Abstract
Chronic kidney disease (CKD) is very common in the elderly. CKD-related metabolic derangements increase the risk of skeletal muscle wasting, so the prevalence of sarcopenia and frailty are substantially higher in CKD patients compared to the general population. Sarcopenia is defined according to the Asian Working Group for Sarcopenia (AWGS), while frailty according to the Japanese version of the Cardiovascular Health Study (J-CHS) in Japan. Sarcopenia and frailty are closely associated with protein-energy wasting. Frailty is also more prevalent in female than in male in CKD patients.
Sarcopenia and frailty are both related to survival prognosis and accelerated progression to end-stage kidney disease in patients with non-dialysis-dependent CKD. In dialysis patients, low muscle strength rather than muscle mass volume is more strongly associated with physical inactivity, inflammation, and total mortality. Frailty is also an independent predictor of cognitive impairment, hospitalization, and mortality in the dialysis population.
Given the convincing relationship between sarcopenia, frailty, and adverse clinical outcomes, we should be more aware of the concept of sarcopenia and frailty and prevent their progressions especially in older patients with advanced CKD.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1.1 Introduction
Sarcopenia is a progressive and generalized “skeletal muscle disease” that is associated with increased likelihood of adverse outcomes including falls, fractures, physical disability, and mortality. In contrast, frailty is “a geriatric syndrome” that is observed as the decline over a lifetime in multiple physiological systems, resulting in negative consequences to physical, cognitive, and social dimensions.
In this chapter, demonstrating after the current trends of CKD epidemiology, I review the epidemiology of sarcopenia and frailty in patients with non-dialysis and dialysis-dependent CKD.
1.2 Current trends in CKD epidemiology
1.2.1 Renal Replacement Therapy (RRT)
More than 2 million people worldwide are being treated for end-stage renal disease (ESRD). The global incidence of dialysis more than doubled from 44 incidents per million people (pmp) in 1990 to 93 pmp in 2010 [1]. The global prevalence of maintenance dialysis has also increased 1.7 times, from 165 pmp in 1990 to 284 pmp in 2010. A systematic review [2] also estimates that 2.6 million people received RRT worldwide in 2010, whereas the number of patients requiring RRT is between 4.9 and 9.7 million. This review also points out that, with rising global incomes, worldwide use of RRT will be more than double to 5.4 million people in 2030, with the most growth in Asia (1.0 million to a projected 2.2 million) and most rapid relative increase projected in Latin America (from 0.37 million in 2010 to 0.90 million by 2030).
In contrast, in already developed nations that provide universal access to maintenance dialysis, there has been a plateauing in rates of ESRD, with recent declines in incidence. In the USA, ESRD incidence adjusted for age, sex, and race/ethnicity was 386 pmp in 2003, but decreased to 356, 352, and 351 pmp in 2011, 2012, and 2013, respectively [3]. In Japan, the actual number of new dialysis patients with diabetic nephropathy has almost been unchanged for the recent few years [4].
1.2.2 Non-dialysis CKD
A meta-analysis of 44 country prevalence studies [5] have demonstrated that the worldwide prevalence of CKD at 13.4% in 2010 (95% confidence interval [95% CI], 11.7–15.1%). A survey of 33 prevalence studies [6] also estimates worldwide prevalence of CKD at 10.4% in men (95%CI, 9.3–11.9%) and at 11.8% in women (95%CI, 11.2–12.6%), with a 15% higher prevalence in low- and middle-income countries compared with high-income countries. The Global Burden of Disease study [7] predicts that there were 21 million incident case of CKD per year, 276 million prevalent cases, and nearly 1.2 million death and 35 million years of healthy life lost due to CKD in 2016.
The prevalence of CKD is especially high in the elderly. Analyses of recent data from the US National Health and Nutrition Examination Survey (NHANES) demonstrated that the crude prevalence of CKD at stages G3 (eGFR from 30 to 59 ml/min/1.73 m2) and G4 (eGFR from 15 to 29 ml/min/1.73 m2) were 4.1% in subjects aged 20–39 years and 10.8% in those aged 40–64 years, while it reached 31.5% in those aged 65–79 years and 65.0% in those over 80 years [8]. Similarly, in Japan, prevalence rates of stage G3 and G4 CKD have been estimated at 43.1% in males and 44.5% in females aged over 80 years old [9].
1.2.3 Clinical Outcomes of CKD
In addition to being a precursor to ESRD, CKD is a potent risk factor for other adverse outcomes, such as acute kidney injury, cardiovascular disease, and mortality. The risk of ESRD, or death related to CKD comorbidities prior to dialysis initiation, varies by age. Analyses of data from a cohort of US veterans [10] demonstrated that younger patients (18–44 years old) were at risk of reaching ESRD before death at eGFR <45 ml/min/1.73 m2, whereas for older patients (65–84 years old), the risk of ESRD first exceeded death at an eGFR of <15 ml/min/1.73 m2. It was also demonstrated that the risk of death always exceeded the risk of ESRD among those 85 years or older. Among patients with CKD stage G4 who were referred to nephrologists, the rate of death without requiring RRT increased from the age of 50 years onwards, and exceeded that of RRT in incident patients aged ≥80 years old [11]. Specifically, older patients with low-grade proteinuria were more likely to die before requiring RRT [11]. A retrospective review of CKD in stage G3 to G5 patients also demonstrated that younger individuals are at higher risk of ESRD, whereas older individuals are more likely to die prior to developing ESRD [12]. The review found that the risk of death prior to ESRD relative to the onset of ESRD was about threefold higher for CKD stage G3, while equal for stage G4, and lower for stage G5, after adjusting for age and other cofounders [12]. It follows from these studies, therefore, that the oldest CKD patients almost died before the initiation of RRT.
In Japan, a cohort study in 461 referred CKD patients (mean age: 67.0 years) demonstrated that the incidence of death before RRT was 2.8/100 patient-years and none had ESRD among CKD stage 3 patients older than 65 years without overt proteinuria during median follow-up was 3.2 years [13]. Newly visiting CKD patients with normal-range proteinuria also did not exhibit a decline of kidney function even in advanced CKD stages 4–5 under specialized nephrology care [14]. The observations suggest that elderly CKD patients with normal-range proteinuria may not exhibit CKD progression even in advanced CKD stage. Therefore, elongation of a healthy life expectancy is more complicated than simply slowing eGFR decline in the elderly with CKD stage G3 and G4.
1.3 Epidemiology of Sarcopenia in CKD
1.3.1 Definition of Sarcopenia
Sarcopenia originally refers to the age-related reduction of appendicular skeletal muscle mass volume. However, in recognition that loss of strength or physical function often accompanies loss of muscle mass, it has been defined to include both low muscle mass and compromised functionality, such as reduced handgrip strength and/or slower gait speed.
Recently, the European Working Group on Sarcopenia in Older People (EWGSOP2) [15] updates the original definition in order to reflect scientific and clinical evidence that has built over the last decade. The working group recognizes sarcopenia, i.e., muscle failure, as “a muscle disease” rooted in adverse muscle changes across a lifetime that may be acute or chronic. Sarcopenia is defined by low levels of measures for three parameters in numerical order: (1) muscle strength, (2) muscle quantity/quality, and (3) physical performance as an indicator of severity (Table 1.1). They also recommend an algorithm for case-finding, diagnosis, and severity determination for systematic and consistent identification of people with sarcopenia or its risk.
In Asia, the Asian Working Group for Sarcopenia (AWGS) [16] defined the cutoff values for muscle mass measurements (7.0 kg/m2 for men and 5.4 kg/m2 for women by using dual X-ray absorptiometry, and 7.0 kg/m2 for men and 5.7 kg/m2 for women by using bioimpedance analysis), handgrip strength (<26 kg for men and <18 kg for women), and usual gait speed (<0.8 m/s) for the elderly. A revised consensus paper of AWGS (AWGS2019) has been recently published (Chen LK, et al. J Am Med Dir Assoc, in press). In this revision, calf circumference (<34 cm for men and <33cm for women) is available for the screening of sarcopenia. In addition, the cutoff of handgrip strength for men is elevated to <28 kg. Low physical perfomance can be diagnosed by either usual gait speed (<1.0 m/s), 5 sit to stand test (> or = 12 sec), or short physical perfprmance battery (< or = 9 points).
1.3.2 Sarcopenia in Non-dialysis CKD
The prevalence of sarcopenia is higher among adult patients with non-dialysis-dependent CKD compared to the general population, ranging from 5.9 to 50.0% [17,18,19,20,21] (Table 1.2). Sarcopenia is more prevalent in men than in women [19, 20]. An increased risk of sarcopenia is associated with age, body mass index, diabetes mellitus, and loop diuretic use [20].
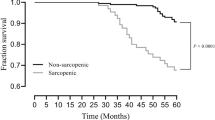
Sarcopenia is related to survival prognosis [17] and GFR decline [19]. A lower bilateral psoas mass area at CT slice is an independent predictor of major adverse cardiovascular events in CKD patients [22].
1.3.3 Sarcopenia in Dialysis Patients
Dialysis patients exhibit more functionally muscle wasting than patient with CKD stage G4 [23]. The prevalence of sarcopenia is substantially high among hemodialysis (HD) patients, ranging from 9.5 to 37.1% (Table 1.3) [24,25,26,27,28,29]. The prevalence of sarcopenia was 8.4% in Japanese peritoneal dialysis (PD) patients using the AWGS criteria [30].
Low muscle strength rather than muscle mass volume was more strongly associated with physical inactivity, inflammation, and mortality than low muscle mass in incident dialysis patients [24]. Physical performance measures, including slow gait speed and weak grip strength, were also associated with mortality even after adjustment for muscle size and other confounders in prevalent HD patients [28]. Low muscle strength was associated with worse quality of life (QOL) domains [29]. So, functional limitations (in strength or speed) are mainly associated with mortality, whereas muscle size appeared to be less important with regard to survival among dialysis patients.
Sarcopenia is also related to accelerated changes of arteriosclerosis. Reduced thigh muscle mass area is independently related to arteriosclerotic parameters such as carotid artery intima-medial thickness, brachial-ankle pulse wave velocity, and ankle-brachial pressure index, indicating that thigh sarcopenia is closely associated with systemic changes of arteriosclerosis in HD patients [31].
1.4 Epidemiology of Frailty in CKD
1.4.1 Definition of Frailty
Frailty is “a multidimensional geriatric syndrome” that is characterized by cumulative decline in multiple body systems or functions. Frailty increases vulnerability to poor health outcomes such as disability, hospital admission, reduced QOL, and even death.
There are two approaches to assessing frailty: one is phenotype model [32] and the other is accumulated deficit model [33]. The physical phenotype of frailty, originally described by Fried and co-workers [32], is characterized by the phenotype according to limitations in three or more of the following five conditions based on Cardiovascular Health Study (CHS): slow gait speed, weakness, exhaustion, low activity, and weight loss. This criteria overlaps with sarcopenia; low grip strength and slow gait speed are characteristic of both.
In contrast, frailty index, a typical accumulated deficit model, is to count deficits in health (which can be symptoms, signs, diseases, disabilities or laboratory, radiographic or electrocardiographic abnormalities) on the grounds that the more deficits a person has, the more likely that person is to be frail. This index is often expressed as a ratio of deficits present to the total number of deficits considered [34].
To date, fundamental differences in the conceptualization of frailty among these approaches result in long-standing hurdles to uniform agreement on a single definition that can be used for identifying those who are at high risk and in need of comprehensive care.
1.4.2 Modified Definition of Frailty in Japan
The Kihon Check List (LCL), which consists of 25 questions to screen participants who require care prevention, is used as a screening tool to assess frailty in Japan [35]. KCL is divided into the eight domains: instrumental activities of daily living (ADL), social ADL, exercise, falling, nutrition, oral function, cognitive function, and depression. Participants are asked to respond either “negative” (score: 1) or “positive” (score: 0), for a total score of 25. Frailty is evaluated by the total points as follows: frail, 8–25 points; pre-frail, 4–7 points; and robust, 0–3 points [35].
Frailty can be also diagnosed by the Japanese version of the Cardiovascular Health Study (J-CHS) [36]. Slow gait speed is established based on a cutoff of <1.0 m/s. Weakness is defined using maximum grip strength and was established according to a sex-specific cutoff (<26 kg for men and <18 kg for women), identical to AWGS criteria [16]. Exhaustion is considered present if a participant responded “yes” to the following question included in the KCL: “In the last 2 weeks, have you felt tired without a reason?” Physical activity is evaluated by asking the following questions about the time spent engaged in exercise: “Do you engage in low levels of physical exercise aimed at health?” If participants answered “no” to the questions, we classified them to the low activity category. Weight loss was assessed by a response of “yes” to the question, “Have you lost 2 kg or more in the past 6 months?”. Participants who do not have any of these components are considered as non-frail (robust), and those with one or two components were considered as pre-frail.
1.4.3 Frailty in Non-dialysis CKD
The prevalence of frailty defined by the Fried phenotype [32] ranges from 7 to 20.9% in pre-dialysis patients [37]. Frailty phenotypes such as body weight loss, low physical activity, and slow gait speed are independently associated with CKD progression and/or total mortality in CKD stages G1 to G4 patients [38]. Physical function such as gait speed and handgrip decreases in ambulant patients with CKD stage 4 or 5 than those with CKD stage 2 or 3 [39].
In community-dwelling Japanese older adults, participants with CKD stage 4 or 5 were more frail (odds ratio [OR] 1.90, 95% confidence interval [CI] 1.01–3.59). In addition, the individuals with a history of diabetes (OR 2.76, 95% CI 1.21–8.24), hypertension (OR 2.53, 95% CI 1.45–5.12), or both (OR 3.67, 95% CI 1.13–14.1) showed a significantly higher risk of frailty [40]. In addition, reduced kidney function (CKD stage 4–5) was associated with a higher risk of weight loss, low physical activity, and slowness [41].
The frailty phenotype was associated with an estimated 2.5 (95% CI, 1.4–4.4)-fold greater risk of death or incident dialysis therapy [42]. Frailty is independently linked to adverse outcomes such as lower physical and metal QOL [43], and limited activity of daily life (ADL) [44].
1.4.4 Frailty in Dialysis Patients
The prevalence of frailty is high in the dialysis populations, ranging from 24 to 78% [37]. However, since several studies have made modification to the frailty phenotype originally proposed, reported prevalence changes depending on the method of frailty assessment [37]. The prevalence of pre-frailty and frailty based on J-CHS criteria [36] was 52.6 and 21.4% in prevalent 413 Japanese HD patients (mean age 67.2 ± 11.9 years old). The 56.6% of the patients were categorized as pre-frailty and 32.7% as frailty among those aged over 75 years old (n = 113) [45]. The prevalence of frailty is reported as 10.9% when diagnosed using the Clinical Frailty Scale [30] in Japanese PD patients.
Frailty is an independent predictor of mortality and hospitalization in maintenance dialysis patients [46]. All five phenotype components are associated with higher mortality, and gait speed was the strongest individual predictor. The number of frailty components met was associated with mortality in a gradient that ranged from a hazard ratio of 2.73 for one component to 10.07 for five components met [47], indicating that measurement of all components was exclusively essential for optimal mortality prediction.
Frailty is also associated with impaired cognitive function using the Modified Mini-Mental State test and Trail Making Tests A and B among patients new to HD [48]. Incident dialysis patients self-reporting frailty experienced nearly twice the risk of medically urgent falls or fractures compared to those who did not report frailty [49].
1.5 Association of Protein-Energy Wasting with Sarcopenia, and Frailty Phenotype
Protein-energy wasting (PEW) is defined by an expert panel of the International Society of Renal Nutrition and Metabolism (ISRNM) in 2008 as the loss of somatic and circulating body protein and energy reserves [50]. PEW develops as the consequence of a combination of insufficient, uremic toxins, systematic inflammation, and superimposed catabolism. PEW can be diagnosed if at least 3 of the 4 listed categories (and at least one test in each of the selected category) are satisfied (Table 1.4).
A systematic review [51] reported that PEW prevalence ranges 11–54% in patients with CKD stages G3 to G5, and 28–54% in dialysis patients. About 15.3–17.1% of Japanese HD patients have PEW based on the ISRNM criteria [52, 53]. Since the hazard ratio for mortality became maximal at BMI <20 kg/m2 in Japanese HD patients, a lower BMI may be more suitable to diagnose the presence of PEW in the Asia population.
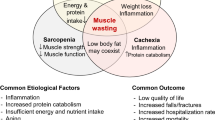
Decreased muscle mass is the same category in sarcopenia and PEW, while anthropometric measurements are different. Unintentional loss of body weight is also applied in the phenotype of frailty and PEW (Fig. 1.1).
1.6 Conclusion
Currently, over 850 million people have been suffering from some form of kidney disease in the world. CKD is a potent risk for fatal cardiovascular events. Annual costs per one patient for HD are expensive, thereby imposing a heavy financial burden on healthcare budgets.
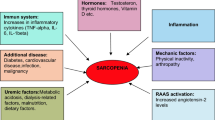
CKD is a predominant disease of the elderly. So, we need to consider the influence of aging, lifestyle, and social factors on renal and overall health, as well as CKD-related comorbidities and complications (Fig. 1.2). Especially, sarcopenia and frailty are very common. Given the convincing relationship between sarcopenia, frailty, and adverse clinical outcomes, we should be more aware of the concept of sarcopenia and frailty in older patients with advanced CKD.
References
Thomas B, Wulf S, Bikbov B, Perico N, Cortinovis M, Courville de Vaccaro K, Flaxman A, Peterson H, Delossantos A, Haring D, Mehrotra R, Himmelfarb J, Remuzzi G, Murray C, Naghavi M. Maintenance dialysis throughout the world in years 1990 and 2010. J Am Soc Nephrol. 2015;26:2621–33.
Liyanage T, Ninomiya T, Jha V, Neal B, Patrice HM, Okpechi I, Zhao MH, Lv J, Garg AX, Knight J, Rodgers A, Gallagher M, Kotwal S, Cass A, Perkovic V. Worldwide access to treatment for end-stage kidney disease: a systematic review. Lancet. 2015;385:1975–82.
United States Renal Data System. USRDS annual data report. Epidemiology of kidney disease in the United States. Bethesda: National Institutes of Health; 2015. p. 2015.
Masakane I, Nakai S, Ogata S, Kimata N, Hanafusa N, Hamano T, Wakai K, Wada A, Nitta K. An overview of regular dialysis treatment in Japan (as of 31 December 2013). Ther Apher Dial. 2015;19:540–74.
Mills KT, Xu Y, Zhang W, Bundy JD, Chen CS, Kelly TN, Chen J, He J. A systematic analysis of worldwide population-based data on the global burden of chronic kidney disease in 2010. Kidney Int. 2015;88:950–7.
Hill NR, Fatoba ST, Oke JL, Hirst JA, O’Callaghan CA, Lasserson DS, Hobbs FD. Global prevalence of chronic kidney disease - a systematic review and meta-analysis. PLoS One. 2016;11:e0158765.
Xie Y, Bowe B, Mokdad AH, Xian H, Yan Y, Li T, Maddukuri G, Tsai CY, Floyd T, Al-Aly Z. Analysis of the Global Burden of Disease study highlights the global, regional, and national trends of chronic kidney disease epidemiology from 1990 to 2016. Kidney Int. 2018;94:567–81.
Murphy D, McCulloch CE, Lin F, Banerjee T, Bragg-Gresham JL, Eberhardt MS, Morgenstern H, Pavkov ME, Saran R, Powe NR, Hsu CY, Centers for Disease Control and Prevention Chronic Kidney Disease Surveillance Team. Trends in prevalence of chronic kidney disease in the United States. Ann Intern Med. 2016;165:473–81.
Imai E, Horio M, Watanabe T, Iseki K, Yamagata K, Hara S, Ura N, Kiyohara Y, Moriyama T, Ando Y, Fujimoto S, Konta T, Yokoyama H, Makino H, Hishida A, Matsuo S. Prevalence of chronic kidney disease in the Japanese general population. Clin Exp Nephrol. 2009;13:621–30.
O’Hare AM, Choi AI, Bertenthal D, Bacchetti P, Garg AX, Kaufman JS, Walter LC, Mehta KM, Steinman MA, Allon M, McClellan WM, Landefeld CS. Age affects outcomes in chronic kidney disease. J Am Soc Nephrol. 2007;18:2758–65.
Demoulin N, Beguin C, Labriola L, Jadoul M. Preparing renal replacement therapy in stage 4 CKD patients referred to nephrologists: a difficult balance between futility and insufficiency. A cohort study of 386 patients followed in Brussels. Nephrol Dial Transplant. 2011;26:220–6.
Sud M, Tangri N, Levin A, Pintilie M, Levey AS, Naimark DM. CKD stage at nephrology referral and factors influencing the risks of ESRD and death. Am J Kidney Dis. 2014;63:928–36.
Obi Y, Kimura T, Nagasawa Y, Yamamoto R, Yasuda K, Sasaki K, Kitamura H, Imai E, Rakugi H, Isaka Y, Hayashi T. Impact of age and overt proteinuria on outcomes of stage 3 to 5 chronic kidney disease in a referred cohort. Clin J Am Soc Nephrol. 2010;5:1558–65.
Iimori S, Naito S, Noda Y, Sato H, Nomura N, Sohara E, Okado T, Sasaki S, Uchida S, Rai T. Prognosis of chronic kidney disease with normal-range proteinuria: The CKD-ROUTE study. PLoS One. 2018;13:e0190493.
Cruz-Jentoft AJ, Bahat G, Bauer J, Boirie Y, Bruyère O, Cederholm T, Cooper C, Landi F, Rolland Y, Sayer AA, Schneider SM, Sieber CC, Topinkova E, Vandewoude M, Visser M, Zamboni M, Writing Group for the European Working Group on Sarcopenia in Older People 2 (EWGSOP2), and the Extended Group for EWGSOP2. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. 2019;48:16–31.
Chen LK, Liu LK, Woo J, Assantachai P, Auyeung TW, Bahyah KS, Chou MY, Chen LY, Hsu PS, Krairit O, Lee JS, Lee WJ, Lee Y, Liang CK, Limpawattana P, Lin CS, Peng LN, Satake S, Suzuki T, Won CW, Wu CH, Wu SN, Zhang T, Zeng P, Akishita M, Arai H. Sarcopenia in Asia: consensus report of the Asian Working Group for Sarcopenia. J Am Med Dir Assoc. 2014;15:95–101.
Pereira RA, Cordeiro AC, Avesani CM, Carrero JJ, Lindholm B, Amparo FC, Amodeo C, Cuppari L, Kamimura MA. Sarcopenia in chronic kidney disease on conservative therapy: prevalence and association with mortality. Nephrol Dial Transplant. 2015;30:1718–25.
Souza VA, Oliveira D, Barbosa SR, Corrêa JODA, Colugnati FAB, Mansur HN, Fernandes NMDS, Bastos MG. Sarcopenia in patients with chronic kidney disease not yet on dialysis: analysis of the prevalence and associated factors. PLoS One. 2017;12:e0176230.
Zhou Y, Hellberg M, Svensson P, Höglund P, Clyne N. Sarcopenia and relationships between muscle mass, measured glomerular filtration rate and physical function in patients with chronic kidney disease stages 3-5. Nephrol Dial Transplant. 2018;33:342–8.
Ishikawa S, Naito S, Iimori S, Takahashi D, Zeniya M, Sato H, Nomura N, Sohara E, Okado T, Uchida S, Rai T. Loop diuretics are associated with greater risk of sarcopenia in patients with non-dialysis-dependent chronic kidney disease. PLoS One. 2018;13:e0192990.
D’Alessandro C, Piccoli GB, Barsotti M, Tassi S, Giannese D, Morganti R, Cupisti A. Prevalence and correlates of sarcopenia among elderly CKD outpatients on tertiary care. Nutrients. 2018;10:E1951.
Harada K, Suzuki S, Ishii H, Aoki T, Hirayama K, Shibata Y, Negishi Y, Sumi T, Kawashima K, Kunimura A, Shimbo Y, Tatami Y, Kawamiya T, Yamamoto D, Morimoto R, Yasuda Y, Murohara T. Impact of skeletal muscle mass on long-term adverse cardiovascular outcomes in patients with chronic kidney disease. Am J Cardiol. 2017;119:1275–80.
McIntyre CW, Selby NM, Sigrist M, Pearce LE, Mercer TH, Naish PF. Patients receiving maintenance dialysis have more severe functionally significant skeletal muscle wasting than patients with dialysis-independent chronic kidney disease. Nephrol Dial Transplant. 2006;21:2210–6.
Isoyama N, Quershi AR, Avesani CM, et al. Comparative associations of muscle mass and muscle strength with morality in dialysis patients. Clin J Am Soc Nephrol. 2014;9:1720–8.
Kim JK, Choi SR, Choi MJ, et al. Prevalence of and factors associated with sarcopenia in elderly patients with end-stage renal disease. Clin Nutr. 2014;33:64–8.
Lamarca F, Carrero JJ, Rodrigues JC, Bigogno FG, Fetter RL, Avesani CM. Prevalence of sarcopenia in elderly maintenance hemodialysis patients: the impact of different diagnostic criteria. J Nutr Health Aging. 2014;18:710–7.
Bataille S, Serveaux M, Carreno E, Pedinielli N, Darmon P, Robert A. The diagnosis of sarcopenia is mainly driven by muscle mass in hemodialysis patients. Clin Nutr. 2017;36:1654–60.
Kittiskulnam P, Chertow GM, Carrero JJ, Delgado C, Kaysen GA, Johansen KL. Sarcopenia and its individual criteria are associated, in part, with mortality among patients on hemodialysis. Kidney Int. 2017;92:238–47.
Giglio J, Kamimura MA, Lamarca F, Rodrigues J, Santin F, Avesani CM. Association of sarcopenia with nutritional parameters, quality of life, hospitalization, and mortality rates of elderly patients on hemodialysis. J Ren Nutr. 2018;28:197–207.
Kamijo Y, Kanda E, Ishibashi Y, Yoshida M. Sarcopenia and frailty in PD: impact on mortality, malnutrition, and inflammation. Perit Dial Int. 2018;38:447–54.
Kato A, Ishida J, Endo Y, Takita T, Furuhashi M, Maruyama Y, Odamaki M. Association of abdominal visceral adiposity and thigh sarcopenia with changes of arteriosclerosis in haemodialysis patients. Nephrol Dial Transplant. 2011;26:1967–76.
Fried LP, Ferrucci L, Darer J, Williamson JD, Anderson G. Untangling the concepts of disability, frailty and comorbidity: implications for improving targeting and care. J Gerontrol A Biol Sci Med Sci. 2004;59:225–63.
Mitnitski AB, Mogilner AJ, Rockwood K. Accumulation of deficits as a proxy measure of aging. Sci World J. 2001;1:323–36.
Searle SD, Mitnitski A, Gahbauer EA, Gill TM, Rockwood K. A standard procedure for creating a frailty index. BMC Geriatr. 2008;8:24.
Satake S, Senda K, Hong YJ, Miura H, Endo H, Sakurai T, Kondo I, Toba K. Validity of the Kihon checklist for assessing frailty status. Geriatr Gerontol Int. 2016;16:709–15.
Satake S, Shimada H, Yamada M, Kim H, Yoshida H, Gondo Y, Matsubayashi K, Matsushita E, Kuzuya M, Kozaki K, Sugimoto K, Senda K, Sakuma M, Endo N, Arai H. Prevalence of frailty among community-dwellers and outpatients in Japan as defined by the Japanese version of the Cardiovascular Health Study criteria. Geriatr Gerontol Int. 2017;17:2629–34.
Carrero JJ, Johansen KL, Lindholm B, Stenvinkel P, Cuppari L, Avesani CM. Screening for muscle wasting and dysfunction in patients with chronic kidney disease. Kidney Int. 2016;90:53–60.
Roshanravan B, Khatri M, Robinson-Cohen C, Levin G, Patel KV, de Boer IH, Seliger S, Ruzinski J, Himmelfarb J, Kestenbaun B. A prospective study of frailty in nephrology-referred patients with CKD. Am J Kidney Dis. 2012;60:912–21.
Hiraki K, Yasuda T, Hotta C, Izawa KP, Morio Y, Watanabe S, Sakurada T, Shibagaki Y, Kimura K. Decreased physical function in pre-dialysis patients with chronic kidney disease. Clin Exp Nephrol. 2013;17:2252–31.
Lee S, Lee S, Harada K, Bae S, Makizako H, Doi T, Tsutsumimoto K, Hotta R, Nakakubo S, Park H, Suzuki T, Shimada H. Geriatr Gerontol Int. 2017;17:1527–33.
Lee S, Lee S, Bae S, Harada K, Jung S, Imaoka M, Makizako H, Doi T, Shimada H. Relationship between chronic kidney disease without diabetes mellitus and components of frailty in community-dwelling Japanese older adults. Geriatr Gerontol Int. 2018;18:286–92.
Roshanravan B, Khatri M, Robinson-Cohen C, Levin G, Patel KV, de Boer IH, Seliger S, Ruzinski J, Himmelfarb J, Kestenbaum B. A prospective study of frailty in nephrology-referred patients with CKD. Am J Kidney Dis. 2012;60:912–21.
Lee SJ, Son H, Shin SK. Influence of frailty on health-related quality of life in pre-dialysis patients with chronic kidney disease in Korea: a cross-sectional study. Health Qual Life Outcomes. 2015;13:70.
Shlipak MG, Stehman-Breen C, Fried LF, Song X, Siscovick D, Fried LP, Psaty BM, Newman AB. The presence of frailty in elderly persons with chronic renal insufficiency. Am J Kidney Dis. 2004;43:861–7.
Takeuchi H, Uchida HA, Kakio Y, Okuyama Y, Okuyama M, Umebayashi R, Wada K, Sugiyama H, Sugimoto K, Rakugi H, Wada J. The prevalence of frailty and its associated factors in Japanese hemodialysis patients. Aging Dis. 2018;9:192–207.
Lee SY, Yang DH, Hwang E, Kang SH, Park SH, Kim TW, Lee DH, Park K, Kim JC. The prevalence, association, and clinical outcomes of frailty in maintenance dialysis patients. J Ren Nutr. 2017;27:106–12.
Johansen KL, Delgado C, Kaysen GA, Chertow GM, Chiang J, Dalrymple LS, Segal MR, Grimes BA. Frailty among patients receiving hemodialysis: evolution of components and associations with mortality. J Gerontol A Biol Sci Med Sci. 2019;74:380–6.
McAdams-DeMarco MA, Tan J, Salter ML, Gross A, Meoni LA, Jaar BG, Kao WH, Parekh RS, Segev DL, Sozio SM. Frailty and cognitive function in incident hemodialysis patients. Clin J Am Soc Nephrol. 2015;10:2181–9.
Delgado C, Shieh S, Grimes B, Chertow GM, Dalrymple LS, Kaysen GA, Kornak J, Johansen KL. Association of self-reported frailty with falls and fractures among patients mew to dialysis. Am J Nephrol. 2015;42:134–40.
Fouque D, Kalantar-Zadeh K, Kopple J, Cano N, Chauveau P, Cuppari L, Franch H, Guarnieri G, Ikizler TA, Kaysen G, Lindholm B, Massy Z, Mitch W, Pineda E, Stenvinkel P, Treviño-Becerra A, Wanner C. A proposed nomenclature and diagnostic criteria for protein-energy wasting in acute and chronic kidney disease. Kidney Int. 2008;73:391–8.
Carrero JJ, Thomas F, Nagy K, Arogundade F, Avesani CM, Chan M, Chmielewski M, Cordeiro AC, Espinosa-Cuevas A, Fiaccadori E, Guebre-Egziabher F, Hand RK, Hung AM, Ikizler TA, Johansson LR, Kalantar-Zadeh K, Karupaiah T, Lindholm B, Marckmann P, Mafra D, Parekh RS, Park J, Russo S, Saxena A, Sezer S, Teta D, Ter Wee PM, Verseput C, Wang AYM, Xu H, Lu Y, Molnar MZ, Kovesdy CP. Global prevalence of protein-energy wasting in kidney disease: a meta-analysis of contemporary observational studies from the International Society of Renal Nutrition and Metabolism. J Ren Nutr. 2018;28:380–92.
Yasui S, Shirai Y, Tanimura M, Matsuura S, Saito Y, Miyata K, Ishikawa E, Miki C, Hamada Y. Prevalence of protein-energy wasting (PEW) and evaluation of diagnostic criteria in Japanese maintenance hemodialysis patients. Asia Pac J Clin Nutr. 2016;25:292–9.
Takahashi H, Inoue K, Shimizu K, Hiraga K, Takahashi E, Otaki K, Yoshikawa T, Furuta K, Tokunaga C, Sakakibara T, Ito Y, Tokai Renal Nutrition Study Group. Comparison of nutritional risk scores for predicting mortality in Japanese chronic hemodialysis patients. J Ren Nutr. 2017;27:201–6.
Disclosures
I declare that I have no competing interests.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Kato, A. (2020). Epidemiology of Sarcopenia and Frailty in CKD. In: Kato, A., Kanda, E., Kanno, Y. (eds) Recent Advances of Sarcopenia and Frailty in CKD. Springer, Singapore. https://doi.org/10.1007/978-981-15-2365-6_1
Download citation
DOI: https://doi.org/10.1007/978-981-15-2365-6_1
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-15-2364-9
Online ISBN: 978-981-15-2365-6
eBook Packages: MedicineMedicine (R0)