Abstract
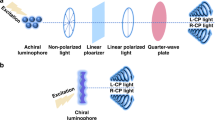
Circularly polarized luminescence (CPL) produced by achiral organic luminophores is described. Achiral organic luminophores can exhibit CPL by the chiral arrangement of the achiral luminophores. Chiral arrangement of achiral luminophores can be constructed through a covalently linked chiral spacer like a binaphthyl moiety. A helical supramolecular assembly also provides chiral environment on an achiral luminophore. The helically stacked assemblies of achiral luminophores are excellent for realizing CPL of the achiral luminophore since the highly assembled structure in the helical assembly provides good CPL activity. The stimuli-responsivity of supramolecular systems provides stimuli-responsive CPL.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
9.1 Introduction
In the field of circularly polarized luminescence (CPL), chiral lanthanide complexes have been dominant due to their large CPL dissymmetry factor, g lum [1]. In recent years, CPL-active organic compounds have attracted growing interest toward their potential applications in optoelectronic devices [2, 3]. The early research on CPL-active organic molecules started with chiral ketones in the 1960s [4]. After that, various CPL-active organic compounds were developed, and some of them exhibit large g lum values comparable to those of chiral lanthanide complexes.
A key issue for constructing CPL-active materials is how to prepare an asymmetric environment on luminophores. A straightforward strategy for realizing CPL-active materials is the direct introduction of chirality on the luminophore. Actually, many lanthanide complexes possessing chiral ligands have been reported to exhibit CPL with the luminescence of the metal center [1]. In the same manner, chiral organic dyes such as helicenes also exhibit CPL [3]. In the field of polymers, chiral polymers are reported as CPL-active materials [5,6,7]. The alternative way to achieve CPL-active organic compounds is the chiral arrangement of achiral luminophores. If two or more luminophores take a chiral orientation, the luminophores chirally interact each other. Then, the electronic state of the luminophore is chirally perturbed. Thus, the emission of the luminophore gets circularly polarized despite the luminophore being achiral. Such chiral orientation can be constructed through a covalently linked chiral spacer. Another way to achieve chiral orientation of achiral luminophores is the use of a chiral supramolecular assembly. In this chapter, CPL properties of small organic compounds and their supramolecular assemblies are described (Fig. 9.1).
9.2 CPL of Chiral Organic Luminophores
The early CPL of organic compounds was produced by fluorescence based on the π∗→n transition of the carbonyl group of chiral ketones (Fig. 9.2) [4, 8,9,10,11]. However, the π∗ → n transition of the carbonyl group is weak and limited at short wavelengths (~360 nm). The use of chiral π-conjugated luminophores that exhibit fluorescence by the π∗ → π transition as CPL-active organic compounds is desirable due to their emission in the visible region with a high emission quantum yield. The most popular chiral π-conjugated molecule is a helicene. A helicene is an ortho-ring-fused polycyclic aromatic compound, in which aromatic rings are angularly annulated to give a helical structure. Helicenes have been widely investigated from the 1950s, and the CPL of a helicene was reported by Katz and coworkers in 2001 [12]. They synthesized [7]helicene-like compound 1 (Fig. 9.3). The molecule exhibited polarized fluorescence at 440 nm in diluted dodecane solution (2 × 10−6 mol L−1), but CPL was not reported under these conditions. However, 1 displayed CPL in the condensed dodecane solution (>1 × 10−3 mol L−1). The authors considered that the formation of aggregated species triggers the CPL.
The first observation of CPL of a nonaggregated helicene was reported by Venkataraman and coworkers in 2003. They synthesized a racemic pair of a helicene, and then, the helicene was reacted with (1S)-camphanate to give a corresponding diastereomeric pair of the helicene ((1S,M)- and (1S,P)-2, Fig. 9.3) [13]. Both of the diastereomers exhibited CPL on the monomeric fluorescence with a g lum of ca. 1 × 10−3. Interestingly, the CPL spectrum of (1S,M)-2 was a mirror-image of that of (1S,P)-2 in spite of a diastereomer, indicating that the (1S)-camphanate attached on the helicene core does not perturb the CPL of the helicene. They also synthesized the more extended [5]helicene-like compounds (1S,M)- and (1S,P)-3 that display CPL too.
After the pioneering work by Venkataraman and coworkers, various chiral helicenes exhibiting CPL have been investigated. Tanaka and coworkers have developed several [7]helicene-like compounds displaying CPL since 2012 (Fig. 9.3) [14, 15]. For example, (M)-4 and (M)-5 exhibited CPL on its monomeric fluorescence in CHCl3 with a g lum of ca. 3 × 10−2, which is a high value for an organic compound [15]. Tanaka and coworkers have also developed the CPL of aza[6]helicene-like compounds [14]. They synthesized aza[6]helicene (M)-6 and S-shaped double aza[6]helicene-like compound (M,M)-7. Interestingly, the CPL of (M,M)-7 (g lum = 1.1 × 10−2) was enhanced compared to that of (M)-6 (g lum < 1 × 10−3). Shinokubo and coworkers reported the CPL of aza[7]helicenes (M)- and (P)-8 in 2012 (Fig. 9.3) [16]. They exhibited CPL with a g lum of 3 × 10−3 in CH2Cl2. Nozaki and coworkers have developed CPL-active sila[7]helicenes (M)- and (P)-9 that exhibited CPL with a g lum of 3.5 × 10−3 in CH2Cl2 (Fig. 9.3) [17].
Takeuchi and coworkers have reported an interesting CPL behavior of a phthalhydrazide-functionalized [7]helicene-like compound 10 (Fig. 9.4) [18]. 10 formed a trimeric disk in chloroform via the hydrogen-bonding interaction of a phthalhydrazide moiety. Further aggregation of the trimeric disk resulted in the formation of a one-dimensional fiber-like assembly. The CPL property of 10 was found in chloroform, but not in methanol, which suggests that the trimeric disk was dissociated by breaking the hydrogen bonds. The g lum values of (M)-10 were estimated to be −3.5 × 10–2 in chloroform and −2.1 × 10–2 in methanol (4 × 10–4 mol L–1), respectively. The formation of the trimeric disk and one-dimensional fiber-like assembly may increase the g lum value of (M)-10 in chloroform compared to that in methanol.
Thus, various CPL-active helicene-like compounds have been reported. However, the fluorescence quantum yields of CPL-active helicenes are moderate (32% for 4, 36% for 8, and 23% for 9, respectively). The distortion of the π-plane may decrease the effective fluorescence of planar π-conjugated luminophores.
9.3 CPL of Chirally Arranged Achiral Luminophores Through Chiral Spacers
An achiral luminophore has an advantage with regard to the fluorescence efficiency compared to a chirally distorted luminophore such as a helicene. A strategy to provide a chiral environment on an achiral luminophore is the chiral arrangement of two or more luminophores. When two luminophores are linked through a chiral spacer, the luminophores take a chiral orientation. Then, the luminophores interact each other, and the electronic state of a luminophore is chirally perturbed by the other luminophore. Various skeletons providing chiral orientation have been developed in the field of asymmetric catalysts. The most famous one is a chiral binaphthyl moiety. A binaphthyl has axial chirality since the free rotation about the bond linking the naphthyl rings is restricted due to the steric effect of hydrogen atoms at the 8 and 8′ positions. Thus, each naphthalene takes a chiral orientation. Fujiki, Imai, and coworkers have developed CPL of simple and extended binaphthyls 11–18 (Fig. 9.5) [19,20,21,22,23,24,25,26,27]. The CPL properties of the binaphthyl derivatives depend on the dihedral angle of binaphthyl, the topology of the neighboring groups, the position of binaphthyl linkage, and the π-extension of the binaphthyl unit. The binaphthyl derivatives other than 14 (nonfluorescent) and 17 (CPL-inactive) exhibited CPL with g lum values within the range of (1.5–0.8) × 10−3 and with fluorescent quantum yields within the range of 15–25%.
The introduction of two luminophores onto binaphthyl is effective for inducing the CPL of the achiral luminophore. The luminophores substituted on each naphthalene take a chiral orientation reflecting the axial chirality of binaphthyl. Kawai and coworkers reported the CPL of perylene bisimide (PBI) linked by a binaphthyl unit (Fig. 9.6) [28]. PBI is one of the most known organic luminophores that exhibits its fluorescence at approximately 550 nm with a high emission quantum yield. They synthesized (R)- and (S)-19, with two PBI moieties attached to the 2 and 2′ positions of the binaphthyl. (R)- and (S)-19 exhibited characteristic π–π∗ absorption and emission for the pair of PBI units. Chiroptical dissymmetry was observed in the circular dichroism (CD) spectrum, indicating that the two PBI moieties take a chiral orientation. (S)-19 displayed CPL with a g lum of 2 × 10−3 in their diluted solution (1 × 10−3 mol L−1). The g lum value increased as the concentration of (S)-19 increased and reached 6 × 10−3 at the concentration of 1 × 10−3 mol L−1, where (S)-19 formed an opaque colloidal solution. The authors suggest that the formation of the aggregate in the condensed solution results in an increase in the g lum value.
Pieters and coworkers developed a CPL-active delayed fluorescence system [29]. In thermally activated delayed fluorescence (TADF) emitters, the energy gap between their singlet and triplet states is so small that reverse intersystem crossing processes easily occur. Thus, both singlet and triplet excitons can be harvested for their fluorescence from the singlet excited state. This property has an advantage in developing motivated organic light emitting diodes (OLEDs) because of the possibility to overcome the theoretical maximum efficiency. They synthesized TADF emitter 20 possessing two achiral carbazoles as luminophores and a binaphthyl (Fig. 9.6). The TADF character of 20 was demonstrated using time-resolved fluorescence analysis. 20 exhibited CPL with a g lum of 1.3 × 10−3. The combination of CPL and TADF may provide a remarkable CPL-OLED material.
The use of a chiral cyclophane is also effective for creating CPL-active organic materials. A cyclophane is a cyclic compound that includes aromatic moieties as an integral part of its structure. [2.2]Paracyclophane has two benzene rings linked by two ethylenes at the 1,1′ and 4,4′ positions. The orientations of two benzene rings of a [2.2]paracyclophane are fixed since the benzene rings cannot invert. Thus, chirally substituted [2.2]paracyclophanes provide a chiral arrangement of luminophores that exhibit chiroptical properties. Recently, CPL-active [2.2]paracyclophanes have been developed by Morisaki, Chujo, and coworkers (Fig. 9.7) [30]. They synthesized propeller-shaped [2,2]paracyclophane derivatives (R)- and (S)-21 and their precursors (R)- and (S)-22. (S)-21 exhibited CPL with a g lum of 1.1 × 10−2 and fluorescence quantum yield of 45%. These values are quite good compared to ordinary chiral organic luminophores. Interestingly, the g lum value of (S)-21 is approximately ten-fold higher than that of (S)-22 (g lum = 1.1 × 10−3). The good CPL activity of (S)-21 comes from its highly extended and crisscrossed delocalized structure. The distortion of the π-conjugated plane in (S)-21 may hinder its fluorescence efficiency, but it is clear that the distortion causes the high g lum value of (S)-21 compared to that of (S)-22. Morisaki, Chujo, and coworkers also synthesized planar chiral tetra-substituted [2.2]paracyclophanes (R)- and (S)-23 and 24 [31]. 23 and 24 have a chirally orientated two para-phenylene-ethynylene luminophore that is not distorted. 24 has a more extended π-conjugated structure compared to 23. 23 and 24 displayed optical dissymmetry in their emissions with good fluorescence quantum yields (65% for 23 and 87% for 24) in diluted chloroform solution (1 × 10−6 mol L−1). The g lum values of (R)-23 and (R)-24 in the diluted solution were −1.7 × 10−3 and −1.2 × 10−3, respectively. Interestingly, the CPL activities of 23 and 24 drastically changed when the molecules formed films. They made spin-coated thin films, drop-casted thin films, and drop-casted thick films of 23 and 24. The films were annealed at 65 °C (for 23) and 90 °C (for 24) for 3 h. The CPL properties of the films are summarized in Table 9.1. The g lum values of 24 were increased after annealing, whereas those of 23 were not increased as much. For example, the g lum value of the drop-casted thick film of (R)-24 was −3.0 × 10−2 before annealing and −2.5 × 10−1 after annealing. The value after annealing is quite large for organic compounds. The authors proposed that the self-assembly of (R)-24 in the annealed film results in the increase of the g lum.
Thus far, the CPL properties of chiral organic luminophores and chirally arranged achiral luminophores through covalently linked spacers have been summarized. Three important findings are obtained from the results: (1) the distortion of the π-plane is effective for CPL activity, but it hinders the fluorescence efficiency of the planar π-conjugated luminophore; (2) a chiral arrangement of an achiral luminophore is effective for CPL activity; and (3) the aggregation of CPL-active organic compounds often increases their CPL activity. These findings encourage the use of the supramolecular assembly of achiral luminophores as CPL-active materials.
9.4 CPL Produced by Helical Supramolecular Assemblies
A supramolecular assembly is a well-defined molecular assembly held by noncovalent bonds such as hydrogen-bonding, π–π stacking, dipole–dipole, and hydrophilic/hydrophobic interactions. A finely designed supramolecular system provides a highly ordered structure of the assembly like the double helix of DNA. Recently, various highly ordered supramolecular assemblies have been developed [32,33,34]. Among them, supramolecular assemblies equipped with chirality have attracted attention due to their potential applications in the fields of asymmetric catalysts, chiral sensors, and chiroptical materials. Here, it is important to remember that the CPL properties of some chiral organic compounds increase in the condensed conditions compared to in the diluted solutions as mentioned above [12, 28, 35]. The increase would result from the formation of aggregates in the condensed conditions. Then, the idea to use supramolecular methods for constructing CPL-active organic materials is attractive since the luminophores form highly assembled structures in highly ordered supramolecular assemblies [2]. The helix is one of the most frequently appearing motifs in chiral supramolecular systems. The right-handed helix is a mirror-image of the left-handed one, and thus, the helix is chiral. Various types of helical assemblies have been developed: chiral and achiral small molecules, oligomers, and polymers form helical assemblies [36]. Considering the CPL properties, helically stacked assemblies of planar π-conjugated luminophores are optimal to balance the highly assembled structure with the chiral orientation of a luminophore.
To form an assembly of a small π-conjugated molecule, the appropriate molecular design is indispensable. If a π-conjugated molecule forms a helical stacked assembly, a racemic mixture of P-helix and M-helix would be obtained without any chiral source. However, the introduction of a chiral source makes the P-helix and M-helix diastereomers. The diastereomers have differences in their Gibbs free-energy, and thus one (P or M) is more stable than the other (M or P) (Fig. 9.8). Therefore, the chiral source, a chiral alkyl side-chain in many cases, must be introduced into the molecule.
The assembled structure of a small π-conjugated molecule is stabilized by π–π stacking interactions, but other intermolecular interactions are needed to form a stable stacked assembly. The hydrogen-bonding interactions of amide moieties are often employed to form stacked assemblies. The hydrogen bonds are formed between hydrogen atoms attached to nitrogen and carbonyl oxygen. The consecutive hydrogen bonds of amide moieties form a one-dimensional network of hydrogen bonds (Fig. 9.9). When the amide moiety is attached to the aromatic rings of a π-conjugated luminophore, the amide moiety takes a twisted conformation to the π-conjugated plane of the aromatic ring. Thus, one-dimensional consecutive hydrogen bonds are formed to stabilize the stacked assembly of the π-conjugated molecules.
Various helical stacked assemblies via hydrogen-bonding interactions have been reported, and some of them exhibited CPL activity. Ajayaghosh and coworkers reported the self-assembly behavior as well as optical and chiroptical properties of 25, an oligo-p-phenylene-ethynylene derivative possessing an amide moiety and chiral side-chains (Fig. 9.10) [37]. The molecule mostly existed as a monomer in tetrahydrofuran (THF) solution (1 × 10−5 mol L−1), whereas 25 formed stacked assemblies in methylcyclohexane (MCH) solution (1 × 10−5 mol L−1). 25 is equipped with an azobenzene moiety that exhibits photo-induced isomerization. In fact, photoisomerization from the (E,E)-isomer to (Z,E)- and (Z,Z)-isomers was observed with the irradiation of ultraviolet (UV) light. The formation of a helical assembly was confirmed by using CD spectroscopy. The assembly of the (E,E)-isomer of (S)-25 exhibited a positive CD signal at 464 nm with two negative CD signals at 407 nm and 322 nm, corresponding to the π → π∗ transition of the oligo-p-phenylene-ethynylene moiety. Interestingly, the CD signals inverted in the CD spectrum of the assembly of the (Z,Z)-isomer of (S)-25. This suggests that the helical sense of the assembly inverted during the photoisomerization of 25. The helical assembly of the (E,E)-isomer of (S)-25 exhibited CPL on its emission at 503 nm with a g lum of 8 × 10−3. The photoisomerization of (S)-25 inverted the CPL as in the case of the CD. The helical assembly of the (Z,Z)-isomer displayed a negative CPL signal with a g lum of −2 × 10−3. (R)-25 gives a mirror-image CD and CPL of (S)-25.
Recently, Takeda, Akutagawa, and coworkers reported a CPL-active assembly of a pyrene derivative 26 (Fig. 9.11) [38]. They synthesized a pyrene possessing four amide moieties, and a chiral side-chain was introduced onto the amides. The simple molecule formed a helical assembly in chloroform, THF, and MCH through the one-dimensional hydrogen-bonding interactions of the amide moieties, which was confirmed by UV-vis and CD spectroscopy techniques. The helical assembly exhibited CPL on its excimer emission band at 500 nm in chloroform, THF, and MCH (1.0 × 10−4 mol L−1). The g lum value of the assembly in MCH (3.0 × 10−2) was one order of magnitude higher than those in chloroform and THF (ca. 2 × 10−3). Furthermore, the CPL signal of the helical assembly in chloroform was inverted compared to those in THF and MCH. The assemblies in chloroform, THF, and MCH exhibited good fluorescence quantum yields (42%, 31%, and 29%, respectively).
A helical assembly can be formed via intermolecular interactions other than hydrogen-bonding interactions. Dipole–dipole interactions are fruitful to form a helical assembly since C 3 symmetrically arranged dipole moments tend to form a helical stacked assembly [39]. Haino and coworkers have reported the helical assembly behavior of C 3 symmetric 1,3,5-tris(4-alkoxyphenylisoxazolyl)benzene 27, which possesses three isoxazoles that provide the local dipole moment (Fig. 9.12) [40, 41]. The directional circular arrangement of the isoxazole rings is directed by the head-to-tail dipole–dipole interaction of the local dipole of isoxazoles. Molecular modeling studies for the hexameric assemblies of 1,3,5-tris(phenylisoxazolyl)benzene revealed that the hexameric assembly has two major geometries, helical and eclipsed. In the former geometry, the local dipoles of isoxazole align in a head-to-tail fashion, whereas they take an antiparallel conformation in the latter geometry. The optical and chiroptical properties of 27 in MCH were considered by using UV-vis and CD spectroscopy techniques. Monomeric 27 in the diluted MCH solution exhibited a monomeric absorption band at 278 nm, whereas the assembly of 27 in the concentrated MCH solution displayed the absorption maximum at 310 nm. The redshift of the absorption suggests the formation of the J-type aggregate. The monomeric 27 displayed no CD signals, indicating that the chiral side-chain of 27 does not perturb the π → π∗ transition. On the other hand, the assembly of (S)- and (R)-27 formed in the concentrated MCH solution exhibited CD spectra that have a mirror-image relationship. This suggests that not an antiparallel but helical assembly is formed, and the helicity was determined by the chirality of the side-chain. By using exciton coupling theory, the helical sense of the assembly of (S)-27 was determined to be right-handedness. From these results, the dipole–dipole interaction of the isoxazole rings drives the helical assembly of small molecules. Unfortunately, the CPL properties of 27 were not reported, but the authors have reported the CPL properties of some luminophores possessing phenylisoxazoles.
(a) Helical assembly of tris(phenylisoxazolyl)benzene derivatives 27. (b, c) Stereoplots of two local minimum geometries for the hexamers obtained from a conformation search by the MacroModel program: (a) helical arrangement of the local dipoles and (b) antiparallel arrangement of them. Adapted with permission from Sato et al. [35]. Copyright 2011 American Chemical Society
Haino and coworkers have reported the helical assembly of PBI possessing phenylisoxazoles 28 and their optical and chiroptical properties (Fig. 9.13) [42]. The formation of the supramolecular assembly of PBI perturbs its π → π∗ absorption and π∗ → π emission. The introduction of tris(phenylisoxazolyl)benzene onto a nitrogen atom of PBI resulted in the formation of a helical assembly, in which the π → π∗ and π∗ → π transitions are chirally perturbed to give chiroptical properties. The self-assembly behavior of 28 was considered by using 1H NMR, UV-vis, and CD spectroscopy techniques. 28 formed a J-type assembly that was confirmed by the redshift of the absorption band of the assembly. The assembly of 28 in decalin solution displayed strong CD with a g abs of 1.4 × 10−3. The right-handedness of the helix was assigned by the plus-to-minus patterns observed in the ascending energy in the CD spectrum of 27. In the emission spectrum of the assembly of 28 in decalin, a broad and redshifted band of the assembly was observed in addition to the sharp emission band of the monomeric species. CPL was observed on the emission of the assembly with a g lum of 7 × 10−3, but no CPL was observed on the monomeric emission. Furthermore, CPL was not observed on the emission of 28 in chloroform, in which most 28 does not form an assembly. (S)- and (R)-28 displayed mirror-image CPL spectra. These results clearly suggest that the formation of a helical assembly leads to the CPL activity of 28.
CPL-active helical assembly of PBI derivatives possessing tris(phenylisoxazolyl)benzene 28. Adapted with permission from Satrijo et al. [5]. Copyright 2012 The Royal Society of Chemistry
A square planar Pt(II) complex tends to form a stacked assembly creating a one-dimensional metal array through metallophilic (Pt–Pt) interactions [43]. The Pt(II) phenylbipyridine complex is known as a luminophore exhibiting phosphorescence that comes from a triplet metal-to-ligand charge transfer (3MLCT) transition. The phosphorescence property of the Pt(II) phenylbipyridine complex is perturbed by the formation of a stacked assembly via Pt–Pt interactions to exhibit a triplet metal-metal-to-ligand charge transfer (3MMLCT) transition [44]. The Pt(II) phenylbipyridine complex does not form a helical assembly without any assistance of other intermolecular interactions, but the complex formed a helical assembly when the phenylisoxazole moiety was introduced onto the ligand. Haino and coworkers have reported the optical and chiroptical properties of a Pt(II) phenylbipyridine complex possessing a 3,5-bis(phenylisoxazolyl)phenylethynyl ligand ((S)- and (R)-29, Fig. 9.14) [45]. 29 effectively formed a stacked assembly via Pt–Pt, dipole–dipole, and π–π stacking interactions. Interestingly, the self-assembly behavior of 29 drastically changed depending on the solvent effect. In chloroform, 29 formed a stacked assembly exhibiting 3MMLCT absorption and emission bands, but the assembly displayed no CD and CPL. It turns out that the assembly of 29 formed in chloroform is not helical, most likely due to the strong solvation that prevents the dipole–dipole interaction between the isoxazole rings. On the other hand, the assembly of 29 formed in toluene displayed completely different characteristics compared to that in chloroform. The assembly formed in toluene was CD active, and mirror-image CD signals were observed upon the assembly of (S)- and (R)-29. Thus, helical assemblies are formed, and the helical senses were directed by the chiral side-chains. The assembly in toluene exhibited aggregation-induced emission enhancement (AIEE); the emission is strongly enhanced in the assembled form compared to that of the monomeric species. The molecular motion, presumably the rotation of the triple bond, is prevented through the formation of the assembly to reduce the nonradiative decay of the excited state, which results in the increase in the emission. The monomeric 29 did not display any CD and CPL even in toluene, but the assembly formed in toluene exhibited CPL with a g lum of 1.0 × 10−2.
CPL-active helical assembly of 29, a Pt(II)phenylbipyridine complex possessing phenylisoxazoles. Adapted with permission from Schippers et al. [10]. Copyright 2015 The Royal Society of Chemistry
Spano, Meskers, and coworkers have developed the self-assembly and chiroptical properties of oligo-p-phenylene-vinylene derivative 30 (Fig. 9.15) [46]. 30 dimerized through the complementary quadruple hydrogen bond of the triazine group, and then, the dimer assembled to form a helical stacked assembly via π–π stacking. The helical assembly exhibited CPL on its fluorescence with a g lum on the order of 10−3. Wong, Tang, and coworkers have developed the CPL of tetraphenylsilole derivatives. Tetraphenylsiloles are known as good aggregation-induced emission (AIE) active luminophores; the luminophore is not emissive in its monomeric form, but the aggregate of the luminophore is highly emissive. For example, 31, a tetraphenylsilole derivative possessing chiral sugar pendants, formed a helical assembly displaying CPL in its suspension and solid state (Fig. 9.15) [47]. The g lum values of 31 depend on the state of the assembly. In a heterogeneous suspension, a neat static cast film, and 10 wt.% Poly(methyl methacrylate) (PMMA) matrix, the g lum values were ca. −0.12, −0.08, and −0.17, respectively. Interestingly, the fabricated pattern obtained by the evaporation of a dichloromethane/toluene solution of 31 in Teflon-based microfluidic channels exhibited CPL with a g lum value of −0.32, which is quite higher than those of other solid samples.
As described herein, supramolecular assemblies that have chirality, especially the helical assemblies, exhibited excellent CPL properties. Highly assembled structures of the luminophore bring about high g lum values [2].
9.5 Stimuli-Responsive CPL Using a Supramolecular Assembly
A notable feature of CPL-active organic compounds is the stimuli-responsiveness of CPL. Organic compounds that exhibit stimuli-responsivity have been widely developed since organic compounds have flexible conformations. For example, 32 and 33, photoresponsive CPL-active polymers possessing the photochromic dithienylethene moiety, have been developed by Akagi and coworkers (Fig. 9.16) [48]. A supramolecular assembly is constructed by reversible intermolecular interactions, and thus, one can control the assembly and disassembly of the supramolecular system by external stimuli. Therefore, stimuli-responsive supramolecular assemblies have been widely investigated so far [49]. Many of the CPL-active supramolecular assemblies mentioned above exhibited concentration- and temperature-dependent CPL; the monomeric form of the luminophore that exists in the low concentration or high temperature shows no CPL, whereas the helical assembly formed in the high concentration or low temperature exhibits CPL [38, 42, 45,46,47]. The CPL signal of compound 25 is photoresponsive, reflecting the photoisomerization of the azobenzene unit [37]. Here, other stimuli-responsive CPL of organic luminophores is described.
Haino and coworkers reported the gelation-induced CPL of Pt(II) phenylbipyridine complex 34 (Fig. 9.17) [50]. The complex has a similar structure to complex 29 mentioned above, but 34 possesses multiple long alkyl side-chains. 34 formed a stacked assembly as 29 did, but the assembly formed in MCH did not display any CD and CPL. The long alkyl side-chains provide the interassembly interaction to afford a three-dimensional network of the one-dimensional stacked assembly. The three-dimensional network holds the solvent molecules in its vacancies to form organogel. 34 formed luminescent organogel in 1-decanol at the concentration of 9.2 × 10−3 mol L−1 with decreasing temperature from 50 to 10 °C. At 50 °C, no CPL signal was observed, but the CPL signals appeared at 500 nm with the gelation. The g lum value at 10 °C was 1.1 × 10−2. It is noteworthy that the gelation of 34 also triggered the AIEE; the emission intensity increased ca. 50-fold from 50 to 10 °C.
Gelation-induced CPL of 34, a Pt(II)phenylbipyridine complex possessing phenylisoxazoles. Adapted with permission from Wilson et al. [6]. Copyright 2018 The Royal Society of Chemistry
Maeda and coworkers have developed pyrrole-based anion receptors, BF2 complexes of 1,3-dipyrrolyl-1,3-propanedione [51]. The two pyrrole NH are located at the side of the carbonyl oxygen without any anion, whereas the molecule forms an anion complex through the N-H···X– interaction of the flipped pyrroles and the C-H···X– interaction. The structural change of the luminophore before and after anion-binding can be applied for the anion-responsive CPL system (Fig. 9.18). Anion-responsive CPL was observed for chiral anion-receptor 35 upon complexation with anions such as Cl– and tetrabutylammonium (TBA) salts [52]. 35 possesses a chiral 1,1′-bi-2-naphthol (BINOL) moiety as the ligand of boron. 35 displayed CPL with a g lum of 1.0 × 10−2 in the presence of Cl–, whereas almost no CPL was observed without Cl–. meta-Phenylene-bridged dimeric anion-receptor 35 also displayed anion-responsive CPL [53]. 36 formed a helical complex with Cl–. Unlike the chiral anion-receptor 35, 36 is an achiral molecule, and thus, the helical complex of 36·Cl– is a racemic mixture that exhibits no CPL without any chiral source. However, once Cl– was added as a salt of chiral binaphthylammonium (RR +), the helicity of the complex was induced since the helical anion complex formed an ion pair with the chiral cation in the dichloromethane solution. The ion pair of 36·Cl–·RR + exhibited CPL with a g lum of 1.8 × 10−2 in dichloromethane/octane solution (1:1, 1 × 10−3 mol L−1) at –70 °C.
9.6 Summary
An achiral π-conjugated luminophore can exhibit CPL by the chiral arrangement of the achiral luminophore. The helical supramolecular assembly of an achiral luminophore is excellent for realizing CPL of the achiral luminophore since the highly assembled structure in the helical assembly provides good CPL activity. Furthermore, the supramolecular assembly has an advantage in its stimuli-responsiveness. The development of CPL-active supramolecular assemblies and their applications are in progress all over the world.
References
Bünzli J-CG, Piguet C (2005) Taking advantage of luminescent lanthanide ions. Chem Soc Rev 34:1048–1077
Kumar J et al (2015) Circularly polarized luminescence in chiral molecules and supramolecular assemblies. J Phys Chem Lett 6:3445–3452
Sánchez-Carnerero EM et al (2015) Circularly polarized luminescence from simple organic molecules. Chem Eur J 21:13488–13500
Emeis CA, Oosterhoff LJ (1967) Emission of circularly-polarised radiation by optically-active compounds. Chem Phys Lett 1:129–132
Satrijo A et al (2006) Probing a conjugated polymer’s transfer of organization-dependent properties from solutions to films. J Am Chem Soc 128:9030–9031
Wilson JN et al (2002) Chiroptical properties of poly(p-phenyleneethynylene) copolymers in thin films: large g-values. J Am Chem Soc 124:6830–6831
Zhao Y et al (2016) Supramolecular chirality in achiral polyfluorene: chiral gelation, memory of chirality, and chiral sensing property. Macromolecules 49:3214–3221
Dekkers HPJM, Closs LE (1976) The optical activity of low-symmetry ketones in absorption and emission. J Am Chem Soc 98:2210–2219
Schippers PH, Dekkers HPJM (1983) Circular polarization of luminescence as a probe for intramolecular 1nπ∗ energy transfer in meso-diketones. J Am Chem Soc 105:145–146
Schippers PH et al (1983) Circular polarization in the fluorescence of β γ-enones: distortion in the 1nπ∗ state. J Am Chem Soc 105:84–89
Steinberg N et al (1981) Measurement of the optical activity of triplet-singlet transitions. The circular polarization of phosphorescence of camphorquinone and benzophenone. J Am Chem Soc 103:1636–1640
Phillips KES et al (2001) Synthesis and properties of an aggregating heterocyclic helicene. J Am Chem Soc 123:11899–11907
Field JE et al (2003) Circularly polarized luminescence from bridged triarylamine helicenes. J Am Chem Soc 125:11808–11809
Nakamura K et al (2014) Enantioselective synthesis and enhanced circularly polarized luminescence of S-shaped double azahelicenes. J Am Chem Soc 136:5555–5558
Sawada Y et al (2012) Rhodium-catalyzed enantioselective synthesis, crystal structures, and photophysical properties of helically chiral 1,1′-bitriphenylenes. J Am Chem Soc 134:4080–4083
Goto K et al (2012) Intermolecular oxidative annulation of 2-aminoanthracenes to diazaacenes and aza[7]helicenes. Angew Chem Int Ed 51:10333–10336
Oyama H et al (2013) Facile synthetic route to highly luminescent sila[7]helicene. Org Lett 15:2104–2107
Kaseyama T et al (2011) Hierarchical assembly of a phthalhydrazide-functionalized helicene. Angew Chem Int Ed 50:3684–3687
Amako T et al (2013) Solid-state circularly polarised luminescence and circular dichroism of viscous binaphthyl compounds. RSC Adv 3:23508–23513
Amako T et al (2013) A comparison of circularly polarized luminescence (CPL) and circular dichroism (CD) characteristics of four axially chiral binaphthyl-2,2′-diyl hydrogen phosphate derivatives. Tetrahedron 69:2753–2757
Amako T et al (2013) Dependence of circularly polarized luminescence due to the neighboring effects of binaphthyl units with the same axial chirality. RSC Adv 3:6939–6944
Kimoto T et al (2012) Control of circularly polarized luminescence by using open- and closed-type binaphthyl derivatives with the same axial chirality. Chemistry 7:2836–2841
Kinuta T et al (2011) Solid-state chiral optical properties of axially chiral binaphthyl acid derivatives. J Photochem Photobiol A Chem 220:134–138
Kinuta T et al (2012) Control of circularly polarized photoluminescent property via dihedral angle of binaphthyl derivatives. Tetrahedron 68:4791–4796
Kitayama Y et al (2014) Enhancing circularly polarised luminescence by extending the π-conjugation of axially chiral compounds. Org Biomol Chem 12:4342–4346
Kitayama Y et al (2015) Circularly polarized luminescence of biaryl atropisomers: subtle but significant structural dependency. RSC Adv 5:410–415
Nakabayashi K et al (2014) Nonclassical dual control of circularly polarized luminescence modes of binaphthyl–pyrene organic fluorophores in fluidic and glassy media. Chem Commun 50:13228–13230
Tsumatori H et al (2010) Observation of chiral aggregate growth of perylene derivative in opaque solution by circularly polarized luminescence. Org Lett 12:2362–2365
Feuillastre S et al (2016) Design and synthesis of new circularly polarized thermally activated delayed fluorescence emitters. J Am Chem Soc 138:3990–3993
Morisaki Y et al (2014) Planar chiral tetrasubstituted [2.2]paracyclophane: optical resolution and functionalization. J Am Chem Soc 136:3350–3353
Gon M et al (2017) Enhancement and controlling the signal of circularly polarized luminescence based on a planar chiral tetrasubstituted [2.2]paracyclophane framework in aggregation system. Macromolecules 50:1790–1802
De Greef TFA et al (2009) Supramolecular polymerization. Chem Rev 109:5687–5754
Haino T (2015) Supramolecular polymerization engineered with molecular recognition. Chem Rec 15:837–853
Hoeben FJM et al (2005) About supramolecular assemblies of pi-conjugated systems. Chem Rev 105:1491–1546
Sato S et al (2017) Chiral intertwined spirals and magnetic transition dipole moments dictated by cylinder helicity. Proc Natl Acad Sci U S A 114:13097–13101
Yashima E et al (2016) Supramolecular helical systems: helical assemblies of small molecules, foldamers, and polymers with chiral amplification and their functions. Chem Rev 116:13752–13990
Gopal A et al (2012) Thermally assisted photonic inversion of supramolecular handedness. Angew Chem Int Ed 51:10505–10509
Anetai H et al (2018) Circular polarized luminescence of hydrogen-bonded molecular assemblies of chiral pyrene derivatives. J Phys Chem C 122:6323–6331
Ikeda T, Haino T (2017) Supramolecular polymeric assemblies of pi-conjugated molecules possessing phenylisoxazoles. Polymer 128:243–256
Haino T, Saito H (2009) A new organogelator based on 1,3,5-tris(phenylisoxazolyl)benzene. Synth Met 159:821–826
Tanaka M et al (2011) Self-assembly and gelation behavior of tris(phenylisoxazolyl)benzenes. J Org Chem 76:5082–5091
Ikeda T et al (2012) Circular dichroism and circularly polarized luminescence triggered by self-assembly of tris(phenylisoxazolyl) benzenes possessing a perylenebisimide moiety. Chem Commun 48:6025–6027
Eryazici I et al (2008) Square-planar Pd(II), Pt(II), and Au(III) terpyridine complexes: their syntheses, physical properties, supramolecular constructs, and biomedical activities. Chem Rev 108:1834–1895
Wong KM-C, Yam VW-W (2011) Self-assembly of luminescent alkynylplatinum(II) terpyridyl complexes: modulation of photophysical properties through aggregation behavior. Acc Chem Res 44:424–434
Ikeda T et al (2015) Novel helical assembly of a Pt(II) phenylbipyridine complex directed by metal-metal interaction and aggregation-induced circularly polarized emission. Dalton Trans 44:13156–13162
Spano FC et al (2007) Probing excitation delocalization in supramolecular chiral stacks by means of circularly polarized light: experiment and modeling. J Am Chem Soc 129:7044–7054
Liu J et al (2012) What makes efficient circularly polarised luminescence in the condensed phase: aggregation-induced circular dichroism and light emission. Chem Sci 3:2737–2747
Hayasaka H et al (2010) Helically π-stacked conjugated polymers bearing photoresponsive and chiral moieties in side chains: reversible photoisomerization-enforced switching between emission and quenching of circularly polarized fluorescence. Adv Funct Mater 20:1243–1250
Maeda H, Bando Y (2013) Recent progress in research on stimuli-responsive circularly polarized luminescence based on pi-conjugated molecules. Pure Appl Chem 85:1967–1978
Ikeda T et al (2018) A circularly polarized luminescent organogel based on a Pt(II) complex possessing phenylisoxazoles. Mater Chem Front 2:468–474
Haketa Y, Maeda H (2017) Dimension-controlled ion-pairing assemblies based on π-electronic charged species. Chem Commun 53:2894–2909
Maeda H et al (2011) Chemical-stimuli-controllable circularly polarized luminescence from anion-responsive pi-conjugated molecules. J Am Chem Soc 133:9266–9269
Haketa Y et al (2012) Asymmetric induction in the preparation of helical receptor-anion complexes: ion-pair formation with chiral cations. Angew Chem Int Ed 51:7967–7971
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Ikeda, T., Haino, T. (2020). Circularly Polarized Luminescence of Chirally Arranged Achiral Organic Luminophores by Covalent and Supramolecular Methods. In: Mori, T. (eds) Circularly Polarized Luminescence of Isolated Small Organic Molecules. Springer, Singapore. https://doi.org/10.1007/978-981-15-2309-0_9
Download citation
DOI: https://doi.org/10.1007/978-981-15-2309-0_9
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-15-2308-3
Online ISBN: 978-981-15-2309-0
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)