intense interest in fungal preparation of metal and metal oxide NPs are due to the simplicity of their scale-up process alongside an efficient extracellular secretion of enzymes (Boroumand Moghaddam et al. 2015; Prasad 2016, 2017; Prasad et al. 2018; Aziz et al. 2016). Utilization of fungi for making NPs is substantially preceded through thin solid substrate fermentation technique, manifesting the optimization of high wall-binding and intracellular metal uptake attributes, owing to their fast growth (Salvadori et al. 2015). Till date, several fungal species, including Verticillium luteoalbum, Fusarium oxysporum, Asperigillus oryzae, Alternataalternata, Trichoderma viride, etc., have been cultured and optimized to yield NPs of different shapes and sizes. An effort worth in this direction by Gawande et al., in 2016, reports the biomimetic mineralization of fungi to synthesize nano- or meso-sized NPs through activity of intra- and extracellularly secreted enzymes (responsible for a range of geometry and sizes of synthesized NPs). Fast growth and easier handling of fungal populations within the laboratory are the key advantages for their preferential usage in such applications (Gawande et al. 2016). The sole constraint behind the use of fungi in nanoparticle preparation is their difficulty in scaling up, hindering the commercial development and application of synthesized nanomaterials. This constraint arises due to higher biomass fraction of fungi (compared to bacteria), and for needful enzymatic activity, the biological activity of cultured microbe has to be adequately expressed in the absence of which the product synthesis becomes impaired or affected (with respect to product quality). Among all the fungal species, Verticillium and Fusarium are most utilized for metal oxide NP synthesis, owing to their possession of versatile enzymes, proteins, and other metabolites (Ovais et al. 2018). One study reports the synthesis of FeO NPs at room temperature using ferrous and ferric salt mixtures as precursors (Mazrouaa et al. 2019). Success has also been obtained regarding the formation of crystalline magnetite NPs through cationic protein enabled hydrolysis of anionic Fe complexes at the extracellular sites. The obtained NPs exhibited a characteristic ferromagnetic transition with an almost insignificant magnetization extent at low temperature. In processes like this, it is very pertinent to take note of the electrostatic proximity of cationic proteins with anionic Fe precursors, and it becomes imperative to have an aggregation preventing capping activities of cationic proteins, which in turn promotes interaction with negatively charged species/molecules (Fig. 7.6).
A rigorous investigation by Latiffah and coworkers reports the formation of spherical FeO NPs using three distinct manglicolous fungal species, namely, Trichoderma asperellum, Phialemoniopsis ocularis, and Fusarium incarnatum, isolated from mangroves. The NPs were, respectively, 25 ± 3.94, 13.13 ± 4.32, and 30.56 ± 8.68 nm in sizes, inferring highest monodispersion with Phialemoniopsis ocularis, a consideration which could be further screened with respect to the biological catalytic action of Phialemoniopsis ocularis (Latiffah et al. 2010).
Many research efforts have explored the bioreducing ability of the fungus Aspergillus niger for making magnetite (Fe3O4) NPs, in view of this fungi’s ability to catalyze the FeSO4 and FeCl3 decomposition to FeS and Fe2O3, respectively. Upon exposure to ethanol-assisted supercritical conditions, the as-formed particles were stored at 300 °C and 850 PSI units of pressure. Subsequent analysis using phase structure and morphology aspects yielded spherical and pure Fe and Fe3O4 NPs having average sizes of 18 and 50 nanometers, respectively. Screening for magnetic properties revealed ferromagnetic behavior with saturation magnetization extent of 68 emu per gram for Fe3O4. The analytical profile provided a novel paradigm shift to optimize biophysical methods for the large-scale synthesis of magnetic NPs optimized for biomedical applications (Abdeen et al. 2016).
Biosynthesis of Fe NPs is also reported using Pleurotus sp. in optimum culture media, where the fungus was allowed to grow in 2x10−4 M FeSO4 solutions up to 72 h. UV-Vis absorbance peaks at 226 and 276 nanometers predicted the formation of NPs, while involvement of proteins in the NP formation (as component of culture medium) was ascertained through spectral analysis of broth containing culture medium at varying time intervals corresponding to 265 nm. Interactions of proteins with NPs were inferred through FTIR spectroscopy through a concomitant involvement of functional groups indicated by shift of functionalities of the treated cells. Morphology inspection through TEM revealed particle deposition at the inner as well as outer surface for completion of the synthesis process. The peculiar role of fungal proteins was confirmed by the absence of these depositions in control samples, where no Pleurotus sp. was furnished. This was also confirmed through X-ray fluorescence, which showed no Fe in control samples but prevailed in mycelium. The presence of few other elements was also noted, which could be due to randomized compositional fluctuations of culture medium. A characteristic aspect of FeO NP synthesis by fungi is the formation of Fe transport molecules like hydroxamates that bind the complex molecules and transport them inside the cells (Mazumdar and Haloi 2011). Similarly, the potential of Aspergillus japonicus isolate AJP01 is also reported for extracellular synthesis of 60–70-nm FeO NPs through hydrolysis of a mixture of Fe cyanide complexes (acting as precursor), under ambient growth conditions. Screening the mechanism, it was noted that hydrolysis of these complexes released Fe+2 and Fe+3, through involvement of protein facilitating coprecipitation and controlled nucleation, ultimately forming FeO NPs. Analysis using FTIR spectroscopy revealed the presence of proteins that confer stability to as-formed NPs, in agreement with the observations of an earlier preliminary investigation (Bhargava et al. 2011).
7.7.3 Microbially Mediated Synthesis of TiO2, ZnO, CuO, SiO2, and Al2O3 NPs
7.7.3.1 Titanium Dioxide (TiO2) NPs
The significance of TiO2 in multidimensional domains of electronics, optics, and environmental remediation is well known. In general, the nontoxic and biocompatible properties of TiO2 impart its suitability for biomedical requirements such as bone or tissue engineering as well as in pharmaceutical industries. Majorly, TiO2 is synthesized using chemical and biological reduction approaches such as sol-gel, hydrothermal, solvothermal, combustion, plant (extract), and microbially assisted biosynthesis. Microbes such as Lactobacillus (bacteria) and Saccharomyces cerevisiae (yeast) are used for TiO2 NP synthesis. Few specific examples of TiO2 NP synthesis using bacterial cultures are notified here. Firstly, Jayaseelan et al. and Kirthi et al. reported synthesis of TiO2 NPs using TiO(OH)2 as a precursor and Bacillus subtilis as a reducing agent. The morphology of the synthesized TiO2 was found to be spherical/oval while the size was in the 66–77-nm range (Kirthi et al. 2011b; Jayaseelan et al. 2012). Secondly, Malarkodi et al. (2013) reported the synthesis of TiO2 NPs of size 100–500 nm, irregularly shaped, using the biomass of Planomicrobium spp. obtained from melted ice. The synthesized NPs exhibited antimicrobial properties against B. subtilis MTCC 3053 and K. planticola MTCC 2727 in agar disk diffusion method (Malarkodi et al. 2013).
Thirdly, a report by Khan and Fulekar focuses on the biological synthesis of TiO2 using Bacillus amyloliquefaciens as a capping agent. The synthesized TiO2 exhibited a spherical morphology in the 22–97-nm size range. The synthesized TiO2 were used further in the photocatalytic degradation of Reactive Red 31 (RR31, a poisonous dye) using platinum-doped TiO2, developing the highest potential (90.98%) for RR31 degradation as compared to native TiO2 (75.83%) (Khan and Fulekar 2016).
7.7.3.2 Zinc Oxide (ZnO) NPs
Owing to its simplified synthesis approach as well as its increasing suitability in the fields of pharmaceuticals, cosmetics rubber industry, biosensor development, optics, solar cells, and environmental remediation, ZnO NPs have emerged as one of the most sought-after metal oxide NPs (Khan et al. 2016, 2018). The specific interest has been to explore the antimicrobial, antibacterial, and antioxidant activities through the inherent nontoxicity of nanoscale forms (Bhuyan et al. 2015). Zinc (Zn) is evidently the second most abundant metal in the earth’s crust (after Fe) and it is the only metal present in all six enzyme classes (lyases, transferases, oxidoreductases, hydrolases, isomerases, and ligases). Similar to Zn, ZnO is the most researched and studied metal oxide after TiO2. Compared to other synthesis methods, the microbial approach offers much advantages emerging helpful for NP synthesis at low pH, temperature, and pressure. In an interesting attempt, Kalpana et al. examined the antibacterial activity of ZnO NPs on Escherichia coli, Salmonella typhi, Bacillus subtilis, Staphylococcus aureus, and Pseudomonas aeruginosa (Kalpana et al. 2018). Further, Kundu et al. synthesized extracellular ZnO NPs of average diameter (100–120 nm) using Rhodococcus pyridinivorans NT2 and applied it for textile finishing and drug delivery in colon carcinoma (Kundu, Hazra) (Kundu et al. 2014). Jayseenlean et al. suggested a novel biological route for the synthesis of spherical, oval-shaped ZnO NPs of size 57.7 nm using bacterium Aeromonas hydrophila. The synthesized ZnO NPs were characterized by all the major sophisticated instruments and its potential was assessed against pathogenic bacteria and fungi (Jayaseelan et al. 2012). Taran et al. (2018) reported a simple, eco-friendly method for the synthesis of ZnO and TiO2 NPs using bacterium Halomonas elongata IBRC-M 10214. The morphological confirmation of NPs by microscopy revealed that the particles were spherical shaped whose average diameter was 104.63 ± 27.75 for TiO2 NPs and 18.11 ± 8.93 nm for ZnO NPs. Both of the NPs were assessed for their antibacterial activity against multidrug-resistant bacteria (MDRB), i.e., E. coli ATCC 25922 and Staphylococcus aureus ATCC 43300 (Taran et al. 2018).
7.7.3.3 Copper Oxide (CuO) NPs
One of the most important transition metal oxides, CuO promises interesting applications in terms of its size-tunable and captivating properties, forming the basis of its inclusion in highly critical temperature gas sensors, superconductors, and catalytic, optical, photoconductive and photoelectronic, electrical, and energy storage purposes. It has been used as an antimicrobial agent recently against various bacterial species (Yadav et al. 2017).
7.7.3.4 Silica (SiO2) NPs
A green technique of SiO2 NP formation, using a thermophilic bacterium (BKH1) as a biological template, is reported using an inorganic precursor (magnesium trisilicate) in an organic (tetraethyl orthosilicate) reducing agent (Show et al. 2015). This novel method of bacterially synthesized SiO2 NPs is eco-friendly and is workable at ambient temperature, thereby not requiring drastic energy inputs. This method avoids the complex protocol of multistep synthesis and is therefore much cost-effective. A notable aspect is the functional activity of thermophilic bacterium (BKH1) at high temperatures, pertaining to which identification of genes conferring survival and needed bioactivities at high temperatures could be an interesting framework for strengthening the impact of similar studies on other microbes. Such activities also create an interest in enzyme/protein stability against denaturation at high temperatures.
The synthesis of silicon/silica nanoparticle composites by the bacterium Actinobacteria sp. is reported by Marikani et al., by exposing the bacterium to K2SiF6 precursor under ambient conditions. The formation of silica NPs is due to the secretion of several reductases and oxidizing enzymes. Further, few scientists have also reported on the synthesis of silica NPs from F. oxysporum isolated from tomato wilt (Marikani et al. 2016). Silica NPs were synthesized from rice husk ash (RSA) by F. oxysporum in malt-glucose (MG) and malt-glucose-yeast-peptone (MGYP) media. The confirmation of size, shape, and purity was analyzed by sophisticated instruments. It was concluded from the results that solubilization of silica was not directly associated with their production of organic acids. The results showed that the production of organic acids was not directly related to the solubilization of silica. It was found that solubility and stability of silica are due to the extracellular proteins released into the medium during the exponential growth phase. Out of both the media, MG media were found more suitable for growth as well as for the formation of semicrystalline, quasi-spherical SiO2 NPs of size 2–8 nm (Pineda-Vásquez et al. 2014).
7.7.4 Virus-Based Synthesis of Metal Oxide Nanoparticles
Plant viruses have been used widely as templates for organic–inorganic hybrid synthesis. However, by simply adjusting the pH, fine-tuning of hybrid nanoparticle structures, especially the control of inorganic particle size and the location where silication occurs (i.e., outside and/or inside of the capsid), remains a challenge. By using the templating effect of the cowpea chlorotic mottle virus (CCMV) protein cage, it was shown that the silication at the outer or inner surface of the protein capsid, and the resulting structures of silica/virus hybrid nanoparticles, can be finely tuned by adjusting the pH (Liu et al. 2017). At pH 4.0, only small silica particles (2.5 nm in diameter) were formed inside the protein cages, whereas at pH 6.0, mainly silication occurred in the protein cages, resulting in monodisperse silica nanoparticles with 14-nm diameter. At pH 7.5, silica deposition was found on both the surfaces, i.e., inner and outer surfaces of the protein cage under aqueous conditions. In these reaction circumstances, multicomponent hybrid virus/nanoparticulate systems, such as CCMVAu/silica and Au/silica nanoparticles, were prepared stepwise. After removal of the CCMV template in thermal degradation, a single gold nanoparticle can be encapsulated within a hollow silica shell to simulate the structure of a baby rattle in which unattached solid particles are in the hollow particles. The Au/silica core-hollow shell nanoparticles can further be used as a stable catalyst. These synthetic methods are expected to provide a versatile method for preparing core-shell nanomaterials with well-designed structures and functions (Liu et al. 2017).
A sol-gel procedure has been developed to integrate bionanoparticles, such as cowpea mosaic virus, turnip yellow mosaic virus, tobacco mosaic virus, and ferritin into silica while maintaining the particle integrity and morphology. The structures of the resulting materials were characterized by TEM, small-angle X-ray scattering (SAXS), and N adsorption-desorption analysis. The results obtained show that the shape and surface morphology of the nanoparticles are largely retained after the incorporation of silica. After removal of the bionanoparticles by calcination, a mesoporous silica having monodisperse pores with well-defined shape and surface morphology of the bionanoparticles was replicated inside the silica (Niu et al. 2010).
7.7.5 Alga-Mediated Synthesis of Metal Oxide Nanoparticles
7.7.5.1 Alumina Nanoparticles (Al2O3) by Algae
Algae are a good source of biomolecules among all other aquatic organisms; this is so because algae contain proteins, carbohydrates, fats, nucleic acids, pigments, and secondary metabolites such as alkaloids, some aromatic compounds, peptides, macrolides, and terpenes (Siddiqi and Husen 2016a). They can act as reducing agents that help in the preparation of nanoparticles from metal salts without producing any toxic by-product. Once the algal biomolecules are identified, the nanoparticles of the desired shape or size may be fabricated. The antimicrobial activity of the thus synthesized metal and metal oxide nanoparticles against several gram-positive and Gram-negative bacterial strains and fungi has been investigated (Shannon and Abu-Ghannam 2016).
The dimensions of synthesized alumina NPs depend on the pH, temperature, incubation time, and concentration of the solution. A new biological methodology is proposed for the production of ceramic α-aluminum oxide nanoparticles using an extract of the algae Sargassum ilicifolium. The algal extract works as a stabilizer as well as a bioreducing agent. The UV-Vis analysis shows the presence of an absorption peak at 227 nm, which confirmed the formation of the aluminum oxide nanoparticles. FTIR analysis has indicated that bioreduction of aluminum ions and stabilization of nanoparticles may be caused by interactions between aluminum and the biofunctional groups of the algal extract. The XRD pattern has confirmed that after calcination at ~1200 °C, the Al2O3 nanoparticles with 35-nm diameter were alpha crystalline in nature and have rhombohedral structure. TEM analysis showed that the alumina nanoparticles were well dispersed and spherical in shape with an average size of 20 ± 2.1 nm, while EDX spectroscopy has confirmed high purity of the alumina nanopowder, as sample contained only aluminum (46.31%) and oxygen (53.69%) (Koopi and Buazar 2018; Siddiqi and Husen 2016b).
7.8 Various Conventional Approaches for Wastewater Treatment
With the continuous advancement of technology, several methods have made huge impact on wastewater treatment. Although there are several conventional wastewater treatment methods, like coagulation, precipitation, electrolysis, filtration, absorption, adsorption (Azimi et al. 2017), etc., most of them are less efficient, expensive, and energy-intensive. Out of these, the most efficient and economical method is adsorption and the process can become cheaper by using waste materials derived from agriculture, industry, domestic, and poultry. The adsorbents developed from such materials have different groups on their surface that act as adsorption sites. These active sites have a role in the removal of multivariate pollutants from the wastewater.
Out of these adsorption is the most reliable technique by nanoparticles due to their economical nature and surface modification by microorganisms (Khan et al. 2019). Generally, nanoparticles adsorb inorganic pollutants on their surface. One more advantage with adsorption method is that the nanoparticles can be treated with 0.1 M NaOH for removal of all the surface-attached pollutants, mainly heavy metals. This helps in the reuse of nanoparticles, whereas if nanoparticles are IONPs, then it can be easily recovered after the reaction is complete, making the whole process economical.
7.9 Applications of Nanoparticles for Wastewater Treatment
7.9.1 Typical Composition of Wastewaters
Before moving on to the mechanisms and efficacies of nanoparticle-enabled wastewater treatment technology, it is quite logical to know about the chemical diversity of wastewater streams in terms of its composition. Multiple research attempts on wastewater streams from different regions of the globe list six major deleterious components, namely, suspended solids, biodegradable organics, pathogens, nutrients, heavy metals, and soluble inorganic salts (Abdel-Raouf et al. 2012). All these constituents pose a significant risk to the pollution level of wastewaters and could be present either in native form or added at various stages of domestic water discharge. For example, the nutrients carbohydrates, fats, and proteins could be present from the source itself (may be due to excretion products of humans and animals), whereas heavy metals could be added to the wastewater stream if it has access to industries. Similarly, the organic constituents act as existence support towards the environmental microbial species, which continuously grow, propagate, and increase their metabolic activities while residing on it (Lareen et al. 2016). Consequently, the microbial load increases, and with waste material as dietary input, these organisms exhibit toxic responses and are easily the opportunistic carriers of communicable infections (Figs. 7.7 and 7.8).
The nitrogen in the discharged fats, proteins, and carbohydrates in the wastewater streams catalyzes the microbial activities through the eutrophication phenomenon, as a consequence of which the toxicity is enhanced (Puyol et al. 2017). The oxidative proximities of proteins, carbohydrates, and fats (summed as biological oxygen demand, BOD) deplete the environmental oxygen whereby serious loss to biodiversity could be driven. Like various other pollutants, inorganically dissolved impurities (such as calcium, sodium, and sulphate) could be added to wastewaters from household activities. These materials make the wastewater unfit for use owing to which its chemical load moderation is mandatory before final discharge as waste streams. Among the major organic materials (OM), fibers were found to prevail in the majority, followed by proteins and sugars, respectively accounting for 20.64, 12.38, and 10.65% of the total organic content (TOC). A certain percentage of endocrine-disrupting chemicals (mostly polyaromatic hydrocarbons [PAHs] and phthalates) were also found to contribute towards the pollutant load of wastewaters (Puyol et al. 2017). Together, volatile fatty acids, soluble proteins, and sugars contribute to nearly 30% of the total chemical oxygen demand (COD) of wastewaters, with the majority of proteins and sugars being >0.001 μm.
Several stages of treatment are employed to reduce the chemical load of wastewater streams, such as removal of impurities and solid materials through sedimentation, froth floatation, or salt-driven precipitation. Each successive stage is intended to reduce the toxic content through treatment procedures implicit towards the chemical diversity of impurities. The microbial decontamination of wastewaters has gained immense popularity in the past few years attributed to powerful chemical breakdown actions of microbial enzymes (Singh et al. 2016b). The advantage with this technology is that microbes can feed on the wastewater as their nutrient base and there is no requirement of maintaining their survival through external controls. The stage is now right to know about the specific bacterial species and strains for specific kinds of wastewater impurities.
Hyperthermophilic microorganisms can help in the migration of metal contaminants by reducing them to less mobile forms. Thermal radioactive or metal-containing industrial wastes may be treated potentially in bioreactors using microorganisms similar to the metabolism of P. islandicum or their enzymes (Kashefi and Lovley 2000). Many mesophilic microorganisms having the ability to use Fe (III) as a terminal electron acceptor can also reduce various metals and metalloids other than Fe (III) (Lovley 2013). Fe (III)-reducing bacteria and archaea were found capable to precipitate gold by reducing Au (III) to Au (0) (Kashefi et al. 2001). The reaction appeared to be enzymatically catalyzed, which was dependent on temperature and the presence of hydrogen (as a specific electron donor). Many Fe (III)-reducing microorganisms were able to reduce the forms of oxidized metals, including radionuclides such as uranium (VI) (Wu et al. 2006) and technetium (VII) and trace metals including arsenic (V), chromium (VI), cobalt (III), manganese (IV), and selenium (VI). Many of these metals and metalloids act as environmental pollutants. Thus, Fe (III)-reducing microorganisms can be used to remove metals from water and waste streams and to immobilize metals in the subsurface environments.
Microbial reduction of metals may play a major role in the formation of metal deposits, which may be especially important in hot environments containing metal-rich waters (Drewniak and Sklodowska 2013). Common products of bacterial iron-reduced nanosized magnetic particles enable early disease detection and accurate prognosis and personalized treatment, monitoring efficacy of prescribed therapy, or study of cellular interaction in a certain biological environment (Chircov et al. 2019). These particles might be used in different radionuclide therapies, drug delivery, magnetic resonance imaging (MRI) (Yadav and Fulekar 2018), diagnostics, immunoassays, molecular biology, DNA and RNA purification, cell separation and purification, cell adhesion studies, hyperthermia, and magnetic ferrofluids for magnetocaloric pumps.
7.10 Specific Metal and Metal Oxide NPs Appropriate for Wastewater Treatment
The suitability of metal or metal oxide NPs for wastewater treatment is manifested in their antimicrobial and antioxidant characteristics since the wastewater streams are rich in oxygen-consuming pathogenic microbes. The NPs are ideal candidates for reducing the chemical complexity of oxygenated aggregations of wastes, facilitating their smoother discharge and disposal (Chen et al. 2015). Among metals, mostly used NPs are Au, Ag, Cu, and Fe, while in oxides, TiO2, ZnO, silica, and nano zerovalent iron (NZVI) NPs are preferred (Ruttkay-Nedecky et al. 2017b). To a smaller extent, MgO, CuO, and Al2O3 conjugates are also employed. The sole mandate of utilizing bacteria-driven NPs for wastewater treatment relies on the interaction enhancing the ability of NPs. In this reference, it is quite interesting to note that not only NPs can reduce the toxicity of wastewater streams but, sometimes, even the bacterial strains as such or in genetically altered forms are added to wastewater streams. Bacteria possess a number of enzymes which work as catalysts to break down the toxic wastes comprising the wastewater stream. With such abilities, sometimes the supplemented bacterial populations themselves become the sources to break down the toxic metal complexes into their salts which are further reduced to their zerovalent states. In the recent past, several interesting studies have reported the addition of genetically modified organisms (GMOs) into toxic waste streams to reduce their recalcitrant and heavy metal loads. This section selectively discusses bacterially synthesized metal and metal oxide NPs and their potential application in wastewater treatment.
In a very interesting 2013 contribution, Yang et al. have compared the wastewater treatment efficacies of nanoscale ZnO, TiO2, zerovalent iron, and AgNPs. They discussed that even though a common toxicity reduction mechanism of all these nanomaterials relies on the generation of reactive oxygen species (ROS), the extent and manner of such activities differ on an individual basis. The important factors affecting the antimicrobial activities of these functionally distinct nanomaterials in wastewaters are their chemical properties and stability (Das et al. 2018). Interestingly, the researchers have confirmed that oxygen is essential for ROS generation by nanoscale Ag and iron particles, whereas for ZnO and TiO2, illumination is needed to elicit the ROS expression. Interestingly, though having similar sizes, ZnO and TiO2 exhibited greater toxicity than nanoscale Ag and Fe, owing to their oxidizing vulnerability and water solubility which result in the generation of toxicity-inducing metal ions. Regarding the toxicity of AgNPs, it is well reported that the toxic response is due to Ag+ formation, which is very sensitively affected by the oxygen concentration of the surroundings.
Under aerobic conditions, the antimicrobial response of AgNPs is due to oxidative dissolution of AgNPs, concomitantly leading to gradual release of Ag ions and the production of ROS (Skandalis et al. 2017). The requirement of oxygen for the toxicity induction of AgNPs is further assured by no Ag+ formation in the anaerobic environment, predicting a necessity of dissolved oxygen to facilitate oxidative dissolution of nanoscale Ag (Abdal Dayem et al. 2017).
Similarly, Fe has comparatively higher reactivity than Cu and Ag, owing to which nano zerovalent Fe (nZVI) can reduce several chemicals in wastewater streams, such as aromatic nitro compounds, chlorinated solvents, and oxidized heavy metals (Yang et al. 2019). In the absence of oxygen (solution phase), water could oxidize nano zerovalent Fe, forming Fe+2 and H2 gas. However, water causes rapid surface oxidation of nano zerovalent Fe under aerobic conditions, after which dissolved oxygen oxidize ferrous state (Fe+2) to ferric (Fe+3) (Yang et al. 2019). So, in totality, the pH and DO concentration regulated the reduction efficacy of nano zerovalent Fe (Park and Dempsey 2005).
Opposed to Fe and Ag, the nanoscale ZnO and TiO2 generate ROS after photon absorption (Park and Dempsey 2005). Under illumination, electrons excited by photon absorption diffuse towards the surface and react with O to form superoxide anions (O2--) and consequentially generated holes diffuse to the surface and react with water to form hydroxyl radicals (OH−) (Liao and Reitberger 2013). A notable aspect is that nanoscale TiO2 is nearly water-insoluble, whereas ZnO can dissolve in water to a higher extent. So unlike TiO2, ZnO on being dissolved in water releases free Zn (as Zn+2) and Zn(OH)2 which are dominant zinc species at neutral pH conditions and exert a toxic response towards aquatic organisms (Reed et al. 2012). Thus, ZnO and TiO2 mediate their toxicity-reducing responses in wastewater treatment through the additive association of sunlight, whereas nanoscale Ag and Fe express their toxicities through mutual oxidation and reduction. It seems reasonable here that a particular wastewater composition might be more specifically suited for treatment by ZnO and TiO2 than Ag and Fe since ionic associations and redox existences are also affected by process temperature and pH. It is of further interest to note here that heterogeneous composition of wastewater streams complicates their detoxification, where one particular material may not be effective to a significant extent. In such situations, combinative approaches like adding microorganisms (preferably in consortium modes) to the wastewater reservoirs or adding two or more antimicrobial agents in simultaneous mode seem to be more effective than conventional technologies. Readers are suggested to refer to the 2016 contribution of Lu et al., where the various problems of nanoscale Ag, Fe, and Zn usage are discussed at length along with their probable resolutions. For example, nanoscale Ag alone often leads to fouling and clogging issues in the aqueous environment, while when AgNPs are attached to filter materials, not only the overall cost of the remediation process moderates but the disinfection efficacy also improves by significant extents (Mourdikoudis et al. 2018). Likewise, NZVI has been found effective in large-scale removal of contaminants (comprising nitroaromatic compounds, halogenated organic compounds, phenols, organic dyes, heavy metals, inorganic anions, nitrates, metalloids, and radio elements) through simultaneous attributes of reduction, oxidation, adsorption, and precipitation, but the complications of aggregation, oxidation, and separation difficulty restrict the long-range disinfection efficacy of nZVI. As a consequence, several modifications are implemented for better nZVI treatment efficacy that includes surface coating, doping with other metals, conjugation with supports, emulsification, and encapsulation in the matrix (Liang et al. 2014). Studies on these modifications suggest enhanced nZVI reactivity via doping with other metals. Similarly, surface coating and conjugation strategies forbid the aggregation and enhance the nZVI dispersion. The modifications of conjugations and matrix encapsulation ensure an optimum nZVI separation from degraded wastewaters, paving the way for their efficient removal from the treated wastewater streams (Ledakowicz et al. 2001). Similarly, the photocatalytic attributes of TiO2 are the reasons for its extraordinary suitability in UV-radiation-driven particle separation. With its reasonable price and long-lasting photo-, chemical, and biological stability alongside a large energy band gap (3.2 eV), TiO2 remains the most sustainable photocatalyst till date (Fujishima et al. 2000). Remarkable photocatalytic efficacy of this material facilitates ROS generation in reasonable time durations, facilitating an efficient removal of contaminants.
The prospect of nanomaterial recovery from the waste stream is definitely a concerning factor for their scale-up suitability in detoxification application (Shan et al. 2009). This challenge can be addressed by careful consideration of the source of these nanomaterials, which critically affects their shape, size, and aggregation stability. For instance, bottom-up approaches offers significantly higher control on their size and shape, owing to which the application stability and suitability would be higher. On the other hand, top-down approaches (mostly used in physical methods of nanomaterial synthesis) are elaborate, utilizing higher energy due to which the end products are less stable and, consequently, more vulnerable towards aggregation.
7.11 Conclusion
Microbial synthesis of nanoparticles has gained tremendous applications in wastewater treatment due to their high surface area to volume ratio, functionalization by biomolecules, etc. Microorganisms have various organic compounds like enzymes, acids, polysaccharides, etc. that act as a reducing agent for metallic nanoparticles, whereas some of biomolecules are also involved in the metal oxide synthesis of nanoparticles. These metallic and metal oxide nanoparticles have adsorption sites on their surface that help in the removal of pollutants from the wastewater. Nano adsorption has several advantages over other pollutant removal process. IONPs not only have diversity in the phases but also are economical, recyclable, and easily available. Microbially synthesized nanoparticles have huge potential for the green, economical, and biocompatible source of nanoparticles that find applications in the field of environmental cleanup.
References
Abdal Dayem A, Hossain MK, Lee SB, Kim K, Saha SK, Yang G-M et al (2017) The role of reactive oxygen species (ROS) in the biological activities of metallic nanoparticles. Int J Mol Sci 18(1):120
Abdeen M, Sabry S, Ghozlan H, El-Gendy AA, Carpenter EE (2016) Microbial-physical synthesis of Fe and Fe3O4 magnetic nanoparticles using Aspergillus Niger YESM1 and supercritical condition of ethanol. J Nanomater 2016:1–7
Abdel-Raouf N, Al-Homaidan AA, Ibraheem IBM (2012) Microalgae and wastewater treatment. Saudi J Biol Sci 19(3):257–275
Agarwal P, Gupta R, Agarwal N (2019) Advances in synthesis and applications of microalgal nanoparticles for wastewater treatment. J Nanotechnol 2019:9
Ali A, Zafar H, Zia M, Ul Haq I, Phull AR, Ali JS et al (2016) Synthesis, characterization, applications, and challenges of iron oxide nanoparticles. Nanotechnol Sci Appl 9:49
Arakaki A, Nakazawa H, Nemoto M, Mori T, Matsunaga T (2008) Formation of magnetite by bacteria and its application. J R Soc Interface 6:977–999
Archana Y, Theivasanthi T, Paul PK, Upadhyay KC (2015) Extracellular biosynthesis of silver nanoparticles from plant growth promoting Rhizobacteria Pseudomonas sp. Int J Curr Microbiol App Sci 4(8):11
Azimi A, Azari A, Rezakazemi M, Ansarpour M (2017) Removal of heavy metals from industrial wastewaters: a review. ChemBioEng Rev 4:1–24
Aziz N, Pandey R, Barman I, Prasad R (2016) Leveraging the attributes of Mucor hiemalis-derived silver nanoparticles for a synergistic broad-spectrum antimicrobial platform. Front Microbiol 7:1984. https://doi.org/10.3389/fmicb.2016.01984
Beveridge TJ, Murray RG (1980) Sites of metal deposition in the cell wall of Bacillus subtilis. J Bacteriol 141(2):876–887
Bhargava A, Jain N, Panwar J (2011) Synthesis and application of magnetic nanoparticles: a biological perspective, pp 117–155
Bhateria R, Jain D (2016) Water quality assessment of lake water: a review. Sustain Water Res Manag 2(2):161–173
Bhuyan T, Mishra K, Khanuja M, Prasad R, Varma A (2015) Biosynthesis of zinc oxide nanoparticles from Azadirachta indica for antibacterial and photocatalytic applications. Mater Sci Semicond Process 32:55–61. https://doi.org/10.1016/j.mssp.2014.12.053
Bilton M, Milne S, Brown A (2012) Comparison of hydrothermal and sol-gel synthesis of nano-particulate hydroxyapatite by characterisation at the bulk and particle level. Open J Inorg Non-metal Mater 2:1–10
Blakemore R (1975) Magnetotactic bacteria. Science 190:377–379
Boroumand Moghaddam A, Namvar F, Moniri M, Tahir P, Azizi S, Mohamad R (2015) Nanoparticles biosynthesized by Fungi and yeast: a review of their preparation, properties, and medical applications. Molecules 20(9):16540–16565
Brock TD, Gustafson J (1976) Ferric iron reduction by sulfur- and iron-oxidizing bacteria. Appl Environ Microbiol 32:567–571
Chang JH, Kang KH, Choi J, Jeong YK (2008) High efficiency protein separation with organosilane assembled silica coated magnetic nanoparticles. Superlattice Microstruct 44(4–5):442–448
Chen D, Zhang X, Lee AF (2015) Synthetic strategies to nanostructured photocatalysts for CO2 reduction to solar fuels and chemicals. J Mater Chem A 3(28):14487–14516
Chintamani RB, Salunkhe KS, Chavan MJ (2018) Emerging use of green synthesis silver nanoparticle: an updated review. Int J Pharmaceut Sci Res 9(10)
Chircov C, Grumezescu AM, Holban AM (2019) Magnetic Particles for Advanced Molecular Diagnosis. Materials 12(13):2158
Das RK, Pachapur VL, Lonappan L, Naghdi M, Pulicharla R, Maiti S et al (2017) Biological synthesis of metallic nanoparticles: plants, animals and microbial aspects. Nanotechnol Environ Eng 2(1):18
Das S, Chakraborty J, Chatterjee S, Kumar H (2018) Prospects of biosynthesized nanomaterials for the remediation of organic and inorganic environmental contaminants. Environ Sci Nano 5(12):2784–2808
Dong H, Huang J, Koepsel RR, Ye P, Russell AJ, Matyjaszewski K (2011) Recyclable antibacterial magnetic nanoparticles grafted with quaternized poly(2-(dimethylamino)ethyl methacrylate) brushes. Biomacromolecules 12:1305–1311
Drewniak L, Sklodowska A (2013) Arsenic-transforming microbes and their role in biomining processes. Environ Sci Pollut Res 20(11):7728–7739
Elcey CD, Kuruvilla AT, Thomas D (2014) Synthesis of magnetite nanoparticles from optimized iron reducing bacteria isolated from iron ore mining sites. Int J Curr Microbiol App Sci 3(8):408–417
El-Saadony MT, El-Wafai N, El-Fattah HIA, Mahgoub S (2019) Biosynthesis, optimization and characterization of silver nanoparticles using a soil isolate of Bacillus pseudomycoides MT32 and their antifungal activity against some pathogenic Fungi. Adv Anim Vet Sci 7:238–249
Fang X, Wang Y, Wang Z, Jiang Z, Dong M (2019) Microorganism assisted synthesized nanoparticles for catalytic applications. Energies 12(1):190
Fijan S (2014) Microorganisms with claimed probiotic properties: an overview of recent literature. Int J Environ Res Public Health 11(5):4745–4767
Fujishima A, Rao T, Tryk D (2000) Titanium dioxide photocatalysis. J Photochem Photobiol C: Photochem Rev 1:1–21
Gahlawat G, Choudhury AR (2019) A review on the biosynthesis of metal and metal salt nanoparticles by microbes. RSC Adv 9(23):12944–12967
Ganachari SV, Banapurmath NR, Salimath B, Yaradoddi JS, Shettar AS, Hunashyal AM et al (2017) Synthesis techniques for preparation of nanomaterials. In: Martínez LMT, Kharissova OV, Kharisov BI (eds) Handbook of ecomaterials. Springer, Cham, pp 1–21
Gawande MB, Goswami A, Felpin F-X, Asefa T, Huang X, Silva R et al (2016) Cu and Cu-based nanoparticles: synthesis and applications in catalysis. Chem Rev 116(6):3722–3811
Gowramma B, Keerthi U, Rafi M, Muralidhara RD (2015) Biogenic silver nanoparticles production and characterization from native stain of Corynebacterium species and its antimicrobial activity. 3 Biotech 5(2):195–201
Gu H, Xu K, Xu C, Xu B (2006) Biofunctional magnetic nanoparticles for protein separation and pathogen detection. Chem Commun 9:941–949
He S, Zhang Y, Guo Z, Gu N (2008) Biological synthesis of gold nanowires using extract of Rhodopseudomonas capsulata. Biotechnol Prog 24(2):476–480
Hoseinnejad M, Mahdi Jafari S, Katouzian I (2017) Inorganic and metal nanoparticles and their antimicrobial activity in food packaging applications. Crit Rev Microbiol 44:1–21
Iravani S (2014) Bacteria in nanoparticle synthesis: current status and future prospects. Int Scholar Res Not 2014:359316
Jayaseelan C, Rahuman AA, Kirthi AV, Marimuthu S, Santhoshkumar T, Bagavan A, Gaurav K et al (2012) Novel microbial route to synthesize ZnO nanoparticles using Aeromonas hydrophila and their activity against pathogenic bacteria and fungi. Spectrochim Acta A Mol Biomol Spectrosc 90:7
Jiang J, Pi J, Cai J (2018) The advancing of zinc oxide nanoparticles for biomedical applications. Bioinorg Chem Appl 2018:18
Kalpana VN, Kataru BAS, Sravani N, Vigneshwari T, Panneerselvam A, Rajeswari VD (2018) Biosynthesis of zinc oxide nanoparticles using culture filtrates of Aspergillus Niger: antimicrobial textiles and dye degradation studies. OpenNano 3:48–55
Karthika D, Vadakkan K, Ashwini R, Shyamala A, Hemapriya J, Vijayanand S (2015) Prodigiosin mediated biosynthesis of silver nanoparticles (AgNPs) and evaluation of its antibacterial efficacy. Int J Curr Microbiol App Sci 4(11):7
Kashefi K, Lovley DR (2000) Reduction of Fe(III), Mn(IV), and toxic metals at 100 degrees C by Pyrobaculum islandicum. Appl Environ Microbiol 66(3):1050–1056
Kashefi K, Tor J, Nevin K, Lovley D (2001) Reductive precipitation of gold by dissimilatory Fe(III)-reducing bacteria and archaea. Appl Environ Microbiol 67:3275–3279
Katayama Y, Kuraishi H (1978) Characteristics of Thiobacillus thioparus and its thiocyanate assimilation. Can J Microbiol 24(7):804–810
Kessi J, Ramuz M, Wehrli E, Spycher M, Bachofen R (1999) Reduction of selenite and detoxification of elemental selenium by the phototrophic bacterium Rhodospirillum rubrum. Appl Environ Microbiol 65(11):4734–4740
Khan R, Fulekar MH (2016) Biosynthesis of titanium dioxide nanoparticles using Bacillus amyloliquefaciens culture and enhancement of its photocatalytic activity for the degradation of a sulfonated textile dye reactive red 31. J Colloid Interface Sci 475:184
Khan SH, Pathak B, Fulekar MH (2016) Development of zinc oxide nanoparticle by sonochemical method and study of their physical and optical properties. In: AIP conference proceedings vol 1724(1), p 020108
Khan S, Pathak B, Fulekar MH (2018) Synthesis, characterization and photocatalytic degradation of chlorpyrifos by novel Fe: ZnO nanocomposite material. Nanotechnol Environ Eng 3
Khan I, Saeed K, Khan I (2019) Nanoparticles: Properties, applications and toxicities. Arab J Chem 12:908–931
Khanna L, Verma NK, Tripathi SK (2018) Burgeoning tool of biomedical applications - Superparamagnetic nanoparticles. J Alloys Compd 752:332–353
Kirthi AV, Rahuman AA, Rajakumar G, Marimuthu S, Santhoshkumar T, Jayaseelan C et al (2011a) Biosynthesis of titanium dioxide nanoparticles using bacterium Bacillus subtilis. Mater Lett 65(17–18):2745–2747
Kirthi V, Rahuman A, Rajakumar G, Marimuthu S, Thirunavukkarasu S, Chidambaram J et al (2011b) Biosynthesis of titanium dioxide nanoparticles using bacterium Bacillus subtilis. Mater Lett 65:2745
Koopi H, Buazar F (2018) A novel one-pot biosynthesis of pure alpha aluminum oxide nanoparticles using the macroalgae Sargassum ilicifolium: a green marine approach. Ceram Int 44(8):8940–8945
Kulkarni RR, Shaiwale NS, Deobagkar DN, Deobagkar DD (2015) Synthesis and extracellular accumulation of silver nanoparticles by employing radiation-resistant Deinococcus radiodurans, their characterization, and determination of bioactivity. Int J Nanomedicine 10:963–974
Kumar A, Ghosh A (2016) Biosynthesis and characterization of silver nanoparticles with bacterial isolate from GangeticAlluvial soil. Int J Biotechnol Biochem 12(2):7
Kumari M, Giri VP, Pandey S, Kumar M, Katiyar R, Nautiyal CS et al (2019) An insight into the mechanism of antifungal activity of biogenic nanoparticles than their chemical counterparts. Pestic Biochem Physiol 157:45–52
Kundu D, Hazra C, Chatterjee A, Chaudhari A, Mishra S (2014) Extracellular biosynthesis of zinc oxide nanoparticles using Rhodococcus pyridinivorans NT2: multifunctional textile finishing, biosafety evaluation and in vitro drug delivery in colon carcinoma. J Photochem Photobiol B Biol 140:194–204
Kuppusamy P, Yusoff MM, Maniam GP, Govindan N (2016) Biosynthesis of metallic nanoparticles using plant derivatives and their new avenues in pharmacological applications – an updated report. Saudi Pharmaceut J 24(4):473–484
Lakshmi Das V, Thomas RT, Varghese R, Vasudevan S, Mathew JK, Radhakrishnan E (2014) Extracellular synthesis of silver nanoparticles by the Bacillus strain CS 11 isolated from industrialized area. 3 Biotech 4:121–126
Lareen A, Burton F, Schäfer P (2016) Plant root-microbe communication in shaping root microbiomes. Plant Mol Biol 90(6):575–587
Latiffah Z, Mah Kok F, Heng Mei H, Maziah Z, Baharuddin S (2010) Fusarium species isolated from mangrove soil in Kampung Pantai Acheh, Balik Pulau, Pulau Pinang, Malaysia. Trop Life Sci Res 21(1):21–29
Ledakowicz S, Solecka M, Zylla R (2001) Biodegradation, decolourisation and detoxification of textile wastewater enhanced by advanced oxidation processes. J Biotechnol 89(2):175–184
Liang D-W, Yang Y-H, Xu W-W, Peng S, Lu S-F, Xiang Y (2014) Nonionic surfactant greatly enhances the reductive debromination of polybrominated diphenyl ethers by nanoscale zerovalent iron: mechanism and kinetics. J Hazard Mater 278:592–596
Liao H, Reitberger T (2013) Generation of free OHaq radicals by black light illumination of Degussa (Evonik) P25 TiO2 aqueous suspensions. Catalysts 3(2):418–443
Lin P-C, Lin S, Wang PC, Sridhar R (2014) Techniques for physicochemical characterization of nanomaterials. Biotechnol Adv 32(4):711–726
Liu Y, Fu J, Chen P, Yu X, Yang P (2000) Studies on biosorption of Au3+ by Bacillus megaterium. Wei Sheng Wu Xue Bao 40(4):425–429
Liu A, Yang L, Verwegen M, Reardon D, Cornelissen JJLM (2017) Construction of core-shell hybrid nanoparticles templated by virus-like particles. RSC Adv 7(89):56328–56334
Lovley D (2013) Dissimilatory Fe(III)- and Mn(IV)-reducing prokaryotes. In: Rosenberg E, EF DL, Lory S, Stackebrandt E, Thompson F (eds) The prokaryotes: prokaryotic physiology and biochemistry. Springer, Berlin, pp 287–308
Lu H, Wang J, Stoller M, Wang T, Bao Y, Hao H (2016) An overview of Nanomaterials for water and wastewater treatment. Adv Mater Sci Eng 2016:10
Luangpipat T, Beattie IR, Chisti Y, Haverkamp R (2011) Gold nanoparticles produced in a microalga. J Nanopart Res 13:6439–6445
Mahdieh M, Zolanvari A, Azimee AS, Mahdieh M (2012) Green biosynthesis of silver nanoparticles by Spirulina platensis. Sci Iranica 19(3):926–929
Malarkodi C, Chitra K, Rajeshkumar S, Gnanajobitha G, Paulkumar K, Vanaja M et al (2013) Novel eco-friendly synthesis of titanium. Der Pharm Sinica 4(3):8
Malik P, Shankar R, Malik V, Sharma N, Mukherjee TK (2014) Green chemistry based benign routes for nanoparticle synthesis. J Nanopart 2014:14
Marikani DK, Uma Sangareswari K, Suganya P, Ganesan R, Rajarathinam K (2016) Biobased approach for the synthesis, characterization, optimization and application of silica nanoparticles by fungus Fusarium oxysporum. Pharmaceut Biol Eval 2:223–233
Mazrouaa AM, Mohamed MG, Fekry M (2019) Physical and magnetic properties of iron oxide nanoparticles with a different molar ratio of ferrous and ferric. Egypt J Pet 28(2):165–171
Mazumdar H, Haloi N (2011) A study on biosynthesis of iron nanoparticles by Pleurotus sp. J Microbiol Biotechnol Res 1(3):39–49
Meng X, Seton HC, Lu LT, Prior IA, Thanh NTK, Song B (2011) Magnetic CoPt nanoparticles as MRI contrast agent for transplanted neural stem cells detection. Nanoscale 3:977–984
Menon S, Shanmugam R, Kumar V (2017) A review on biogenic synthesis of gold nanoparticles, characterization, and its applications. Resource-Efficient Technol 3:516
Mohammed B, Abdulsattar J, Adnan W (2016) Biosynthesis of iron oxide nanoparticles using Lactobacillus rhamnosus and its effect against pathogenic bacteria
Mohd Yusof H, Mohamad R, Zaidan UH, Abdul Rahman NA (2019) Microbial synthesis of zinc oxide nanoparticles and their potential application as an antimicrobial agent and a feed supplement in animal industry: a review. J Anim Sci Biotechnol 10(1):57
Mohseniazar M, Barin M, Zarredar H, Alizadeh S, Shanehbandi D (2011) Potential of microalgae and lactobacilli in biosynthesis of silver nanoparticles. Bioimpacts 1:149–152
Mourdikoudis S, Pallares RM, Thanh NTK (2018) Characterization techniques for nanoparticles: comparison and complementarity upon studying nanoparticle properties. Nanoscale 10(27):12871–12934
Nalenthiran P, Sambandam A, Kathiravan G, Kannaian N, Prakash U, Crawford S, Muthupandian AK (2009) Microbial synthesis of silver nanoparticles by Bacillus sp. J Nanopart Res 11:1811–1815. https://doi.org/10.1007/s11051-009-9621-2
Nangia Y, Wangoo N, Goyal N, Shekhawat G, Suri CR (2009) A novel bacterial isolate Stenotrophomonas maltophilia as living factory for synthesis of gold nanoparticles. Microb Cell Fact 8(1):39
Nath D, Banerjee P (2013) Green nanotechnology – a new hope for medical biology. Environ Toxicol Pharmacol 36(3):997–1014
Niu Z, Kabisatpathy S, He J, Lee LA, Rong J, Yang L et al (2010) Synthesis and characterization of bionanoparticle—silica composites and mesoporous silica with large pores. Nano Res 2(6):474–483
Ovais M, Khalil AT, Ayaz M, Ahmad I, Nethi SK, Mukherjee S (2018) Biosynthesis of metal nanoparticles via microbial enzymes: a mechanistic approach. Int J Mol Sci 19(12):4100
Park B, Dempsey BA (2005) Heterogeneous oxidation of Fe(II) on ferric oxide at neutral pH and a low partial pressure of O2. Environ Sci Technol 39(17):6494–6500
Patel V, Berthold D, Puranik P, Gantar M (2014) Screening of cyanobacteria and microalgae for their ability to synthesize silver nanoparticles with antibacterial activity. Biotechnol Rep (Amst) 5:112–119
Patra JK, Baek K-H (2014) Green nanobiotechnology: factors affecting synthesis and characterization techniques. J Nanomater 2014:12
Philipse AP, Maas D (2002) Magnetic colloids from magnetotactic bacteria: chain formation and colloidal stability. Langmuir 18(25):9977–9984
Pineda-Vásquez TG, Casas-Botero AE, Ramírez-Carmona ME, Torres-Taborda MM, Soares CHL, Hotza D (2014) Biogeneration of silica Nanoparticles from Rice husk ash using Fusarium oxysporum in two different growth media. Ind Eng Chem Res 53(17):6959–6965
Pourali P, Badiee SH, Manafi S, Noorani T, Rezaei A, Yahyaei B (2017) Biosynthesis of gold nanoparticles by two bacterial and fungal strains, Bacillus cereus and Fusarium oxysporum, and assessment and comparison of their nanotoxicity in vitro by direct and indirect assays. Electron J Biotechnol 29:86–93
Prasad M, Lambe UP, Brar B, Shah I, Manimegalai J, Ranjan K et al (2018) Nanotherapeutics: an insight into healthcare and multi-dimensional applications in medical sector of the modern world. Biomed Pharmacother 97:1521–1537
Prasad R (2016) Advances and applications through fungal nanobiotechnology. Springer International Publishing, Cham. ISBN 978-3-319-42989-2. http://www.springer.com/us/book/9783319429892
Prasad R (2017) Fungal nanotechnology: applications in agriculture, industry, and medicine. Springer International Publishing, Cham. ISBN 978-3-319-68423-9. https://www.springer.com/gb/book/9783319684239
Prasad R, Kumar V, Kumar M, Shanquan W (2018) Fungal nanobionics: principles and applications. Springer Singapore, Singapore. ISBN 978-981-10-8666-3. https://www.springer.com/gb/book/9789811086656
Prasad R, Pandey R, Barman I (2016) Engineering tailored nanoparticles with microbes: quo vadis. WIREs Nanomed Nanobiotechnol 8:316–330. https://doi.org/10.1002/wnan.1363
Prema P, Iniya PA, Immanuel G (2016) Microbial mediated synthesis, characterization, antibacterial and synergistic effect of gold nanoparticles using Klebsiella pneumoniae (MTCC-4030). RSC Adv 6(6):4601–4607
Puyol D, Batstone DJ, Hülsen T, Astals S, Peces M, Krömer JO (2017) Resource recovery from wastewater by biological technologies: opportunities, challenges, and prospects. Front Microbiol 7:2106
Reed RB, Ladner DA, Higgins CP, Westerhoff P, Ranville JF (2012) Solubility of nano-zinc oxide in environmentally and biologically important matrices. Environ Toxicol Chem 31(1):93–99
Ruttkay-Nedecky B, Krystofova O, Nejdl L, Adam V (2017a) Nanoparticles based on essential metals and their phytotoxicity. J Nanobiotechnol 15(1):33
Ruttkay-Nedecky B, Krystofova O, Nejdl L, Adam V (2017b) Nanoparticles based on essential metals and their phytotoxicity. J Nanobiotechnol 15(1):33
Sabri MA, Umer A, Awan GH, Hassan MF, Hasnain A (2016) Selection of suitable biological method for the synthesis of silver nanoparticles. Nanomater Nanotechnol 6:29
Sakaguchi T, Burgess JG, Matsunaga T (1993) Magnetite formation by a sulphate-reducing bacterium. Nature 365(6441):47–49
Salvadori MR, Ando RA, Nascimento CAO, Corrêa B (2015) Extra and intracellular synthesis of nickel oxide nanoparticles mediated by dead fungal biomass. PLoS One 10(6):e0129799
Sarker PK, Talukdar SA, Deb P, Sayem SA, Mohsina K (2013) Optimization and partial characterization of culture conditions for the production of alkaline protease from Bacillus licheniformis P003. Springerplus 2:506
Sehgal N, Soni K, Gupta N, Kohli K (2018) Microorganism assisted synthesis of gold nanoparticles: a review. Asian J Biomed Pharmaceut Sci 8(64):–8
Senthil M, Ramesh C (2012) Biogenic synthesis of Fe3O4 nanoparticles using Tridax Procumbens leaf extract and its antibacterial activity on Pseudomonas aeruginosa. Dig J Nanomater Biostruct 7(3):6
Seqqat R, Blaney L, Quesada D, Kumar B, Cumbal L (2019) Nanoparticles for environment, engineering, and nanomedicine. J Nanotechnol 2019:2
Shah M, Fawcett D, Sharma S, Tripathy S, Poinern G (2015a) Green synthesis of metallic nanoparticles via biological entities. Materials 8(11):7278–7308
Shah M, Fawcett D, Sharma S, Tripathy SK, GEJ P (2015b) Green Synthesis of Metallic Nanoparticles via Biological Entities. Materials 8:7278–7308. (1996-1944 (Print))
Shan G, Yan S, Tyagi R, Surampalli R (2009) Applications of nanomaterials in environmental science and engineering: review. Pract Period Hazard Toxic Radioact Waste Manage 13:110
Shannon E, Abu-Ghannam N (2016) Antibacterial derivatives of marine algae: an overview of pharmacological mechanisms and applications. Mar Drugs 14(4):81
Sharma N, Pinnaka AK, Raje M, Ashish A, Bhattacharyya M, Choudhury AR (2012) Exploitation of marine bacteria for production of gold nanoparticles. Microb Cell Fact 11:86
Show S, Tamang A, Chowdhury T, Mandal D, Chattopadhyay B (2015) Bacterial (BKH1) assisted silica nanoparticles from silica rich substrates: a facile and green approach for biotechnological applications. Colloids Surf B Biointerfaces 126:245–250
Siddiqi KS, Husen A (2016a) Fabrication of metal and metal oxide nanoparticles by algae and their toxic effects. Nanoscale Res Lett 11(1):363
Siddiqi KS, Husen A (2016b) Fabrication of metal and metal oxide nanoparticles by algae and their toxic effects. Nanoscale Res Lett 11(1):363
Siddiqi KS, Husen A, Rao RAK (2018) A review on biosynthesis of silver nanoparticles and their biocidal properties. J Nanobiotechnol 16(1):14
Singh P, Kim Y-J, Zhang D, Yang D-C (2016a) Biological synthesis of nanoparticles from plants and microorganisms. Trends Biotechnol 34:588
Singh R, Kumar M, Mittal A, Mehta PK (2016b) Microbial enzymes: industrial progress in 21st century. 3 Biotech 6(2):174
Singh J, Dutta T, Kim K-H, Rawat M, Samddar P, Kumar P (2018) ‘Green’ synthesis of metals and their oxide nanoparticles: applications for environmental remediation. J Nanobiotechnol 16(1):84
Sintubin L, de Gusseme B, van der Meeren P, Pycke BF, Verstraete W, Boon N (2011) The antibacterial activity of biogenic silver and its mode of action. Appl Microbiol Biotechnol 91(1):10
Skandalis N, Dimopoulou A, Georgopoulou A, Gallios N, Papadopoulos D, Tsipas D et al (2017) The effect of silver nanoparticles size, produced using plant extract from Arbutus unedo, on their antibacterial efficacy. Nano 7(7):178
Srinath BS, Keerthiraj DN, Byrappa K (2017) Eco-friendly synthesis of gold nanoparticles by gold mine bacteria Brevibacillus formosus and their antibacterial and biocompatible studies. J Pharm 7:53–60
Srivastava SK, Constanti M (2012) Room temperature biogenic synthesis of multiple nanoparticles (Ag, Pd, Fe, Rh, Ni, Ru, Pt, Co, and Li) by Pseudomonas aeruginosa SM1. J Nanopart Res 14:831
Suman J, Neeraj S, Rahul J, Sushila K (2014) Microbial synthesis of silver nanoparticles by actinotalea sp. MTCC 10637. Am J Phytomed Clin Therapeut 2(8):7
Sundaram PA, Augustine R, Kannan M (2012) Extracellular biosynthesis of iron oxide nanoparticles by Bacillus subtilis strains isolated from rhizosphere soil. Biotechnol Bioprocess Eng 17(4):835–840
Syed B, Prasad NMN, Satish S (2016) Endogenic mediated synthesis of gold nanoparticles bearing bactericidal activity. J Microscopy Ultrastruct 4(3):162–166
Tamer U, Gündoğdu Y, Boyacı İH, Pekmez K (2009) Synthesis of magnetic core–shell Fe3O4–Au nanoparticle for biomolecule immobilization and detection. J Nanopart Res 12(4):1187–1196
Taran M, Rad M, Alavi M (2018) Biosynthesis of TiO2 and ZnO nanoparticles by Halomonas elongata IBRC-M 10214 in different conditions of medium. Bioimpacts 8(2):9
Tchounwou PB, Yedjou CG, Patlolla AK, Sutton DJ (2012) Heavy metal toxicity and the environment. Experientia Suppl 101:133–164
Thomas R, Janardhanan A, Varghese RT, Soniya EV, Mathew J, Radhakrishnan EK (2015) Antibacterial properties of silver nanoparticles synthesized by marine Ochrobactrum sp. Braz J Microbiol 45(4):1221–1227
Torabian P, Ghandehari F, Fatemi M (2018) Biosynthesis of iron oxide nanoparticles by cytoplasmic extracts of bacteria lactobacillus casei. Asian J Green Chem 1(2):181–188
Wang L, Hu C, Shao L (2017) The antimicrobial activity of nanoparticles: present situation and prospects for the future. Int J Nanomed 12:1227–1249
Wu Q, Sanford RA, Löffler FE (2006) Uranium(VI) reduction by Anaeromyxobacter dehalogenans strain 2CP-C. Appl Environ Microbiol 72(5):3608–3614
Wu B, Hussain M, Zhang W, Stadler M, Liu X, Xiang M (2019) Current insights into fungal species diversity and perspective on naming the environmental DNA sequences of fungi. Mycology 10(3):127–140
Xie J, Chen K, Chen X (2009) Production, modification and bio-applications of magnetic nanoparticles gestated by magnetotactic bacteria. Nano Res 2(4):261–278
Xue W, Liu Y, Zhang N, Yao Y, Ma P, Wen H et al (2018) Effects of core size and PEG coating layer of iron oxide nanoparticles on the distribution and metabolism in mice. Int J Nanomedicine 13:5719–5731
Yadav L, Tripathi RM, Prasad R, Pudake RN, Mittal J (2017) Antibacterial activity of cu nanoparticles against E. coli, Staphylococcus aureus and Pseudomonas aeruginosa. Nano Biomed Eng 9(1):9–14. https://doi.org/10.5101/nbe.v9i1.p9-14
Yadav VK, Fulekar MH (2018) Biogenic synthesis of maghemite nanoparticles (γ-Fe2O3) using Tridax leaf extract and its application for removal of fly ash heavy metals (Pb, Cd). Mater Today Proc 5(9, Part 3):20704–20710
Yang J, Hou B, Wang J, Tian B, Bi J, Wang N et al (2019) Nanomaterials for the removal of heavy metals from wastewater. Nanomaterials 9(3):424
You H, Yang S, Ding B, Yang H (2013) Synthesis of colloidal metal and metal alloy nanoparticles for electrochemical energy applications. Chem Soc Rev 42(7):2880–2904
Zargar M, Hamid AA, Bakar FA, Shamsudin MN, Shameli K, Jahanshiri F et al (2011) Green synthesis and antibacterial effect of silver nanoparticles using Vitex negundo L. Molecules 16(8):6667–6676
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Yadav, V.K. et al. (2020). Microbial Synthesis of Nanoparticles and Their Applications for Wastewater Treatment. In: Singh, J., Vyas, A., Wang, S., Prasad, R. (eds) Microbial Biotechnology: Basic Research and Applications. Environmental and Microbial Biotechnology. Springer, Singapore. https://doi.org/10.1007/978-981-15-2817-0_7
Download citation
DOI: https://doi.org/10.1007/978-981-15-2817-0_7
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-15-2816-3
Online ISBN: 978-981-15-2817-0
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)