Abstract
Since time immemorial, essential oils (EOs) have been utilized for their therapeutic properties. However, during recent decades, a renewed interest has been experienced in EOs and their individual constituents due to their remarkable pharmacological potentials evidenced by various experimental studies. For instance, they are broadly acknowledged for their antimicrobial, antidiabetic, anti-inflammatory, antispasmodic and anticancer properties. Also, EOs have demonstrated to improve conditions associated with brain and cardiovascular diseases amongst others. Multiple studies have identified and isolated EOs phytocomponents possessing therapeutic activities. In addition, studies have elucidated their underlying mechanisms of actions involved in the treatment and management of several ailments. Thus, given the wide spectrum of biological activities exhibited by these active compounds, this chapter endeavours to provide a mechanistic overview of some common EOs constituents with regard to their pharmacological properties.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
18.1 Introduction
Essential oils (EOs) are volatile aromatic, hydrophobic and oily liquids obtained from plants. They may be produced from cells or groups of cells specialized within the specific parts of plants, such as leaves, bark or wood, foliage, fruits, seeds, stems and rhizomes (Miguel 2010). EOs play a significant role in protecting the plants against bacterial, viral, fungal and insect attacks, and also herbivores by decreasing their appetite for the plants. Besides, they serve in attracting some insects for seeds and pollen dispersal or help to keep away unwanted insects (Bakkali et al. 2008).
EOs can be obtained by a number of different techniques, including fermentation, expression or enfleurage, although hydrodistillation is the most commonly used one (Speranza and Corbo 2010). A large number of herbal plants have been studied for their EOs and exploited for commercial applications (Mohammadreza 2008), particularly in cosmetics, perfumery, agriculture and food industries (Burt 2004).
EO constituents can be categorized into two distinct classes of chemicals, namely terpenes and phenylpropanoids. Even though terpenes and their derived oxygenated compounds (terpenoids) are more common and rich in EOs, certain species are composed of high amounts of shikimates, notably phenylpropanoids, which confer a specific flavour and odour to the plant (Tisserand and Young 2013; Baser and Buchbauer 2015; Zuzarte and Salgueiro 2015). However, it is important to note that EOs composition can vary depending on factors, such as variety of plants, parts of the plant, growth area, time of harvest, climatic changes, conditions of storage as well as the chemotype of each constituent (Pauli and Schilche 2009).
EOs and their active components have been extensively studied for their vast range of biological activities along with their underlying mechanisms of actions. For instance, they have been reported to act as natural antimicrobials (Edris and Farrag 2003; Burt 2004; Swamy et al. 2016) and have even been evaluated for their potential uses as substitute antidotes for the treatment of several infectious diseases (Freires et al. 2015). Additionally, they are regarded as potent antioxidants, anti-inflammatory (Miguel 2010; Pérez et al. 2011) and antidiabetic agents (Tahir et al. 2016; Ya’ni et al. 2018), amongst others. EOs have also demonstrated anticarcinogenic effects through a range of mechanisms, including acting directly on the tumour cells or by interaction with the microenvironment (Edris 2007; Sitarek et al. 2017). Moreover, their importance in aromatherapy has been widely documented. For instance, in medical aromatherapy, EOs have been reported to help in health promotion and treatment of clinically diagnosed medical diseases (Maeda et al. 2012).
Even though EOs major constituents are crucial for their bioactivities, minor components can play a significant role as well, since they can increase the effects of the major constituents. However, some of these EOs compounds have been shown to exhibit both additive and antagonistic effects (Bassolé and Juliani 2012). Therefore, EOs bioactivity can be regarded as the sum of its components acting either synergistically or antagonistically (Baser and Buchbauer 2010; Elshafie et al. 2015). This chapter aims to discuss different mechanisms of actions demonstrated by EOs constituents (in synergism, as single agents or as potentiating agents in tandem with conventional drugs) in relation to their pharmacological properties.
18.2 Essential Oils (EOs) Major Compounds and Their Mechanisms of Actions
18.2.1 Antimicrobial Property
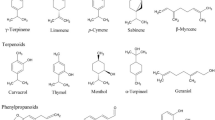
The antimicrobial potentials of EOs and their components have been extensively studied in the search for more effective antimicrobials to combat antibiotic-resistant pathogenic microorganisms (Swamy et al. 2016). In that aspect, EOs that are abundant with phenols or aldehydes, such as cinnamaldehyde, citral, thymol, eugenol or carvacrol (Fig. 18.1) were found to exhibit the highest antibacterial potential, followed by those composed of terpene alcohols like terpineol, fenchyl alcohol, and borneol. On the other hand, volatile oils consisting of esters or ketones like geranyl acetate, α-thujone or β-myrcene demonstrated much weaker activity, while EOs containing terpene hydrocarbons were generally not active (Davidson 1997; Dorman and Deans 2000). Besides, EOs rich in phenolic compounds like eugenol, carvacrol and thymol (Fig. 18.1) are usually characterized by significant antibacterial activities (Knobloch et al. 1986; Dorman and Deans 2000; Lambert et al. 2001; Swamy et al. 2016). In addition, these compounds have been held accountable for the disruption of plasma membrane, protons driving force, active transport, electron flow as well as the coagulation of cellular contents (Sikkema et al. 1994; Denyer and Hugo 1991; Pauli 2001; Swamy et al. 2016). Thus, the antimicrobial activity of EO constituents is determined by the lipophilicity of their hydrocarbon skeleton as well as the hydrophilicity of their major functional groups, and accordingly it has been classified in the following order: phenols > aldehydes > ketones > alcohols > ethers > hydrocarbons (Kalemba and Kunicka 2003).
A major compound of EOs, eugenol (Fig. 18.1) has been evaluated for its antibacterial effect and its mechanism of action against Salmonella typhi has been elucidated (Devi et al. 2010). According to them, eugenol was observed to decrease the viability of cells and cause absolute inhibition of the bacterium with the minimum inhibition concentration (MIC) and minimum bactericidal concentration (MBC) of 0.0125% and 0.025%, respectively. Additionally, inactivation of S. typhi was induced within 60 min of exposure to eugenol. Eugenol’s chemoattractant property along with the observed strong antibacterial activity at alkaline pH suggests that this compound can act more effectively when given in vivo. Furthermore, the interaction of eugenol on S. typhi cell membrane was found to be responsible for its antibacterial action. In this regard, eugenol was noted to cause increased membrane permeability, disruption of bacterial cell membrane and distortion of membrane macromolecules. Likewise, other studies have confirmed the antibacterial activity of eugenol to be indicative of its disruptive action on cytoplasmic membrane, thus enhancing its non-specific permeability (Gill and Holley 2006; Swamy et al. 2016). It has also been highlighted that the hydrophobic characteristic of eugenol permits its penetration in the lipopolysaccharide of the gram-negative bacterial cell membrane, hence modifying the structure of the cell and resulting into the escape of intracellular components (Burt 2004). Also, the hydroxyl group of eugenol is believed to attach to proteins, which then prevents enzyme action in Enterobacter aerogenes (Burt 2004).
The mechanism of action of (−)-α-pinene (Fig. 18.1) in relation to its modulation of antibiotic resistance in Campylobacter jejuni has also been reported by Kovač et al. (2015). Remarkably, (−)-α-pinene was found to effectively modulate antibiotic resistance in C. jejuni by reducing the MIC of erythromycin, triclosan and ciprofloxacin by up to 512-fold. Further, they used insertion mutagenesis method using ethidium bromide to identify the target antimicrobial efflux systems, and DNA microarrays were used to evaluate C. jejuni adaptability to (−)-α-pinene. The results showed that (−)-α-pinene promoted the expression of cmeABC as well as Cj1687, a putative antimicrobial efflux gene. The accumulation of ethidium bromide was found higher in the wild-type strain as compared to the antimicrobial efflux mutant strains. Thus, it confirmed that these antimicrobial efflux systems were the target of action of (−)-α-pinene. Furthermore, a decrease in membrane integrity induced by (−)-α-pinene implied that the enhanced microbial influx was the secondary mode of action of (−)-α-pinene. It was also revealed from the findings that (−)-α-pinene caused the disruption of several metabolic pathways, in particular those implicated in heat-shock retorts. Therefore, the inhibition of microbial efflux and reduced membrane integrity including metabolic disruption were amongst the mechanisms of (−)-α-pinene involved in the modulation of antibiotic resistance in C. jejuni.
Kannappan and co-authors (2019) demonstrated the combinatorial antibiofilm efficacy of geraniol (Fig. 18.1) and cefotaxime against S. epidermidis (ATCC 35984) and MRSA (ATCC 33591). Cefotaxime, a broad-spectrum third-generation cephalosporin antibiotic, acts by binding to one or more of the penicillin-binding proteins and hence causes inhibition of bacterial cell wall formation (Luthy et al. 1979). Significant diminution in biofilm biomass and slime formation was observed following treatment with geraniol and cefotaxime combination (GCC), yielding a minimal biofilm inhibitory concentration of 100 μg/mL and 2 μg/mL of geraniol and cefotaxime respectively. The results also revealed that GCC targeted the initial attachment of the cells associated with biofilm formation. Moreover, treatment of the test pathogens to GCC reduced the production of staphyloxanthin pigment in MRSA and consequently rendered the pathogenic cells vulnerable to the host immune responses. In addition to inhibiting biofilm formation, GCC was found to suppress the production of extracellular polymeric substance (EPS) and slime. GCC treatment was also found to induce reduced expression of surface adhesin genes, and in MRSA, the gene responsible for the production of virulence factor such as staphylococcal enterotoxin A was downregulated. Microscopic analysis made on GCC-treated EPS corroborated with the findings of the in vitro biofilm inhibition assay, thus establishing the destructive effect of GCC on the evaluated pathogens’ biofilm formation. Besides, GCC considerably increased the vulnerability of the test pathogens towards human blood. In vivo assay conducted on Caenorhabditis elegans also revealed the antibiofilm potential of GCC in the control of biofilm-associated infections caused by Staphylococcus species. For instance, while the nematodes exposed to the test pathogens demonstrated an enhanced colonization together with deformed pharynx and internal hatching of eggs, those treated with GCC and test pathogens cells displayed reduced internal colonization as well as healthy pharynx (Kannappan et al. 2019).
Moreover, Yuan and Yuk (2019) investigated the adaptive responses of E. coli O157:H7 with regard to its virulence gene expression and virulence properties at the sublethal concentrations of trans-cinnamaldehyde (TC) (Fig. 18.1), carvacrol (Car) (Fig. 18.1) and thymol (Thy) (Fig. 18.1). E. coli O157:H7 grown to the early stationary phase in the presence of sublethal EOs demonstrated notable reduction in motility (reversible subsequent to stress elimination), biofilm formation capacity and efflux pump activities, without inducing antibiotic resistance or infection, since no marked changes were noted with regard to the invasive and adhesive capacity of the test pathogens on human colon adenocarcinoma (Caco-2) cells. Reduced expression of related virulence genes, together with those encoding biosynthesis and functioning of flagella, biofilm formation regulators and multidrug efflux pumps, in addition to type III secretion system components, was reported. Hence, TC, Thy and Car at sublethal doses did not seem to cause potentiation of virulence in adapted E. coli O157:H7 (Yuan and Yuk 2019).
Further, Guimarães et al. (2019) investigated the antibacterial activity of several terpenoids and terpenes found in EOs. In general, the results showed that oxygenated functional groups in terpenes showed better antibacterial action compared to hydrocarbons. Out of 16 compounds identified to possess antibacterial activity, carvacrol, eugenol and thymol were found to be the most potent; even greater than sulfanilamide against the four strains tested (B. cereus, E. coli, S. aureus and S. typhimurium). Contrastingly, compounds such as borneol, camphor, m-cymene, (±)-linalool and (+)- and R-(+)-limonene (Fig. 18.1) displayed the least antibacterial activity. Nonetheless, only six of the 16 compounds that showed antimicrobial action were seen to be bactericidal (absence of growth) in the study. For instance, none of compounds tested were found to be bactericidal against B. cereus, while only terpineol and thymol demonstrated bactericidal activity against S. aureus. However, the lowest MBC value against S. typhimurium (0.06 mg/mL) was presented by eugenol. Moreover, although thymol exhibited the lowest MIC values against the tested strains, it did not show any bactericidal action at concentrations of MIC, 2× MIC as well as 4× MIC, but rather at concentration 0.12 mg/mL. Eugenol and thymol were observed to cause inhibition (IC100) of S. aureus and S. typhimurium growth. The morphological changes detected in E. coli, S. aureus and S. typhimurium that were treated with β-citronellol, l-carveol and trans-geraniol were also analysed. The treated E. coli cells were of irregular sizes with the presence of debris, probably due to interrupted cell division or cell membrane dysfunction compared to the control cells which had smooth surfaces and bacillary shapes. Besides, cells treated with geraniol and citronellol were smaller, had considerably rough surfaces and the cells were adhered to one another. On the other hand, S. aureus treatment with terpineol disrupted cell division and were found to have a distorted “grape bunch” shape, a typical morphology of the colonies. As for S. typhimurium, terpineol and eugenol treatment revealed the loss of cell membrane integrity or function as the death mechanisms involved, whereby the cell membrane was completely destroyed along with the presence cell debris. Further, carveol, citronellol, eugenol, geraniol and terpineol were found to be fast-acting compounds, given that they caused E. coli and S. typhimurium inactivation within 2 h. The authors also pointed out that the compounds that exhibited the best activity in relation to their MIC and time-kill kinetics had low molecular weights as well as polar functional groups. Such features could enhance antimicrobial potential by facilitating permeation via the outer cell membrane as confirmed by eugenol action, a low-molecular-weight phenolic compound that displayed fast time-kill kinetics leading to the death of S. typhimurium at all concentrations in only 2 h (Guimarães et al. 2019).
Carvacrol (Fig. 18.1), a major monoterpene of oregano (Origanum vulgare) and thyme (Thymus vulgaris) volatile oils, has been reported to possess a strong antifungal activity against Candida albicans (Chaillot et al. 2015). In the attempt of understanding the underlying mechanism of action, they demonstrated that fungal cells need the UPR (unfolded protein response) signalling path to be able to counterattack carvacrol. Moreover, carvacrol was found to act as an ER (endoplasmic reticulum) stress inducer, disturbing the morphology as well as the integrity and protein-folding capacity of the ER leading UPR activation. Carvacrol also enhanced the antifungal action of fluconazole, echinocandin and caspofungin (antifungal drugs) as well as UPR inducers, such as tunicamycin and dithiothreitol against C. albicans.
Furthermore, Xia et al. (1999) demonstrated in their study that α-pinene (Fig. 18.1) exerts a significant antifungal activity against C. albicans. The antifungal mechanisms that were put forward included the rupture of C. albicans cell wall and cytoplasmic membrane, the release of intracellular components and fusion of cell residues into irregular masses. Besides, there was the inhibition of RNA, DNA, cell wall polysaccharide and cell membrane ergosterol synthesis.
Linalool and geraniol (Fig. 18.1), two major oxygenated monoterpenes present in EOs of various medicinal plants such as Pelargonium graveolens, Peperomia pellucida and Acorus calamus (Souza et al. 2016; Okoh et al. 2017; Parki et al. 2017), have also been tested for their anticandidal potential against five Candida species of ATCC strains (C. albicans, C. glabrata, C. parapsilosis, C. krusei and C. tropicalis) (Singulani et al. 2018). Although both linalool and geraniol were found to inhibit candidal growth in vitro, geraniol was observed to be more effective. Furthermore, while C. albicans was mostly resistant to the compounds (MIC ≥ 1000 μg/mL), C. parapsilosis was susceptible (MIC 37.5 and 125 μg/mL for geraniol and linalool, respectively) (Singulani et al. 2018).
18.2.2 Antidiabetic Property
Citronellol (Fig. 18.1), which is a monoterpene alcohol commonly found in citrus species, such as oranges, lemons, and pomelos, has been evaluated for its antidiabetic effect in streptozotocin (STZ)-induced diabetic rats (40 mg/kg body weight (b.w.)) (Srinivasan and Muruganathan 2016). According to them, citronellol administered orally for 30 days (50 mg/kg) was observed to improve the insulin, hepatic glycogen and haemoglobin levels along with a substantial decline in the levels of glucose and glycated haemoglobin (HbA1c). Additionally, changes in enzyme activities involved in carbohydrate metabolism, kidney and hepatic biomarkers were brought to near-normal levels in citronellol-treated rats. Hypoplasia and extensive damages of the islets of langerhans in STZ-induced diabetic rats were decreased in groups treated with citronellol. Moreover, citronellol supplement enhanced insulin immunoreactivity and augmented the number of immunoreactive β-cells in the treated group. Thus, citronellol demonstrated the capacity to improve the secretion of insulin or regeneration of the β-cells in the diabetic animals (Srinivasan and Muruganathan 2016).
The effect of carvone (Fig. 18.1), a monoterpene ketone, on carbohydrate metabolic enzymes in the liver of STZ-induced diabetic rats (40 mg/kg b.w.) was also studied in a similar way by Muruganathan and Srinivasan (2016). Carvone (50 mg/kg b.w.) daily oral administration in diabetic rats for 30 days resulted into a significant decrease in the levels of HbA1c and plasma glucose, while a considerable amelioration in haemoglobin and insulin levels was noted. Carvone administration also caused a reversal in the activities of the carbohydrate metabolic enzymes, enzymatic antioxidants and hepatic marker enzymes that were eventually restored close to normal levels in the diabetic rats. Gliclazide was also used as a standard oral hypoglycaemia drug for comparison. Moreover, carvone treatment was found to reduce the STZ-induced damage in hepatic and pancreas β-cells (Muruganathan and Srinivasan 2016).
The anti-hyperglycaemic property of eugenol was also determined by assessing the activities of key enzymes of glucose metabolism in STZ-induced diabetic rats (40 mg/kg b.w.) (Srinivasan et al. 2014). Eugenol was intragastrically administered in diabetic rats for 30 days at 2.5, 5 and 10 mg/kg b.w. At an effective dose of 10 mg/kg b.w., remarkable decrease in blood glucose and HbA1c levels and increase in plasma insulin level were reported. Eugenol also caused altered activities of key enzymes involved in carbohydrate metabolism, for instance, pyruvate kinase, glucose-6-phosphate dehydrogenase, hexokinase, fructose-1,6-bisphosphatase, glucose-6-phosphatase and liver marker enzymes (serum aspartate aminotransferase, alanine aminotransferase and alkaline phosphatase) and creatine kinase including blood urea nitrogen in serum and blood to significantly reverse to near-normal levels in diabetic rats. In furtherance, eugenol improved body weight and hepatic glycogen level in the treated group, thus demonstrating the potential of eugenol as an anti-hyperglycaemic agent in STZ-induced diabetic rats (Srinivasan et al. 2014).
Likewise results were obtained by myrtenal (Fig. 18.1), a natural monoterpene that has been evaluated for its efficacy as an anti-hyperglycaemic agent as well as its β-cell protective properties in STZ-induced diabetic rats (Rathinam et al. 2014). Myrtenal was orally administered to diabetic rats for 28 days resulting into a significant rise in insulin and haemoglobin levels and reduction in the levels of HbA1c and plasma glucose. The diabetic rats subjected to myrtenal treatment were also protected from loss of body weight. The altered activities of the key metabolic enzymes implicated in carbohydrate metabolism and hepatic enzymes in the diabetic rats were considerably improved by myrtenal administration. Myrtenal also caused an improvement in hepatic and muscle glycogen content in diabetic rats. Further, histopathological analysis unveiled the restoration of reduced islet cells to near-normal conditions, and alteration in the liver architecture was also averted following myrtenal treatment (Rathinam et al. 2014).
The acyclic monoterpene alcohol, geraniol occurring in several medicinal plants exhibits numerous medicinal properties including antidiabetic effect. According to a study by Babukumar et al. (2017), the administration of geraniol at its effective dose of 200 mg/kg b.w. for 45 days relatively enhanced the levels of insulin, haemoglobin and reduced HbA1c and plasma glucose in diabetic-treated rats. Geraniol ameliorated the key enzymes involved in glucose metabolism. Further, the content of hepatic glycogen was improved in diabetic rats that were treated with geraniol. This suggests that geraniol possessed anti-hyperglycaemic potential.
Several EO constituents have also been evidenced to exhibit enhanced antidiabetic effects in combination with antidiabetic drugs. For instance, carvacrol, was evaluated for its anti-hyperglycaemic activity and potential to improve dysregulated carbohydrate metabolism in combination with rosiglitazone in high-fat diet (HFD)-induced type 2 diabetic C57BL/6 J mice (Ezhumalai et al. 2014). The post-oral administration of carvacrol and rosiglitazone was given at 20 mg/kg b.w. and 4 mg/kg b.w., respectively, for 35 days. The HFD mice demonstrated increased levels of insulin, glycosylated haemoglobin and plasma glucose and a declined level of haemoglobin. Besides, there were higher activities of enzymes involved in carbohydrate metabolism in the liver of HFD mice. However, the treatment of diabetic mice with carvacrol and rosiglitazone resulted in increased glucokinase activity due to improved sensitivity of insulin. Likewise, glucose-6-phosphate dehydrogenase activity was augmented causing enhanced utilization of glucose. In addition, activities of gluconeogenic enzymes (fructose-1,6-bisphosphatase and glucose-6-phosphatase) were significantly reduced in carvacrol- and rosiglitazone-treated mice resulting into reduced levels of blood glucose. Furthermore, the activities of hepatic marker enzymes (aspartate aminotransferase, alanine aminotransferase, alkaline phosphatase and gamma-glutamyl transpeptidase), which were in elevated levels in HFD mice, were restored to normal levels through improved insulin resistance. These results were also supported by histopathological analysis of pancreas, which was in line with the biochemical findings.
18.2.3 Cardioprotective Property
The cardioprotective activities of EOs and their derived components have been widely documented. Included amongst them is 1,8-cineole (Fig. 18.1) a major monoterpenic oxide present in many EOs, which has been evaluated for its effects on systolic blood pressure (SBP) as well as oxidative stress in rats that have been constantly exposed to nicotine via intraperitoneal injection (Moon et al. 2014). A remarkable decline in SBP was observed along with a significant increase in the levels of plasma nitrite in 1,8-cineole-treated rats compared to those exposed to nicotine alone. Besides, a significant rise in the levels of lipid peroxidation and plasma corticosterone was detected in untreated rats. The elevated levels of plasma corticosterone were not reduced by 1,8-cineole; however the increase in lipid peroxidation levels was considerably antagonized in 1,8-cineole-treated rats (0.01 and 0.1 mg/kg). Thus, the results were indicative that the antihypertensive effect of 1,8-cineole led to reduced SBP, which may be linked to the control of nitric oxide levels and oxidative stress in rats that were chronically exposed to nicotine (Moon et al. 2014).
The cardiovascular effects of 1,8-cineole have also been reported by other authors in both in vitro and in vivo experimental models. For instance, Lahlou et al. (2002) demonstrated that 1,8-cineole administration via bolus injections (0.3–10 mg/kg, i.v.) in both conscious and anaesthetized rats induced comparable and dose-dependent declines in mean aortic pressure. Additionally, at the highest dose, i.e., 10 mg/kg, 1,8-cineole caused a significant reduction in heart rate. Moreover, 1,8-cineole-induced hypotension (10 mg/kg) was found to be related to significant bradycardia in both conscious and anaesthetized rats. This resulting effect seemed to be from vagal origin, given that it was significantly decreased with methylatropine pretreatment (i.v.) or by bilateral vagotomy. Furthermore, in the same study, in vitro experiments performed using isolated rat aorta preparations revealed that 1,8-cineole at 0.006–2.6 mM elicited a concentration-dependent decrease in potassium-induced contractions (60 mM). Hence, this study showed that 1,8-cineole displayed hypotensive effects in conscious and anaesthetized rats, which appeared to be associated with active vascular relaxation.
Furthermore, El-Bassossy et al. (2017) showed that geraniol provided effective protection against cardiac dysfunction caused by diabetes. Oral administration of geraniol (150 mg kg−1 day−1) in STZ-injected rats considerably caused the alleviation of the attenuated cardiac systolic function associated with diabetes indicated by hindering the reduction in the rate of rise (dP/dtmax) in ventricular pressure and the rise in systolic duration detected in diabetic rats. Additionally, geraniol alleviated impaired diastolic function as demonstrated by restraining the decrease in the rate of fall (dP/dtmin) in ventricular pressure and isovolumic relaxation constant (Tau) increase in diabetic rats. Additionally, geraniol averted any boost in QTc and T-peak-T-end intervals, left ventricular (LV) ischaemia and arrhythmogenesis markers in diabetic rats. Geraniol also suppressed the exaggerated oxidative stress by preventing 8-isoprotane increase. Moreover, geraniol was able to prevent the inhibition in catalase (CAT) activity although it did not affect the superoxide dismutase (SOD) activity in the heart. Besides, geraniol was observed to partly reduce hyperglycaemia and prevent hypercholesterolemia, but did not have any effect on the adiponectin serum level in diabetic rats.
Moreover, Menezes et al. (2010) investigated the effects of five terpenes, namely, (−)-β-pinene, (+)-α-pinene, (±)-linalool, (±)-citronellol (monoterpenes) and (−)-α-bisabolol (a sesquiterpene) on the blood pressure and heart rate in non-anaesthetized normotensive rats. The monoterpenes were observed to display hypotension associated with tachycardia that could be indicative of an effect on the peripheral vascular resistance with resultant baroreflex response. Alternatively, (−)-α-bisabolol (Fig. 18.1) induced hypotension linked to intense bradycardia, probably as a result of reduced cardiac output. In addition, although all terpenes demonstrated hypotensive effects, terpene hydrocarbons were less efficient as compared to the terpene alcohols.
Linalyl acetate (Fig. 18.1) has also been evaluated for its possible cardiovascular effects on adolescent rats acutely exposed to nicotine (Kim et al. 2017). The levels of nitric oxide, heart rate, systolic blood pressure, vascular contractility and lactate dehydrogenase (LDH) activity were the parameters measured in this study. Significant reductions in both heart rate and LDH activities were noted in linalyl acetate-treated rats. Furthermore, acute nicotine exposure resulted into a minor relaxation effect that was followed by a sustained recontraction stage in contracted mouse aortic rings, while nicotine and linalyl acetate demonstrated a steady relaxation effect. Additionally, treatment with linalyl acetate was found to reduce elevated nitrite levels induced by nicotine. Other authors have also reported the cardiovascular activities of linalyl acetate. For instance, Koto et al. (2006) showed in their study that linalyl acetate as the main component of lavender EO caused vascular smooth muscle relaxation in rabbit carotid arteries, which was partly due to the activation of the nitric oxide/cGMP pathway. Furthermore, linalyl acetate-rich lavender (linalyl acetate 43.73%) aromatherapy was established to cause a rise in coronary flow velocity reserve, hence enhancing coronary circulation in healthy men (Shiina et al. 2008).
Carvacrol and thymol have also been observed to induce vasodilatory effects in isolated rat thoracic aorta preparations (Peixoto-Neves et al. 2010). Both terpenoids caused relaxation of the KCl- and phenylephrine (PHE)-induced contractions of the aortic rings in a concentration-dependent manner. Moreover, carvacrol and thymol were found to totally terminate the phasic component of PHE-provoked endothelium-containing ring contractions in Ca2+-free medium with 2 mM ethylene glycol-bis(2-aminoethylether)-N,N,N′,N′-tetraacetic acid. In Ca2+-free medium, carvacrol and thymol considerably decreased the CaCl2-induced contractions at 400 μM. In addition, both carvacrol (1000 μM) and thymol (300 μM) significantly diminished the contraction evoked by phorbol dibutyrate (an activator of protein kinase C) at 1 μM. Furthermore, an enhancement was observed in the magnitude of the inhibitory activity in the presence of the thapsigargin, Ca2+ pump inhibitor (1 μM). However, none of the two terpenoids were found to modify the resting potential of vascular smooth muscle cells at 1000 μM. Hence, relaxation in rat isolated aorta induced by carvacrol and thymol was endothelium independent, an effect apparently mediated through some mechanisms involving a transduction pathway between release of Ca2+ from sarcoplasmic reticulum and by regulating Ca2+ sensitivity of the contractile system. It was also proposed that thymol and carvacrol caused blockage of Ca2+ influx through the membrane, at low concentrations.
The cardioprotective effects of carvacrol and thymol have also been highlighted by other studies. For instance, Chen et al. (2017) demonstrated in their study that carvacrol provided significant protection of the heart function as well as reduced the myocardial infarct size in myocardial ischaemia/reperfusion (I/R) injured rats. In addition, the levels of SOD and CAT were increased, while the malondialdehyde (MDA) level and cardiomyocytes apoptosis were decreased. Carvacrol treatment also caused the upregulation of phosphorylated ERK without having any effect on p38 mitogen-activated protein kinase (p38MAPK) and c-Jun N-terminal kinase (JNK). Besides, the protective efficiency of carvacrol on cardiomyocytes hypoxic reperfusion (H/R) injury was observed in vitro. Furthermore, carvacrol pretreatment markedly increased the activation of Akt/eNOS pathway in cardiomyocytes subjected to H/R, and carvacrol protective effects were terminated in the presence of the Akt inhibitor LY294002. Hence, the cardioprotective effect of carvacrol was attributable to its anti-apoptotic and antioxidant activities through the activations of the Akt/eNOS and MAPK/ERK signalling pathways. El-Sayed et al. (2016) as well demonstrated that carvacrol and thymol exerted protective effects against doxorubicin (DOX)-induced cardiotoxicity in rats (10 mg/kg i.v.). Administration of carvacrol and thymol (25 and 20 mg/kg p.o., respectively) was found to ameliorate the heart function and oxidative stress parameters. However, thymol was more cardioprotective than carvacrol. A synergistic cardioprotective effect was achieved when carvacrol and thymol were combined together, which might have resulted from its anti-inflammatory including antioxidant and anti-apoptotic activities.
18.2.4 Anticancer Property
In the search for novel anticancer agents derived from plant sources, EOs and their active constituents have been found to be a promising alternative to surmount chemotherapy drug resistance as well as cancer recalcitrance. For instance, thymoquinone (TQ) (Fig. 18.1), a bioactive component obtained from Nigella sativa L. seeds and other EOs, has demonstrated notable anticancer potential (Arafa et al. 2011). Interestingly, TQ was found to significantly restrain doxorubicin-resistant human breast cancer (MCF-7/DOX) cell proliferation. Amongst the different mechanisms proposed, TQ was reported to increase cellular levels of phosphatase and tensin homolog (PTEN) proteins that led to a considerable decline of the phosphorylated Akt cell survival protein. Expression of PTEN was accompanied with increase in PTEN mRNA. Additionally, TQ caused G2/M phase arrest in MCF-7/DOX cells as well as augmented the cellular levels of p53 and p21 proteins. TQ-induced apoptosis was allied with the disruption of mitochondrial membrane potential, caspases activation and cleavage of poly-(ADP-ribose) polymerase-1 (PARP). Upregulation of Bax and downregulation of Bcl2 proteins, resulting in an increase in Bax/Bcl2 ratio, were also observed (Arafa et al. 2011). Mauro et al. (2013) also elucidated the mechanism of action involved in the chemotherapeutic property of D-limonene which was found to be cytotoxic to V79 Chinese hamster cells. It was revealed that D-limonene affected the dividing cells by preventing the assemblage of mitotic spindle microtubules caused by tubulin depolymerization in the early phase of mitosis. Furthermore, both chromosomal segregation and cytokinesis were affected leading to aneuploidy, which consequently resulted into the death of the cell or even genomic instability.
Thymol was also investigated for its chemoprotective effect against genotoxicity induced by bleomycin in human normal lymphocytes as well as anti-proliferative effect on human ovarian cancer cells (SKOV-3) (Arab et al. 2015). Bleomycin (BLM) is an anticancer agent that causes tissue toxicities through DNA damage and death of cells. Samples of blood were treated with BLM following 2 h incubation with thymol at different concentrations (50, 100 and 150 μM). In order to determine the frequency of micronuclei in cytokinesis-blocked binucleated lymphocytes, lymphocytes were cultured with a mitogenic stimulation. A marked decline in the frequency of micronuclei in lymphocytes treated with thymol and BLM was noted compared to blood samples incubated with only BLM (frequency of micronuclei in BLM-treated lymphocytes: 7.79 ± 2.00). Thymol was observed to significantly mitigate micronuclei frequency at doses 50 and 100 μM in BLM-treated lymphocytes (frequency: 2.81 ± 0.99, 2.42 ± 0.16, respectively). Thymol did not demonstrate any genotoxicity in cultured lymphocytes at 150 μM without BLM treatment. Moreover, neither cell protective effect nor improved cell death was observed with thymol pretreatment of SKOV-3 cells. Therefore, this study suggested that thymol provided selective protection to human lymphocytes against DNA damage that was BLM induced without any protection on the killing effect of BLM on cancerous cells (Arab et al. 2015). The effects of thymol on several other cancer lines have been studied as well, whereby their mechanisms responsible for their anticancer or chemopreventive properties have been elucidated (Table 18.1). Likewise, farnesol (Fig. 18.1), an acyclic sesquiterpene alcohol found in the EOs of a variety of plants such as citronella, lemon grass, rose, balsam and neroli (Goossens and Merckx 1997; Azanchi et al. 2014; Krupčík et al. 2015), has been studied for their anticancer effect on different cancer cell lines (Table 18.1).
The anticancer property of patchouli alcohol (PA) (Fig. 18.1), a component isolated from the Pogostemon cablin EO, has been tested by Jeong et al. (2013) against several cancer lines such as prostate cancer cells (PC3), breast cancer cells (MCF7), human umbilical vein endothelial cells (HUVEC) and pancreatic cancer cells (BxPC3) including human colorectal cancer cells (SW480 and HCT116). PA was noted to exhibit a suppressive activity on cell growth and promote apoptosis dose dependently in the cancer cells. With regard to human colorectal cancer, exposure of PA to SW480 and HCT116 cells caused the increased expression of p21 and downregulated the expression of cell cycle regulatory proteins (cyclin D1 and cyclin-dependent kinase 4 (CDK4)) in a dose-dependent way. Additionally, PA inhibited the expressions of c-myc, HDAC2 (histone deacetylase 2) and HDAC enzyme activity. c-Myc, a transcription factor that mediates progression of cancer, is greatly overexpressed in 60% of colorectal cancer (Smith and Goh 1996), while HDACs are involved in the regulation of cell signalling and gene expression, and their overexpression can result into several diseases including cancers (Haberland et al. 2009). PA also induced activation of the transcriptional activity of NF-κB by increasing the translocation of p65 into the nucleus in human colorectal cancer cells. NF-κB has been reported to cause sensitization of cells to apoptosis as well as promote proapoptotic response (Gibson et al. 2000; Baud and Karin 2009). Moreover, the potent antitumor properties of sesquiterpenes such as β-elemene (Fig. 18.1), has been extensively studied. In this context, it was found to exert anti-glioblastoma effect by restraining cell proliferation and arresting cells in the G0/G1 mediated by the mutually compensatory activation of mitogen-activated protein kinase kinase-3 and -6 (MKK3 and MKK6) (Zhu et al. 2011). Similarly, Liang et al. (2012) showed that β-elemene inhibited viability osteosarcoma cells as a result of apoptosis. However, since treatment with β-elemene also caused upregulation of hypoxia-inducible factor 1α (HIF-1α) protein inducing partial inhibition of apoptosis, expression of HF-1α was reduced with tiny interfering RNA or co-treatment with an HIF-1α inhibitor (3-(5′-hydroxymethyl-2′-furyl)-1-benzyl indazole). This in turn was observed to greatly improve the antitumor potential of β-elemene. Additionally, more recently Wang et al. (Wang et al. 2018b) demonstrated that β-elemene was able to suppress human cervical cancer SiHa cell proliferation, migration and invasion, as well as promote apoptosis by reducing the Wnt/β-catenin signalling pathway which is associated with tumour formation, invasion and metastasis of different kinds of cancer (Polakis 2000; Hoffmeyer et al. 2012).
EO constituents have also been shown to improve the cytotoxicity of chemotherapy drugs in several cancer cell lines, resulting in the use of reduced concentration of the drug to provide same effect (Legault and Pichette 2007; Rabi and Bishayee 2009). Such synergism has been observed in the case of β-caryophyllene (Fig. 18.1) which, although did not exert any cytotoxic effect alone at the tested concentration, was reported to clearly increase cytotoxicity in combination with paclitaxel in numerous cancer cell lines. It also was pointed out that accumulation of β-caryophyllene in the lipid bilayer resulted into altered permeability that eventually contributed to enhance the permeability of cell membrane for uptake of paclitaxel (Legault and Pichette 2007).
Another such example is a monoterpene alcohol, linalool (Fig. 18.1), which has been tested both individually and combined with doxorubicin, for its effects on two breast cancer adenocarcinoma cell lines (MCF7 WT and MCF7 AdrR). While linalool was found to inhibit cell proliferation only moderately, subtoxic levels of linalool were able potentiate doxorubicin-induced cytotoxic and proapoptotic effects in both cell lines. Strikingly, a considerable synergistic interaction could be observed in MCF7 AdrR (adriamycin-resistant) cells (IC50 of doxorubicin, 16.16 ± 0.94 μM; IC50 of doxorubicin + 50 μM linalool, 1.24 ± 0.26 μM), which could be at least partly attributed to the capacity of linalool to boost doxorubicin buildup and to induce a decline in Bcl-xL levels (Ravizza et al. 2008).
Furthermore, geraniol has been reported to cause sensitization of cancer cells to the 5-fluorouracil, a conventional chemotherapeutic drug, including an increase uptake of the drug (Carnesecchi et al. 2002, 2004). Geraniol also demonstrated to exhibit a chemoprotective action towards rat normal colon cells when a potent carcinogen dimethylhydrazine was administered; this effect was thought to be mediated by reducing DNA damage in contrast with the control where no EO was used (Vieira et al. 2011).
β-Bisabolene (Fig. 18.1), a sesquiterpene present in EO of plants such as Commiphora guidotti, was tested for their selective cytotoxicity in both human and murine mammary tumour cells (Yeo et al. 2016). Treatment with β-bisabolene was found to lead to loss of viability of mammary cancer cells as a result of apoptosis. β-Bisabolene was also efficient in decreasing the growth of transplanted 4 T1 mammary tumours in female Balb/C mice in vivo (37.5% decrease). In addition, histological studies performed on the β-bisabolene-treated tumours showed an apparent increase in cell death. Conversely, the number of proliferating cells was decreased in β-bisabolene-treated tumours (Yeo et al. 2016).
18.2.5 Anticonvulsant Activity
Anticonvulsant effects of EOs components have also been reported. As example, Quintans-Júnior et al. (2010) investigated the anticonvulsant effect of three EO components, namely, (-)-borneol, citral and carvacrol in rodents using two animal models of convulsion, i.e., pentylenetetrazole (PTZ)-induced convulsion and maximal electroshock (MES) tests. In their study, the mice were pretreated with the EO components, followed by injection of PTZ at 60 mg/kg after 30 min. Similarly, for MES test, the mice received electroconvulsive shock (130 V, 150 pulses/s, 0.5 s) 30 min after the monoterpenes were injected. The latency for developing convulsions including the percentage protection were then noted. While (-)-borneol and citral were able to stimulate an increase of latency for the development of convulsions by PTZ induction in all doses, carvacrol was effective in only high dose. Besides, these monoterpenes were observed to be efficient in the prevention of tonic convulsions caused by MES. Flumazenil (FLU), an explicit antagonist of the benzodiazepine (BZD) site in the γ-aminobutyric acid (GABAA)-BZD receptor complex (File and Pellow 1986), was employed to examine the role of the participation of GABAA-BZD receptors in the monoterpene-prompted anticonvulsant properties. Interestingly, the presence of FLU was not capable to inverse the anticonvulsant effects of the two monoterpenes, carvacrol and citral, thus implying that there was no direct activation of the BZD site of the GABAA-BZD receptor involved as the mechanism of action. On the contrary, (-)-borneol produced significant antagonistic effect indicating the possible modulation of the GABAergic system via the improvement of GABAA-BZD receptor (Quintans-Júnior et al. 2010).
The effect of isopulegol (a monoterpene alcohol) (Fig. 18.1), in PTZ-encouraged convulsions, was also examined and its possible mechanism of action elucidated (Silva et al. 2009). Intraperitoneal injection of saline, diazepam or isopulegol was administered 30 min before PTZ. The latency for developing convulsions and mortality rate were recorded in mice. Moreover, the antioxidant activity of catalase and the concentrations of lipid peroxidation and reduced glutathione in brain hippocampus were determined. Isopulegol was found to significantly extend mortality and the latency for convulsions in mice in the same way to diazepam. In addition, high dose of isopulegol was observed to induce protection to all animals. FLU was also used in order to investigate on the participation of GABAergic system. The pretreatment of FLU resulted in reduced seizure prolongation latency elicited by both isopulegol and diazepam, even if it was unable to reverse the latency and mortality percentage protection. Also, the monoterpene alcohol significantly averted PTZ-induced rise in lipid peroxidation and maintained normal levels of catalase activity along with the prevention of the PTZ-induced loss of reduced glutathione in the treated mice hippocampus. These findings were indicative that the anticonvulsant and bioprotective activities of isopulegol against convulsions induced by PTZ were probably associated with positive modulation of BZD-sensitive GABAA receptors together with antioxidative properties.
Terpinen-4-ol (4TRP) (Fig. 18.1), a constituent of EOs obtained from several aromatic plants including Melaleuca alternifolia (Carson et al. 2006), was examined for its anticonvulsant potential by studying the electrophysiological as well as the behavioural activities in rats and mice models (Nóbrega et al. 2014). For this purpose, 4TRP was administered intracerebroventricularly (i.c.v.) (10, 20 and 40 ng/2 μL) and intraperitoneally (i.p.) (25–200 mg/kg) in the animals, while for in vitro experiments, 4TRP was used at 0.1 and 1.0 mM. Based on the results, 4TRP (i.p.) was found to inhibit PTZ-evoked seizures, thus demonstrating anticonvulsant effects. In addition, the protection provided by 4TRP (i.c.v.) against PTZ-induced seizures were found to corroborate with the behavioural results. 3-Mercapto-propionic acid-induced convulsions were used to confirm the involvement of the GABAergic neurotransmission in 4TRP anticonvulsant activity. Moreover, since FLU was not able to reverse the anticonvulsant effect, it could be concluded that 4TRP did not bind to the BZD-binding site although its action was directly or indirectly related to the GABAergic system. Besides, 4TRP inhibited sodium current through voltage-dependent sodium channels and hence its anticonvulsant activity may be associated with changes in neuronal excitability as a result of regulation of these channels (Nóbrega et al. 2014).
As per the study of Sancheti et al. (2014), thymol was found to exhibit potent anticonvulsant as well as antiepileptogenic effects in several experimental models. For instance, the anticonvulsant potential of thymol at a dose of 5–100 mg/kg i.p. was studied using MES-, PTZ-, 4-aminopyridine- (4-AP) and strychnine-induced seizures in rats/mice models. Thymol treatment demonstrated anticonvulsant effect against MES (66.66% death protection at both 50 and 100 mg/kg) and PTZ models (66.66 and 83.33% death protection at 50 and 100 mg/kg, respectively) unlike strychnine and 4-AP models. Thymol capacity to obstruct neuronal voltage-gated Na+ channels and its positive allosteric modulation of GABAA receptor could possibly be responsible for thymol anticonvulsant effect in PTZ and MES models (Haeseler et al. 2002; García et al. 2006). Furthermore, thymol was also found to induce marked decrease in locomotor activity (16–80% reduction in locomotion at the doses 25–100 mg/kg, respectively). PTZ-induced kindling model and measurement of MDA and glutathione levels were employed to assess the antiepileptogenic activity of thymol (5–25 mg/kg). Thymol (25 mg/kg, i.p.) was observed to cause significant decrease in the seizure score and MDA level and increase in glutathione level in PTZ-induced kindling animal model (Sancheti et al. 2014).
18.2.6 Antispasmodic Property
EOs and their individual components have also been investigated for their antispasmodic potential. For example, Magalhães and co-workers (1998) showed that Croton nepetaefolius EO as well as its components, namely, methyl eugenol (Fig. 18.1), terpineol and cineole, possessed myorelaxant and antispasmodic effects. Both in vivo (mice) and in vitro (guinea pig isolated ileum, cardiac, pyloric and ileocaecal sphincters) models were used to investigate their effects on intestinal motility and mechanical action of intestinal smooth muscle, respectively. The EO was found to be potent modulator of intestinal smooth muscle. Additionally, EO-induced modulation of gastrointestinal smooth muscle activity was indicative that it was able to assist digestion via its relaxant activities without causing gut stasis. More specifically, the EO was found to increase the intestinal transit of charcoal marker delivered to the mice stomach, while it preferentially reduced basal tonus compared with the amplitude of spontaneous contractions in segments of guinea pig ileum and the sphincters. Similar to the EO, methyleugenol, cineole and terpineol induced concentration-linked relaxation activity of basal tonus and also caused obstruction of 60 mM [K+]-induced contraction when applied individually (Magalhães et al. 2004). The major compound of C. nepetaefolius EO, methyleugenol seemed to play a significant role with better relaxant potency (EC50 for reduction of basal tonus, 9.4 ± 3.3 μg/mL; IC50 for the inhibition of potassium contraction, 12.3 ± 2.7 μg/mL) in comparison with C. nepetaefolius EO (EC50, 15.7 ± 4.4 μg/mL; IC50, 18.2 ± 2.3 μg/mL) in isolated intestinal smooth muscle. Cineole (25.37%), the major component found in the EO of C. nepetaefolius, comparatively appeared to provide the least contribution (EC50, 322.1 ± 29.8 μg/mL; IC50, 418.9 ± 58.7 μg/mL) as it had only weak relaxant and antispasmodic effect to that of the EO. On the other hand, terpineol by itself induced a higher maximal relaxation of the isolated ileum than the other two constituents (EC50, 70.7 ± 10.7 μg/mL; IC50, 95.4 ± 7.5 μg/mL). However, its presence in the EO did not affect the maximal relaxant response due to its limited amount (4.96%) in the EO, or probably the presence of other constituent(s), which might have inhibited terpineol-induced depressant effect of the basal intestinal tonus.
Likewise, Lima et al. (2000) investigated the effects of methyleugenol (Fig. 18.1) on guinea pig isolated ileum, whereby it was found to reversibly cause relaxation of the basal tonus (EC50: 52.2 ± 18.3 μM), which remained unchanged even by hexamethonium (0.5 mM) or tetrodotoxin (0.5 μM). In addition, relaxation of the ileum pre-contracted with 60 mM KCl was reported. Besides, even though a slight hyperpolarization of the ileum was induced by methyleugenol, it did not have any effect on the depolarized tissues. Moreover, contractions elicited by acetylcholine, KCl and histamine were inhibited by methyleugenol (IC50: 82, 65 and 124 μM, respectively). Thus, it was deduced that methyleugenol caused ileum relaxation by acting directly on the smooth muscle via mechanism mostly independent of membrane potential modifications.
Furthermore, the spasmolytic activity of several monoterpenes present in Mentha x villosa leaves EO has been studied by De Sousa et al. (2008). Limonene oxide, pulegone oxide and carvone epoxide, pulegone, (−)-carvone, (+)-carvone, (+)-limonene and rotundifolone were amongst the monoterpenes investigated. Rotundifolone (Fig. 18.1), a component present in many EOs, is known to be spasmolytic. The relationship between the structure and spasmolytic action of rotundifolone and its monoterpene analogues in ileum obtained from guinea pig was examined. With the exception of (+)-limonene and pulegone, all the other analogues were observed to exert spasmolytic effect that was stronger than rotundifolone. The results also revealed that the functional groups and their respective position at the ring of rotundifolone contributed to the ileum relaxation activity and the absence of the oxygenated molecular structure did not really determine the molecule bioactivity. Carvone epoxide (Fig. 18.1) was observed to exhibit significantly stronger spasmolytic behaviour compared to rotundifolone.
Eugenol present in the EOs of many aromatic plants, has also been studied by Leal-Cardoso et al. (2002) for their inhibitory activities on isolated rat ileum. The findings of this study revealed that eugenol was able to cause relaxation of the isolated ileum and inhibit contractions caused by both receptor-independent and -dependent mechanisms. Moreover, eugenol was able to hinder responses of different contractile stimuli, for instance, elevated [K+] (depolarizing stimulus) and acetylcholine (neurotransmitter) in the absence and presence of a concentration of nifedipine that prevents [K+]-induced contractions. As a result, a complete relaxation of the tonus increased by 60 mM K+ with a maximal effect comparable to nifedipine was noted by eugenol. Also, the contraction caused by acetylcholine agonists in solution with nifedipine and Ca2+-free solutions was blocked by eugenol, thus demonstrating its capacity to depress the contraction of the ileal smooth muscle at a certain stage of the signal transduction cascade away from receptors of sarcoplasmic membrane. Therefore, given that the blockage of contractions elicited by 60 mM K+ and acetylcholine did not cause significant changes to the membrane potential of muscles in normal nutrient solution and complete relaxation of contractions induced by high K+ was brought about by eugenol without modifying the membrane potential, it was implied that eugenol had a depressant effect on isolated ileum independent of the participation of membrane potential and voltage-dependent Ca2+ channels.
The mechanism responsible for the antispasmodic action of menthol (Fig. 18.1), the major constituent in peppermint oil, on the human distal colon has also been evaluated in vitro by Amato et al. (2014). In a concentration-dependent manner, menthol (0.1 mM–30 mM) was found to reduce the amplitude of the spontaneous contractions without having an effect on the frequency and the resting basal tone. Tetrodotoxin (a neural blocker), tetraethylammonium (a blocker of potassium (K+)-channels), 5-benzyloxytryptamine (a transient receptor potential-melastatin8 channel antagonist) or 1H-[1,2,4] oxadiazolo[4,3-a]quinoxalin-1-one (inhibitor of nitric oxide (NO)-sensitive soluble guanylyl cyclase) at concentrations 1 μM, 10 mM, 1 μM and 10 μM, respectively, showed no effect on the inhibitory action of menthol. In contrast, significant reduction was obtained in menthol inhibitory actions in the presence of 3 nM nifedipine (a voltage-activated L-type Ca2+ channel blocker). Menthol also decreased the contractile responses resulting from the exogenous application of Ca2+ (75–375 μM) in a Ca2+-free solution, or caused by KCl (40 mM) in a concentration-dependent manner. Besides, menthol (1–3 mM) potently reduced both the electrical field stimulation (EFS)-induced atropine-sensitive contractions and contractile responses induced by carbachol. Therefore, menthol was observed to exhibit spasmolytic effects in circular muscle of human colon by the direct inhibition of contractility of the gastrointestinal smooth muscle, via the blockage of Ca2+ influx through the sarcolemma L-type Ca2+ channels (Amato et al. 2014).
18.2.7 Anti-Inflammatory Property
Various plants and their active components utilized in traditional medicine have been evidenced to be valuable in the treatment of inflammatory disorders (Bernstein et al. 2018; Oguntibeju 2018). Likewise, a series of in vivo, in vitro and in silico studies have been conducted on the anti-inflammatory properties of EOs and their compounds (Pérez et al. 2011; Yadav et al. 2013; Maurya et al. 2014; Andrade 2015; Yang et al. 2016). For instance, Hirota et al. (2010) assessed the anti-inflammatory potential of limonene (Fig. 18.1) obtained from Citrus junos Tanaka peel in the treatment of bronchial asthma. For this purpose, the level of monocyte chemoattractant protein-1 (MCP-1), p38 mitogen-activated protein kinase (MAPK), nuclear factor (NF) kappa B as well as the reactive oxygen species (ROS) on human eosinophilic leukaemia HL-60 clone 15 cells was measured. Interestingly, at low concentration (7.34 mM), limonene was found to be effective in hindering the production of ROS for eotaxin-stimulated HL-60 clone 15 cells. Furthermore, a significant reduction was observed in the production of MCP-1 at 14.68 mM of limonene. This was achieved by activating NF-kappa B similar to when the proteasomal inhibitor MG132 was added. Inhibition of cell chemotaxis in a p38 MAPK-dependent manner comparable to the addition of SB203580 (inhibitor of p38 MAPK) was also noted at a concentration of 14.68 mM of limonene.
Two sesquiterpenic constituents, namely, α-humulene and (−)-trans-caryophyllene (Fig. 18.1) isolated from Cordia verbenacea EO, were assessed by Fernandes et al. (2007) for their anti-inflammatory properties. Several inflammatory experimental models involving mice and rats and different inflammation inducers were used. For instance, paw oedema caused by carrageenan in mice was strikingly repressed by these two sesquiterpenes (50 mg/kg, oral administration). Besides, both active compounds were found to effectively decrease bradykinin-, ovalbumin- and platelet-activating factor-induced mouse paw oedema. Similarly, the formation of prostaglandin E2, expression of inducible cyclooxygenase (COX) and nitric oxide synthase caused by carrageenan intraplantar injection in rats were also diminished by α-humulene and (−)-trans-caryophyllene. However, oedema induced by histamine injection was reduced solely by α-humulene. Furthermore, while (−)-trans-caryophyllene was able to reduce only the release of TNFα (tumour necrosis factor-α), α-humulene mostly averted both TNFα and IL-1β (interleukin-1β) production in carrageenan-injected rats upon systemic treatment. The results also revealed that the two sesquiterpenes demonstrated anti-inflammatory activities comparable to those experienced by dexamethasone-treated rats (positive control drug).
Moreover, thymol isolated from EO obtained from the leaves of Lippia gracilis (32.68%) is thought to be a chief phytoconstituent accountable for its antinociceptive as well as its anti-inflammatory effects (Mendes et al. 2010). Thymol has been demonstrated to hinder the discharge of arachidonic acid, COX and prostaglandins biosynthesis like prostaglandin E2 in visceral pain model (Mendes et al. 2010). Likewise, thymol at 100 mg/kg was observed to minimize inflammation and encourage wound healing in rodent models by inhibiting leucocytes influx to the wounded regions and as a result prevented oedema (Riella et al. 2012).
Liang et al. (2014) also evaluated the anti-inflammatory property of thymol (10, 20, 40 μg/mL) in lipopolysaccharide (LPS)-stimulated mouse mammary epithelial cells (mMECs). Thymol was seen to significantly hold back the production of IL-6 and TNF-α in LPS-stimulated mMECs. Thymol was also found to suppress the expression of COX-2 and iNOS dose dependently. In addition, the phosphorylation of NF-κB p65, IκBα, JNK, ERK and p38 mitogen-activated protein kinases (MAPKs) in LPS-stimulated mMECs was blocked by thymol. These findings thus pointed out that thymol anti-inflammatory activity in LPS-stimulated mMECs was brought about by hindering the activation of MAPK and NF-κB signalling pathways (Liang et al. 2014).
Furthermore, Xia et al. (2019) investigated the anti-inflammatory potential of trans-cinnamaldehyde (TCA) (Fig. 18.1), a compound present in Cinnamomum cassia Presl on cartilage chondrocytes in vitro and in rat models of osteoarthritis (OA). SW1353 cells and human primary chondrocytes were exposed to varying concentrations of TCA (2–20 μg/mL) for 2 h followed by stimulation of IL-1β, an inflammatory cytokine. TCA showed insignificant effect on cell viability at 10 μg/mL. TCA treatment at concentration 2–10 μg/mL was observed to reduce the levels of expression of matrix metalloproteinases (MMP-1, MMP-3, MMP-13) as well as a disintegrin and metalloproteinase with thrombospondin motifs (ADAMTS-4 and ADAMTS-5) dose dependently in IL-1β-stimulated SW1353 cells. TCA-treated IL-1β-stimulated SW1353 cells at concentration 20 μg/mL also showed reduction in matrix metalloproteinases expression levels, significantly lower than that of 10 μg/mL TCA. Similarly, increase in TCA up to 20 μg/mL also reduced ADAMTS-5, involved in the degradation of cartilaginous matrix, while contrarily ADAMTS-4 showed a significant increase in expression level at 20 μg/mL in IL-1β-stimulated SW1353 cells. Pretreatment of IL-1β-stimulated SW1353 cells with TCA inhibited NF-κB activation, IκBα degradation and increased p-IκBα expression, suggesting that nuclear factor-kappa B (NF-κB) inactivation was caused by TCA. NF-κB activation by stimulation of IL-1β can lead to the expression of proinflammatory mediators thereby causing inflammation (Marcu et al. 2010). Interestingly, TCA was also found to suppress the activation of p-p38 and p-JNK1/2, while p-ERK levels were not considerably affected. IL-1β-Stimulated human primary chondrocytes treated with TCA at 10 μg/mL, considered to be the effective concentration, also demonstrated comparable results to IL-1β-stimulated SW1353 cells. In vivo evaluation to examine the TCA chondrocyte protective effects involved the intraperitoneal injection of TCA (50 mg/kg) in monosodium iodoacetate-induced OA rats. Based on the results, TCA was established to reduce OA progression and the Osteoarthritis Research Society International (OARSI) scores, therefore confirming its cartilage protecting effect and anti-inflammatory property vis-à-vis OA.
18.3 Conclusion and Future Prospects
Plant-derived EOs are complex mixtures of bioactive components responsible for a wide spectrum of biological activities. Several studies have been conducted on EOs, including their individual constituents to evaluate their pharmacological properties. Moreover, studies have been focusing on their underlying mechanisms of actions involved in showing those effects. However, their isolated active compounds, occurring at high amounts have been of particular interests as they are believed to play a major role in the pharmacological actions demonstrated by the EOs.
Nevertheless, it has been reported that EOs minor components can also exert synergistic or antagonistic effects, and as a result contribute to the EOs bioactivities. Therefore, the evaluation of the isolated EO constituents and a better understanding of their corresponding mechanistic actions can aid in more specific and targeted approach in the treatment of several diseases. In addition to acting as a substitute for synthetic drugs with low toxicity, many of the EO constituents have been seen to have drug potentiating effect, resulting into more enhanced effects and concomitantly reducing the concentration of drug being used as well as their possible adverse effects. Thus, this is a very interesting feature that can be further explored by the scientific community given the vast panoply of bioactive components present in volatile oils, and in this way they can be used in combination with synthetic drugs for better treatment and management of diseases.
References
Amato A, Liotta R, Mulè F (2014) Effects of menthol on circular smooth muscle of human colon: analysis of the mechanism of action. Eur J Pharmacol 740:295–301
Andrade LN (2015) Sesquiterpenes from essential oils and anti-inflammatory activity. Nat Prod Commun 10:1767–1774
Arab HA, Fathi M, Mortezai E, Hosseinimehr SJ (2015) Chemoprotective effect of thymol against genotoxicity induced by bleomycin in human lymphocytes. Pharm Biomed Res 1(1):26–31. https://doi.org/10.18869/acadpub.pbr.1.1.26
Arafa ESA, Zhu Q, Shah ZI, Wani G, Barakat BM, Racoma I, El-Mahdy MA, Wani AA (2011) Thymoquinone up-regulates PTEN expression and induces apoptosis in doxorubicin-resistant human breast cancer cells. Fund Mol Mech Mut 706:28–35
Azanchi T, Shafaroodi H, Asgarpanah J (2014) Anticonvulsant activity of Citrus aurantium blossom essential oil (neroli): involvement of the GABAergic system. Nat Prod Res 6:1615–1618
Babukumar S, Vinothkumar V, Sankaranarayanan C, Srinivasan S (2017) Geraniol, a natural monoterpene, ameliorates hyperglycemia by attenuating the key enzymes of carbohydrate metabolism in streptozotocin-induced diabetic rats. Pharm Biol 55:1442–1449
Bakkali F, Averbeck S, Averbeck D, Idaomar M (2008) Biological effects of essential oils—a review. Food Chem Toxicol 46:446–475
Baser KHC, Buchbauer G (2010) Handbook of essential oils: science, technology, and applications. CRC Press, Boca Raton, FL
Baser KHC, Buchbauer G (2015) Handbook of essential oils: science, technology, and applications. CRC Press, USA
Bassolé IHN, Juliani HR (2012) Essential oils in combination and their antimicrobial properties. Molecules 17:3989–4006. https://doi.org/10.3390/molecules17043989
Baud V, Karin M (2009) Is NF-κB a good target for cancer therapy? Hopes and pitfalls. Nat Rev Drug Discov 8:33
Bernstein N, Akram M, Daniyal M, Koltai H, Fridlender M, Gorelick J (2018) Antiinflammatory potential of medicinal plants: a source for therapeutic secondary metabolites. In: Sparks D (ed) Advances in agronomy, vol 150. Academic Press, USA, pp 131–183
Burt S (2004) Essential oils: their antibacterial properties and potential applications in foods—a review. Int J Food Microbiol 94:223–253
Carnesecchi S, Langley K, Exinger F, Gosse F, Raul F (2002) Geraniol, a component of plant essential oils, sensitizes human colonic cancer cells to 5-fluorouracil treatment. J Pharmacol Exp Ther 301:625–630
Carnesecchi S, Bras-Gonçalves R, Bradaia A, Zeisel M, Gossé F, Poupon MF, Raul F (2004) Geraniol, a component of plant essential oils, modulates DNA synthesis and potentiates 5-fluorouracil efficacy on human colon tumor xenografts. Cancer Lett 215:53–59
Carson CF, Hammer KA, Riley TV (2006) Melaleuca alternifolia (tea tree) oil: a review of antimicrobial and other medicinal properties. Clin Microbiol Rev 19:50–62
Chaillot J, Tebbji F, Remmal A, Boone C, Brown GW, Bellaoui M, Sellam A (2015) The monoterpene carvacrol generates endoplasmic reticulum stress in the pathogenic fungus Candida albicans. Antimicrob Agents Chemother 59:4584–4592
Chang HT, Hsu SS, Chou CT, Cheng JS, Wang JL, Lin KL, Fang YC, Chen WC, Chien JM, Lu T, Pan CC (2011). Effect of thymol on Ca2+ homeostasis and viability in MG63 human osteosarcoma cells. Pharmacology 88:201–212
Chauhan AK, Bahuguna A, Paul S, Kang SC (2017) Thymol elicits HCT-116 colorectal carcinoma cell death through induction of oxidative stress. Anticancer Agents Med Chem 17:1942–1950
Chen Y, Ba L, Huang W, Liu Y, Pan H, Mingyao E, Shi P, Wang Y, Li S, Qi H, Sun H (2017) Role of carvacrol in cardioprotection against myocardial ischemia/reperfusion injury in rats through activation of MAPK/ERK and Akt/eNOS signaling pathways. Eur J Pharmacol 796:90–100
Davidson PM (1997) Chemical preservatives and natural antimicrobial compounds. In: Doyle MP, Beuchat LR, Montville TJ (eds) Food microbiology: fundamental and Frontiers. ASM Publications, Washington, DC, pp 520–556
De Sousa DP, Junior GA, Andrade LN, Calasans FR, Nunes XP, Barbosa-Filho JM, Batista JS (2008) Structure and spasmolytic activity relationships of monoterpene analogues found in many aromatic plants. Zeitschrift für Naturforschung C 63:808–812
Deb DD, Parimala G, Devi SS, Chakraborty T (2011) Effect of thymol on peripheral blood mononuclear cell PBMC and acute promyelotic cancer cell line HL-60. Chemico-biological interactions 193:97–106
Denyer SP, Hugo WB (1991) Biocide-induced damage to the bacterial cytoplasmic membrane. In: Denyer SP, Hugo WB (eds) Mechanisms of action of chemical biocides, the Society for Applied Bacteriology, technical series no 27. Oxford Blackwell Scientific Publication, Oxford, pp 171–188
Devi KP, Nisha SA, Sakthivel R, Pandian SK (2010) Eugenol (an essential oil of clove) acts as an antibacterial agent against Salmonella typhi by disrupting the cellular membrane. J Ethnopharmacol 130:107–115
Dorman HJD, Deans SG (2000) Antimicrobial agents from plants: antibacterial activity of plant volatile oils. J Appl Microbiol 88:308–316
Edris AE (2007) Pharmaceutical and therapeutic potentials of essential oils and their individual volatile constituents: a review. Phytother Res 21:308–323
Edris AE, Farrag E (2003) Antifungal activity of peppermint and sweet basil essential oils and their major aroma constituents on some plant pathogenic fungi from the vapor phase. Food Nahrung 47:117–121
El-Bassossy HM, Ghaleb H, Elberry AA, Balamash KS, Ghareib SA, Azhar A, Banjar Z (2017) Geraniol alleviates diabetic cardiac complications: effect on cardiac ischemia and oxidative stress. Biomed Pharmacother 88:1025–1030
El-Sayed ESM, Mansour AM, Abdul-Hameed MS (2016) Thymol and carvacrol prevent doxorubicin-induced cardiotoxicity by abrogation of oxidative stress, inflammation, and apoptosis in rats. J Biochem Mol Toxicol 30:37–44
Elshafie HS, Mancini E, Sakr S, De Martino L, Mattia CA, De Feo V, Camele I (2015) Antifungal activity of some constituents of Origanum vulgare L. essential oil against postharvest disease of peach fruit. J Med Food 18:929–934
Ezhumalai M, Radhiga T, Pugalendi KV (2014) Antihyperglycemic effect of carvacrol in combination with rosiglitazone in high-fat diet-induced type 2 diabetic C57BL/6J mice. Mol Cell Biochem 385:23–31
Fernandes ES, Passos GF, Medeiros R, da Cunha FM, Ferreira J, Campos MM, Pianowski LF, Calixto JB (2007) Anti-inflammatory effects of compounds alpha-humulene and (−)-trans-caryophyllene isolated from the essential oil of Cordia verbenacea. Eur J Pharmacol 569:228–236
File SE, Pellow S (1986) Intrinsic actions of the benzodiazepine receptor antagonist Ro 15-1788. Psychopharmacology 88:1–11
Freires IA, Denny C, Benso B, De Alencar SM, Rosalen PL (2015) Antibacterial activity of essential oils and their isolated constituents against cariogenic bacteria: a systematic review. Molecules 20:7329–7358
García DA, Bujons J, Vale C, Suñol C (2006) Allosteric positive interaction of thymol with the GABAA receptor in primary cultures of mouse cortical neurons. Neuropharmacology 50:25–35
Gibson SB, Oyer R, Spalding AC, Anderson SM, Johnson GL (2000) Increased expression of death receptors 4 and 5 synergizes the apoptosis response to combined treatment with etoposide and TRAIL. Mol Cell Biol 20:205–212
Gill AO, Holley RA (2006) Disruption of Escherichia coli, Listeria monocytogenes and Lactobacillus sakei cellular membranes by plant oil aromatics. Int J Food Microbiol 108:1–9
Goossens A, Merckx L (1997) Allergic contact dermatitis from farnesol in a deodorant. Contact Dermatitis 37:179–180
Guimarães AC, Meireles LM, Lemos MF, Guimarães MCC, Endringer DC, Fronza M, Scherer R (2019) Antibacterial activity of terpenes and Terpenoids present in essential oils. Molecules 24:2471
Haberland M, Montgomery RL, Olson EN (2009) The many roles of histone deacetylases in development and physiology: implications for disease and therapy. Nat Rev Gen 10:32
Haeseler G, Maue D, Grosskreutz J, Bufler J, Nentwig B, Piepenbrock S, Dengler R, Leuwer M (2002) Voltage-dependent block of neuronal and skeletal muscle sodium channels by thymol and menthol. Eur J Anaesthesiol 19:571–579
Hirota R, Roger NN, Nakamura H, Song HS, Sawamura M, Suganuma N (2010) Anti-inflammatory effects of limonene from yuzu (Citrus junos Tanaka) essential oil on eosinophils. J Food Sci 75:H87–H92
Hoffmeyer K, Raggioli A, Rudloff S, Anton R, Hierholzer A, Del Valle I, Hein K, Vogt R, Kemler R (2012) Wnt/β-catenin signaling regulates telomerase in stem cells and cancer cells. Science 336:1549–1554. https://doi.org/10.1126/science.1218370
Hsu SS, Lin KL, Chou CT, Chiang AJ, Liang WZ, Chang HT (2011) Effect of thymol on Ca2+ homeostasis and viability in human glioblastoma cells. Eur J Pharmacol 670:85–91. https://doi.org/10.1016/j.ejphar.2011.08.017
Jaafari A, Tilaoui M, Mouse HA, M’bark LA, Aboufatima R, Chait A, Lepoivre M, Zyad A (2012) Comparative study of the antitumor effect of natural monoterpenes: relationship to cell cycle analysis. Rev Bras 22:534–540. https://doi.org/10.1590/s0102-695x2012005000021
Jeong JB, Choi J, Lou Z, Jiang X, Lee SH (2013) Patchouli alcohol, an essential oil of Pogostemon cablin, exhibits anti-tumorigenic activity in human colorectal cancer cells. Int Immunopharmacol 16:184–190
Joo JH, Liao G, Collins JB, Grissom SF, Jetten AM (2007) Farnesol-induced apoptosis in human lung carcinoma cells is coupled to the endoplasmic reticulum stress response. Cancer Res 67:7929–7936
Kalemba D, Kunicka A (2003) Antibacterial and antifungal properties of essential oils. Curr Med Chem 10:813–829
Kang SH, Kim YS, Kim EK, Hwang JW, Jeong JH, Dong X, Lee JW, Moon SH, Jeon BT, Park PJ (2016) Anticancer effect of thymol on AGS human gastric carcinoma cells. J Microbiol Biotechnol 26:28–37
Kannappan A, Balasubramaniam B, Ranjitha R, Srinivasan R, Packiavathy IASV, Balamurugan K, Pandian SK, Ravi AV (2019) In vitro and in vivo biofilm inhibitory efficacy of geraniol-cefotaxime combination against Staphylococcus spp. Food Chem Toxicol 125:322–332
Kim JR, Kang P, Lee HS, Kim KY, Seol GH (2017) Cardiovascular effects of linalyl acetate in acute nicotine exposure. Env Health Prevent Med 22:42
Knobloch K, Weigand H, Weis N, Schwarm HM, Vigenschow H (eds) (1986) Action of terpenoids on energy metabolism. Walter de Gruyter, Berlin, Germany
Koto R, Imamura M, Watanabe C, Obayashi S, Shiraishi M, Sasaki Y, Azuma H (2006) Linalyl acetate as a major ingredient of lavender essential oil relaxes the rabbit vascular smooth muscle through dephosphorylation of myosin light chain. J Cardiovasc Pharmacol 48:850–856
Kovač J, Šimunović K, Wu Z, Klančnik A, Bucar F, Zhang Q, Možina SS (2015) Antibiotic resistance modulation and modes of action of (−)-α-pinene in campylobacter jejuni. PLoS One 10:e0122871
Krupčík J, Gorovenko R, Špánik I, Sandra P, Armstrong DW (2015) Enantioselective comprehensive two-dimensional gas chromatography. A route to elucidate the authenticity and origin of Rosa damascena Miller essential oils. J Sep Sci 38:3397–3403
Lahlou S, Figueiredo AF, Magalhães PJC, Leal-Cardoso JH (2002) Cardiovascular effects of 1, 8-cineole, a terpenoid oxide present in many plant essential oils, in normotensive rats. Can J Physiol Pharmacol 80:1125–1131
Lambert RJW, Skandamis PN, Coote PJ, Nychas GJ (2001) A study of the minimum inhibitory concentration and mode of action of oregano essential oil, thymol and carvacrol. J Appl Microbiol 91:453–462
Leal-Cardoso JH, Lahlou S, Coelho-de-Souza AN, Criddle DN, Pinto Duarte GI, Santo MA, Magalhães PJ (2002) Inhibitory actions of eugenol on rat isolated ileum. Can J Physiol Pharmacol 80:901–906
Lee JH, Kim C, Kim SH, Sethi G, Ahn KS (2015) Farnesol inhibits tumor growth and enhances the anticancer effects of bortezomib in multiple myeloma xenograft mouse model through the modulation of STAT3 signaling pathway. Cancer Lett 360:280–293
Legault J, Pichette A (2007) Potentiating effect of β-caryophyllene on anticancer activity of α-humulene, isocaryophyllene and paclitaxel. J Pharm Pharmacol 59:1643–1647
Liang D, Yang M, Guo B, Yang L, Cao J, Zhang X (2012) HIF-1α induced by β-elemene protects human osteosarcoma cells from undergoing apoptosis. J Cancer Res Clin Oncol 138:1865–1877
Liang D, Li F, Fu Y, Cao Y, Song X, Wang T, Wang W, Guo M, Zhou E, Li D, Yang Z (2014) Thymol inhibits LPS-stimulated inflammatory response via down-regulation of NF-κB and MAPK signaling pathways in mouse mammary epithelial cells. Inflammation 37:214–222
Lima CC, Criddle DN, Coelho-de-Souza AN, Monte FJ, Jaffar M, Leal-Cardoso JH (2000) Relaxant and antispasmodic actions of methyleugenol on Guinea-pig isolated ileum. Planta Med 66:408–411
Lüthy R, Münch R, Blaser J, Bhend H, Siegenthaler W (1979) Human pharmacology of cefotaxime (HR 756), a new cephalosporin. Antimicrob Agents Chemother 16:127–133
Maeda K, Ito T, Shioda S (2012) Medical aromatherapy practice in Japan. Essence 10:14–16
Magalhães Pedro JC, Criddle DN, Tavares RA, Melo EM, Mota TL, Leal-Cardoso JH (1998) Intestinal myorelaxant and antispasmodic effects of the essential oil of Croton nepetaefolius and its constituents cineole, methyl-eugenol and terpineol. Phytother Res 12:172–177
Magalhães PJ, Lahlou S, Leal-Cardoso JH (2004) Antispasmodic effects of the essential oil of Croton nepetaefolius on Guinea-pig ileum: a myogenic activity. Fund Clin Pharmacol 18(5):539–546
Marcu BK, Otero M, Olivotto E, Maria Borzi R, Goldring BM (2010) NF-κB signaling: multiple angles to target OA. Curr Drug Targ 11:599–613
Mauro M, Catanzaro I, Naselli F, Sciandrello G, Caradonna F (2013) Abnormal mitotic spindle assembly and cytokinesis induced by D-limonene in cultured mammalian cells. Mutagenesis 28:631–635
Maurya AK, Singh M, Dubey V, Srivastava S, Luqman S, Bawankule DU (2014) α-(−)-bisabolol reduces pro-inflammatory cytokine production and ameliorates skin inflammation. Curr Pharm Biotechnol 15:173–181
Mendes SS, Bomfim RR, Jesus HCR, Alves PB, Blank AF, Estevam CS, Antoniolli AR, Thomazzi SM (2010) Evaluation of the analgesic and anti-inflammatory effects of the essential oil of Lippia gracilis leaves. J Ethnopharmacol 129:391–397. https://doi.org/10.1016/j.jep.2010.04.005
Menezes IA, Barreto CM, Antoniolli ÂR, Santos MR, de Sousa DP (2010) Hypotensive activity of terpenes found in essential oils. Zeitschrift für Naturforschung C 65:562–566
Miguel MG (2010) Antioxidant and anti-inflammatory activities of essential oils: a short review. Molecules 15:9252–9287
Mohammadreza VR (2008) Variation in the essential oil composition of Artemisia annua L. of different growth stages cultivated in Iran. Bot Res J 1:33–35
Moon HK, Kang P, Lee HS, Min SS, Seol GH (2014) Effects of 1, 8-cineole on hypertension induced by chronic exposure to nicotine in rats. J Pharm Pharmacol 66:688–693
Muruganathan U, Srinivasan S (2016) Beneficial effect of carvone, a dietary monoterpene ameliorates hyperglycemia by regulating the key enzymes activities of carbohydrate metabolism in streptozotocin-induced diabetic rats. Biomed Pharmacother 84:1558–1567
Nóbrega FF, Salvadori MG, Masson CJ, Mello CF, Nascimento TS, Leal-Cardoso JH, De Sousa DP, Almeida RN (2014) Monoterpenoid terpinen-4-ol exhibits anticonvulsant activity in behavioural and electrophysiological studies. Oxid Medicine Cell Longev 2014:703848. https://doi.org/10.1155/2014/703848
Oguntibeju OO (2018) Medicinal plants with anti-inflammatory activities from selected countries and regions of Africa. J Inflamma Res 11:307
Okoh SO, Iweriebor BC, Okoh OO, Okoh AI (2017) Bioactive constituents, radical scavenging, and antibacterial properties of the leaves and stem essential oils from Peperomia pellucida (L.) Kunth. Pharmacog Mag 13(Suppl 3):S392
Park JS, Kwon JK, Kim HR, Kim HJ, Kim BS, Jung JY (2014) Farnesol induces apoptosis of DU145 prostate cancer cells through the PI3K/Akt and MAPK pathways. Int J Mol Med 33:1169–1176
Parki A, Chaubey P, Prakash O, Kumar R, Pant AK (2017) Seasonal variation in essential oil compositions and antioxidant properties of Acorus calamus L. accessions. Medicines 4:81
Pauli A (2001) Antimicrobial properties of essential oil constituents. Int J Aromather 11:126–133
Pauli A, Schilche H (2009) In vitro antimicrobial activities of essential oils monographed in the European pharmacopoeia. In: Hüsnü K, Baser C, Buchbauer G (eds) Handbook of essential oils: science, technology, and applications. CRC Press, USA, pp 353–547
Peixoto-Neves D, Silva-Alves KS, Gomes MDM, Lima FC, Lahlou S, Magalhães PJC, Ceccatto VM, Coelho-de-Souza AN, Leal-Cardoso JH (2010) Vasorelaxant effects of the monoterpenic phenol isomers, carvacrol and thymol, on rat isolated aorta. Fund Clin Pharmacol 24:341–350
Pérez GS, Zavala SM, Arias GL, Ramos LM (2011) Anti-inflammatory activity of some essential oils. J Essent Oil Res 23:38–44
Pfister C, Pfrommer H, Tatagiba MS, Roser F (2013) Detection and quantification of farnesol-induced apoptosis in difficult primary cell cultures by TaqMan protein assay. Apoptosis 18:452–466
Polakis P (2000) Wnt signaling and cancer. Genes Dev 14:1837–1851
Quintans-Júnior LJ, Guimarães AG, Araújo BE, Oliveira GF, Santana MT, Moreira FV, Santos MR, Cavalcanti SC, Júnior WL, Botelho MA, Ribeiro LA (2010) Carvacrol, (−)-borneol and citral reduce convulsant activity in rodents. Afr J Biotechnol 9:6566–6572
Rabi T, Bishayee A (2009) D-limonene sensitizes docetaxel-induced cytotoxicity in human prostate cancer cells: generation of reactive oxygen species and induction of apoptosis. Mol Carcinog 8. https://doi.org/10.4103/1477-3163.51368
Rathinam A, Pari L, Chandramohan R, Sheikh BA (2014) Histopathological findings of the pancreas, liver, and carbohydrate metabolizing enzymes in STZ-induced diabetic rats improved by administration of myrtenal. J Physiol Biochem 70:935–946
Ravizza R, Gariboldi MB, Molteni R, Monti E (2008) Linalool, a plant-derived monoterpene alcohol, reverses doxorubicin resistance in human breast adenocarcinoma cells. Oncol Rep 20:625–630
Riella KR, Marinho RR, Santos JS, Pereira-Filho RN, Cardoso JC, Albuquerque-Junior RLC, Thomazzi SM (2012) Anti-inflammatory and cicatrizing activities of thymol, a monoterpene of the essential oil from Lippia gracilis, in rodents. J Ethnopharmacol 143:656–663
Sancheti J, Shaikh MF, Chaudhari R, Somani G, Patil S, Jain P, Sathaye S (2014) Characterization of anticonvulsant and antiepileptogenic potential of thymol in various experimental models. Naunyn Schmiedeberg’s Arch Pharmacol 387:59–66
Shiina Y, Funabashi N, Lee K, Toyoda T, Sekine T, Honjo S, Hasegawa R, Kawata T, Wakatsuki Y, Hayashi S, Murakami S (2008) Relaxation effects of lavender aromatherapy improve coronary flow velocity reserve in healthy men evaluated by transthoracic Doppler echocardiography. Int J Cardiol 129:193–197
Sikkema J, de Bont JAM, Poolman B (1994) Interactions of cyclic hydrocarbons with biological membranes. J Biol Chem 269:8022–8028
Silva MIG, Silva MAG, De Aquino Neto MR, Moura BA, De Sousa HL, De Lavor EPH, De Vasconcelos PF, Macêdo DS, De Sousa DP, Vasconcelos SMM, De Sousa FCF (2009) Effects of isopulegol on pentylenetetrazol-induced convulsions in mice: possible involvement of GABAergic system and antioxidant activity. Fitoterapia 80:506–513
Singulani JL, Pedroso RS, Ribeiro AB, Nicolella HD, Freitas KS, Damasceno JL, Vieira TM, Crotti AE, Tavares DC, Martins CH, Mendes-Giannini MJ (2018) Geraniol and linalool anticandidal activity, genotoxic potential and embryotoxic effect on zebrafish. Fut Microbiol 13(15):1637–1646
Sitarek P, Rijo P, Garcia C, Skała E, Kalemba D, Białas AJ, Szemraj J, Pytel D, Toma M, Wysokińska H and Śliwiński T (2017). Antibacterial, anti-inflammatory, antioxidant, and antiproliferative properties of essential oils from hairy and normal roots of Leonurus sibiricus L. and their chemical composition. Oxid Med Cell Longev 2017
Smith DR, Goh HS (1996) Overexpression of the c-myc proto-oncogene in colorectal carcinoma is associated with a reduced mortality that is abrogated by point mutation of the p53 tumor suppressor gene. Clin Cancer Res 2:1049–1053
Souza CM, Pereira Junior SA, Moraes TD, Damasceno JL, Amorim Mendes S, Dias HJ, Stefani R, Tavares DC, Martins CH, Crotti AE, Mendes-Giannini MJ (2016) Antifungal activity of plant-derived essential oils on Candida tropicalis planktonic and biofilms cells. Sabouraudia 54(5):515–523
Speranza B, Corbo MR (2010) Essential oils for preserving perishable foods: possibilities and limitations. Application of alternative food-preservation technologies to enhance food safety and stability 23, pp. 35–37
Srinivasan S, Muruganathan U (2016) Antidiabetic efficacy of citronellol, a citrus monoterpene by ameliorating the hepatic key enzymes of carbohydrate metabolism in streptozotocin-induced diabetic rats. Chem Biol Interact 250:38–46
Srinivasan S, Sathish G, Jayanthi M, Muthukumaran J, Muruganathan U, Ramachandran V (2014) Ameliorating effect of eugenol on hyperglycemia by attenuating the key enzymes of glucose metabolism in streptozotocin-induced diabetic rats. Mol Cell Biochem 385:159–168
Swamy MK, Akhtar MS, Sinniah UR (2016) Antimicrobial properties of plant essential oils against human pathogens and their mode of action: an updated review. Evid Based Complement Alternat Med 2016:21. https://doi.org/10.1155/2016/3012462
Tahir HU, Sarfraz RA, Ashraf A, Adil S (2016) Chemical composition and antidiabetic activity of essential oils obtained from two spices (Syzygium aromaticum and Cuminum cyminum). Int J Food Prop 19:2156–2164
Tisserand R, Young R (eds) (2013) Essential oil safety: a guide for health care professionals. Elsevier Health Sciences, United Kingdom
Vieira A, Heidor R, Cardozo MT, Scolastici C, Purgatto E, Shiga TM, Barbisan LF, Ong TP, Moreno FS (2011) Efficacy of geraniol but not of β-ionone or their combination for the chemoprevention of rat colon carcinogenesis. Braz J Med Biol 44:538–545
Wang YL, Liu HF, Shi XJ, Wang Y (2018a) Antiproliferative activity of Farnesol in HeLa cervical cancer cells is mediated via apoptosis induction, loss of mitochondrial membrane potential (ΛΨm) and PI3K/Akt signalling pathway. J BUON 23:752–757
Wang L, Zhao Y, Wu Q, Guan Y, Wu X (2018b) Therapeutic effects of β-elemene via attenuation of the Wnt/β-catenin signaling pathway in cervical cancer cells. Mol Med Rep 17:4299–4306
Xia Z, Mao X, Luo Y (1999) Study on antifungal mechanism of alpha-pinene. Hunan yi ke da xue xue bao= Hunan yike daxue xuebao= Bullet Hum Med Univ 24:507–509
Xia T, Gao R, Zhou G, Liu J, Li J, Shen J (2019) Trans-Cinnamaldehyde inhibits IL-1β-stimulated inflammation in chondrocytes by suppressing NF-κB and p38-JNK pathways and exerts chondrocyte protective effects in a rat model of osteoarthritis. Biomed Res Int 2019, 1
Ya’ni AA, Eldahshan OA, Hassan SA, Elwan ZA, Ibrahim HM (2018) Antidiabetic effects of essential oils of some selected medicinal Lamiaceae plants from Yemen against α-glucosidase enzyme. J Phytochem Biochem 2:2
Yadav DK, Mudgal V, Agrawal J, Maurya AK, Bawankule DU, Chanotiya CS, Khan F, Thul ST (2013) Molecular docking and ADME studies of natural compounds of agarwood oil for topical anti-inflammatory activity. Curr Comput-Aid Drug 9:360–370
Yang H, Woo J, Pae AN, Um MY, Cho NC, Park KD, Yoon M, Kim J, Lee CJ, Cho S (2016) α-Pinene, a major constituent of pine tree oils, enhances non-rapid eye movement sleep in mice through GABAA-benzodiazepine receptors. Mol Pharmacol 90:530–539
Yeo SK, Ali AY, Hayward OA, Turnham D, Jackson T, Bowen ID, Clarkson R (2016) β-Bisabolene, a Sesquiterpene from the essential oil extract of Opoponax (Commiphora guidottii), exhibits cytotoxicity in breast Cancer cell lines. Phytother Res 30:418–425
Yuan W, Yuk HG (2019) Effect of sublethal thymol, carvacrol and trans-cinnamaldehyde adaptation on virulence properties of Escherichia coli O157: H7. Appl Environ Microbiol AEM-00271. https://doi.org/10.1128/AEM.00271-19
Zhu T, Zhao Y, Zhang J, Li L, Zou L, Yao Y, Xu Y (2011) β-Elemene inhibits proliferation of human glioblastoma cells and causes cell-cycle G0/G1 arrest via mutually compensatory activation of MKK3 and MKK6. Int J Oncol 38:419–426
Zuzarte M, Salgueiro L (eds) (2015) Essential oils chemistry. Springer, Switzerland
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Jugreet, B.S., Mahomoodally, M.F. (2020). Pharmacological Properties of Essential Oil Constituents and their Mechanisms of Action. In: Swamy, M. (eds) Plant-derived Bioactives. Springer, Singapore. https://doi.org/10.1007/978-981-15-2361-8_18
Download citation
DOI: https://doi.org/10.1007/978-981-15-2361-8_18
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-15-2360-1
Online ISBN: 978-981-15-2361-8
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)