Abstract
Adequate understanding of a microbial-mediated process is the key to its successful operation. It involves the study of microbes, process metabolism, substrate degradation, and by-product formation. As compared to aerobic process, the anaerobic process is more complex as it involves multistep interdependent stages of metabolism in series. Thus an anaerobic process is carried out by syntrophic microbial consortium which altogether makes the metabolic reactions thermodynamically feasible. Among all the bacteria, methanogens are highly vulnerable and sensitive to variations in the conditions of environment and thus they are considered rate-limiting agents for the overall process. These methanogens thus demand a meticulous process control for the stable operation. In this chapter, various metabolic stages of anaerobic processes and related microbiological aspects are presented in detail.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Microbial production
- Organic acids
- Anaerobic processes
- Acidogenesis
- Methanogenesis
- Metabolic products
- Shock imposition
- Hydrogen partial pressure
- Microbial diversity
- Syntrophic relationship
10.1 Introduction
Anaerobic production of organic acids is a multistep biological process having complex combination of various processes involving several groups of microorganisms which collectively act to convert organic carbon into organic acids. Fundamental understanding of these various processes, their energetics, and microorganisms involved and their interdependence shall be essential to critically analyze and design the process requirements to overcome the limitations commonly faced by any anaerobic processes. Production of organic acids using microbe-mediated anaerobic acidogenesis process is a sustainable approach. However, the various by-products produced along with organic acids affect the overall stability of the process. Thus, to control these various by-products becomes highly important for the acidogenesis process. Therefore, it is highly desired to control the operating conditions practically in order to selectively produce various organic acids and other by-products. pH has been reported as the indicator parameter by various authors as it is great influencer in shifting and driving the bioprocess in a particular direction (Zoetemeyer et al. 1982). Further, the pH trends not only present the progress of acidogenesis in anaerobic processes but also an important parameter for the selective production of a specific organic acid during the acidogenesis. It can be easily understood from the scenario that pH plays a vital role in selective production of a variety of organic acids. Thus the understanding of the significance of pH on the organic acid production during anaerobic process is of utmost important.
10.2 Anaerobic Biotechnology
Anaerobic processes are broadly categorized into four sub-processes which are well known as hydrolysis, fermentation/acidogenesis, acetogenesis, and methanogenesis, respectively. Process of anaerobic metabolism starts with hydrolysis which basically refers to the formation of simpler/low molecules weight compounds such as oligomers and monomers from its precursor molecules of complex/high-molecular-weight organic compounds. These simpler compounds have the tendency to be easily consumed/absorbed inside their cells of fermenting bacteria. More specifically, simple sugars, amino acids, and long-chain fatty acids (LCFA) and glycerol are produced from proteins, carbohydrates, and lipids, respectively. While oxidation of LCFA obligately requires an electron acceptor, sugar and amino acid are capable of getting oxidized in the absence of electron acceptors such as oxygen, nitrate, nitrite, sulfate through a process called fermentation (Pandey and Sarkar 2017; Stanbury et al. 2013). The most common factors responsible for different pathways in fermentation of sugars such as glucose, include pH, concentration of substrate, and dissolved hydrogen (Rodriguez et al. 2006; Murto et al. 2004). Such factors are responsible for quantitative as well as qualitative production of different end products, i.e., hydrogen (H2), volatile fatty acids (VFAs), alcohols. Furthermore, energy associated with different pathways is also reported to be the function of these factors (Pandey and Sarkar 2019a; Thauer et al. 1977; Rodriguez et al. 2006). The process of fermentation begins with conversion of glucose, a 6-carbon (C6) molecule, into pyruvic acid (C3) in an oxygen-independent pathway where the electrons released from glucose molecule are taken up by NAD+ which in turn reduces to NADH. The Gibbs free energy for the conversion of NAD+ to NADH is positive, meaning that energy must be taken from the organic molecule being oxidized. In order to keep the process running sustainably, NADH should be re-oxidized back to NAD+ so that it can be reused again. Re-oxidation of NADH into NAD+ involves extraction of electrons and passing them onto another electron acceptor or to another carrier, with a concomitant release of chemical energy which may be converted to other useful forms. When the electron acceptor such as oxygen is absent, nitrate or sulfate, the electron is channelized to reduce protons to form H2. Pyruvate formed from the glucose molecules can be oxidized to acetate through reaction with acetyl coA enzyme while ferredoxin ferries the electrons to H2. Other pathways are possible in which much reduced end products such as butyric acid, propionic acid, valeric acid, lactic acid, and ethanol may be produced. Eventually, these end products which have more than two carbon atoms are again metabolized to form acetate through a process known as acetogenesis where along with acetate, H2 is also produced. Furthermore, there are two possible different pathways for the conversion of acetic acid into methane. In one of pathways where methanogenesis occurs directly is known as aceticlastic methanogens. In this pathway, CH4 and CO2 are directly produced from the carboxyl and methyl groups of acetic acid. Hydrogenotrophic methanogenesis is an alternate pathway for methane production. CO2, produced as a by-product of the fermentation, hydrolysis, or acetogenesis process reacts with H2 to form methane gas; this process is known as hydrogenotrophic methanogenesis. It is also possible that through a process known as syntrophic acetate oxidation, acetic acid can be oxidized to carbon dioxide and hydrogen, which are further transformed into CH4 via hydrogenotrophic methanogenesis. The CO2 and H2 thus formed can also led to the production of acetic acid through a process called homoacetogenesis. Compared to all the other processes that the anaerobic degradation of organic compounds consists of, the methanogenesis process, more specifically the aceticlastic methanogenesis where decomposition of acetic acid takes place to form methane, is more sensitive to changes in temperature, organic matters composition, pH, reactor configuration, and organic loading rate. In case of anaerobic degradation of wastewater containing simpler organic molecules, the aceticlastic methanogenesis is the slowest process compared to other processes; hence, it is the rate-limiting process in the anaerobic treatment of wastewater. However, in case of wastewater containing particulate organic material or complex soluble substrate hydrolysis becomes the slowest step, making it the rate-controlling step. Being major pathway, aceticlastic methanogenesis contributes to methane production up to 72–77% (Khanal 2011; Wang et al. 2013). Figure 10.1 shows the different metabolic pathways of anaerobic degradation.
10.3 Thermodynamic Basis of Various Processes Constituting Anaerobic Production of Organic Acid
Redox reactions, accomplished by the microorganisms, helps in maintaining the energy requirements for cell growth and maintenance. In most, if not all, of the biological processes, the electron(s) are removed from the primary donor molecule and are ferried to a terminal electron accepting molecule via one or more number of electron carriers. The donor molecule thus gets oxidized whereas the electron acceptor gets reduced, while the electron carrier being a simple transporter does not undergo any net change. The transfer steps are associated with a free-energy release that is captured by the cells of the microorganisms in the form of energy carriers. In anaerobic treatment process, first group of bacteria (hydrolytic bacteria) converts the complex/high molecular compounds into monomers/low-molecular-weight compounds such as glucose, amino acids, glycerol, and fatty acids. Second group of bacteria (fermentative or acidogenic bacteria) further convert these monomers through redox reactions.
For each molecule of glucose, four moles of H2 is produced along with the formation of two moles of carbon dioxide as per the following equation:
The change in free energy (ΔG) of the reaction is given by
where, [] stands for the molar concentration of the component and p stands for the partial pressure. The high negative value of ΔG0 indicates that the reaction is spontaneous and significant amount of energy would be possible to be extracted from the reaction even when the reactants and products are present in unit concentrations. However, it is also evident from eq. 10.2 that Gibbs free-energy change, ΔG, is directly proportional (fourth power of the partial pressure of hydrogen) to the partial pressure of hydrogen. So, the system where hydrogen scavenging activity is not present or has been compromised, reaction 10.1 may get inhibited and hydrogen ion concentration increases. The maintenance of low hydrogen partial pressure is accomplished by hydrogenotrophic organisms, such as hydrogenotrophic methanogens which produce methane as per the following reaction:
As per reactions 10.1 and 10.3, it may also be observed that accumulation of other reaction end products such as acetate also may cause rise in the ΔG value, making the forward reaction less favorable. However, impact of accumulation of acetate shall not have that great an impact on the forward reaction as accumulation of hydrogen would have. Therefore, evacuation of acetate is also important, but more important is the evacuation of hydrogen from the system. Acetate eventually gets converted to CH4 by the aceticlastic microorganism as per the following reaction:
The above reaction pathway is known as aceticlastic methanogenesis. Maintenance of low concentration or low partial pressure of hydrogen by hydrogenotrophic microorganisms results in faster fermentation rates (Schink 1997; Kuntze et al. 2008; Zhang et al. 2009). At the attainment of the threshold, the H2 production being faster than its consumption, fermenting organisms should slow down its H2 production. This causes a shift in fermentation reaction in such a way that metabolites which are in more reduced form such as propionate (reaction 10.5), butyrate (reaction 10.6), ethanol (reaction 10.7), and lactate (reaction 10.8) are produced. In fact, the Gibbs free-energy changes for the reactions are more energetic than the acetate reaction, the highest being for propionate (−358 kJ/mol), butyrate (−255 kJ/mol), ethanol (−226 kJ/mol), etc. For lactate fermentation, ΔG0 is lower than that for acetate fermentation (−198 kJ/mol). The relevant reactions are indicated below as follows:
At low partial pressure of hydrogen (<10 Pa), electrons are released as hydrogen molecules following reaction 10.1, through which more acetate, hydrogen, and CO2 will be produced rather than formation of butyrate or ethanol, or other more reduced products following the other fermentation reactions. In a well-balanced anaerobic system, it is must to achieve low hydrogen partial pressure through hydrogen scavenging microorganisms; the mass diffusion of carbon and electrons takes place exclusively through the acetate, carbon dioxide, and hydrogen pathway. Whereas, the minor role is played by the reduced fermentation products such as fatty acids. Lactate is formed when the concentration of the substrate or glucose is significantly high. Normally such a situation is not encountered during wastewater treatment processes. Therefore, while undergoing anaerobic decomposition of wastewater containing COD mainly due to the presence of intermediate metabolites of treatment process such as glucose and other volatile fatty acids (acetate, propionate, butyrate, etc.). Please note that out of the VFA intermediates, formation of propionate does not produce any H2, rather consumes two moles of H2. Fermentation of butyrate produces two moles of H2 as compared to four moles H2 produced during the fermentation of acetate. Thus, it may be concluded that the role of the H2 scavengers is important for the sustainable production of VFA intermediates like acetate and butyrate, however not so much for the production of propionic acid. Thus, the products from the first stage, i.e., fermentation, generally consist of approximately acetate (51%), H2 (19%), and other reduced products like alcohols, higher VFA, or lactate (Angelidaki and Sanders 2004). The reduced intermediates such as VFAs would become extremely important if elevated concentration levels of hydrogen are observed. These levels can be attributed to the increased concentration of substrate for fermentation, inhibition of hydrogenotrophic methanogens probably due to pH drop to a level of <6.0, and availability of toxic substances.
The methanogenesis process cannot directly consume the fermentation products or VFA having carbon atoms three or more, alcohols having more than one carbon atom, fatty acids having branched chains or aromatic fatty acids. These complex compounds first need to be oxidized into acetate and hydrogen through acetogenesis stage before the onward conversion of the products to methane through either aceticlastic or hydrogenotrophic methanogenesis pathway. Propionate is considered as a dominant volatile fatty acid and a precursor to about 35% (by mol) of total methane produced during anaerobic digestion.
Below reactions show the reaction pathways through which propionic and butyric acids are oxidized to acetic acid.
The changes in free energy of the above reactions are calculated as per the following equations:
It can be inferred from reactions 10.9 and 10.10 that the change in Gibbs free energy, i.e., ΔG0, is mainly positive. The positive value indicates that under unit activity (concentration) of the reactants and the products, under standard conditions the forward reaction is not spontaneously possible. Further, the negative values of change in Gibbs free energy (ΔG) favor the forward reaction. Eqs. 10.11 and 10.12 suggest that in order that the overall value of ΔG becomes negative, it is required that concentration or partial pressure of H2 should be as low as possible. In other words, sustaining the low values of hydrogen partial pressure can shift the reaction stoichiometry of propionate and butyrate degradation into acetate. The eqs. 10.9 through 10.12 also suggest that due to greater amount of hydrogen produced, oxidation of propionate is more susceptible to the changes in partial pressure of H2, as compared to butyrate. Calculations show that for the oxidation of propionate to acetic acid to take place, the partial pressure of H2 should be kept between 10−4 and 10−6 bar (Azbar et al. 2001). Such low pressure of H2 can be maintained through hydrogenotrophic methanogens, and methane formation will take place via reaction 10.3.
Aceticlastic methanogens cleave the acetic acid produced during the fermentation and acetogenesis process and convert it into methane and carbon dioxide according to reaction 10.4:
10.4 Interspecies Hydrogen Transfer and its Implications
From the reactions and eqs. 10.9 through 10.1, 10.2, 10.3, and 10.4; it may be concluded that in order to achieve the oxidation of VFAs into acetic acid, the driving force between acetogenic bacteria and hydrogenoptrophic methanogenic bacteria is interspecies hydrogen transfer. This is a kind of symbiotic relationship between two or more otherwise dissimilar group of bacteria in the interest of gaining energy from the degradation of a common substrate and is known as syntrophic relationship (Schink 1997). Such association helps the species involved to gain energy from reactions, which are not thermodynamically feasible under standard/normal conditions. One such example of syntrophic association is the anaerobic oxidation of propionate. The complete mineralization of propionate in anaerobic condition requires syntrophic participation of three groups of bacteria, namely (1) acetogenic bacteria for conversion of propionate to acetate, (2) hydrogenotrophic bacteria for scavenging of hydrogen from the acetogenesis reaction to convert it into methane, and (3) aceticlastic bacteria which help to convert acetate into methane. Individually, even at low hydrogen concentration, the degradation of propionate would not generate enough energy for the sustenance of the acetogens unless both the processes, i.e., degradation of propionate with hydrogenotrophic and aceticlastic methane production, take place simultaneously. Same can be observed in reactions 10.9, 10.3, and 10.4. Combination of these reactions results in a net ΔG0 value of about −56 kJ/mol which is approximately equivalent to the energy required for generation of a molecule of ATP from ADP. Therefore, each of the syntrophic partners at the end of degradation get a share of energy of approximately one-third of ATP if it is equally shared. However, it was later proven that there is no such minimum quantum of energy requirement; the reactions can run at the availability of smallest possible energy also. (Jackson and McInerney 2002). Figure 10.2 illustrates a schematic on the importance of hydrogen transfer in various stages of anaerobic treatment.
Working under a severe energy limited condition demands that the participating microorganisms must organize themselves in order to overcome the inconveniences in growth. The minimum requirement is efficient evacuation of hydrogen gas from the producer (acetogens), and to ensure its maximum availability to the consumers (hydrogenotrophic methanogens). In other words, the flux of hydrogen from the producer to the consumer should be the maximum. Fick’s first law of diffusion states that the hydrogen flux from producer to the consumer can be formulated as the following equation:
where, J = flux, A = surface area of propionate degrading microorganism; d = distance; D = diffusion constant in water, [] stands for concentration and the subscripts H2, subscripts P and C stand for hydrogen, producer, and consumer, respectively.
It follows from the above equation that the flux of hydrogen will be the maximum at the minimum distance between interspecies, i.e., syntrophic acetogen and hydrogenotrophic methanogen. Therefore, it is likely that in order to maximize the flux of hydrogen, the syntrophic partners would like to maintain the interspecies distance as small as possible. Such small distances between interspecies can be maintained through aggregate forms of microbes such as granules. It was demonstrated that bacterial cultures offered a higher rate of propionate degradation in aggregated form as compared to those in suspended form (Cobb and Hill 1991). A biofilm or granular anaerobic reactor could be found to degrade propionate efficiently (Singh et al. 2016; Tatara et al. 2008; Zheng et al. 2009; Zellner and Neudörfer 1995). In this configuration, abundance of syntrophs remains unexposed to high partial pressure of H2 of the reactor indirectly. Kus and Wiesmann (1995) reported that in a mixed culture grown on a porous support and effectively degrading propionate could resist the inhibitory effect of high concentration of H2 which was added from outside. Figure 10.3 is an evidence of close packing of syntrophic association within a granular structure which was collected from a reactor where syntrophic reactions were taking place (De Bok et al. 2005). The De Bok et al. (2005) showed in his study that one large syntrophic acetogen remains surrounded by a number of hydrogenotrophic methanogenic archaea. This is a direct proof that the syntrophic partners should form aggregates in such a way that there is maximization of hydrogen flux during interspecies hydrogen transfer between the participating organisms.
It is also possible that the granules formed in continuously stirred tank reactor may also help in the complete degradation of carbohydrate into the end products. Fig. 10.4 provides a conceptual structure of a granule for optimal complete anaerobic degradation of carbohydrates, where routes through propionic acid and butyric acid are also possible (Liu et al. 2003).
It is also possible that being small in size, formic acid can also at as carrier of electron. Further, it can be easily diffused, just like hydrogen. Literature also revealed that CO2/formate couple has nearly similar value of redox potential as H+/H2 combination. Because of similarities, it is difficult to predict whether formate or hydrogen acts as electron carrier and takes part in interspecies transfer. However, some recent experimental data have pointed out to the abundance of hydrogen as electron carrier (Schink 1997). The diffusion kinetics of anaerobic processes suggested that suspended growth systems are expected to offer formate/CO2 couple as a preferred electron transfer system, where carrier has the tendency to diffuse a long distance in an aqueous medium. On the other hand, hydrogen is reported to be offering a better electron transfer system in attached/biofilm growth systems.
10.5 Microbial Diversity within an Anaerobic Reactor
The overall anaerobic process is governed by the functioning of the four trophic groups of bacteria, which are named as hydrolytic, acidogenic, acetogenic bacteria, and methanogenic archaea. Figure 10.5 illustrates the different groups of bacteria that are involved in different processes that an anaerobic treatment process generally consists of. During the hydrolysis stage of the anaerobic metabolism, low molecular weight soluble compounds (sugars, long-chain fatty acids, amino acids, glycerin, etc.) are formed from their high molecular weight precursors like carbohydrates, proteins, and lipids. These conversions are facilitated by the action of extracellular enzymes, excreted by the hydrolytic bacteria. On the basis of the nature of substrate, there may be diverse group of the hydrolytic bacteria. Clostridium, Bacteroides, Cellulomonas, Acetivibrio, etc. produce extracellular enzymes such as cellulase, amylase, and xylanase to convert the carbohydrate polymers into monomers such as simple sugar or glucose. Clostridium, Bacillus, Peptococcus, etc. are responsible for breaking down of protein into amino acids with or without concomitant generation of simple sugar. Species like Clostridium, Mycobacterium, and Staphylococcus are responsible for the release of enzymes like lipase and phospholipase to convert fats or lipids into simple sugar and long-chain fatty acids (LCFAs) with or without the concomitant formation of alcohols. In fermentation or acidogenesis process, the simple monomers are converted into short-chain fatty acids or volatile fatty acids such as acetate, butyrate, propionate, and valerate along with the formation of other compounds such as lactate, ethanol, butanediol, formate, and succinate to a smaller extent. A vast diversity of bacteria is capable of such transformation. Major acetate-producing bacteria belong to the genera Acetobacterium, Clostridium, and Sporomusa. Although the main domain of ethanol fermentation is by yeast, such as Saccharomyces, other genera such as Erwinia, Sarcina, and Zymomonas can produce ethanol due to the fermentation. At low pH values, Enterobacter and Serratia are two genre that produce alcohol from glucose. Strictly anaerobic bacteria of the genera Clostridium and Butyrivibrio are known to ferment sugars into butyric acid. Under low pH, some clostridium bacteria are known for the small amount of production of n-butanol and acetone. The lactate-producing genera are generally Bifidobacterium, Lactobacillus, Streptococcus, Leuconostoc. Propionate and succinate are produced by the genera like Bacteroides, Clostridium, Peptostreptococcus, and Selenomonas. Various bacteria of the genera Salmonella, Escherichia, Serratia, Enterobacter, Erwinia, and Shigella are very well known to carry out mixed acid fermentation—acetate, formate, lactate, and succinate from sugar. Acidogenesis or fermentation is the most diverse phase of the whole anaerobic treatment process, where plethora of intermediate reduced end product can be formed by diverse group of microbes which become dominant under different specific environmental and concentration conditions.
In the acetogenesis process, the acetogenic bacteria oxidize the VFAs and other reduced products into acetates and hydrogen. Such class of bacteria also includes the genera Syntrophobacter and Syntrophomonas. The end products of this process, i.e., acetic acid and H2, are then utilized by the methanogens, which helps in sustaining the low hydrogen concentration (essential for sustaining the otherwise thermodynamically unfavorable reactions) (Schink 1997). The primary alcohols such as ethanol are oxidized into acetate by the bacterial class of genera Desulfovibrio, Thermoanaerobium, and Pelobacter. The genera Syntrophomonas is predominantly responsible for the oxidation of propionic acid, butyric acid, and higher homologs. Syntrophomonass apovorans, Syntrophomonas wolfei and Syntrophomonas bryanti can convert butyrate and higher homologs into acetate, while Syntrophobacter wolinii is responsible for the conversion of propionate into acetate. Pentanoic acids are converted into propionic acid by the bacteria like Methanobacterium suboxydans, on the other hand Methanobacterium propionicum are mainly related to the conversion of propionic acid into acetic acid. The release of hydrogen occurs during acetogenesis, which exerts inhibitory effects on the responsible bacteria involved in the process. Therefore, a symbiotic relationship between the acetogenic bacteria with autotrophic methanogenic bacteria is necessary as they consume hydrogen. The acetogenic phase dominate in qualitative as well as quantitative production of biogas, as approximately 70% of methane is expected to produce during the acetate reduction. Consequently, methane digestion process produces acetates as a key intermediate product. During acetogenic phase, approximately 25% of the formation of acetic acid takes place and about 11% of hydrogen is produced in the decomposition of organic compounds.
Syntrophic bacteria produce hydrogen and thus these bacteria cannot form a pure culture. The metabolic processes of syntrophic bacteria are dependent upon the bacteria which can consume hydrogen. Methanogenic archaea is an example for consumption of hydrogen produced by syntrophic bacteria and subsequent methane production, these bacteria perform metabolic processes in association. During the oxidation process of propionic acid, many species of the Syntrophobacter genus of bacteria can utilize sulfate as terminal electron acceptor. Desulfotomaculum may use sulfate as electron acceptors. Desulfo vibrio may also use sulfate and lactate to form acetate and H2, through syntrophic association with Methanobacterium genus. Desulpho vibrio may compete with methanogens by using the same substrate and producing H2S and thereby hindering the methane formation.
It is well understood that acetate, H2, and CO2 are utilized mainly by the methanogenic bacteria for methane. Based on chemical oxygen demand (COD), decarboxylation of acetic acid is responsible for about 72% of production of methane, whereas the remaining 28% of methanogenesis takes places from carbon dioxide reduction (McCarty 1964). Aceticlastic methanogens are primarily responsible for the conversion of acetic acid into methane which consists of major part of the methanogenesis process. Remainder part of methanogenesis takes place by utilizing H2 and CO2 to form methane by the hydrogenotrophic methanogens.
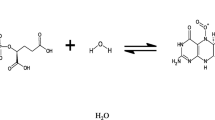
Previously, methanogens were classified as bacteria; but with the increasing understanding of anaerobic processes, these microorganisms were classified as archaea which are bit different from the microbes. In particular, these comprise of membrane lipids and distinctive ribosomal RNA (Pandey and Sarkar 2019b; Boone et al. 1993), whereas the lack of basic cellular characteristics (e.g., peptidoglycan) are observed in such class of bacteria. Methanogens are obligate anaerobes. There are three major pathways known for the methanogenesis: acetotrophic or aceticlastic methanogenesis, CO2 reducing or hydrogenotrophic methanogenesis, and methylotrophic pathways. The splitting of acetic acid to form methane according reaction 10.4 is known as aceticlastic pathway, and the methanogens taking part in such a pathway are called aceticlastic methanogens or aceticlastic archaea. Example of aceticlastic methanogens are Methanosarcina and Methanosaeta. The other most commonly encountered pathway of methane formation is anaerobic oxidation of hydrogen where carbon dioxide acts as the terminal electron acceptor. Reaction 10.3 is the representation of such pathway. In anaerobic treatment system ~28% of the methane generation is achieved through hydrogenotrophic pathways. Example of such hydrogenotrophic methanogens are Methanobacterium, Methanobrevibacterium, and Methanospirillum. Hydrogenotrophic methanogens are the hydrogen scavangers and help in maintaining a low partial pressure of H2 which is a prerequisite for the acetogenesis process to proceed in the forward direction. There is a wide class of hydrogenotrophic methanogens which are known to use formate as electron source to reduce CO2 to methane. Few numbers of methanogens are known to oxidize alcohols to reduce carbon dioxide to methane. Methanosarcina typically forms granules of spherical cell units and it is known to many other substrates like methylamines, methanol, and also H2/CO2 at several occasions. Utilizing acetate, Methanosarcina typically doubles its number in a time of 1–2 days. On the other hand, the rod-shaped Methanosaeta can only utilize acetate for its growth and doubles its number in a time of 4–9 days (Lee and Zinder 1988). Therefore, for a short solid retention time (SRT) and a completely mixed anaerobic reactor, Methanosaeta would easily wash out from the system making Methanosarcina as the predominant genera. In the reports of Raskin et al. (1995), where he used anaerobic digesters in which SRT was set to 20 days or more, Methanosaeta were observed to be the dominant methanogens. As per the reports of Conklin et al. (2006), Methanosaeta is predominantly found in a majority of anaerobic reactors. As per the reports, Methanosarcina is the most dominant methanogen in anaerobic bioreactors in which acetate is the primary substrate for utilization with a concentration greater about 236 mg/L having HRTs of 10 days or less. Noike et al. (1985) predicted that in continuous stirred tank reactors, the most dominant methanogen at an SRT of 6.5 days or less would be Methanosarcina, whereas for an SRT of 9.6 days or more, the bioreactor would be dominated by Methanosaeta.
Compounds like methanol, mono-, di-, and trimethylamine, and dimethyl sulfide, which contain methyl groups, are catabolized by methylotrophic pathways. Through this pathway, the methyl carrier takes up the methyl group to reduce it to methane. For the reduction of methyl, the required electron can be gained by either using H2 as an electron donor or by the oxidation of methyl groups (Boone et al. 1993).
Homoacetogens play a vital role during anaerobic digestion process as they produce acetate as the end product, which is an essential precursor for the aceticlastic methanogenesis. Either of the autotrophic or heterotrophic bacteria can mediate the process. The autotrophic homoacetogens are capable of utilizing CO2 and H2, with CO2 being the only source of the carbon for cell synthesis. Few homoacetogens can also make use of CO as the sole source of carbon. On the other hand, heterotrophic homoacetogens produce acetate as the end by-product by using organic substrates such as methanol and formate. In the reports of Novaes 1986 the two isolates from the sewage sludge were identified as Acetobacterium woodii and Clostridium aceticum which are homoacetogenic bacteria thriving in mesophilic environment. These homoacetogenic bacteria are known to have a high thermodynamic efficiency; due to this attribute the accumulation of hydrogen and carbon dioxide does not take place even when multicarbon substrates are fed to the reactor for the bacterial growth. The Gibb’s free-energy changes are analogous to hydrogenotrophic methanogenesis, which very closely competes for the available electron donor (H2). During the stress conditions of low temperature or low pH, aceticlastic methanogens may compete very successfully with the hydrogenotrophic methanogens and can adapt better. However, extensive research is needed to explore more about the metabolism of aceticlastic and hydrogenotrophic methanogenesis.
10.6 Major External Factors Affecting Anaerobic Digestion
The anaerobic degradation of organic compound is affected by a variety of external factors. For enhancement of the activity of the microorganisms and increase in the overall efficiency of anaerobic treatment process, the process should be optimized by controlling the operating parameters so that the effect of the external factors can be minimized.
10.6.1 Nutrient
Just like any other biological process, the requirement for the macronutrients (nitrogen and phosphorus) and micronutrients (trace elements) is also inevitable for the anaerobic bacteria to support their growth activities. Compared to aerobic process, nutrient requirements in anaerobic process are significantly different. The major reason behind such difference is that fermentative and methane-forming bacteria have significantly lower cell yield as compared to aerobic bacteria (Uke and Stentiford 2013; Ortner et al. 2014; Long et al. 2012). In general, municipal wastewater has a balance of macronutrients so that there is no need of extraneous addition of macronutrients. Sometimes, industrial wastewaters may not possess sufficiently balanced nutrients, and it may be highly desirable to add nitrogen and phosphorus for the maintenance of C, N, P at sufficient ratios for the efficient biological treatment of wastewater. The suitable C:N:P ratios of about 100:5:1 and 100:1.8:0.28 have been reported for anaerobic microorganisms (Diez-Gonzalez et al. 1998; Yilmaz et al. 2008). Besides nitrogen and phosphorus, other trace elements are essential at low concentration stimulating the activity of anaerobic microorganisms. The micronutrients of importance are magnesium, molybdenum, iron, nickel, and cobalt. Out of these, cobalt was shown to be the most critical micronutrient (Florencio et al. 1993) for efficient anaerobic degradation of wastewater. Kayhanian and Rich (1995) reported that molybdenum, cobalt, and nickel are essential for the growth of Methanobacterium.
10.6.2 pH
Fundamentally, the anaerobic digestion process consists of two major processes: acid formation through breakdown of substrates and methane formation by conversion of acid generated in acid formation stage. Therefore, essentially acidogenesis process runs at acidic pH, while methanogenesis process should operate at near neutral pH. It is well known that the range of pH for the optimum activity of acidogenic bacteria is 5.5–7.2, while the optimum range of pH value for methanogenic archaea is 6.6–7.6 (Visvanathan and Abeynayaka 2012). A closer examination of the fermentation or acidogenesis reactions mentioned earlier in this review should reveal that the characteristics and loading of the substrate have immediate effect on the pH of the reactor during acidogenesis reaction. The acid-forming bacteria are kinetically faster than the methanogenic bacteria. An increase in the loading of simple and easy-to-be-degraded substrate would allow for a spike in the production of VFAs, but the methanogens, having slower kinetics, might not be able to convert the acetate or hydrogen into methane. Thus, there will be accumulation of VFAs and excess production of CO2 within the reactor. Consequently, for both the changes, there should be concomitant lowering of the pH of the system. The pH may fall below the threshold range of values required for the methanogens to work effectively. This is when the reactor is considered to have become sour along with the washout of methanogens from the reactor. In order to circumvent the problem of acidification of the reactor as a result of increased organic load, anaerobic treatment processes should require sufficient capacity of buffering or alkalinity to minimize the effects of pH variations caused by either inlet conditions or by the increased substrate loading. Typically, pH of anaerobic system is maintained by natural alkalinity or self-producing alkalinity. The use of sodium bicarbonate, sodium hydroxide, or lime can efficiently control the lowering pH. While lime can cause scaling problem in the reactor with a precipitation of CaCO3, sodium bicarbonate is preferred due to its high buffering capability. Metcalf (2003) suggested that the alkalinity should be maintained within the range of 1–5 g/L as CaCO3.
10.6.3 Temperature
Like all other biological processes, temperature variations affect the metabolic rate, bacterial growth, and the activity of the bacteria in anaerobic wastewater treatment. As the microorganisms involved in the anaerobic reactions have to thrive on the small energy budget as compared to other type of degradation, their activities are quite sensitive to small variations in temperature at which the reactions take place. Thus, in many cases it is possible to correctly predict the effect of the change in temperature on these reactions if the thermodynamic basis for the availability of free energy from the reactions is known. Hydrolysis process is favored at elevated temperature. Bouallagui et al. (2004) reported that thermophilic hydrolysis rate of cellulose is higher than mesophilic hydrolysis rate around 5–6 times. Acid formation reactions have highly negative ΔG0 values which indicate the spontaneity of the reactions and also availability of high amount of free energy from these reactions for meeting the metabolic requirements of the fermenting microorganisms. Acetogenesis reactions have positive values of ΔG0 which indicate that in order for the reactions to take place, one of the reaction products should be present in very low concentrations. The reaction schemes and discussions mentioned in previous sections clearly indicate that partial pressure of hydrogen has to be maintained to be minimum through mutual cooperation with other syntrophic partner who utilize hydrogen by oxidizing it through hydrogenotrophic methanogenesis to form methane in order to release the energy that will be required for carrying out of metabolic activities. It has been reported that under psychrophilic conditions (<15 °C), homoacetogenesis leading to the formation of acetate from CO2 and H2 dominates, and it helps in the removal of hydrogen. During this process, the aceticlastic methanogenesis takes over as compared to hydrogenotrophic methanogenesis (Kotsyurbenko et al. 2001). Hydrogenotrophic methanogenesis reactions are thermodynamically favorable at low and high partial pressure of H2. However, with the increase of temperature, the ΔG0 value increases considerably, indicating that less free energy shall be available for metabolic activities at higher temperature. Alternatively, acetic acid can be converted to other forms in two possible ways: aceticlastic cleavage to form methane and acetic acid oxidation to form CO2 and hydrogen. At a temperature of 35 °C or lower aceticlastic methanogenesis produces more energy than hydrogenotrophic methanogenesis.
Under standard conditions, hydrogenotrophic methanogenesis yields more energy than homoacetogenic hydrogen oxidation in which hydrogen and carbon dioxide react to form acetate. Thus at standard conditions, homoacetogens would not compete with hydrogenotrophic methanogens. However, the situation dramatically changes at slightly acidic situation under low temperature, low acetate concentration and low partial pressure of H2. When the acetic acid concentration becomes 10 mM and H2 partial pressure reaches lower than 10 Pa, the process of homoacetogenesis reaches the same gain of energy at 5 °C as hydrogenotrophic methanogenesis does at 35 °C. Therefore, at low temperature, the pathway of homoacetogenesis and aceticlastic methanogenesis would be predominant over the hydrogenotrophic methanogenesis. The opposite scenario takes place at high temperature under which aceticlastic methanogenesis becomes less significant, homoacetogenesis operates in the opposite direction making the electron to flow from acetate through either formate or carbon dioxide and driving hydrogen toward methane though hydrogenotrophic methanogenesis. Nielsen et al. (2004) indicated that the thermophilic hydrolytic and fermentation bacteria and hydrogen consuming methanogens work efficiently in the range of 55 °C–75 °C and 55 °C–70 °C, respectively. Ahring et al. (1995) reported that conversion of acetate, butyrate, and propionate to methane had an optimum temperature range at 55–60 °C. It has been reported that the anaerobic reactors operated at thermophilic condition have produced more methane than that at mesophilic condition (Ramakrishnan and Surampalli 2013). Prokaryotic microorganisms can better adapt to higher temperature than eukaryotes do. Madigan et al. (2003) showed that for eukaryotes the limiting temperature for growth is around 60 °C, which for prokaryotes are much higher: 70 °C (for bacteria) and 113 °C (for archaea).
10.6.4 Toxic Compounds
Anaerobic microorganisms and its activity can be inhibited by anaerobic inhibitors present in wastewater or by-products from metabolic activities of anaerobic microorganisms. Furthermore, anaerobic inhibitors largely depend on wastewater characteristics. Ammonia, heavy metals, phenol, and halogenated compounds are the examples for toxic materials of anaerobic microorganisms. Generally, varying concentrations of different toxic compounds are reported by many researchers. The probable reason is investigation on reactors of different configurations as well as time and approach of seed sludge acclimatization. One of the interesting findings of anaerobic system-based research studies is that many anaerobic microorganisms have also the potential to degrade the refractory organics or recalcitrant compounds (Singh et al. 2018; Stronach et al. 2012). This toleration/degradation can be expected through the acclimation of microorganism to such toxic compounds. These findings can also be helpful in exploring the possibilities of anaerobic treatment of industrial wastewaters laden with varying concentrations of toxic/recalcitrant compounds (Pandey et al. 2016; Chen et al. 2008; Basri et al. 2010).
10.7 Conclusions
Being a complex system with a variety of bacteria involved in an anaerobic system, it needs a meticulous control over the processes for stable operation. Despite of all the advantages of anaerobic systems, lack of understanding about the processes makes it less used technology as compared to aerobic systems. The performance of the reactors under various adverse physicochemical conditions like pH, nutrient stress, toxic substances, detergents, and varying hydraulic loading rates affect the performance of the reactor heavily. These parameters should be meticulously taken care of in order to keep the bioprocess under control. A complete understanding should be a pre-condition before scaling up the process to industrial-level application. Accurate control of the anaerobic reactor may help in maximizing and recovering target intermediates which may prove to be commercially more attractive than methane production. Along with organic acids, hydrogen gas production is also one established and lucrative intermediate which can be economically beneficial and sustainable.
References
Ahring BK, Sandberg M, Angelidaki I (1995) Volatile fatty acids as indicators of process imbalance in anaerobic digestors. Appl Microbiol Biotechnol 43(3):559–565
Angelidaki I, Sanders W (2004) Assessment of the anaerobic biodegradability of macropollutants. Rev Environ Sci Bio Technol 3(2):117–129
Azbar N, Ursillo P, Speece RE (2001) Effect of process configuration and substrate complexity on the performance of anaerobic processes. Water Res 35(3):817–829
Basri MF, Yacob S, Hassan MA, Shirai Y, Wakisaka M, Zakaria MR, Phang LY (2010) Improved biogas production from palm oil mill effluent by a scaled-down anaerobic treatment process. World J Microbiol Biotechnol 26(3):505–514
Boone DR, Whitman WB, Rouvière P (1993) Diversity and taxonomy of methanogens. In: Methanogenesis. Springer, Boston, MA, pp 35–80
Bouallagui H, Haouari O, Touhami Y, Cheikh RB, Marouani L, Hamdi M (2004) Effect of temperature on the performance of an anaerobic tubular reactor treating fruit and vegetable waste. Process Biochem 39(12):2143–2148
Chen Y, Cheng JJ, Creamer KS (2008) Inhibition of anaerobic digestion process: a review. Bioresour Technol 99(10):4044–4064
Cobb SA, Hill DT (1991) Volatile fatty acid relationships in attached growth anaerobic fermenters. Trans ASAE 34(6):2564–2572
Conklin A, Stensel HD, Ferguson J (2006) Growth kinetics and competition between Methanosarcina and Methanosaeta in mesophilic anaerobic digestion. Water Environ Res 78(5):486–496
De Bok FA, Harmsen HJ, Plugge CM, de Vries MC, Akkermans AD, de Vos WM, Stams AJ (2005) The first true obligately syntrophic propionate-oxidizing bacterium, Pelotomaculumschinkii sp. nov., co-cultured with Methanospirillumhungatei, and emended description of the genus Pelotomaculum. Int J Syst Evol Microbiol 55(4):1697–1703
Diez-Gonzalez F, Callaway TR, Kizoulis MG, Russell JB (1998) Grain feeding and the dissemination of acid-resistant Escherichia coli from cattle. Science 281(5383):1666–1668
Florencio L, Jeniček P, Field JA, Lettinga G (1993) Effect of cobalt on the anaerobic degradation of methanol. J Ferment Bioeng 75(5):368–374
Jackson BE, McInerney MJ (2002) Anaerobic microbial metabolism can proceed close to thermodynamic limits. Nature 415(6870):454
Kayhanian M, Rich D (1995) Pilot-scale high solids thermophilic anaerobic digestion of municipal solid waste with an emphasis on nutrient requirements. Biomass Bioenergy 8(6):433–444
Khanal SK (2011) Anaerobic biotechnology for bioenergy production: principles and applications. John Wiley & Sons, Hoboken
Kotsyurbenko OR, Glagolev MV, Nozhevnikova AN, Conrad R (2001) Competition between homoacetogenic bacteria and methanogenic archaea for hydrogen at low temperature. FEMS Microbiol Ecol 38(2–3):153–159
Kuntze K, Shinoda Y, Moutakki H, McInerney MJ, Vogt C, Richnow HH, Boll M (2008) 6-Oxocyclohex-1-ene-1-carbonyl-coenzyme A hydrolases from obligately anaerobic bacteria: characterization and identification of its gene as a functional marker for aromatic compounds degrading anaerobes. Environ Microbiol 10(6):1547–1556
Kus F, Wiesmann U (1995) Degradation kinetics of acetate and propionate by immobilized anaerobic mixed cultures. Water Res 29(6):1437–1443
Lee MJ, Zinder SH (1988) Carbon monoxide pathway enzyme activities in a thermophilic anaerobic bacterium grown acetogenically and in a syntrophic acetate-oxidizing coculture. Arch Microbiol 150(6):513–518
Liu Y, Xu HL, Yang SF, Tay JH (2003) Mechanisms and models for anaerobic granulation in upflow anaerobic sludge blanket reactor. Water Res 37(3):661–673
Long JH, Aziz TN, Francis L III, Ducoste JJ (2012) Anaerobic co-digestion of fat, oil, and grease (FOG): a review of gas production and process limitations. Process Saf Environ Prot 90(3):231–245
Madigan MT, Martinko JM, Parker J (2003) Microbial growth. In: Brock biology of microorganisms. Prentice-Hall, Upper Saddle River, NJ, pp 137–166
McCarty PL (1964) Anaerobic waste treatment fundamentals. Public Works 95(9):107–112
Metcalf INC (2003) Wastewater engineering; treatment and reuse. McGraw-Hill, New York
Murto M, Björnsson L, Mattiasson B (2004) Impact of food industrial waste on anaerobic co-digestion of sewage sludge and pig manure. J Environ Manag 70(2):101–107
Nielsen HB, Mladenovska Z, Westermann P, Ahring BK (2004) Comparison of two-stage thermophilic (68 C/55 C) anaerobic digestion with one-stage thermophilic (55 C) digestion of cattle manure. Biotechnol Bioeng 86(3):291–300
Noike T, Endo G, Chang JE, Yaguchi JI, Matsumoto JI (1985) Characteristics of carbohydrate degradation and the rate-limiting step in anaerobic digestion. Biotechnol Bioeng 27(10):1482–1489
Novaes RF (1986) Microbiology of anaerobic digestion. Water Sci Technol 18(12):1–14
Ortner M, Leitzinger K, Skupien S, Bochmann G, Fuchs W (2014) Efficient anaerobic mono-digestion of N-rich slaughterhouse waste: influence of ammonia, temperature and trace elements. Bioresour Technol 174:222–232
Pandey S, Sarkar S (2017) Anaerobic treatment of wastewater using a two-stage packed-bed reactor containing polyvinyl alcohol gel beads as biofilm carrier. J Environ Chem Eng 5(2):1575–1585
Pandey S, Sarkar S (2019a) Performance evaluation and substrate removal kinetics of an anaerobic packed-bed biofilm reactor. Int J Environ Res 13(2):223–233
Pandey S, Sarkar S (2019b) Spatial distribution of major bacterial species and different volatile fatty acids in a two-phase anaerobic biofilm reactor with PVA gel beads as bio-carrier. Prep Biochem Biotechnol 49:704–717
Pandey S, Singh NK, Bansal AK, Arutchelvan V, Sarkar S (2016) Alleviation of toxic hexavalent chromium using indigenous aerobic bacteria isolated from contaminated tannery industry sites. Prep Biochem Biotechnol 46(5):517–523
Ramakrishnan A, Surampalli RY (2013) Performance and energy economics of mesophilic and thermophilic digestion in anaerobic hybrid reactor treating coal wastewater. Bioresour Technol 127:9–17
Raskin L, Zheng D, Griffin ME, Stroot PG, Misra P (1995) Characterization of microbial communities in anaerobic bioreactors using molecular probes. Antonie Van Leeuwenhoek 68(4):297–308
Rodriguez J, Kleerebezem R, Lema JM, van Loosdrecht MC (2006) Modeling product formation in anaerobic mixed culture fermentations. Biotechnol Bioeng 93(3):592–606
Schink B (1997) Energetics of syntrophic cooperation in methanogenic degradation. Microbiol Mol Biol Rev 61(2):262–280
Singh NK, Pandey S, Singh S, Singh S, Kazmi AA (2016) Post treatment of UASB effluent by using inorganic coagulants: role of zeta potential and characterization of solid residue. J Environ Chem Eng 4(2):1495–1503
Singh NK, Pandey S, Singh RP, Dahiya S, Gautam S, Kazmi AA (2018) Effect of intermittent aeration cycles on EPS production and sludge characteristics in a field scale IFAS reactor. J Water Process Eng 23:230–238
Stanbury PF, Whitaker A, Hall SJ (2013) Principles of fermentation technology. Elsevier, Amsterdam
Stronach SM, Rudd T, Lester JN (2012) Anaerobic digestion processes in industrial wastewater treatment, vol 2. Springer Science & Business Media, Berlin
Tatara M, Makiuchi T, Ueno Y, Goto M, Sode K (2008) Methanogenesis from acetate and propionate by thermophilic down-flow anaerobic packed-bed reactor. Bioresour Technol 99(11):4786–4795
Thauer RK, Jungermann K, Decker K (1977) Energy conservation in chemotrophic anaerobic bacteria. Bacteriol Rev 41(1):100
Uke MN, Stentiford E (2013) Enhancement of the anaerobic hydrolysis and fermentation of municipal solid waste in leachbed reactors by varying flow direction during water addition and leachate recycle. Waste Manag 33(6):1425–1433
Visvanathan C, Abeynayaka A (2012) Developments and future potentials of anaerobic membrane bioreactors (AnMBRs). Membr Water Treat 3(1):1–23
Wang M, Sahu AK, Rusten B, Park C (2013) Anaerobic co-digestion of microalgae Chlorella sp. and waste activated sludge. Bioresour Technol 142:585–590
Yilmaz T, Yuceer A, Basibuyuk M (2008) A comparison of the performance of mesophilic and thermophilic anaerobic filters treating papermill wastewater. Bioresour Technol 99(1):156–163
Zellner G, Neudörfer F (1995) Stability and metabolic versatility of a propionate-degrading biofilm operating in an anaerobic fluidised bed reactor. J Ferment Bioeng 80(4):389–393
Zhang H, Banaszak JE, Parameswaran P, Alder J, Krajmalnik-Brown R, Rittmann BE (2009) Focused-pulsed sludge pre-treatment increases the bacterial diversity and relative abundance of acetoclastic methanogens in a full-scale anaerobic digester. Water Res 43(18):4517–4526
Zheng M, Li X, Li L, Yang X, He Y (2009) Enhancing anaerobic biogasification of corn stover through wet state NaOH pretreatment. Bioresour Technol 100(21):5140–5145
Zoetemeyer RJ, Van den Heuvel JC, Cohen A (1982) pH influence on acidogenic dissimilation of glucose in an anaerobic digestor. Water Res 16(3):303–311
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Pandey, S. et al. (2020). Bacterial Production of Organic Acids and Subsequent Metabolism. In: Singh, V., Singh, A., Bhargava, P., Joshi, M., Joshi, C. (eds) Engineering of Microbial Biosynthetic Pathways. Springer, Singapore. https://doi.org/10.1007/978-981-15-2604-6_10
Download citation
DOI: https://doi.org/10.1007/978-981-15-2604-6_10
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-15-2603-9
Online ISBN: 978-981-15-2604-6
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)