Abstract
This chapter focuses on the molecular designs of Small Organic Molecules (SOM) merging Circularly Polarized Luminescence (CPL) and Thermally Activated Delayed Fluorescence (TADF) properties. In Introduction, the benefits associated with the combination of these properties into SOM for their application as emitting materials to construct Circularly Polarized Organic Light Emitting Diodes (CPOLED) are presented. Next, the different molecular designs leading to CPTADF SOM are described depending on the nature of the chirality of these molecules (point, axial, or planar chirality). The synthesis, photophysical, and chiroptical properties of these molecules and the performance of related CPOLED devices are discussed.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Circularly Polarized Luminescence (CPL)
- Thermally Activated Delayed Fluorescence (TADF)
- Small Organic Molecules (SOM)
- Chirality
- Organic Light Emitting Diodes (OLED)
13.1 Introduction
Among all the potential applications of Circularly Polarized Luminescent active Simple Organic Molecules (CPL-SOMs), their use as emitters in Organic Light Emitting Diodes (OLED) is one of the most exciting applications. Indeed, the development of CP-OLED has emerged as an interesting direction to substantially increasing the energy efficiency of conventional OLED displays in which 50% of the light emitted is suppressed using a polarizer and a quarter-wave plate to reduce the external light reflection [1, 2]. This high energy loss can be overcome by using CP-OLED which generates circularly polarized electroluminescence that can pass through these filters without any attenuation, resulting in higher display performances in terms of autonomy and contrast. Although this type of device presents undeniable advantages over conventional OLED, described examples are still rare in the literature [3]. Among those research works, chiral lanthanides [4, 5] and cholesteric-based [6,7,8] CP-OLED devices have shown high degree of circular polarized electroluminescence (g El up to 1 and 1.6 for lanthanide and cholesteric CPL emitters, respectively), but these devices were strongly limited in efficiency (EQE~0.05%, notably because of the low luminescence quantum yield of these types of chiral emitter). Impressive results have also been reported by Fuchter et al. [9], using phosphorescent organometallic helicenic complex as emitters which has allowed to generate a degree of CP-electroluminescence (g El = 0.4) sufficient to give a 19% increased brightness compared to unpolarized OLED of similar performances. Nevertheless, the corresponding luminescence and power efficiencies were still low (EQE < 1%), highlighting the need of molecular engineering to design more efficient chiral emitters. In the field related to the development of OLED technology, the design of Thermally Activated Delayed Fluorescence (TADF) materials is an exploding area of research (For recent reviews see: [10,11,12]). Indeed, in such materials both singlet and triplet excitons can be harvested for light emission by a reverse intersystem crossing process thanks to a small energy gap between their singlet and triplet states (ΔE ST). This property has recently motivated numerous research works because of the possibility to develop, in theory, OLEDs with maximum efficiency. Therefore, the design of new molecular architectures presenting both TADF and CPL emission properties appears as a cornerstone for enhancement of the performances of OLED devices. In order to merge these two properties, several molecular designs have been imagined during last 4 years. This chapter covers the design principles allowing to combine TADF and CPL emissions in simple organic molecules, the synthesis and photophysical performances of such compounds and their ability to generate CP electroluminescence once incorporated in OLED devices.
13.2 Principles of TADF and Design Rules
Fluorescence and electroluminescence can be produced in organic molecules upon and photo- and electrical excitation, respectively. Generally, fluorescent small organic molecules exhibit prompt fluorescence (PF), and in this case, emission takes place following photon absorption (S 0 → S n) and excited-state relaxation to the lowest excited singlet state (S 1). On the other hand, delayed fluorescence can be generated via another mechanism involving the participation of the triplet-excited state. Once S 1 is attained, if the energy difference between the two lowest excited states S 1 and T 1 (ΔE ST) is sufficiently small (ΔE ST < 100 meV), the molecule undergoes intersystem crossing (ISC) to the lowest triplet-excited state. Then due to thermal activation, the molecule may go back to S1 via a process named RISC for Reverse InterSystem Crossing and generates delayed fluorescence owning the same emission spectrum than PF but with a longer lifetime, generally in the micro- to millisecond timeframe (see Fig. 13.1). In the emissive layer of an OLED, electron-hole recombination in the organic emitters creates 25% singlet excitons and 75% triplet excitons. This means that in the case of an OLED using a classical fluorophore as emitter the maximal internal efficiency is limited to 25%. Because in TADF molecules both singlet and triplet excitons can be harvested for light emission, they appear as a solution of choice to build more efficient OLED devices. But because of the atypical conditions that are required to generate such a delayed fluorescence: small ΔE ST, high ISC, and RISC probabilities along with rather long T 1 lifetime, efficient TADF emitters are difficult to design.
In order to minimize the ΔE ST in small organic molecules, one of the most reliable approaches described so far involves the design of Donor–Acceptor (D-A) molecules in which a steric hindrance allows to twist D and A moieties around the D-A axis. The origin of this design rule is arising from the fact that such geometry in D-A molecules reduces the HOMO and LUMO overlap, which is proportional to the ΔE ST. We will see in the following lines that most of the chiral TADF molecules developed so far adhere to this design paradigm. However, this has the potential to reduce the oscillator strength of the transition and therefore to be detrimental to the fluorescence quantum yield (Φ PL). Please see references [10,11,12] for further information about the design of TADF molecules.
13.3 CPTADF Emitters: Design, Synthesis, Photophysical, and Chiroptical Properties
13.3.1 TADF Molecules Possessing Point Chirality
Shuzo Hirata and co-workers were the first to report the design and synthesis of a molecule merging both TADF and CPL properties (named CPTADF molecules for Circularly Polarized Thermally Activated Delayed Fluorescence molecules in the rest of the document) [13]. This first approach toward such molecules was based on the construction of a chiral carbon center bearing an electron donor, a triarylamine unit, and an acceptor unit, naphthacen-5(12H)-one (see Fig. 13.2).
The target molecule, 12-(2-(diphenylamino)phenyl)-12-hydroxynaphthacen-5(12H)-one (DPHN), was obtained by the addition of the 2-litiumtriphenylamine, prepared by a classical halogen-lithium exchange on the 2-bromotriphenylamine A, to the 5,12-naphthacenequinone B using THF as solvent giving the target compound in 47% yield (see Scheme 13.1). Thereafter, the two enantiomers of the racemic mixture were separated using preparative chiral high-pressure liquid chromatography (HPLC).
Both enantiomers of compound 1 display green emission in toluene solution with a maximum wavelength centered on 513 nm. DFT and TD-DFT calculations at the B3LYP/6-31G(d,p) level have revealed some interesting insight concerning the photophysical properties of this molecule which can be resumed as follows: at the fundamental state (S 0), the transition that generates the first absorption band showed a weak charge transfer (CT) character with rather high spatial separation of the HOMO and LUMO. At the lowest singlet state (S 1), the character of the HOMO-LUMO transition was pure CT with a better spatial separation of the HOMO (located predominantly on triarylamine substructure) and LUMO (mainly located on the acceptor unit) compared to the S 0 state. The calculated energy difference between the lowest singlet state (S 1) and the lowest triplet state (T 1), ΔE ST, was found to be very small with a value of 0.07 eV indicating the potential of such molecular design to generate TADF. Unfortunately, this molecule possesses a relatively small quantum yield (Φ PF = 4%) in toluene solution and the TADF emission in solution state was not demonstrated. The photophysical properties of compound 1 were then studied in film state by spin casting a chloroform solution containing 9% wt of compound 1 in N,N′-4,4′-dicarbazole-3,5-benzene (mCP) on quartz substrate. Interestingly at the solid state, higher quantum yields were obtained for prompt fluorescence (Φ PF = 11%) and more importantly, TADF emission was observed (Φ DF = 15%) with a lifetime in the order of milliseconds. This moderate photophysical performances can be explained by quite large ΔE ST values (experimentally determined to be 0.19 eV) and the small fluorescent rate constant (k F) resulting in a deactivation of excitons from T 1. Concerning chiroptical properties, such molecules possess interesting performances with a dissymmetry factor, |g lum| of 1.1 × 10−3 measured in toluene solution, which is in the range of the value found for purely organic chromophores (10−5 to 10−2). However, the possibility to use such molecules as dopants in CP-OLED was not demonstrated.
13.3.2 TADF Molecules Possessing Axial Chirality
The second example of CPTADF-SOM has been reported by Pieters et al. few months after the pioneer work from Hirata [14]. Conceptually, this other molecular design involves the tethering of a chiral unit (derived from BINOL) to an active TADF chromophore (see Fig. 13.3). Here, the proximity of the chiral unit is anticipated to induce chiroptical properties (induced circular dichroism and circularly polarized luminescence) to the TADF emitter, like in the chiral O-BODIPY dyes described by De la Moya et al. in 2015 [15]. The TADF unit used in this design is a donor–acceptor system composed of carbazoles as electron donor and a terephthalonitrile unit as acceptor. Such type of D-A systems has been previously used to construct very efficient TADF molecules by Adachi and coworkers [16].
The target enantiomers, (R)-2 and (S)-2, were synthesized through a one-pot sequential procedure at room temperature involving commercially available compounds (Scheme 13.2). The first sequence consists in a highly selective desymmetrization of the starting tetrafluoroterephthalonitrile C, involving enantiopure BINOL D and K2CO3 as a base, which led to the formation of a chiral difluorinated intermediate. Then, carbazole E (2.1 equiv) and additional amount of base were added to the reaction mixture, leading to the targeted molecule 2 with high yield (83%) and enantiomeric excess (>99%, based on chiral HPLC) after purification over silica gel (see Scheme 13.2).
DFT calculations performed at the B3LYP/6-31G level have revealed some interesting information about such compounds. First, the spatial separation of HOMO and LUMO is significant with a HOMO mainly located on carbazolyl units and a LUMO centered onto the terephthalonitrile substructure. Second, at the ground state at least, no orbital coefficient has been found of the BINOL moiety. This means that the BINOL unit plays, at least at the ground state, a role of chiral perturbator for the potentially TADF active chromophore. Such compounds own quite interesting photophysical properties with a strong positive solvatochromism with Φ PF values between 0.74 and 0.06 depending on the solvent polarizability. By contrast with the first example of CPTADF molecules described earlier, TADF properties were clearly observed in toluene solution with an important increase in the quantum yield in degassed toluene compared to the one in aerated solution (from 0.28 to 0.53). A bi-exponential fluorescence decay was observed with lifetime of 20 ns for prompt fluorescence and of 2.9 μs for the delayed component. The chiral perturbation from the BINOL unit to the TADF active emitter was found to be effective, both at ground and exited state, with CD signal located at the wavelengths corresponding to the Internal Charge Transfer band (ICT band) of the TADF emitter (centered at 420 nm) and a |g lum| value of 1.3 × 10−3 recorded in degassed toluene solution (C = 1 mM). These compounds were successfully applied as emissive dopant in OLED devices built via Chemical Vapor Deposition (CVD). Using a nonoptimized OLED architecture, a maximal external quantum efficiency of 9.1% has been measured. Although the authors have confirmed the enantiopurity of the compounds after the CVD process, no data were communicated about the potential polarization light emanating from the device.
The potential of this molecular design to generate efficient emitters for construction of CP-OLED was later confirmed by Tang and coworkers [17]. Indeed, following the same design rules, they have synthesized four novel chiral TADF emitters, involving carbazole, 3,6-ditertbutylcarbazole and 9,10-Dihydro-9,9-dimethylacridine as electron donors units (see Fig. 13.4). Those compounds were synthesized using the same synthetic procedure described for compound 2, except for compound 6, another couple base-solvent was used (tBuOK as base and ACN as solvent). In terms of photophysical properties, molecules 3–6 exhibit broad and relatively weak absorption bands centered at 400, 420, 440, and 450 nm, respectively, which can be associated with intramolecular charge transfer (ICT) process derived from the Donor-Acceptor (D-A) active chromophore units. Emission spectra were measured in toluene solution and maximum emissions were observed between 495 nm and 595 nm depending on the nature of electron donor moiety (see Table 13.1). Low to good quantum yields from 0.05 for compound 6 to 0.4 for compound 3 were determined in neat thin film fabricated by CVD. Fluorescence decays were also investigated for each molecule in thin film. All compounds were found to be TADF emitters and show bi-exponential fluorescent decays with nanosecond-order prompt fluorescence (lifetimes from 11.3 to 162 ns) and microsecond-order delayed fluorescence (lifetimes from 0.04 to 24 μs). Chiroptical properties were also investigated in toluene solution. Compounds 3–6 possess g lum values in the same order of magnitude than compound 2 (from 0.5 × 10−3 to 1.2 × 10−3).
Importantly, Ben Zhong Tang and coworkers have demonstrated the Aggregation Induced Enhancement Emission (AIEE) properties of such chiral compounds by studying their fluorescence emissions in THF-H2O mixtures with different water fractions (f w). Here, upon the formation of aggregates in water, the intramolecular rotational and vibrational motions occurring in solution state are limited and nonradiative decay processes from the excited states are decreased, leading to an important increase in the fluorescence upon aggregation.
The authors have also evidenced a strong amplification of the chiroptical properties of this kind of emitters in thin films. Indeed, analyzing CD responses of neat and doped films fabricated by CVD, a slight enhancement of Kuhn’s dimensionless anisotropy factors (g abs multiplied by 2 up to 5 depending on the compound) was observed for all emitters 3–6. Surprisingly, the enhancement of the g lum values was found to be much higher, with an impressive 40-fold increase in the case of compound 3, exhibiting a glum value of 1.3 × 10−3 in toluene solution compared to a value of 4.1 × 10−2 in neat film state (see Fig. 13.5). The authors have explained this important amplification of the dissymmetry factors by the formation of chiral aggregates during the formation of the thin film. Interestingly, this amplification also occurs when the molecules 3–6 are used as emissive dopants in a mCP matrix. In order to understand the origin of this amplification, more detailed characterization of the postulated chiral aggregates would be necessary.
(a) g abs values in toluene solutions and in thin films measured at 405 nm for 3, 436 nm for 4, 440 nm for 5, and 450 nm for 6; (b) g lum values in toluene solutions and in thin films. Measured at 493 nm for 3, 530 nm for 4, 531 nm for 5, and 585 nm for 6 in solution (wavelengths used to measure gabs and glum in thin film were not indicated by the authors)
Nevertheless, based on those impressive chiroptical properties in thin film state, CP-OLED were fabricated through CVD using neat and doped film involving molecules 3–6 as emissive layers. This has led to the fabrication of the most performant CP-OLEDs reported so far using CPL-SOMs in terms of circular polarization with gel up to 0.08 and External Quantum Efficiency (EQE) up to 3.5% for OLED using neat films as emissive layers.
The third example of molecular design allowing the synthesis of molecules merging CPL and TADF properties has been described by the group of Man-Keung Fung and Chuan-Feng Chen [18]. Their original approach relies on the linkage of two active TADF units, composed of a D-A-D system involving carbazoles as donors and an aromatic-imide as acceptors, by chiral unit derived from the commercially available trans-1,2-diaminocyclohexane (see Fig. 13.6).
Both enantiomers of this molecule were prepared in two steps starting from commercially available (−)-(R,R)- or (+)-(S,S)-diaminocyclohexane F (see Scheme 13.3). This straightforward synthesis involved the lactamization of 4,5-difluorophthalic anhydride G with the chiral diamine F in AcOH as a first step, followed by a nucleophilic aromatic substitution reaction on the fluoroaromatic imide intermediate H with carbazole anions formed in THF using NaH as base. Overall, the target molecule was obtained with a high enantiomeric excess (ee > 99% measured using chiral HPLC) in almost 40% yield over the two synthetic steps.
In terms of photophysical properties, the absorption spectrum in THF of (+)-(S,S)-7 exhibited an intense intramolecular charge-transfer absorption band at about 380 nm in THF. As expected for such D-A-D chromophores, this molecule exhibited a strong positive solvatochromism with maximum emission wavelengths ranging from 508 nm in hexane to 562 nm in acetonitrile. Interestingly, although the quantum yields were rather low in solution (0.18 in toluene), they were found to be much higher in thin film state, with values up to 0.78 in doped film using 3,3′-di(9H-carbazol-9-yl)biphenyl (mCBP) as the host matrix. By studying temperature-dependent transient photoluminescence characteristics of the co-doped films, the authors confirmed that these chiral compounds were TADF active and measured a very small ΔE ST value of 0.06 eV. The transient photoluminescence decays of molecule 7 in the co-doped film under vacuum show an expected biexponential distribution. The fitting gave a short lifetime of 44 ns and a long one of 130 μs which confirms that 7 emits both prompt and delayed fluorescence. The efficient induction of chiroptical properties from the chiral 1.2-diaminocyclohexane unit to the TADF active chromophores was next confirmed by the study of the chiroptical properties in thin film state. Indeed, circular dichroism spectra show a clear CD signal for the band centered on 400 nm corresponding to the ICT transition of the TADF active chromophores. At the excited state, the induction of chiroptical properties was also demonstrated with the measurement of luminescence dissymmetry factors of +/−1.1 × 10−3. Using 7 as dopant in the emitting layers (15 wt % (+)-(S,S)-7 or (−)-(R,R)-7 in mCBP matrix), CP-OLED possessing a high maximum EQE of 19.7% and electroluminescent dissymmetry factors (g El) of 2.2 × 10−3 (close to the photoluminescence dissymmetry factor measured in the thin film state, 1.1 × 10−3) was fabricated. Based on the same molecular design, Chen’s research group has also synthesized the red emitting CPTADF molecule 8 (see Fig. 13.7) [19].
In molecule 8, the red emitting TADF active units are composed of a 1,8-naphthalimide moiety as electron acceptor (in blue Fig. 13.7) and a 9,9-dimethyl-9,10-dihydroacridine (DMAC, in red) as electron donor. The tethering of these active chromophores by a chiral 1,2 diaminocyclohexane unit also allows to induce CD and CPL properties to the TADF emitters. As a matter of fact, enantiomers of those molecules exhibited a mirror-image CD and a CPL signals with |g lum| value of 9.2 × 10−4 in thin film state. These molecules were also successfully used as dopant in the emissive layer of an OLED owning an orange-red emission (centered at 592 nm), and exhibiting a maximum EQE value of 12.4%. Although the authors have demonstrated the circular polarization of the emitters’ photoluminescence in thin film state, no information was given concerning the potential circular polarization of the light emanating from the constructed OLED devices.
13.3.3 TADF Molecules Possessing Planar Chirality
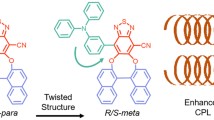
At the end of 2018, a fourth molecular design allowing to merge TADF and CPL properties in a small molecule has been reported by Cui-Hua Zhao et al. [20] In the first three examples of CPTADF molecules described in this chapter, the HOMO-LUMO spatial separation requisite for TADF activity was attained using D-A chromophores possessing a highly twisted relative conformation. Here, in order to obtain a molecule owning a small exchange integral between the two frontier orbitals, they have embedded a Donor and an Acceptor units in a chiral[2.2]-paracyclophane skeleton (see Fig. 13.8).
In these [2.2]paracyclophane derivatives, g-9 and m-10, electron-donating dimethylamine group and the electron-accepting dimesytylboryl moiety are introduced at the pseudo-gem and pseudo-meta positions. In order to synthetize the emitter g-9 (see Scheme 13.4), 4-bromo-13-amino[2.2]paracyclophane g-I was methylated using dimethyl sulfate as methylation agent in acetone using potassium carbonate as base. Then lithiation of the bromide g-J followed by addition of dimesitylboron fluoride was achieved to give g-9 a 59% yield.
The synthesis of the m-10 isomer was initiated by the amination of the 4,15-dibromo-[2.2]paracyclophane m-K via copper-assisted coupling reaction using hydroxyproline as ligand and aqueous ammoniac. Then, the same reaction sequence used for the synthesis of g-9 gave racemate m-10 with an overall yield of 16% over the three synthetic steps (see Scheme 13.5).
In terms of photophysical properties, compound g-9 displays a broad and moderately intense absorption band centered at 374 nm, which was assigned using theoretical calculations to the intramolecular charge transfer transition from the HOMO, located on dimethylaminophenyl moiety, to the LUMO, localized on dimesitylborylphenyl unit. For m-10, the assignation of this ICT band was found to be more difficult because of the very small oscillator strength (0.0209) of the first excited state (corresponding to the ICT band). In terms of emissive properties, both isomers emit green fluorescence with a maximum emission wavelength of 521 nm for g-9 and of 531 nm for m-10. Interestingly, the values of the quantum yields were found to be highly dependent on the presence of oxygen in the toluene solution. Indeed, g-9 and m-10 possess quantum yields of 0.46 and 0.34 in degassed toluene solution, respectively, whereas these values drop to 0.068 and 0.049 in presence of oxygen. This oxygen dependence on fluorescence efficiencies can be explained by the oxygen triplet state quenching and preludes therefore the possibility of exciton exchanges via efficient intersystem crossing. TADF properties were confirmed by the measurement of fluorescent decays in toluene solution. Biexponential decays were observed for both molecules with prompt fluorescence lifetimes of 17 ns for g-9 and 22 ns for m-10 and delayed fluorescence lifetimes of 0.38 μs for g-9 and 0.22 μs for m-10. Those TADF active molecules show also good quantum yields at the solid state with a value of 0.53 for m-10 and 0.33 for g-9. Because of its better photophysical performances, g-9 was selected to study the chiroptical properties of such chiral TADF paracyclophanes. The enantiomers of g-9 were synthesized starting from enantiopure pseudo-gem-substituted bromo amines g-H (see Scheme 13.4), which were obtained through optical resolution of its racemic form with (R)-(−)-10-camphorsulfonyl chloride. Both enantiomers display a small CD signal for the ICT band centered at 374 nm and a larger signal around 313 nm. The dissymmetry factor (g abs) of absorbance at the longest wavelength (374 nm) was determined to be 1.44 × 10−3. These compounds were also proved to be CPL active and possess a g lum of 4.24 × 10−3, the highest value measured in solution so far for CPTADF molecules. Despite their promising photophysical and chiroptical properties, the authors did not report so far about the use of such chiral paracyclophanes as emitters to construct efficient CP-OLED.
13.4 Conclusion and Perspectives
After the first report about CPTADF molecules in mid-2015, only three other molecular designs were described in the literature so far (see Fig. 13.9).
This small number of effective compounds can be explained by the inherent difficulty related to the merging of CPL and TADF properties in a small organic molecule. Indeed, such molecules should possess a low ΔE ST and a large magnetic dipole transition moment while maintaining a strong oscillator strength of the transition involved in the fluorescence emission. More systematic studies are needed in order to establish general guidelines for the design of efficient CPTADF emitters, as well as other approaches to combine CPL and TADF properties should be sought. So far, only two of these four efficient molecular designs have furnished molecules successfully used as emitters to construct CP-OLEDs. This highlights another important requirement to integrate in the future design of such molecules that is related their configurational stability. Indeed, in order to be incorporated in OLED stacks fabricated through CVD (the fabrication process affording the highest level of performance so far), these chiral molecules should also possess very high racemization barriers. Because of the fundamental challenge that represents their molecular design and their high potential in terms of application in the context of display devices, there is no doubt that numerous future research works will be dedicated to the discovery of novel CPTADF molecules.
References
Schadt M (1997) Liquid crystal materials and liquid crystal displays. Annu Rev Mater Sci 27:305–379
Singh R, Unni KNN, Solanki A (2012) Improving the contrast ratio of OLED displays: an analysis of various techniques. Opt Mater 34(4):716–723
Han J, Guo S, Lu H, Liu S, Zhao Q, Huang W (2018) Recent progress on circularly polarized luminescent materials for organic optoelectronic devices. Adv Opt Mater 6(17):1800538
Zinna F, Giovanella U, Di Bari L (2015) Highly circularly polarized electroluminescence from a chiral europium complex. Adv Mater 27(10):1791
Zinna F, Pasini M, Galeotti F, Botta C, Di Bari L, Giovanella U (2017) Design of lanthanide‐based oleds with remarkable circularly polarized electroluminescence. Adv Funct Mater 27(1):1603719
Peeters E, Christiaans MPT, Janssen RAJ, Schoo HFM, Dekkers HPJM, Meijer EW (1997) Circularly polarized electroluminescence from a polymer light-emitting diode. J Am Chem Soc 119(41):9909–9910
Geng Y, Trajkovska A, Culligan SW, Ou JJ, Chen HMP, Katsis D, Chen SH (2003) origin of strong chiroptical activities in films of nonafluorenes with a varying extent of pendant chirality. J Am Chem Soc 125(46):14032–14038
Lee DM, Song JW, Lee YJ, Yu CJ, Kim JH (2017) Control of circularly polarized electroluminescence in induced twist structure of conjugate polymer. Adv Mater 29(29):1700907
Brandt JR, Wang X, Yang Y, Campbell AJ, Fuchter MJ (2016) Circularly polarized phosphorescent electroluminescence with a high dissymmetry factor from PHOLEDs based on a Platinahelicene. J Am Chem Soc 138(31):9743–9746
Yang Z, Mao Z, Xie Z, Zhang Y, Liu S, Zhao J, Xu J, Chi Z, Aldred MP (2017) Recent advances in organic thermally activated delayed fluorescence materials. Chem Soc Rev 46:915–1016
Wong MY, Zysman-Colman EC (2017) Purely organic thermally activated delayed fluorescence materials for organic light-emitting diodes. Adv Mater 29(22):1605444
Czerwieniec R, Leitl MJ, Homeier HHH, Yersin H (2016) Cu(I) complexes – Thermally activated delayed fluorescence. Photophysical approach and material design. Coord Chem Rev 325:2–28
Imagawa T, Hirata S, Totani K, Watanabe T, Vacha M (2015) Thermally activated delayed fluorescence with circularly polarized luminescence characteristics. Chem Commun 51:13268–13271
Feuillastre S, Pauton M, Gao L, Desmarchelier A, Riives AJ, Prim D, Tondelier D, Geffroy B, Muller G, Clavier G, Pieters G (2016) Design and synthesis of new circularly polarized thermally activated delayed fluorescence emitters. J Am Chem Soc 138(12):3990–3993
Sanchez-Carnerero EM, Moreno F, Maroto BL, Agarrabeitia AR, Ortiz MJ, Vo B, Muller G, de La Moya S (2014) Circularly polarized luminescence by visible-light absorption in a chiral O-BODIPY dye: unprecedented design of CPL organic molecules from achiral chromophores. J Am Chem Soc 136(9):3346–3349
Uoyama H, Goushi K, Shizu K, Nomura H, Adachi C (2012) Highly efficient organic light-emitting diodes from delayed fluorescence. Nature 492:234–238
Song F, Xu Z, Zhang Q, Zhao Z, Zhang H, Zhao W, Qiu Z, Qi C, Zhang H, Sung HHY, Williams ID, Lam JWY, Zhao Z, Qin A, Ma D, Tang BZ (2018) Highly efficient circularly polarized electroluminescence from aggregation-induced emission luminogens with amplified chirality and delayed fluorescence. Adv Funct Mater 28(17):1800051
Li M, Li SH, Zhang D, Cai M, Duan L, Fung MK, Chen CF (2018) Stable enantiomers displaying thermally activated delayed fluorescence: efficient OLEDs with circularly polarized electroluminescence. Angew Chem Int Ed 57(11):2889
Wang Y-F, Lua H-Y, Chen C, Li M, Chen C-F (2019) 1,8-Naphthalimide-based circularly polarized TADF enantiomers as the emitters for efficient orange-red OLEDs. Org Electron 70:71–77
Zhang M-Y, Li Z-Y, Lu B, Wang Y, Ma Y-D, Zhao C-H (2018) Solid-state emissive triarylborane-based [2.2]paracyclophanes displaying circularly polarized luminescence and thermally activated delayed fluorescence. Org Lett 20(21):6868–6871
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Pieters, G., Frederic, L. (2020). Design of Circularly Polarized Thermally Activated Delayed Fluorescence Emitters. In: Mori, T. (eds) Circularly Polarized Luminescence of Isolated Small Organic Molecules. Springer, Singapore. https://doi.org/10.1007/978-981-15-2309-0_13
Download citation
DOI: https://doi.org/10.1007/978-981-15-2309-0_13
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-15-2308-3
Online ISBN: 978-981-15-2309-0
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)